Abstract
Both in vitro and in vivo studies established that interleukin 7 (IL-7) is essential for differentiation of immature T cells and B cells but not natural killer (NK) cells in the mouse. In humans, although both T-cell and B-cell progenitors express the functional IL-7 receptor that consists of IL-7Rα and the γcommon (γc) chain, this lymphocyte receptor system is critical for T lineage but not for B lineage development. Indeed, complete γc deficiency like IL-7Rα deficiency results in the arrest of T-cell but not B-cell development (T−B+ SCID). However, partial deficiency of γc caused by missense mutations results in a T+B+ phenotype and a delay of clinical presentation. It was therefore plausible to assume that partial deficiency of IL-7Rα, like partial γc deficiency may lead to a milder clinical and immunologic phenotype. A P132S mutation in the IL-7Rα was identified in 3 patients with severe combined immunodeficiency (SCID) within an extensively consanguineous family. Substitution of proline with serine in the extracellular portion of IL-7Rα did not affect IL-7Rα messenger RNA (mRNA) and protein expression, but severely compromised affinity to IL-7, resulting in defective signal transduction. In response to IL-7 stimulation, Jak-3 phosphorylation was markedly reduced in both patient cells as well as in COS cells reconstituted with mutant IL-7Rα. Surprisingly, this partial deficiency of IL-7Rα resulted in a severe phenotype, including markedly reduced circulating T cells while sparing B-cell numbers similar to γc chain deficiency. However, unlike the previously reported cases, serum immunoglobulins were virtually absent. Further, unlike γc deficiency, NK cell numbers and function was preserved. Despite the partial deficiency, clinical presentation was indistinguishable from a complete γc deficiency, including severe and persistent viral and protozoal infections and failure to thrive. Unlike partial γc deficiency, a partial deficiency of IL-7Rα results in an arrest of T-cell development, leading to typical severe combined immunodeficiency. This underscores the critical role of IL-7Rα chain in the differentiation of T cells.
Introduction
The generation of lymphocytes with a wide variety of antigen receptor specificity is a central event in lymphocyte differentiation. It provides the immune system with the capacity to respond to a vast array of different antigens. This is accomplished by somatic gene rearrangement of the variable, diversity, and joining gene segments of the T-cell and B-cell receptors.1,2 This process is lineage specific and follows a defined temporal sequence, with T-cell receptor beta (TCRβ) locus rearrangement preceding that of TCRα in T cells. In the thymus, early triple negative (TN) cells (CD3−CD4−CD8−) rearrange the TCRβ gene, and express this protein in conjunction with an invariant pre-TCRα chain and components of the CD3 complex. Signaling through this complex is essential to the progression of differentiation. Subsequently, these TN cells rearrange and express the TCRα, leading to the creation of a complete αβ T-cell receptor and the expression of both CD4 and CD8 coreceptors (double positive [DP] thymocytes).3-5
This critical transition from TN to DP thymocytes is dependent on interleukin 7 (IL-7) secreted by thymic stromal cells. This cytokine stimulates the proliferation of TN cells6-8 and promotes TCRβ rearrangement in vitro.9 These signals are transmitted through an IL-7 receptor consisting of the IL-7Rα and the γ common (γc) chains. Much has been learned about the function of these receptor chains from null mutations in mice and humans. Null mutations of γc in humans have been identified as causing X-linked severe combined immunodeficiency (X-SCID)10 and typically show severely reduced T-cell and natural killer (NK) cell populations, but normal numbers of B cells. In addition to reduced T- and NK-cell numbers, γc knockout mice also have dramatically reduced B-cell populations,11 12 indicating that γc is more critical for B-cell development in mice than in humans.
The γc chain would appear to have 2 important roles in cytokine receptor complexes. The first is to increase the affinity of ligand binding, whereas the second is to recruit the cytoplasmic Jak-3 tyrosine kinase. The importance of Jak-3 to γc function was also realized through studies of deficiencies in mice and humans. Jak-3–deficient mice have a severe defect in T- and B-cell development, similar to γc-deficient mice.13-15Consistently, human Jak-3 deficiency, like human γc deficiency (X-SCID), presents with normal numbers of circulating B cells, but no mature T cells.16 17
A comparison of murine knockouts of cytokine receptors using γc suggests that the IL-7R is the most important in early lymphocyte development. Thus, the phenotype of γc deficiency would appear to be mainly due to an absence of functional IL-7R complexes. Similar to γc-deficient mice, IL-7 and IL-7Rα knockout mice have markedly reduced numbers of B and T cells. Although the arrest of T-cell development in γc-, IL-7–, and IL-7Rα–deficient mice is early and profound,18 the lack of IL-7Rα appears most severe; suggesting that IL-7Rα may mediate developmental signals not arising from the IL-7Rα/γc complex. This may involve TSLP, an IL-7–like factor secreted by thymic stromal cells, which supports the growth of both T- and B-cell precursors.19
One significant difference between the IL-7Rα and γc knockouts in mice is the lack of NK-cell development in γc deficiency. This observation, in particular, predicted that a subgroup of human SCID with reduced T-cell populations, but normal NK-cell numbers, might arise from aberrant IL-7Rα expression or function. Indeed, Puel et al21 recently reported on 2 patients with T−B+ NK+ that was caused by IL-7Rα deficiency. Only in one of the patients were null mutations in the coding region of IL-7Rα found. We demonstrate here a missense mutation in the IL-7Rα extracellular portion in 3 related patients. This mutation leads to impaired ligand binding but does not affect IL-7Rα messenger RNA (mRNA) or protein expression. This partial deficiency is sufficient to block T-cell development and lead to a SCID phenotype.
Materials and methods
Peripheral lymphocyte function assays
Cell surface markers of peripheral blood cells were determined by immunofluorescent staining and flow cytometry (Epics V; Coulter Electronics, Hialeah, FL) with antibodies purchased from Coulter Diagnostics. To assay lymphocyte proliferation, peripheral blood mononuclear cells (isolated by Ficoll-Hypaque gradient centrifugation) were incubated at 37°C (5% CO2) in complete culture medium RPMI-1640, supplemented with 10% (vol/vol) fetal calf serum (FCS) (Gibco/BRL, Gaitherburg, MD). Cells were incubated in round bottom tissue culture plates with or without phytohemagglutinin (PHA) (Difco Laboratories Inc, Detroit, MI), anti-CD3 (Ab-1 antibody, Oncogene Science Inc, Mineola, NY), or formalin-treated SAC (Calbiochem Corp, La Jolla, CA). Four hours before termination of the culture, 0.037 MBq (1 μCi) [3H]thymidine (24.79 × 1010 Bq/mmol [6.7 Ci/mmol]) was added to each well. The cells were then harvested and samples counted in a liquid scintillation counter.
Western blotting
The 2 × 106 cells were pelleted and lysed in 50 μL lysis buffer (20 mmol/L Tris pH 7.4, 150 mmol/L NaCl, 1% Triton X-100, 5 mmol/L EDTA, 2 mmol/L Na3VO4, 1 mmol/L PMSF), incubated on ice for 15 minutes, followed by centrifugation for 10 minutes at 12 000g. The supernatant was collected, 2 × SDS-gel sample buffer added, and the samples separated on SDS-polyacrylamide gel. Proteins were electrophoretically transferred to nitrocellulose membrane (Hybond-C; Amersham Corp, Arlington Heights, IL), and blocked overnight with 5% milk in 10 mmol/L Tris, 150 mmol/L NaCl pH 8.0 (TBS). The blots were incubated with the primary antibody in TBS-T (0.05% Tween-20) + 1% milk for 2 hours at room temperature, washed, and incubated with secondary detecting antibodies directly conjugated to horseradish peroxidase (donkey-antirabbit-horseradish peroxidase [HRP] or sheep-antimouse-HRP; Amersham Corp). The filters were then incubated with enhanced chemiluminescence reagents from Gibco, BRL, as per manufacturer's instructions and exposed to film. Antibodies to the IL-7Rα, IL-2Rγ chains and Jak-3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunoprecipitation
The 1 × 106 transfected COS-7 cells or 107 Epstein-Barr virus (EBV) transformed B cells per sample were stimulated with rIL-7 (Genzyme Corp) and 1 mL 1% Triton lysates prepared. Anti-Jak-3 and protein-A were added to the lysates, after clarification by centrifugation, and incubated overnight at 4°C with agitation. The immunoprecipitates were washed 3 times in lysis buffer, SDS-sample buffer added, and electrophoresed as above.
RNA and DNA preparation
DNA and RNA were isolated from peripheral blood mononuclear cells after Ficoll-Hypaque gradient centrifugation. To isolate RNA, cells were resuspended in Trizol reagent (Gibco/BRL) and total RNA isolated per the manufacturer's instructions. DNA was isolated by proteinase K digestion of cells in lysis buffer (100 mN Tris pH 8.0, 5 mmol/L EDTA, 0.2% SDS, 200 mmol/L NaCl) at 37°C for 4 hours. DNA was purified by 2 phenol/chloroform extractions, followed by ethanol precipitation.
Polymerase chain reaction and sequencing of the IL-7Rα transcript and genomic sequence
Oligo-dT primed first strand complementary DNA (cDNA) synthesis was performed on total RNA. Aliquots were used in polymerase chain reactions (PCRs) designed to isolate the entire coding region of the IL-7 and Jak-3 message. The IL-7Rα coding sequence was isolated using the primers 5′-CGCAGACCATGTTCCAT and 5′-GACTTGAATGGCACTCGCTG. To amplify exon 4 from genomic DNA, the primers 5′-CCTTGGCTGCCCTTTAGACA and 5′-GTTGATGTAATTATTTTATTTTTGAAT were used, corresponding to the 5′ and 3′ ends of the exon, respectively. For PCR from genomic DNA template, the cycle conditions 94°C (30 seconds), 45°C (30 seconds), and 72°C (90 seconds) were used for 5 cycles, followed by 30 cycles with an annealing temperature of 65°C. All PCR products were electrophoresed on agarose gels, purified, and directly sequencing using Thermosequenase and 33P-ddNTPs (Amersham).
Transfection of COS cells
COS-7 cells were maintained in DMEM with 10% FCS. The eucaryotic expression vector pcDNA3 (Invitrogen Corp, San Diego, CA) was used to express complete cDNAs for Jak-3, IL-2Rγ (a gift from Dr G. Mills, MD Anderson Cancer Center, Houston, TX), IL-7Rα, and the mutated IL-7Rα isolated from the patient. COS cells were transfected using the cationic lipid reagent lipofectamine and given 72 hours to express the transfected cDNAs before harvesting.
125I-labeled IL-7 binding assay
125I-labeled IL-7 was prepared using carrier-free IL-7 (Medicorp) and Iodogen (Pierce) as per the manufacturer's instructions. Iodination reactions were desalted and the fraction of highest specific activity determined by TCA precipitation. Control and patient EBV-transformed B cells (1 × 106) were resuspended in RPMI (5% FCS) at 4°C and varying amounts of125I-labeled IL-7 added. To determine the level of nonspecific binding, parallel samples were incubated for 2 hours at 4°C with a 100-fold excess of unlabeled IL-7. To remove unbound125I-labeled IL-7, samples were then spun through 200 μL of oil and the supernatant aspirated. The bottom of the tube containing the cell pellet was taken and bound 125I-labeled IL-7 measured by γ-radiation counting.
Results and discussion
Clinical presentation
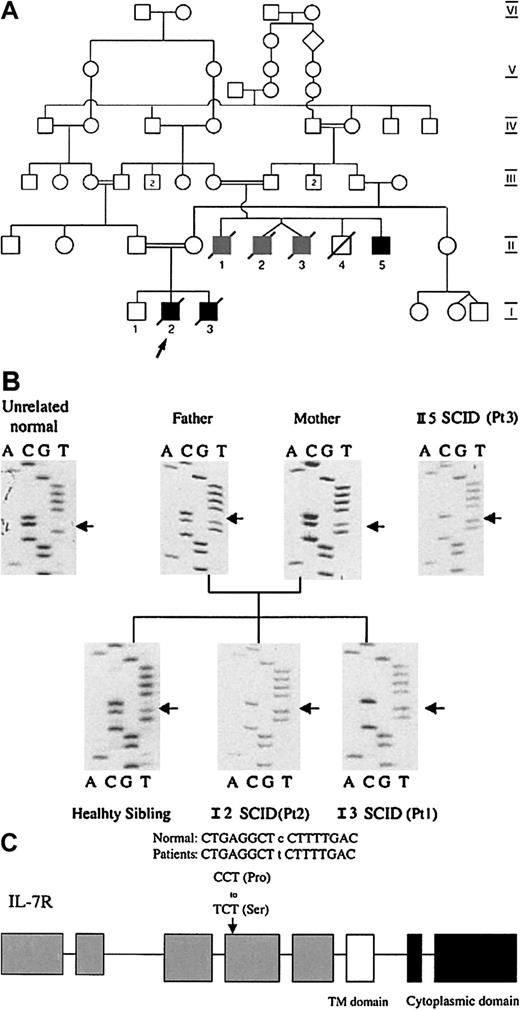
The proband, patient 1 (I-2) was the second child born at term to consanguineous parents of Sicilian descent (Figure1). At the age of 4 months, he presented with persistent oral thrush, oral ulcers, and failure to thrive. He had no palpable lymph nodes and no thymus shadow on a chest radiograph. Patient 2 (I-3) was diagnosed soon after birth and is the younger brother of patient 1. A third sibling (I-1), now 5 years old, has always been healthy. The proband's mother had 5 first cousins (II-1 through 5), born to the brother of the proband's maternal grandfather and the sister of his paternal grandfather. Three of them (II-1 through 3) died in infancy from failure to thrive, diarrhea, and fungal and bacterial infections. An autopsy on one of them (II-3) demonstrated findings consistent with SCID. Another brother (II-4) was born with esophageal atresia and died shortly after birth, his autopsy reportedly showed normal lymphoid tissue. The remaining sibling, patient 3 (II-5) presented with oral candidiasis at the age of 2 weeks and failure to thrive. No thymic shadow was detected on chest x-ray film and peripheral blood showed persistent lymphopenia. The patient had no lymph nodes, failed to reject a skin allograft and did not show an increase in the blood IgG and IgM antibodies for DTP after 3 vaccinations.20 He had a successful HLA matched bone marrow transplantation 25 years ago and is alive. The parents of these 5 children were first cousins. This family history of primary severe immunodeficiency with multiple affected male infants strongly suggested an X-linked inheritance, and raises the possibility of X-SCID caused by mutations of the γc chain.10 Nevertheless, the consanguinity in this family suggested an alternative, autosomal recessive inheritance.
The family pedigree and the IL-7Rα sequences.
(A) The family pedigree showing an inbred family with consanguinity across 5 generations. Information concerning early infant deaths or severe combined immunodeficiency could only be obtained for the 2 most recent generations. Patients 1(I-2) and 2 (I-3) and one of their cousins (II-5) (solid black symbols) were diagnosed with SCID. Three other male cousins (II-1-3) (gray symbols) died in infancy from severe infections consistent with SCID. (B) IL-7Rα sequences from the affected family. IL-7Rα exons were isolated from genomic DNA by PCR and directly sequenced. Patients 1, 2, and 3 were homozygous for a C to T transition at nucleotide 394 in exon 4, leading to a proline to serine substitution (P132S) in the extracellular domain of IL-7R. The sibling of patient 1 and 2 and their parents harbored both wild and mutant alleles. In contrast to this family, IL-7R sequences from 60 unrelated control individuals failed to show any alteration to the normal sequence in exon 4.
The family pedigree and the IL-7Rα sequences.
(A) The family pedigree showing an inbred family with consanguinity across 5 generations. Information concerning early infant deaths or severe combined immunodeficiency could only be obtained for the 2 most recent generations. Patients 1(I-2) and 2 (I-3) and one of their cousins (II-5) (solid black symbols) were diagnosed with SCID. Three other male cousins (II-1-3) (gray symbols) died in infancy from severe infections consistent with SCID. (B) IL-7Rα sequences from the affected family. IL-7Rα exons were isolated from genomic DNA by PCR and directly sequenced. Patients 1, 2, and 3 were homozygous for a C to T transition at nucleotide 394 in exon 4, leading to a proline to serine substitution (P132S) in the extracellular domain of IL-7R. The sibling of patient 1 and 2 and their parents harbored both wild and mutant alleles. In contrast to this family, IL-7R sequences from 60 unrelated control individuals failed to show any alteration to the normal sequence in exon 4.
Immunologic phenotype
Typically, γc deficient patients have low numbers of circulating T cells, but normal B-cell numbers. Consistent with this, all 3 patients (I-2 and 3 II-5) had markedly reduced T cells, as determined by flow cytometry analysis of peripheral blood lymphocyte CD2 and CD3 expression (Table 1; Puel et al21). In contrast, B cells (CD19) were relatively abundant. Not surprisingly, PHA and anti-CD3 responses were barely above background, whereas B cells appeared functionally mature, as they expressed surface Ig and responded normally to SAC stimulation. However, immunoglobulin production appeared to be depressed, as patients had undetectable levels of serum IgA or IgM (Table 1; Puel et al21). IgG levels were also below normal in patient 1, but within normal limits for patient 2. It is likely, however, given his tender age, that the normal IgG level represented maternally transferred immunoglobulin.
Humoral and cellular immunity
| . | Patient 1 . | Patient 2 . | Control or normal range . |
|---|---|---|---|
| Markers; % (count) | |||
| CD2 | 8 (143) | 8.4 (421) | 75-96 |
| CD3 | 0.2 (4) | 0.4 (9) | 60-85 |
| CD4 | 0 (0) | 0.5 (4) | 30-60 |
| CD8 | 0.1 (2) | 0 (0) | 15-35 |
| CD56 | 6 (125) | 14 (711) | 5-20 |
| CD16 | 15 (277) | 7 (352) | 5-20 |
| CD19 | 81 (1493) | 76 (4074) | 2-20 |
| Mitogenic responses; counts × 10−3 | |||
| Phytohemagglutinin (PHA) | 1.3 | 0.7 | 90-130 |
| Anti-CD3 | 2.5 | 1.0 | 35-55 |
| Staphylococcus aureus Cowan A | 11.0 | 90.6 | 10-60 |
| Serum immunoglobulins; g/L | |||
| IgG | 0.5 | 1.7 | 2.3-14.1 |
| IgM | 0.1 | < 0.1 | 0.0-1.4 |
| IgA | 0.1 | 0.1 | 0.0-0.8 |
| . | Patient 1 . | Patient 2 . | Control or normal range . |
|---|---|---|---|
| Markers; % (count) | |||
| CD2 | 8 (143) | 8.4 (421) | 75-96 |
| CD3 | 0.2 (4) | 0.4 (9) | 60-85 |
| CD4 | 0 (0) | 0.5 (4) | 30-60 |
| CD8 | 0.1 (2) | 0 (0) | 15-35 |
| CD56 | 6 (125) | 14 (711) | 5-20 |
| CD16 | 15 (277) | 7 (352) | 5-20 |
| CD19 | 81 (1493) | 76 (4074) | 2-20 |
| Mitogenic responses; counts × 10−3 | |||
| Phytohemagglutinin (PHA) | 1.3 | 0.7 | 90-130 |
| Anti-CD3 | 2.5 | 1.0 | 35-55 |
| Staphylococcus aureus Cowan A | 11.0 | 90.6 | 10-60 |
| Serum immunoglobulins; g/L | |||
| IgG | 0.5 | 1.7 | 2.3-14.1 |
| IgM | 0.1 | < 0.1 | 0.0-1.4 |
| IgA | 0.1 | 0.1 | 0.0-0.8 |
In contrast to the typical presentation of γc deficiency in both mice and humans, both patients demonstrated normal NK-cell populations (CD56) (Table 1) and normal NK function (not shown). Normal γc gene sequences and protein expression levels (not shown) confirmed that this cytokine receptor chain was not responsible for the phenotype of these patients.
IL-7Rα gene sequences in the patients and family
In both γc and Jak-3 deficient mice, there is an ablation of B-cell development that is not apparent in the respective human deficiencies. Similar to these murine deficiencies, both IL-7 and IL-7Rα deficient mice lack B and T cells. We therefore reasoned that a similar species-specific difference might occur in IL-7Rα deficiency and entertained the possibility that aberrant IL-7 or IL-7Rα function might cause our patients' phenotype. Indeed, a recent report described an IL-7Rα mutation in a patient causing a similar phenotype.
A phenotype comprised of an absence of T cells, combined with normal to elevated B-cell and NK-cell numbers, was identified in 15 of our patient population. IL-2Rγ, Jak-3, and IL-7 were analyzed for mutations in all these patients, but were found to be normal (not shown). Although no mutations were detected in the IL-7Rα cDNA coding sequences of 12 of the 15 patients, sequencing of samples from patients 1 and 2 revealed a C to T transition at nucleotide 394 within exon 4. This is predicted to replace a proline residue with serine, at position 132 in the extracellular domain of the IL-7Rα receptor chain. This mutation was confirmed by direct sequencing of exon 4 genomic DNA PCR product, revealing the presence of only the mutant T nucleotide in both patients (Figure 1B). This implied that the mutation was homozygous, as the IL-7Rα gene is autosomal. The identical change was identified in genomic DNA extracted from fibroblasts of patient 3. Sequencing of genomic DNA from the parents, and the healthy sibling, revealed both the wild-type C and mutant T nucleotides at this position, consistent with heterozygosity. Eleven other family members were subsequently screened and found to carry either one mutant allele (6 heterozygotes) or a completely normal sequence. Homozygosity was associated with the SCID phenotype in all cases, whereas heterozygotes had no clinical symptoms or immunologic aberrations. Genomic DNA obtained from 60 unrelated individuals failed to show a similar C to T transition, further suggesting that this change is the disease-causing mutation.
IL-7Rα messenger RNA and protein expression in patient Epstein-Barr virus transformed B cells
The IL-7 receptor is expressed on immature B and T cells as well as in mature T cells, NK cells and macrophages. However, the patients had few circulating T lymphocytes, making it difficult to obtain sufficient primary cells for functional studies. We therefore used EBV transformed B lymphoblasts derived from one of the patients (P1). Unlike normal mature B cells, all EBV transformed cells appear to express high levels of the IL-7R. It is not immediately clear why these cells should express the IL-7 receptor, but this may be related to the transformation event, or alternatively, EBV may preferentially infect IL-7R+ B cells and prevent receptor down-regulation.
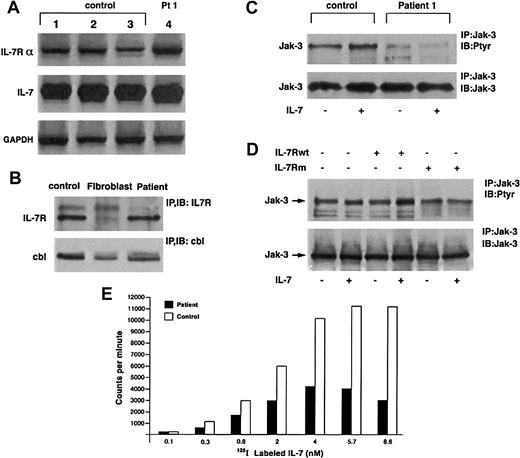
Both IL-7 and IL-7Rα mRNA could be detected in patient and control cells by reverse transcriptase-polymerase chain reaction (RT-PCR) (Figure 2A). Furthermore, semiquantitation analysis using serial dilutions of RNA revealed no significant difference between the patient and control samples (not shown).
IL-7 and IL-7Rα.
(A) IL-7 and IL-7Rα RNA expression is normal in patient cells. PCR was performed with IL-7 and IL-7Rα specific primers on cDNA from patient 1 and 3 control EBV cell lines. Control GAPDH products are also shown. (B) IL-7R expression is not altered. Il-7Rα was immunoprecipitated from lysates of control and patient EBV cell lines and human fibroblasts (negative control) and Western blotted with anti–IL-7R antibody. Anti-Cbl immunoprecipitation and Western blot were performed as a positive control. (C) Absence of Jak-3 tyrosine phosphorylation after IL-7 stimulation in patient cells. Patient and control cells were incubated for 10 minutes ± 30 ng/mL rIL-7. Lysates were precipitated with anti–Jak-3 antibody and blotted with antiphosphotyrosine (top panel) and anti–Jak-3 after stripping (bottom panel). (D) COS-7 cells were transfected with expression vectors encoding γc, Jak-3, and either wild-type or mutant IL-7Rα as indicated (top). Cells were incubated ± IL-7 for 10 minutes, lysed, and immunoprecipitated with anti–Jak-3 antibody and subsequently blotted with either antiphosphotyrosine (top) or anti–Jak-3 (bottom) antibodies. (E) Specific binding of125I-labeled IL-7 to EBV-transformed B cells derived form patient or healthy donor. IL-7 binding is presented as counts after subtraction of the background binding measured in the presence of a 100-fold excess of unlabeled IL-7.
IL-7 and IL-7Rα.
(A) IL-7 and IL-7Rα RNA expression is normal in patient cells. PCR was performed with IL-7 and IL-7Rα specific primers on cDNA from patient 1 and 3 control EBV cell lines. Control GAPDH products are also shown. (B) IL-7R expression is not altered. Il-7Rα was immunoprecipitated from lysates of control and patient EBV cell lines and human fibroblasts (negative control) and Western blotted with anti–IL-7R antibody. Anti-Cbl immunoprecipitation and Western blot were performed as a positive control. (C) Absence of Jak-3 tyrosine phosphorylation after IL-7 stimulation in patient cells. Patient and control cells were incubated for 10 minutes ± 30 ng/mL rIL-7. Lysates were precipitated with anti–Jak-3 antibody and blotted with antiphosphotyrosine (top panel) and anti–Jak-3 after stripping (bottom panel). (D) COS-7 cells were transfected with expression vectors encoding γc, Jak-3, and either wild-type or mutant IL-7Rα as indicated (top). Cells were incubated ± IL-7 for 10 minutes, lysed, and immunoprecipitated with anti–Jak-3 antibody and subsequently blotted with either antiphosphotyrosine (top) or anti–Jak-3 (bottom) antibodies. (E) Specific binding of125I-labeled IL-7 to EBV-transformed B cells derived form patient or healthy donor. IL-7 binding is presented as counts after subtraction of the background binding measured in the presence of a 100-fold excess of unlabeled IL-7.
We examined next whether the P132S mutation affected the level of IL-7Rα protein expression in patient cells, as substitution of proline with serine might be expected to destabilize the protein. However, immunoblotting of cell lysates with an anti–IL-7Rα antibody revealed similar levels of IL-7Rα expression in patient and control cells (Figure 2B). Fibroblasts, which do not express IL-7Rα, were used as a negative control. Thus, the mutation did not appear to affect either IL-7Rα mRNA or protein expression.
Absence of Jak-3 activation by patient IL-7Rα/γc receptors
IL-7Rα must form a complex with the γc chain to transmit intracellular growth signals, primarily because of its ability to bind the tyrosine kinase Jak-3. Recruitment of Jak-3 is essential, as its activation appears to be required for most of the signaling events downstream of the IL-7R receptor complex.22
To determine whether the P132S IL-7Rα chain could activate Jak-3, we stimulated patient and control EBV-transformed B-cell lines with recombinant human IL-7. Immunoprecipitation of Jak-3 and subsequent immunoblotting with antiphosphotyrosine revealed increased Jak-3 tyrosine phosphorylation in the control, but not in the patient sample (Figure 2C). The apparent difference in Jak-3 phosphorylation was not due to decreased Jak-3 expression in patient cells, as subsequent blotting with anti–Jak-3 revealed equivalent expression in both samples. Therefore, the mutated IL-7Rα appeared to be incapable of activating Jak-3.
To confirm this observation, we reconstituted the IL-7Rα/γc receptor complex in fibroblasts. COS-7 cells were transfected with Jak-3, γc, and either wild-type or mutant IL-7Rα cDNA in the pcDNA3 eucaryotic expression vector. Wild-type IL-7Rα and the mutated receptor were found to express equally well (not shown). On stimulation with IL-7, Jak-3 tyrosine phosphorylation increased in fibroblasts transfected with wild-type receptor, but not in those transfected with the mutant, despite equal expression of Jak-3 protein (Figure 2D). Thus, in agreement with the observations in EBV-transformed B cells, the mutant IL-7R was incapable of activating Jak-3 in response to cytokine.
Altered binding of 125I-labeled IL-7 to patient IL-7 receptors
As the P132S mutation localized to the extracellular domain of the IL-7Rα chain, it was possible that the defect in Jak-3 activation might stem from insufficient IL-7 binding. Although the mutated proline residue is not predicted (on the basis of related receptor crystal structures) to form a part of the ligand binding domain, substitution of the hydrophobic proline ring with serine may disrupt hydrogen bonding and alter receptor conformation. To explore this possibility, we measured the binding of 125I-labeled IL-7 to control and patient EBV-transformed B cells (Figure 2E). Binding of radiolabeled cytokine to patient cells was significantly decreased relative to the control. The level of 125I-labeled IL-7 binding is shown after subtraction of the background registered in the presence of a 100-fold excess of unlabeled IL-7. Scatchard analysis revealed high affinity-specific binding to control cells (Kd = 1.3 × 104 nmol/L), with patient cells demonstrating lower affinity binding (Kd = 3.9 × 104 nmol/L). Patient cells also demonstrated somewhat fewer (10% ± 6% in 3 separate experiments) binding sites than control cells.
The apparent decrease in receptor affinity for IL-7 is likely to result from minor structural alterations. It is highly likely that replacement of the rigid proline ring has altered hydrogen-bonding patterns, potentially destabilizing the folded protein structure. The link between the proline residue side chain and its amino group normally induces a bend in the protein backbone, that would be absent on serine substitution. Thus, although not a direct participant in the ligand-binding pocket, steric alterations caused by substitution of Pro132 are likely to be transmitted through the structure. These may contain secondary structure elements undergoing minor alterations in their relative positioning, thus leading to both reduced ability to bind IL-7 and a failure of triggering signal transduction.
Acknowledgment
C.M.R. is the Donald and Audrey Campbell Chair of Immunology.
Supported by the Medical Research Council of Canada and the Reichman Immunodeficiency Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chaim Roifman, The Hospital for Sick Children, IIIR Program, 555 University Ave, Toronto ON M5G 1X8, Canada; e-mail: croifman@sickkids.on.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal