Abstract
Two dendritic cell (DC) subsets have been identified in the murine system on the basis of their differential CD8α expression. CD8α+ DCs and CD8α− DCs are considered as lymphoid- and myeloid-derived, respectively, because CD8α+ but not CD8α− splenic DCs were generated from lymphoid CD4low precursors, devoid of myeloid reconstitution potential. Although CD8α− DCs were first described as negative for CD4, our results demonstrate that approximately 70% of them are CD4+. Besides CD4− CD8α− and CD4+CD8α− DCs displayed a similar phenotype and T-cell stimulatory potential in mixed lymphocyte reaction (MLR), although among CD8α− DCs, the CD4+ subset appears to have a higher endocytic capacity. Finally, experiments of DC reconstitution after irradiation in which, in contrast to previous studies, donor-type DCs were analyzed without depleting CD4+ cells, revealed that both CD8α+ DCs and CD8α− DCs were generated after transfer of CD4low precursors. These data suggest that both CD8α+ and CD8α− DCs derive from a common precursor and, hence, do not support the concept of the CD8α+ lymphoid-derived and CD8α−myeloid-derived DC lineages. However, because this hypothesis has to be confirmed at the clonal level, it remains possible that CD8α− DCs arise from a myeloid precursor within the CD4low precursor population or, alternatively, that both CD8α+ and CD8α− DCs derive from an independent nonlymphoid, nonmyeloid DC precursor. In conclusion, although we favor the hypothesis that both CD8α+ and CD8α− DCs derive from a lymphoid-committed precursor, a precise study of the differentiation process of CD8α+ and CD8α− DCs is required to define conclusively their origin.
Introduction
Two main dendritic cell (DC) subsets have been described in the mouse, which can be distinguished on the basis of their differential CD8α expression,1,2 The finding that murine thymic DCs expressing CD8α derived from CD4lowlymphoid precursors, devoid of myeloid differentiation potential,3 lead to the definition of the lymphoid DC lineage.
Over the past few years, numerous reports dealing with the phenotype, localization, and function of murine CD8α+ DCs and CD8α− DCs have been published, contributing enormously to our understanding of DC biology.4-15 However, certain issues dealing with CD8α+ versus CD8α−DCs, such as their differential T-cell stimulatory capacity, production of cytokines, responsiveness to chemokines, induction of TH1 versus TH2 responses, and, in particular, the definition of their respective precursor populations, are still controversial or remain unknown.
These studies were based on a variety of experimental approaches performed both in vivo and in vitro and were undertaken by using different DC purification techniques. Consequently, some published controversial results regarding the phenotype and/or the function of the different DC subsets might reflect differences in the DC isolation method used. The protocols of DC isolation used by different research groups differ mainly in the enzymatic digestion performed, in the way of obtaining a DC-enriched low-density fraction, and, more importantly, in the antibodies used to eliminate contaminating cells by magnetic or flow cytometry separation procedures. This negative selection of contaminating cells is of special relevance because certain DC populations can be completely or partially lost, depending on the antibodies used. Therefore, the design of an appropriate negative selection protocol requires an exhaustive phenotypic study of the DC subset to be purified. In this sense, the majority of the reports dealing with the function of CD8α+ versus CD8α− DC subsets4-7,9 and, more importantly, the article establishing that CD8α+ DCs but not CD8α− DCs derived from lymphoid committed precursors,16 which has been the basis of the concept of murine lymphoid versus myeloid DCs, rely on a DC isolation method using anti-CD4 and anti-F4/80 antibodies to deplete CD4+ T cells and F4/80+ macrophages. In the present report, we have performed a precise phenotypic analysis of murine splenic CD8α+ and CD8α− DCs with regard to the expression of certain conflictive markers, and we have re-addressed the question of the derivation of both DC subsets from CD4lowprecursors. Our results revealed that CD8α− DCs, but not CD8α+ DCs, expressed CD4 and F4/80 and, therefore, that the use of antibodies against those molecules during CD8α− DC isolation might lead to the loss of a significant proportion of them. More importantly, CD8α−DCs together with CD8α+ DCs were generated from lymphoid-committed CD4low precursors in irradiation chimeras in which the development of donor-type DCs was analyzed on DC-enriched splenic low-density fractions, without depleting cells expressing CD4 and/or F4/80. In irradiation chimeras donor-type CD8α+ and CD8α− DCs displayed an equivalent phenotype to that of their control counterparts and were generated from CD4low precursors at a similar CD8+-to-CD8− ratio to that found in control mice. These results suggest that both CD8α+ and CD8α− DCs derive from a common precursor and do not support the concept in the murine system of the CD8α+lymphoid-derived and CD8α− myeloid-derived DC lineages.
Materials and methods
Mice
For phenotypic and functional experiments 5- to 6-week-old C57 BL/6 and BALB/c mice were used. In reconstitution experiments, donors were 5- to 6-week-old C57 BL/Ka Ly 5.2 mice and recipients were 8-week-old C57 BL/6 Ly 5.1 Pep3b mice.
Preparation of DC-enriched very low density cell fractions
Spleens were cut into small fragments and digested with collagenase A (0.5 mg/mL; Boehringer-Mannheim, Mannheim, Germany) and Dnase I (40 μg/mL; Boehringer-Mannheim) in RPMI 1640 medium supplemented with 5% fetal calf serum (FCS) for 10 minutes at 37°C with continuous agitation. Digested fragments were filtered through a stainless-steel sieve, and cell suspensions were washed twice in phosphate-buffered saline (PBS) supplemented with 5% FCS and 5 mmol/L EDTA (PBS-EDTA-FCS) containing 5 μg/mL Dnase I. The cells were resuspended in cold isoosmotic Optiprep solution (Nyegaard Diagnostics, Oslo, Norway), pH7.2, density 1.061 g/cm3, containing 5 mmol/L EDTA to dissociate DC-thymocyte complexes, and a very low-density cell fraction (1.061-density fraction), accounting for less than 1% of the starting cell population, obtained by centrifugation at 1700g for 10 minutes, and washed twice in PBS-EDTA-FCS.
Preparation of CD8α− DC-enriched cell fractions
CD8α− DC-enriched cell fractions were obtained by treating DC-enriched 1.061-density fractions for 50 minutes at 4°C with a monoclonal antibody (mAb) mixture, including anti-CD3 (clone KT3-1.1), anti-CD8α (clone 53-6.72), anti-B220 (clone RA3-6B2), and anti-granulocyte antigen Gr1 (clone RB6-8C5). The unwanted cells were removed magnetically after incubation for 30 minutes at 4°C with anti-rat immunoglobulin-coated magnetic beads (Dynabeads, Dynal, Oslo, Norway) at a 7:1 bead-to-cell ratio. Analysis of CD11c versus CD8α expression of CD8α− DC-enriched cell fractions revealed that they were composed of more than 80% CD11c+CD8α− DCs and approximately 20% CD11c−CD8α− contaminants, CD11c+CD8α+ DCs, representing less than 1% (data not shown).
In vivo depletion with anti-CD4 antibodies
Depletion of CD4+ cells was achieved by intraperitoneal injection of the in vivo depleting anti-CD4 antibody GK1.5. For this purpose, mice received three injections of 300 μg of GK1.5 on 3 consecutive days and were analyzed 24 hours after the last injection.
Modulation of cell surface marker expression by CD8α− DCs on culture
CD8α− DC-enriched cell fractions prepared as described above were cultured for 24 hours or 48 hours at 37°C in the presence 100 μg/mL anti-CD40 mAb (clone FGK45) or anti-CD43 (clone S7). The culture medium was RPMI 1640 supplemented with 10% FCS, 10 mmol/L Hepes, 50 μmol/L 2-mercaptoethanol, 100 U/mL penicillin-streptomycin, and 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF).
MLR assay
Splenic CD4− CD8α− DCs, CD4+ CD8α− DCs, total CD8α−DCs, or CD8α+ DCs from C57BL/6 (H-2b) mice were cultured with purified T cells obtained from mesenteric lymph nodes of BALB/c (H-2d) mice in flat-bottom 96-well plates (1 × 105 cells per well) at different APC-to-T cell ratios. T-cell proliferation was assessed after 5 days by [3H] thymidine (1 μCi/well) uptake in a 4-hour pulse or by CD25 expression. For this assay, CD4−CD8α− DCs, CD4+ CD8α− DCs, and total CD8α− DCs were sorted from CD8α− DC-enriched cell fractions. CD8α+DCs were sorted from DC-enriched 1.061-density fractions. The sorted populations had a purity of more than 97%.
Isolation of CD4low and CD44+CD25+ precursor populations
CD4low precursors were isolated from C57 BL/Ka Ly 5.2 donor thymuses by depleting pre-T cells, double-positive and single-positive thymocytes, B cells, DCs, macrophages, and granulocytes by complement-mediated cytotoxicity by using anti-CD3 (clone Y-CD3) and anti-CD8 (clone 31M) and then immunomagnetic bead depletion after incubation with anti-CD3 (clone KT3), anti-CD8 (clone 53.6.7-2), anti-CD25 (clone PC61.5), anti-B220 (clone RA3-6B2), anti-MHC class II (MHC II) (clone FD11), anti-macrophage antigen F4/80 (clone C1.A3.1), and anti-granulocyte antigen Gr-1 (clone RB6-8C5). CD4lowprecursors were then sorted as Thy-1low CD44+cells after double immunofluorescent staining fluorescein isothiocyanate (FITC)-conjugated anti-Thy-1 (clone AT15) and phycoerythrin (PE)-conjugated anti-CD44 (clone IM7, Pharmingen, San Diego, CA). Flow cytometric cell sorting was carried out on a FACSort instrument (Becton Dickinson, Mountain View, CA). The sorted preparation had a purity of more than 98% and contained less than 1% CD11c+ cells as assessed after staining with biotinylated anti-CD11c (clone N418; data not shown) followed by streptavidin-tricolor (Caltag, San Francisco, CA).
CD44+ CD25+ precursors were isolated by complement-mediated cytotoxicity by using anti-CD3 (clone Y-CD3), anti-CD4 (clone 172.4), and anti-CD8 (clone 31M) and then sorting after double immunofluorescent staining with PE-conjugated anti-CD44 and biotinylated anti-CD25 followed by streptavidin-tricolor. The sorted population had a purity of more than 97%.
Reconstitution experiments with CD4low precursors or CD44+ CD25+ precursors
Thymic CD4low precursors (3 × 104 ) or thymic CD44+ CD25+ precursors (3 × 104) from C57 BL/Ka Ly 5.2 donor mice were injected intravenously into γ-irradiated (7 Gy) C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 3 × 104 Ly 5.1 bone marrow (BM) cells to ensure survival of recipients.
Reconstitution experiments with BM cells
BM cells (2 × 106) from C57 BL/Ka Ly 5.2 donor mice were injected intravenously into γ-irradiated (7 Gy) C57 BL/6 Ly 5.1 Pep3b mice.
Flow cytometry
DC-enriched very low density cell fractions (Figure1) were analyzed after triple staining with FITC-conjugated anti-CD11c (clone N418), PE-conjugated anti-CD8α (clone CT-CD8a, Caltag), and biotin-conjugated anti-DEC-205 (clone NLDC-145); anti-macrophage antigen F4/80 (clone 31-A3-1); or anti-CD4 (clone GK1.5) followed by streptavidin-tricolor (Caltag). The phenotypic analysis of CD4− CD8α− DCs and CD4+ CD8α− DC subsets (Figure2) was performed after triple staining with FITC-conjugated anti-CD11c, PE-conjugated anti-CD4 (clone CT-CD4, Caltag), and biotin-conjugated anti-DEC-205; anti-macrophage antigen F4/80; anti-Mac-1 (clone M1/70); anti-FcγRII/III (clone 2-4G2); anti-LFA-1α (clone FD441.8); anti-CD69 (clone H.1.2F3); anti-B7-2 (clone GL1, Pharmingen); anti-CD40 (clone FGK45); or anti-MHC class II (clone FD11-54.3), followed by streptavidin-tricolor. Analysis of cell surface marker expression by CD8α− DCs on culture (Figure 3) was performed after triple staining with FITC-conjugated anti-CD11c, PE-conjugated anti-CD8α, and biotin-conjugated anti-CD4; anti-macrophage antigen F4/80; or anti-DEC-205 followed by streptavidin-tricolor. Analysis of T-cell proliferation (Figure 4) was performed after triple staining with FITC-conjugated anti-CD8, PE-conjugated anti-CD4, and biotin-conjugated anti-CD25 (clone PC61.5) followed by streptavidin-tricolor. Analysis of DC reconstitution (Figures5 and 6) was performed on splenic DC-enriched 1.061-density fractions after triple staining with FITC-conjugated anti-CD11c, PE-conjugated anti-Ly 5.2 (clone AL1-4A2, Pharmingen), and tricolor-conjugated anti-CD8α (clone CT-CD8a, Caltag). Because, after transfer of BM precursors, more than 95% of DCs were of donor origin, the phenotypic analysis of Ly 5.2+ CD8α− DCs (Figure 6) was performed after triple staining with FITC-conjugated anti-CD11c, PE-conjugated anti-CD8α, and tricolor-conjugated anti-CD4 (clone CT-CD4, Caltag) or biotin-conjugated anti-macrophage antigen F4/80 followed by streptavidin-tricolor. Analysis was performed on a FACSort flow cytometer.
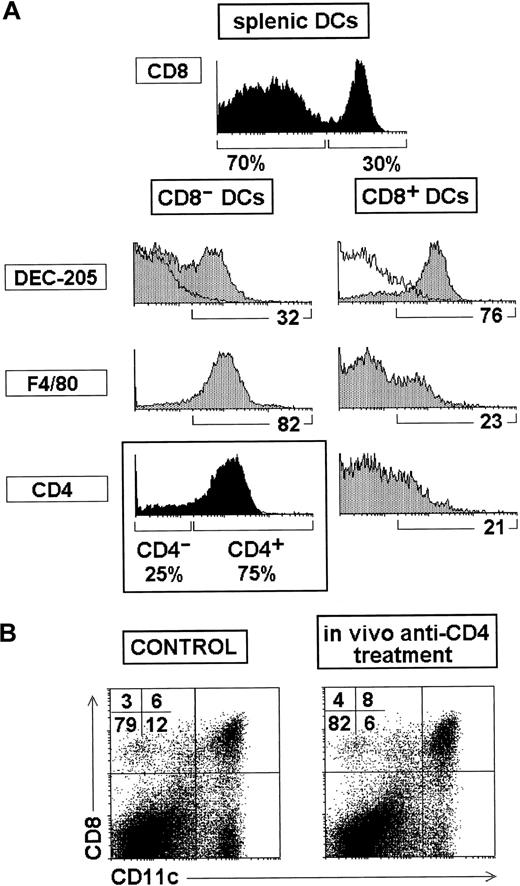
CD4 and F4/80 expression by splenic DCs.
(A) Phenotype of CD8α+ and CD8α− DCs analyzed on splenic DC-enriched very low density fractions obtained without antibody-mediated depletion. Black histograms show the proportion of CD8α− and CD8α+ DCs after gating for CD11c+ cells and the proportion of CD4− and CD4+ DCs within the CD8α− subset. White profiles represent control stainings. The percentage of cells positive for the indicated markers is shown under the gray histograms. (B) Splenic CD11c versus CD8α profiles of control mice and mice injected intravenously with in vivo depleting anti-CD4 antibodies, showing that most CD8α−DCs were eliminated after anti-CD4 treatment. Data are representative of 3 experiments with similar results.
CD4 and F4/80 expression by splenic DCs.
(A) Phenotype of CD8α+ and CD8α− DCs analyzed on splenic DC-enriched very low density fractions obtained without antibody-mediated depletion. Black histograms show the proportion of CD8α− and CD8α+ DCs after gating for CD11c+ cells and the proportion of CD4− and CD4+ DCs within the CD8α− subset. White profiles represent control stainings. The percentage of cells positive for the indicated markers is shown under the gray histograms. (B) Splenic CD11c versus CD8α profiles of control mice and mice injected intravenously with in vivo depleting anti-CD4 antibodies, showing that most CD8α−DCs were eliminated after anti-CD4 treatment. Data are representative of 3 experiments with similar results.
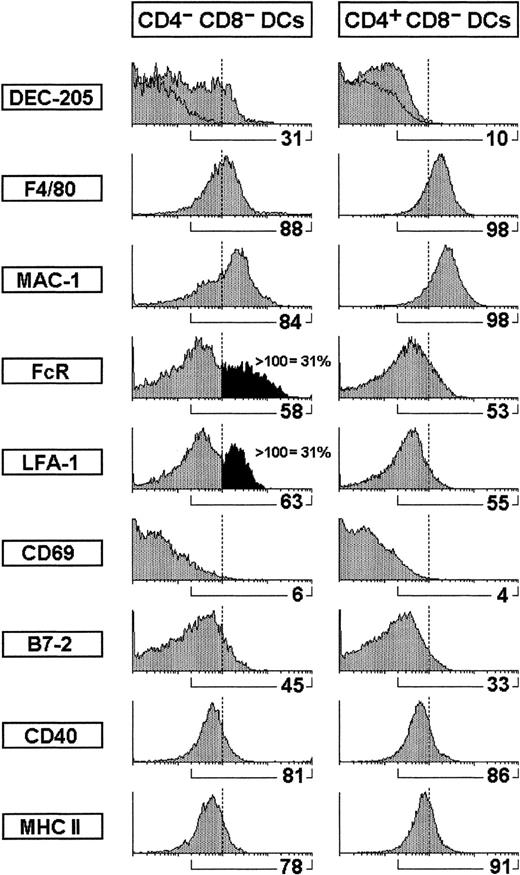
Comparative phenotypic analysis of CD4−CD8α− versus CD4+ CD8α− DCs.
The phenotype of CD4− CD8α− versus CD4+ CD8α− DCs was performed on CD8α− DC-enriched fractions after gating for CD11c+ CD4− or CD11c+CD4+ cells. The percentage of cells positive for the indicated markers is shown. In addition, the percentage of CD4− CD8α− DCs expressing high levels of FcR or LFA-1 is also indicated.
Comparative phenotypic analysis of CD4−CD8α− versus CD4+ CD8α− DCs.
The phenotype of CD4− CD8α− versus CD4+ CD8α− DCs was performed on CD8α− DC-enriched fractions after gating for CD11c+ CD4− or CD11c+CD4+ cells. The percentage of cells positive for the indicated markers is shown. In addition, the percentage of CD4− CD8α− DCs expressing high levels of FcR or LFA-1 is also indicated.
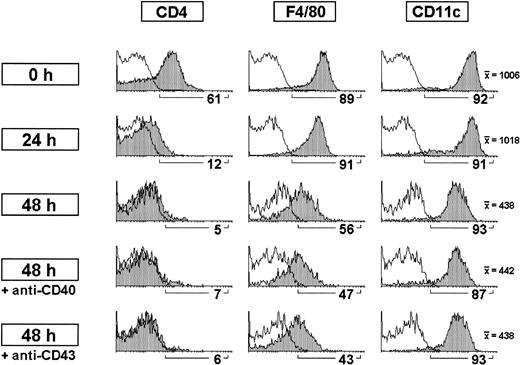
Modulation of CD4 expression by CD8α− DCs on culture.
CD8α− DC-enriched fractions were cultured for 24 hours or 48 hours alone or in the presence of anti-CD40 or anti-CD43 antibodies. CD8α− DCs were analyzed after gating for CD11c+ CD8α− cells. The percentage of cells positive for the indicated markers as well as the mean fluorescence intensity for CD11c expression is shown. White profiles represent control stainings. Data are representative of 3 experiments with similar results.
Modulation of CD4 expression by CD8α− DCs on culture.
CD8α− DC-enriched fractions were cultured for 24 hours or 48 hours alone or in the presence of anti-CD40 or anti-CD43 antibodies. CD8α− DCs were analyzed after gating for CD11c+ CD8α− cells. The percentage of cells positive for the indicated markers as well as the mean fluorescence intensity for CD11c expression is shown. White profiles represent control stainings. Data are representative of 3 experiments with similar results.
T-cell stimulation capacity of CD8α+ DCs versus CD8α− DCs.
T-cell stimulatory capacity of CD8α+ versus CD8α− DC (A) and CD4− CD8α−versus CD4+ CD8α− DC (B). DCs from C57BL/6 mice were cultured with purified allogeneic T cells from BALB/c mice at the indicated APC:T cell ratios. After 5 days, T-cell proliferation was determined by [3H] thymidine uptake or CD25 expression. Data are representative of 3 experiments with similar results. In the [3H] thymidine incorporation assay, error bars represent the SD for triplicate cultures.
T-cell stimulation capacity of CD8α+ DCs versus CD8α− DCs.
T-cell stimulatory capacity of CD8α+ versus CD8α− DC (A) and CD4− CD8α−versus CD4+ CD8α− DC (B). DCs from C57BL/6 mice were cultured with purified allogeneic T cells from BALB/c mice at the indicated APC:T cell ratios. After 5 days, T-cell proliferation was determined by [3H] thymidine uptake or CD25 expression. Data are representative of 3 experiments with similar results. In the [3H] thymidine incorporation assay, error bars represent the SD for triplicate cultures.
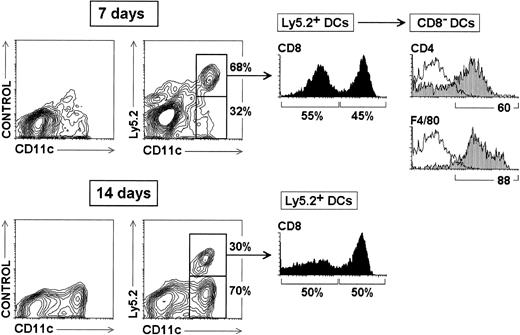
Reconstitution of splenic CD8α+ and CD8α−DCs from CD4low lymphoid precursors.
Thymic CD4low precursors (3 × 104) from C57 BL/Ka Ly 5.2 donor mice were injected intravenously into γ-irradiated (7 Gy) C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 4 × 104 Ly 5.1 BM cells to ensure survival of recipients. At the indicated times, mice were analyzed for donor-derived DCs, identified as Ly 5.2+ CD11c+cells, in splenic DC-enriched 1.061-density fractions. The percentage of Ly5.2− and Ly5.2+ CD11c+ DCs (contour plots), as well as the percentage of CD8α− and CD8α+ DCs among Ly5.2+ DCs (black histograms) are indicated. Grey profiles show the expression of CD4 and F4/80 by CD8α− DCs. White profiles represent control stainings. These results are representative of four independent experiments with similar results.
Reconstitution of splenic CD8α+ and CD8α−DCs from CD4low lymphoid precursors.
Thymic CD4low precursors (3 × 104) from C57 BL/Ka Ly 5.2 donor mice were injected intravenously into γ-irradiated (7 Gy) C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 4 × 104 Ly 5.1 BM cells to ensure survival of recipients. At the indicated times, mice were analyzed for donor-derived DCs, identified as Ly 5.2+ CD11c+cells, in splenic DC-enriched 1.061-density fractions. The percentage of Ly5.2− and Ly5.2+ CD11c+ DCs (contour plots), as well as the percentage of CD8α− and CD8α+ DCs among Ly5.2+ DCs (black histograms) are indicated. Grey profiles show the expression of CD4 and F4/80 by CD8α− DCs. White profiles represent control stainings. These results are representative of four independent experiments with similar results.
Reconstitution of splenic CD8α+ and CD8α− DCs from CD44+ CD25+precursors.
Thymic CD44+ CD25+ precursors (3 × 104) were injected intravenously into 7 Gy γ-irradiated 8-week-old C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 4 × 104 Ly 5.1 BM cells to ensure survival of recipients. Ten days after transfer of precursors, mice were analyzed for donor-derived DCs. The percentage of Ly5.2+ CD11c+ DCs as well as the percentage of CD8α− and CD8α+ DCs among Ly5.2+ DCs are indicated. Data are representative of 2 independent experiments with similar results.
Reconstitution of splenic CD8α+ and CD8α− DCs from CD44+ CD25+precursors.
Thymic CD44+ CD25+ precursors (3 × 104) were injected intravenously into 7 Gy γ-irradiated 8-week-old C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 4 × 104 Ly 5.1 BM cells to ensure survival of recipients. Ten days after transfer of precursors, mice were analyzed for donor-derived DCs. The percentage of Ly5.2+ CD11c+ DCs as well as the percentage of CD8α− and CD8α+ DCs among Ly5.2+ DCs are indicated. Data are representative of 2 independent experiments with similar results.
Results
Phenotypic profile of mouse splenic DC revisited: CD4 and F4/80 expression by CD8α− splenic DCs
The most comprehensive analyses of mouse DC phenotype reported so far1 2 have been performed on highly enriched DC preparations obtained after immunomagnetic depletion of contaminating cells with specific mAbs. Those contaminants comprise essentially T and B lymphocytes, macrophages, and granulocytes, and, therefore, mAbs used for depleting them included usually at least anti-CD3, anti-CD4, anti-B220, anti-FcR, anti-F4/80 (macrophage marker), and anti-Gr-1 (granulocyte marker). Obviously, the selection of these mAbs was originally made on the basis of the assumption that DCs did not express those cell surface molecules.
However, DCs can be accurately analyzed on DC-enriched very low-density cell fractions, obtained without including the mAb-mediated magnetic bead depletion step, by using a centrifugation medium adjusted at 1.061 g/mL. As shown in Figure 1A, the phenotypic analysis of splenic DCs performed under those conditions revealed that CD8α− DCs and CD8α+ DCs represented approximately 70% and 30% of all splenic DCs, respectively. As shown in the Table, estimation of the absolute DC number revealed that the spleen of 5- to 6-week-old C57 BL/6 mice contained 180 000-190 000 CD8α− DCs and 80 000-90 000 CD8α+ DCs. Interestingly, CD8α− DCs expressed the macrophage marker F4/80 and more interestingly 70%-75% of them were CD4+. Moreover, CD8α+ DCs were essentially negative for F4/80 and CD4, although approximately 20% of them expressed low levels of these markers. Consequently, in the mouse spleen CD8α+, DCs were CD4− F4/80− DEC-205+, whereas CD8α− DCs were CD4+F4/80+ DEC-205−. However, within the lymph nodes, an additional DC subset expressing intermediate levels of CD8 exists, which has been related to Langerhans cells.2 These cells displayed the same phenotype as splenic CD8α+ DCs regarding the markers considered above, that is, they were CD4− F4/80− DEC-205+ (data not shown).
To check whether CD4 was functionally expressed at the surface of splenic CD8α− DCs, BALB/c mice were injected intraperitoneally with the in vivo depleting anti-CD4 antibody GK1.5. As shown in Figure 1B, the spleen of mice treated in vivo with GK1.5 suffered a profound depletion of CD8α− DCs, whereas the CD8α+ DC subset was unaffected. CD8α− DCs remaining after GK1.5 injection most likely corresponded to CD8α− DCs negative for CD4 or expressing low levels of this molecule. This result indicates that the CD4 molecule was functionally expressed by splenic CD8α− DCs.
In conclusion, the phenotypic analysis of splenic DCs performed on DC-enriched very low-density cell fractions revealed that expression of CD8α and CD4 by splenic DC subsets is mutually exclusive, in that CD8α+ DCs are CD4− and, inversely, CD8α− DCs are CD4+.
Comparative phenotypic analysis of CD4−CD8α− DCs versus CD4+CD8α− DCs
To investigate whether the CD4− and CD4+cells CD8α− DC corresponded to distinct DC populations or to different CD4 expression levels within the CD8α−DC subset, we performed a comparative phenotypic study of CD4− CD8α− DCs versus CD4+CD8α− DCs. For this purpose, a CD8α−DC-enriched fraction was obtained from a splenic 1.061-density fraction after immunomagnetic bead depletion with anti-CD3, anti-CD8α, anti-B220, and anti-Gr-1 mAbs. The analysis was performed on this CD8α− DC-enriched fraction by gating on CD4− or CD4+ cells (as shown in Figure 1A) after triple immunofluorescence staining with FITC-conjugated anti-CD11c, PE-conjugated anti-CD4, and biotin-conjugated antibodies against the cell surface markers indicated in Figure 2. Our data show that CD4− CD8α− and CD4+CD8α− DCs have a very similar phenotypic profile with regard to a variety of cell surface molecules, including DC and macrophage markers, adhesion, activation, and costimulatory molecules. There was, however, a slight but significant difference with regard to the expression of FcR (CD16-CD32) and LFA-1, because approximately 30% CD4− CD8α− DCs but not CD4+CD8α− DCs expressed high levels of these markers.
Modulation of CD4 expression by CD8α− DCs on culture
The data derived from the comparative phenotypic analysis of CD4− versus CD4+ CD8α− DC subsets suggest that they belong to a unique DC category with differential expression of CD4. Therefore, it can be speculated that CD4 expression levels correlate with different activation and/or maturation states. To test this hypothesis, CD8α−DC-enriched populations were cultured alone or in the presence of antibodies against the molecules CD40 or CD43, known to induce DC activation on ligation.17-19 As illustrated in Figure 3, CD4 expression was strongly down-regulated in CD8α− DCs after 24 hours in culture and was almost undetectable after 48 hours. Moreover, addition of anti-CD40 or anti-CD43 antibodies did not prevent CD4 down-regulation, suggesting that CD4 expression by CD8α− DCs was not related to the activation of these cells. Under the same experimental conditions, F4/80 expression was also down-regulated after 48 hours, although a significant proportion of CD8α− DCs remained positive for this marker. However, CD11c underwent only a slight down-regulation on culture. As for CD4, anti-CD40 or anti-CD43 antibodies had no effect on F4/80 or CD11c expression on 48-hour culture.
T-cell stimulation capacity of CD8α+ versus CD8α− DCs
To test whether CD4 expression by CD8α− DCs was correlated with their functional potential as antibody-presenting cells, we tested their capacity to induce T-cell stimulation in an MLR assay. For these experiments, CD4− and CD4+subsets of CD8α− DCs, as well as total CD8α− DCs and CD8α+ DCs, were FACS-sorted and cultured with purified allogeneic T cells. After 5 days, T-cell proliferation was determined by [3H] thymidine uptake or by CD25 expression. We first tested the differential T-cell stimulatory potential of CD8α− versus CD8α+ DCs. Our data revealed that CD8α+ DCs induced a higher [3H] thymidine uptake and CD25 up-regulation in an allogeneic MLR than CD8α− DCs (Figure 4A). Because, as shown above, there was a direct correlation between [3H] thymidine incorporation and expression of CD25, the latter was subsequently used to assess, in the same in vitro assay, the capacity of CD4− CD8α− DCs versus CD4+CD8α− DCs to stimulate CD8+ or CD4+ T cells. As illustrated in Figure 4B, CD4− and CD4+ CD8α− DCs displayed a similar T-cell stimulatory potential in MLR, although the CD4+ CD8α− DC subset induced a slightly higher response of both CD8+ and CD4+ T cells. Interestingly, preliminary results indicate that the endocytic capacity of CD8α− DCs appears to be restricted to the CD4+ subset (data not shown).
Reconstitution of splenic CD8α+ DCs and CD8α− DCs from CD4low lymphoid precursors
As discussed above, in the murine system the concept that CD8α− DCs and CD8α+ DCs represent the myeloid and lymphoid DC subsets, respectively, derives essentially from a report analyzing the DC reconstitution potential of CD4low precursors on intravenous injection.16In that report, only CD8α+ DCs were found among the progeny of CD4low precursor after 2 weeks. However, in this study DC reconstitution was analyzed after DC purification, using an immunomagnetic bead depletion protocol employing anti-CD4 and anti-F4/80 antibodies, and, therefore, CD8α− DCs could have been excluded from the analysis.
To test this hypothesis and thus to investigate whether CD8α− DCs derive from the lymphoid lineage-committed CD4low precursor population, CD4low precursors isolated from Ly 5.2 mice were transferred intravenously into γ-irradiated Ly 5.1 recipient mice. These mice were subsequently analyzed for donor-derived DCs, identified as Ly 5.2+CD11c+ cells, in DC-enriched 1.061-density fractions, obtained without including the mAb-mediated magnetic bead depletion step, as described above (Figure 1A). Our data, shown in Figure 5, demonstrate that 7 days after the transfer approximately 70% of DCs were donor derived and, interestingly, that among those cells both CD8α+ and CD8α− DCs were found. At this time point, Ly 5.2+ CD8α+ and Ly 5.2+ CD8α− DCs represented 55% and 45% of all Ly 5.2+ DCs, respectively. Importantly, donor-derived CD8α− DCs derived from CD4low precursors displayed the phenotypic profile characteristic of CD8α−DCs found in control spleen (Figure 1), that is, they expressed high levels of CD4 and F4/80. After 14 days, only 30% Ly 5.2+DCs were found, indicating that the DC reconstitution potential of the CD4low precursor population was partially extinguished and replaced by that of Ly 5.1+ BM precursors. Both Ly 5.2+ CD8α+ and Ly 5.2+CD8α− DCs were also found after 14 days, each subset constituting approximately 50% of all Ly 5.2+ DCs. Therefore, as postulated above, in the report by Wu et al,16 CD8α− DCs of donor origin could have been missed in the analysis because of their CD4 and/or F4/80 expression that could have determined their depletion, because antibodies against these molecules were used for immunomagnetic depletion in this study. In conclusion, these data indicate that both CD8α− and CD8α+ DCs are generated from CD4low lymphoid-committed precursors. To exclude that CD8α− DCs could have been transferred with the CD4low precursors, the CD4low precursor population was analyzed before transfer for the presence of CD11c+ cells and no CD11c+ cells were found (data not shown). In addition, mice reconstituted with CD4low precursors were analyzed 2 days after transfer for Ly 5.2+ DCs, but no CD11c+ were found in the spleen at this time point (data not shown). Therefore, these data reinforce the view that Ly 5.2+ CD8α− and CD8α+ DCs derived from the transferred CD4lowprecursors.
To further strengthen our data with CD4low precursors, we tested the ability of the next downstream precursor population, namely the CD44+ CD25+ pro-T cell precursors that have lost the capacity to form B cells but still form DCs16 to generate CD8α− and CD8α+ DCs on intravenous transfer. As shown in Figure 6, 10 days after the transfer of CD44+ CD25+ precursors, approximately 30% of CD11c+ DCs were donor derived, and both CD8α+ and CD8α− DCs were found. Interestingly, CD8α+ and CD8α− DCs represented 58% and 42% of Ly 5.2+ DCs, respectively, indicating that CD4low and CD44+CD25+ precursors generated the two splenic DC subsets at comparable CD8α− DC-to-CD8α+ DC ratios.
Kinetics of splenic DC differentiation and phenotypic variations of CD8α− DCs during reconstitution after transfer of BM cells
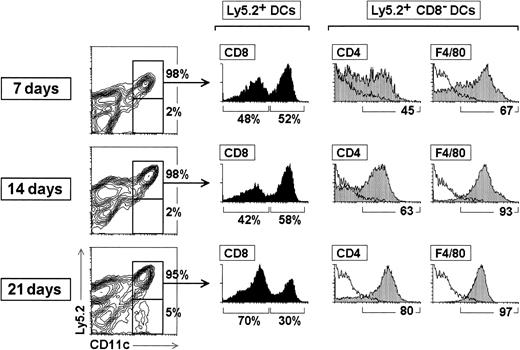
To study the phenotype of CD8α− DCs during the process of DC reconstitution after irradiation, BM cells from Ly 5.2 donor mice were transferred intravenously into γ-irradiated Ly 5.1 recipient mice. These mice were subsequently analyzed for donor-derived DCs at 7, 14, and 21 days after transfer. For this purpose, the experiments were carried out with BM precursor cells instead of CD4low precursors because the progressive loss of DC reconstitution capacity of the latter, as a result of their extinction, does not allow the analysis of the phenotypic variations undergone by CD8α− DCs during the reconstitution process.
As shown in Figure 7, during the reconstitution period analyzed (from 7 to 21 days after transfer), virtually all the DCs present in the spleen of reconstituted mice were of donor origin; only after 21 days a small proportion of Ly 5.1+ CD11c+ cells (approximately 5% of all CD11c+ cells) was detected. With regard to the proportion of the CD8α− and CD8α+ subsets, at 7 and 14 days CD8α+ DCs represented approximately 50% of splenic DCs, but after 21 days the proportion of CD8α−DCs increased, with the CD8α− DC-to-CD8α+DC ratio being 70:30 (ie, as in control spleens; Table 1).
Kinetics of splenic DC differentiation and phenotypic variations of CD8α− DCs during reconstitution after transfer of BM cells.
BM cells (2 × 106) from C57 BL/Ka Ly 5.2 donor mice were injected intravenously into γ-irradiated (7 Gy) C57 BL/6 Ly 5.1 Pep3b recipient mice. At the indicated times, mice were analyzed for donor-derived DCs in splenic DC-enriched 1.061-density fractions. The percentage of Ly5.2− and Ly5.2+CD11c+ DCs (contour plots), as well as the percentage of CD8α− and CD8α+ DCs among Ly5.2+ DCs (black histograms) are indicated. Grey profiles show the expression of CD4 and F4/80 by Ly5.2+CD8α− DCs; the percentage of CD4+ or F4/80+ cells is indicated. White profiles represent control stainings. These results are representative of three independent experiments with similar results.
Kinetics of splenic DC differentiation and phenotypic variations of CD8α− DCs during reconstitution after transfer of BM cells.
BM cells (2 × 106) from C57 BL/Ka Ly 5.2 donor mice were injected intravenously into γ-irradiated (7 Gy) C57 BL/6 Ly 5.1 Pep3b recipient mice. At the indicated times, mice were analyzed for donor-derived DCs in splenic DC-enriched 1.061-density fractions. The percentage of Ly5.2− and Ly5.2+CD11c+ DCs (contour plots), as well as the percentage of CD8α− and CD8α+ DCs among Ly5.2+ DCs (black histograms) are indicated. Grey profiles show the expression of CD4 and F4/80 by Ly5.2+CD8α− DCs; the percentage of CD4+ or F4/80+ cells is indicated. White profiles represent control stainings. These results are representative of three independent experiments with similar results.
Analysis of splenic CD8α+ DC and CD8α− DC reconstitution after transfer of BM precursor cells*
| . | CD8α− DC cell number . | CD8α+ DC cell number . | CD8− to CD8+ DC ratio . | Spleen total cell number . |
|---|---|---|---|---|
| Control | ||||
| 1 | 189 000 | 81 000 | 70:30 | 86 × 106 |
| 2 | 181 000 | 71 000 | 72:28 | 70 × 106 |
| 7 days after transfer | ||||
| Experiment 1 | 129 000 | 171 000 | 43:57 | 45 × 106 |
| Experiment 2 | 223 000 | 190 000 | 54:46 | 22 × 106 |
| 14 days after transfer | ||||
| Experiment 1 | 505 000 | 485 000 | 51:49 | 137 × 106 |
| Experiment 2 | 345 000 | 390 000 | 47:53 | 100 × 106 |
| 21 days after transfer | ||||
| Experiment 1 | 405 000 | 135 000 | 75:25 | 91 × 106 |
| Experiment 2 | 639 000 | 261 000 | 71:29 | 116 × 106 |
| . | CD8α− DC cell number . | CD8α+ DC cell number . | CD8− to CD8+ DC ratio . | Spleen total cell number . |
|---|---|---|---|---|
| Control | ||||
| 1 | 189 000 | 81 000 | 70:30 | 86 × 106 |
| 2 | 181 000 | 71 000 | 72:28 | 70 × 106 |
| 7 days after transfer | ||||
| Experiment 1 | 129 000 | 171 000 | 43:57 | 45 × 106 |
| Experiment 2 | 223 000 | 190 000 | 54:46 | 22 × 106 |
| 14 days after transfer | ||||
| Experiment 1 | 505 000 | 485 000 | 51:49 | 137 × 106 |
| Experiment 2 | 345 000 | 390 000 | 47:53 | 100 × 106 |
| 21 days after transfer | ||||
| Experiment 1 | 405 000 | 135 000 | 75:25 | 91 × 106 |
| Experiment 2 | 639 000 | 261 000 | 71:29 | 116 × 106 |
DC indicates dendritic cell; BM, bone marrow.
With regard to kinetics of reconstitution of the two DC subsets after BM transfer, the estimation of the absolute numbers of CD8α− and CD8α+ DCs of donor origin revealed that 7 days after transfer both DC compartments were fully reconstituted, with the absolute number of CD8α+ DCs being 2-fold higher than in control spleens (Table 1). The absolute number of both DC subpopulations increased considerably during the second week of reconstitution, and after 3 weeks, even though the CD8α− DC-to-CD8α+ DC ratio was similar to that of control spleens, the absolute number of both DC subsets was still 2-fold to 3-fold higher than in controls.
Globally, the data derived from our experiments of reconstitution after irradiation show that CD8α− and CD8α+ DC reconstitution is faster than that of T and B lymphocytes, that the process of DC reconstitution occurs simultaneously for CD8α− and CD8α+ DCs, and that both subsets are produced in equal numbers during the first 2 weeks after transfer of precursors. In addition, the data reveal that CD8α−and CD8α+ DCs are generated at the same ratio during reconstitution with both CD4low precursors and BM precursors.
Concerning the phenotype of reconstituted DCs, from the shortest time point analyzed, CD8α+ DCs displayed the same phenotypic profile as their control counterparts (not shown). However, as illustrated in Figure 7, CD8α− DCs underwent variations in their CD4 and F4/80 expression during reconstitution because the percentage of CD4+ CD8α− DCs and F4/80+ CD8α− DCs increased significantly from 7 to 21 days after transfer. As for the CD8α−DC-to-CD8α+ DC ratio, after 21 days the CD4 and F4/80 expression profile of CD8α− DCs was similar to that of control mice. These data indicate that F4/80 and particularly CD4 expression was subjected to variations during the reconstitution process, suggesting that CD4 expression levels by CD8α−DCs might be related with their differentiation and/or physiological state. In addition, these results demonstrate that with 14-day transfer, the majority of CD8α− DCs expressed CD4 and F4/80, supporting the hypothesis in the study by Wu et al16that most likely CD8α− DCs were missed in the analysis of CD4low progeny, because the majority or all of them were depleted with anti-CD4 and anti-F4/80 antibodies.
Discussion
CD8α+and CD8α− DCs differ in their location, migratory capacity, T-cell stimulation potential, and phenotype. In the mouse spleen CD8α− DCs appear to be located in the marginal zone bridging channels and extend from the marginal zone into the red pulp, whereas CD8α+ DCs correspond with the interdigitating DCs located in the T-cell zone of the white pulp.10 However, CD8α+ DCs have been demonstrated to have a reduced migratory capacity compared with CD8α− DCs.14,15 With regard to the function of CD8α− versus CD8α+ DCs, several reports are consistent with the notion that CD8α− DCs have a higher phagocytic capacity than their CD8α+counterparts,6 10 but, as discussed below, the data published so far regarding their antigen processing and T-cell stimulatory capacity remain controversial.
Regarding their phenotypic characteristics, CD8α+ and CD8α− DCs have been reported to be DEC-205+CD11b− and DEC-205− CD11b+, respectively. Both subsets have been considered negative for the T-cell marker CD4 and the macrophage marker F4/80.2,20 Therefore, as stated in the “Introduction” section, on the basis of this assumption antibodies against these molecules have been used to deplete contaminating T cells and macrophages in functional studies that involve the isolation of CD8α+ and CD8α−DCs. However, as illustrated in the present report, a careful examination of spleen CD8α− DC phenotype revealed that a majority of these cells, but not CD8α+ DCs, expressed both molecules. CD4 expression by a proportion of CD8α−DCs has been previously reported by Pulendran et al6 in FLT3 ligand-treated mice and recently by Vremec et al.21This observation is of special relevance because a number of reports analyzing the differential function of CD8α− versus CD8α+ DCs were performed with purified CD8α− DC subsets from which CD4+F4/80+ DCs were depleted (ie, biased toward the CD4−F4/80− fraction). Therefore, these data could not be representative of the functional capacity of the whole CD8α− DC population, if CD4− and CD4+ CD8α− DCs do not represent two independent DC subsets, but rather different activation, maturation, and/or differentiation stages of the CD8α− DC population, as suggested by the data presented in the present study. In particular, our data regarding CD4 expression by CD8α−DCs indicate that this molecule is upregulated during CD8α− DC reconstitution after irradiation, that CD4 undergoes a dramatic down-regulation on culture, and that the CD4+ fraction of CD8α− DCs has a higher T-cell stimulatory and endocytic capacity (data not shown) than the CD4− fraction. In addition, some controversial results regarding the function of CD8α− versus CD8α+ DCs reported so far might be related to the DC isolation protocol employed and then to differences in the proportion of CD4+ F4/80+ cells among the CD8α− DC subset. In this sense, whereas some authors found that CD8α− DCs had a much higher T-cell stimulatory potential than CD8α+ DCs, which were consequently proposed to exert an immunosuppressor/tolerogenic role,4,5,11 several lines of evidence support the view that both subsets are equally capable of inducing T-cell stimulation.12,14 Moreover, in relation with this issue, it has been proposed that the T-cell response mediated by the CD8α+ DCs and CD8α− DCs is biased toward a TH1 and TH2 profile, respectively.12,22 In addition, it has been reported that splenic CD8α+ but not CD8α− DCs produce interleukin 12 (IL-12) after in vivo microbial stimulation.23 More recently, Ohteki et al13 have shown that CD8α+ DCs are more efficient than CD8α− DCs in producing interferon-γ in response to IL-12 during bacterial infection, suggesting that the CD8α+ DC subset may play an essential role as initiators of innate immunity and subsequent TH1 responses. Therefore, additional studies are needed to define more precisely the role of CD8α+ and CD8α− DCs in the induction of T-cell responses.
With regard to the origin of CD8α+ and CD8α− DCs, the fact that CD8α+ DCs were generated along with T cells after intrathymic transfer of CD4low precursors, devoid of myeloid reconstitution potential,3 led to the widely held hypothesis that CD8α+ DCs were lymphoid-derived DCs, although this point remains to be confirmed by clonal assays. Because CD8α+DCs but not CD8α− DCs were found in the progeny of CD4low precursors transferred intravenously,16CD8α− DCs were, therefore, considered as myeloid DCs. However, our results revealed that CD8α− DCs were generated from CD4low precursors and suggest, as mentioned above, that, in the study stating that CD8α− DCs do not derive from CD4low precursors, CD8α− DCs could have been missed because of the depletion of the CD4+and/or F4/80+ cells. Moreover, the data presented here support that CD8α+ and CD8α− DCs derive from a common precursor because both CD4low precursors and BM precursors generated the two DC types at a similar CD8α− DC-to-CD8α+ DC ratio than that existing in nonreconstituted control spleens. However, it remains possible that CD8α− DCs are derived from a myeloid precursor, present among the CD4low precursor population transferred in reconstitution experiments, which would be devoid of reconstitution potential for granulocytes and macrophages, because these cell types were not found among the progeny of CD4lowprecursors.24 However, assuming as previously proposed that CD8α+ DCs derive from CD4low lymphoid precursors, it seems unlikely that this precursor population could contain an unrelated CD8α− DC precursor different from the T cell-CD8α+ DC lymphoid precursor population, because, if this was the case, this precursor should be present among the CD4low precursors at the definite frequency to allow a correct reconstitution of the CD8α+ and CD8α− DC subsets. Alternatively, both CD8α+ and CD8α− DCs could derive from an independent nonlymphoid, nonmyeloid DC precursor. According to this hypothesis, CD8α+ DCs would not belong to the lymphoid lineage. This view is supported by two recent reports that describe a developmental dissociation between thymic (CD8α+) DCs and T cells in mice deficient for c-kit and the common cytokine receptor gamma chain25 and for Notch1.26 Nevertheless, against this differentiation model, and supporting the existence of a lymphoid DC lineage, a committed DC precursor expressing pTα messenger has been recently described in the human thymus.27
In conclusion, we favor the hypothesis that both CD8α+and CD8α− DCs have a common origin and derive from a lymphoid-committed precursor. This hypothesis is supported by the analysis of mice homozygous for an Ikaros dominant-negative mutation (Ikaros DN−/−) that lack T cells, B cells, natural killer cells, and both CD8α+ and CD8α− DCs but have a normal myeloid development.8 In addition, it has been demonstrated that the development of CD8α+ and CD8α− DCs does not depend on the presence of GM-CSF,28 which has been claimed to be an essential cytokine for the differentiation of myeloid DCs (reviewed in Shortman and Caux29). With regard to the concept that CD8α+ and CD8α− DCs derive from a common precursor, two independent studies analyzing the redistribution of DCs to T-cell areas of the mouse spleen after in vivo microbial stimulation23,30 suggest that CD8α+ and CD8α− DCs represent different maturation stages of splenic DCs. Nevertheless, the analysis of mice homozygous for an Ikaros null mutation (Ikaros C−/−),8 or deficient for RelB31 or PU.132 in which CD8α+ but not CD8α− DCs developed, does not allow one to draw any decisive conclusion with regard to their origin but supports that they represent two independent DC subsets. Therefore, a precise definition of the immediate precursors and of the differentiation process of both CD8α+ and CD8α− DCs is required to define conclusively their origin.
Finally, if CD8α+ and CD8α− DCs derive from a common lymphoid precursor as suggested by the data presented here, the majority of murine DCs would be of lymphoid origin, as previously proposed by Shortman and Caux,29 and, therefore, in the murine system myeloid DCs would correspond to monocyte-derived DCs recently described by Randolph et al.33
Acknowledgment
The authors would like to thank Dr A. Rolink (Basel Institute for Immunology, Basel, Switzerland) for the anti-CD40 hybridoma FGK45.
Supported by a grant from DGICYT (PB95-0376) and a grant from CAM (08.1/0018/1998) to C.A.
P.M., G.M.d.H., and F.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carlos Ardavı́n, Department of Cell Biology, Faculty of Biology, Complutense University, 28040 Madrid, Spain; e-mail: ardavin@bio.ucm.es.

![Fig. 4. T-cell stimulation capacity of CD8α+ DCs versus CD8α− DCs. / T-cell stimulatory capacity of CD8α+ versus CD8α− DC (A) and CD4− CD8α−versus CD4+ CD8α− DC (B). DCs from C57BL/6 mice were cultured with purified allogeneic T cells from BALB/c mice at the indicated APC:T cell ratios. After 5 days, T-cell proliferation was determined by [3H] thymidine uptake or CD25 expression. Data are representative of 3 experiments with similar results. In the [3H] thymidine incorporation assay, error bars represent the SD for triplicate cultures.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/7/10.1182_blood.v96.7.2511/5/m_h81900210004.jpeg?Expires=1765885862&Signature=QGHqIbpSeD4lCQ8yaPSNJVN2vGU1rrEqRRayZw95sIAQ84JD3trn4fkuYvnV7aqXFAFM3BdpemIc3qu3Hyu1p~kleoXSlhpxhXrRX7Ybqny2wgBdaIQfuYOQBuLb~bHnTRiTuuacVLh9KV3LmcZzi5lYaVimleBfS~hxxoNs42ZrctsbimuTDgsZdfQb5BQfzbwB8rJ58a~2ulha2iOOhgt7tm1~M75CSD2OEMRz~3clcZa4j5FL9JDVd4qcFgzDIfbv0fP7cq418bfoynkveRAadeF8uZ-3m-o7zEIAfw5Jkx07TEb~fI-u71Y~oIya78EEOJC9PiCXkFqzKyCPpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal