Abstract
Immune thrombocytopenic purpura (ITP) is an autoimmune disease related to the presence of elevated levels of platelet-associated immunoglobulin, or autoantibodies. In recent years the importance of macrophage Fcγ receptors in the uptake of platelets in ITP has been confirmed. Although in patients with ITP the platelet destruction occurs in liver and spleen, in this present experimental mouse model the liver was the principal organ of sequestration of sensitized platelets. The uptake in the spleen, bone marrow, lung, and kidneys was negligible and not different from that in control animals. In addition, the trapped platelets did not return to circulation, and new cells derived from the platelet-storage pool or new thrombocytogenesis were necessary to restore the platelet count. The depletion of splenic and hepatic murine macrophages by liposome-encapsulated clodronate (lip-clod) was studied as a new strategy for ITP treatment. Lip-clod inhibits, in a dose-dependent manner, the antibody-induced thrombocytopenia. Moreover, lip-clod treatment rapidly restored (24 hours) the platelet count in thrombocytopenic animals to hematologic safe values, and despite additional antiplatelet antiserum treatment, mice were able to maintain this level of platelets at least up to 48 hours. The bleeding times in lip-clod–treated animals was not different from those in controls, demonstrating that the hemostasis was well controlled in these animals. The results presented in this study demonstrate that lip-clod treatment can be effective in the management of experimental ITP.
Introduction
Immune thrombocytopenic purpura (ITP) is an autoimmune disease in which platelet antibodies cause increased platelet consumption sometimes leading to hemorrhage. The destruction of platelets is mediated by the reticuloendothelial system (RES), particularly by splenic and hepatic macrophages,1,2and management of the disease is associated with the concept of decreasing the RES activity. In fact, it includes splenectomy, corticosteroids; intravenous high-dose gamma globulin (IVIg); anti-D immunoglobulins, danazol, and immunosuppression.3-7 In recent years the importance of the receptors for the Fc portion of IgG (FcγRs) in the uptake of platelets in ITP has been confirmed.8,9 Moreover, FcγRI- and FcγRIII-deficient mice are resistant to the development of experimental ITP.10
Oral corticosteroids are the first-line treatment of ITP, and about 60% to 80% of patients respond with an elevation of the platelet count.11 When this treatment fails, IVIg has been recommended as second-line therapy.12 However, patients refractory to conventional treatments comprise about 25% to 30% of patients with ITP13-15 with a risk of fatal hemorrhage of 16%,13 indicating that the treatment of refractory ITP is still unsolved, and new therapeutic approaches are needed. Thus, although the American Society of Hematology has developed guidelines for the initial management of ITP, there is no consensus on how to manage refractory cases.16-18
All these data encouraged us to search for a new approach in the management of ITP. Our strategy was based on the depletion of macrophages by delivering liposome-encapsulated clodronate (lip-clod) into the cells. Although a similar approach was initially reported by Ahn and colleagues19 using vinblastine-loaded platelets as a Trojan horse for macrophages, they never proved the blockade of phagocytic function and the method has not fulfilled its early promise.20 Moreover, the available data do not support its use.21
In this report we analyze the role of hepatic and splenic macrophages in a mouse model of ITP induced by antiplatelet antibodies (Ab). The depletion of these cells was carried out by the intravenous injection of lip-clod. This macrophage “suicide” method22 has been used in different experimental models23,24 and has important characteristics such as: (1) lip-clod inhibits the clearance of IgG-sensitized autologous red cells in mice25; (2) the intravenous injection of lip-clod ensures that clodronate is efficiently trapped by splenic and hepatic macrophages, killing the cells without affecting other organs22-24; (3) the free drug (from leakage of liposomes) has an extremely short half-life in circulation and body fluids. In addition, free clodronate is a nontoxic bisphosphonate already in use for the treatment of osteoporosis, osteolytic bone metastases, and bone resorption including Paget disease.26 27
Materials and methods
Mice
BALB/c mice were bred in the animal facility of the Academia Nacional de Medicina, Buenos Aires. Male and female mice aged 14 to 16 weeks and weighing 23 to 26 g were used throughout the experiments. They were maintained under a 12-hour light-dark cycle at a temperature of 22°C ± 2°C and fed with standard diet and water ad libitum. The experiments were conducted according to principles set forth in the Guide for the Care and Use of Laboratory Animals.28
Liposome-encapsulated clodronate
Clodronate (dichloromethylene bisphosphonate) was provided by Roche Diagnostics, Mannheim, Germany. Lip-clod was prepared using 86 mg of phosphatidylcholine (Lipoid EPC; LIPOID, Ludwigshafen, Germany), 8 mg of cholesterol (Sigma Chemical Co, St Louis, MO), and clodronate (0.7 mol/L), in a final volume of 4 mL as previously described.23 This lip-clod will be referred to in the text also as original preparation or lip-clod 1:1. Empty liposomes were prepared under the same conditions in phosphate-buffered saline (lip-PBS). The intravenous injection of 0.1 mL/10g body weight of this lip-clod suspension induces the complete depletion of splenic and hepatic macrophages within 24 hours.23
Preparation of platelets and platelet counts
Platelet-rich plasma (PRP) was prepared as previously outlined.29 Briefly, blood from mice was collected in plastic tubes containing sodium citrate (3.8% w/v), pH 7.4. After centrifugation at 200g for 10 minutes, PRP was removed and pooled. Platelet-poor plasma (PPP) was obtained by centrifugation of platelets at 1800g for 10 minutes. When necessary, the concentration of platelets was adjusted by addition of PPP. Platelets were counted in a hemocytometer using a buffer containing ammonium oxalate 1% for erythrocyte lysis. In agreement with others,30 31 the range for platelet count in our BALB/c mice was from 350 to 500 × 103/μL (n = 75).
Rabbit-antimouse platelet antiserum
Polyclonal antibody against mouse platelets was prepared in rabbits. For this purpose, a pool of PRP of BALB/c mice was centrifuged at 200g for 10 minutes to eliminate erythrocytes. The supernatant was centrifuged at 1800g for 20 minutes, the platelets resuspended, and injected intravenously into rabbits (1 × 109 platelets/dose) at days 1, 15, 30, and 40. Ten days later the animals were bled. The sera were adsorbed with BALB/c mice red blood cells to remove a weak antimouse erythrocyte activity. The antiplatelets antibodies belong to the IgG class (purified by Sepharose G; Sigma). To avoid a partial blockade of the RES by IgG aggregates we used, like others,32-34 dilutions of whole serum instead of purified IgG.
Labeling of platelets with 111In-oxine
Platelet labeling was performed carefully to avoid cell damage. A pool of blood from 10 BALB/c mice was drawn in acid-citrate-dextrose buffer (ACD-A) (9 vol blood to 1 vol ACD-A). The platelet pellet obtained by centrifugation of PRP at 1800g for 10 minutes (approximately 0.5-1 × 1010 platelets) was washed with ACD-A-saline, prior to adjusting to pH 6.8 with NaOH 1 mol/L. The platelets were resuspended in the same buffer and incubated with 200 μCi (7.4 MBq) of 111In-oxine at 24°C for 20 minutes. The reaction was terminated by addition of 4 mL of PPP. After 7 minutes the sample was centrifuged 5 minutes at 1800g to remove free111In-oxine and the platelets were suspended in PPP. Labeling efficiency measured by counting radioactivity of the pellet and supernatant was 82%.
Clearance studies
Mice were injected intravenously with 6 × 108111In-oxine labeled platelets (111In-Plat).35 After 90 minutes (time necessary for 111In-Plat to equilibrate with the splenic pool) the animals were injected with Ab or saline. The distribution of111In-Plat was determined by serial bleedings. The amount of counts per minute (cpm) in peripheral blood at 1 minute after Ab or saline injection was considered as 100% of cpm in circulation (t = 0 minute). Blood samples were diluted in saline, centrifuged, and free and intracellular 111In-oxine was determined. The total number of counts in the bone marrow was estimated assuming that one sixth of the marrow space was within the femur.35 The111In-oxine in the blood at 24 hours was obtained considering the total volume as 0.09 mL/g body weight. This value plus the radioactivity present in the organs was considered the total radioactivity. Taking into account the short half-life of111In (67.4 hours), the radioactivity of different samples from kinetics experiments was evaluated simultaneously at the end of experiments.
Platelet survival study
Mice were injected with 200 μL of 111In-Plat as described above. Blood samples were taken at 1, 3, 24, 48, 72, and 120 hours after injection. Free and intracellular radioactivity was distinguished by counting packed platelets and supernatants after washing the cells. Mean platelet survival was determined as previously described.36
Ex vivo platelet aggregation
Platelet aggregation studies were performed by the standard turbidimetric technique using a Chrono Log Corporation aggregometer. Aggregation was initiated by addition of 5 to 20 μL of aggregating agents: (1) adenosine diphosphate (ADP) 50 μmol/L, (2) collagen 20 μg/mL, or (3) arachidonic acid 1 nmol/L. Aggregation was quantified as percentage of maximal amplitude 5 minutes after addition of aggregating agents.
Bleeding time test
The assay was done by making a standardized incision of a depth of 3 mm in parallel to the tail veins of mice, at a site where no visible vessel was seen. The blood was carefully removed at exactly 30-second intervals with a filter paper, until bleeding stopped completely. The time taken for the blood flow to stop was recorded. The normal bleeding time of mice was less than 2 minutes (n = 15).
Statistical analysis
Intergroup contrasts of dimensional variables were compared with one-way analysis of variance (ANOVA) followed by Bonferronit test. All statistical tests were interpreted in a 2-tailed fashion to estimate P values.
Results
Effect of lip-PBS and lip-clod on platelet, red blood cell, and leukocyte counts
Mice were injected intravenously with 200 μL of either saline, lip-PBS, or lip-clod, and the platelet count was evaluated at 24, 48, 72, and 96 hours after injection. Lip-clod induced a slight but significant thrombocytopenia in the animals during the period from 48 to 72 hours after treatment, returning to the normal platelet count at 96 hours after treatment. The results, expressed as mean ± SEM of platelets × 103/μL were the following: 48 hours, saline, 410 ± 10; lip-PBS, 420 ± 1; lip-clod, 376 ± 3* (*P < .01 versus saline and lip-PBS treated groups, n = 6); 72 hours, saline, 411 ± 4; lip-PBS, 440 ± 6; lip-clod, 320 ± 10* (*P < .001 versus saline and lip-PBS treated groups, n = 6). However, this effect on the platelet count was not observed when lip-clod diluted 1:8 to 1:32 was administered (not shown). The number of peripheral red blood cells and leukocytes was not significantly different from controls at any time after treatment.
Effect of rabbit antimouse platelet antiserum on the platelet count
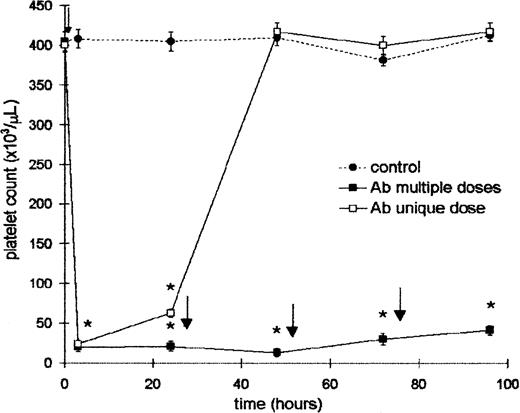
Mice were injected intraperitoneally with a unique dose of 25 μL (100 μL diluted 1:4) of Ab and the platelet count was evaluated at the times indicated in Figure 1. A severe thrombocytopenia was induced at 4 hours after injection. The effect was platelet specific because the Ab did not modify either the leukocyte count or hematocrit at any time after treatment. However, 24 hours later this value was slightly increased, and the recovery of control levels was achieved 48 hours after Ab injection. Taking into account this result, another group of mice was injected with a daily dose of Ab to maintain the platelet count at low levels. This schedule was used to obtain a sustained thrombocytopenia throughout the experiments. In addition, the treatment with normal rabbit serum did not modify either the platelet and leukocyte count or hematocrit (not shown). For the ITP model it is important to know whether rabbit Ab at a concentration equivalent to that obtained in vivo did not induce platelet aggregation. Thus, taking into account that the amount of whole blood in normal mice is approximately 7% of the body weight (range, 1.6-1.8 mL),37 25 μL of Ab (amount that induces thrombocytopenia) was incubated with 0.5, 1, and 1.5 mL of whole blood during 1 hour at 37°C. After this period the microscopic aggregation of platelets was not observed. In addition, the platelet counts before and after Ab treatment were similar, confirming that aggregates were not formed. Complement-dependent lysis of platelets was not observed.
Effect of rabbit ant-mouse platelet antiserum on the platelet count.
Two groups of 6 BALB/c mice were injected intraperitoneally with 25 μL (100 μL dilution 1:4) of Ab. One of them received a single dose (time = 0) (■), and the other received a daily dose of Ab (▪). Control mice were injected with 25 μL of saline (●). Control and Ab-treated groups were bled at 4, 24, 48, 72, and 96 hours for platelet count. The arrows represent the time (after bleeding) at which the Ab was administered. Values represent mean ± SEM. *P < .0001, significantly different from control group at 4, 24, 48, 72, and 96 hours, Bonferroni test (2-tailed).
Effect of rabbit ant-mouse platelet antiserum on the platelet count.
Two groups of 6 BALB/c mice were injected intraperitoneally with 25 μL (100 μL dilution 1:4) of Ab. One of them received a single dose (time = 0) (■), and the other received a daily dose of Ab (▪). Control mice were injected with 25 μL of saline (●). Control and Ab-treated groups were bled at 4, 24, 48, 72, and 96 hours for platelet count. The arrows represent the time (after bleeding) at which the Ab was administered. Values represent mean ± SEM. *P < .0001, significantly different from control group at 4, 24, 48, 72, and 96 hours, Bonferroni test (2-tailed).
Lip-clod inhibits the Ab-induced thrombocytopenia in a dose-dependent manner
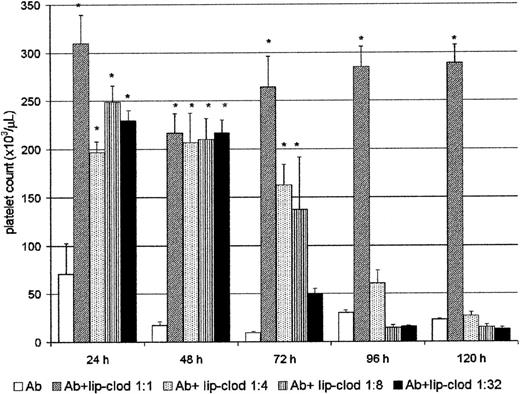
Clearance of immune complexes is one of the most important functions carried out by the RES, and it is mostly dependent on the FcγR present on the surface of hepatic and splenic macrophages.38 We have previously shown that clearance of immune complexes can be strongly inhibited by lip-clod in a mice model of sensitized autologous erythrocytes.25 Then, we further analyzed whether lip-clod inhibits the Ab-induced thrombocytopenia. For this purpose, groups of mice were injected intravenously with a unique dose of 200 μL of different amounts of lip-clod. Twenty-four hours later the animals were injected intraperitoneally with Ab. The administration of Ab was repeated at 24-hour intervals during the course of the experiment, and the mice were bled daily for platelet count (before Ab administration). As shown in Figure2, whereas lip-clod 1:1 inhibits the Ab-induced thrombocytopenia up to 120 hours, a decreasing and dose-dependent effect was observed with lip-clod 1:4, 1:8 and 1:32, respectively. In the lip-clod 1:1-treated group, the inhibition of thrombocytopenia was partial at 172 hours (Ab plus lip-clod: 145 × 103 ± 17 × 103 versus Ab: 16 × 103 ± 4 × 103 platelets/μL,P < .001, n = 6), and at 200 hours the level of platelets was similar in both groups (Ab plus lip-clod: 22 × 103 ± 5 × 103 versus Ab: 11 × 103 ± 2 × 103 platelets/μL). On the other hand, lip-PBS did not alter the Ab-induced thrombocytopenia at all (not shown). Then, taking into account the long-lasting effect of lip-clod 1:1 (original preparation, see “Materials and methods”), we used this dose throughout the experiments.
Lip-clod inhibits the Ab-induced thrombocytopenia in a dose-dependent manner.
Five groups of 6 BALB/c mice were injected intravenously with 200μL of saline, or different amounts of lip-clod (1:1 ; 1:4 ; 1:8 or 1:32). After this treatment the animals were injected at 24, 48, 72, 96, and 120 hours with a daily intraperitoneal dose of 25μL of Ab. The mice were bled daily for platelet counting as described in “Materials and methods.” Bars represent mean ± SEM *P < .0001, significantly different from the control Ab-treated group at 24, 48, 72, 96, and 120 hours, Bonferroni test (2-tailed).
Lip-clod inhibits the Ab-induced thrombocytopenia in a dose-dependent manner.
Five groups of 6 BALB/c mice were injected intravenously with 200μL of saline, or different amounts of lip-clod (1:1 ; 1:4 ; 1:8 or 1:32). After this treatment the animals were injected at 24, 48, 72, 96, and 120 hours with a daily intraperitoneal dose of 25μL of Ab. The mice were bled daily for platelet counting as described in “Materials and methods.” Bars represent mean ± SEM *P < .0001, significantly different from the control Ab-treated group at 24, 48, 72, 96, and 120 hours, Bonferroni test (2-tailed).
Reversal of antibody-induced thrombocytopenia by lip-clod
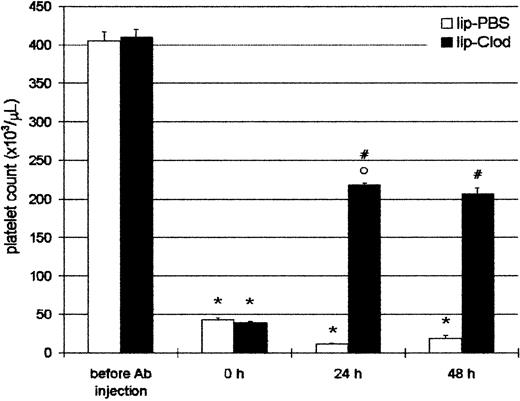
Taking into account that ITP patients are usually admitted to the hospital with thrombocytopenia, we further investigated if thrombocytopenic animals were capable of recovering the platelet count to a hemostatically safe level after lip-clod treatment. For this purpose, animals were injected intraperitoneally with Ab, and 4 hours later (time = 0 hour, Figure 3) the mice had a platelet count less than 5 × 104/μL. After this, the mice were injected intravenously with 200 μL of lip-PBS or lip-clod. The animals were bled daily for platelet count and reinjected with Ab to obtain a sustained thrombocytopenia. As shown in Figure 3, lip-clod treatment rapidly restored (24 hours) the platelet count to approximately 50% of normal values. Moreover, despite additional Ab treatment, mice were able to maintain this level of platelets (48 hours). Although to a lesser extent, the recovery of platelet count was also observed with lip-clod diluted up to 1:64 (24 hours: 11.2% ± 0.3% and 48 hours: 20% ± 1.0% of control, n = 6).
Reversal of antibody-induced thrombocytopenia by lip-clod.
Two groups of 6 BALB/c mice were injected intraperitoneally with 25μL of Ab. After 4 hours of Ab treatment the mice were bled for platelet counting (time = 0 hours) and 15 minutes later the mice were injected intravenously with 200 μL of lip-PBS or lip-clod. At 24 hours the animals were bled for platelet count and reinjected with Ab. Finally, the platelet number was evaluated at 48 hours. Bars represent mean ± SEM. *P < .001 and °P < .01, significantly different from control group (before Ab injection).#P < .001, significantly different from lip-PBS–treated group, Bonferroni test (2-tailed).
Reversal of antibody-induced thrombocytopenia by lip-clod.
Two groups of 6 BALB/c mice were injected intraperitoneally with 25μL of Ab. After 4 hours of Ab treatment the mice were bled for platelet counting (time = 0 hours) and 15 minutes later the mice were injected intravenously with 200 μL of lip-PBS or lip-clod. At 24 hours the animals were bled for platelet count and reinjected with Ab. Finally, the platelet number was evaluated at 48 hours. Bars represent mean ± SEM. *P < .001 and °P < .01, significantly different from control group (before Ab injection).#P < .001, significantly different from lip-PBS–treated group, Bonferroni test (2-tailed).
Effect of lip-clod in the organ uptake of 111In-oxine labeled platelets
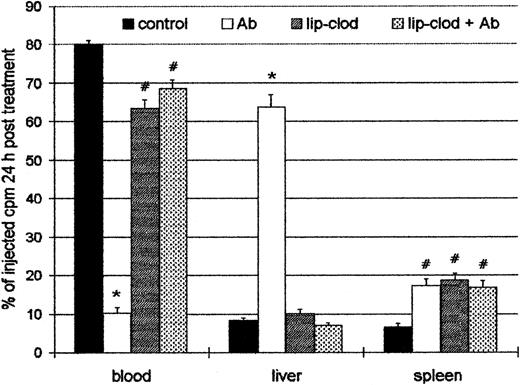
Because the injection of Ab induced a profound thrombocytopenia, the organ localization of platelets in experimental ITP was analyzed. For this purpose, control and lip-clod–treated mice were injected intravenously with 6 × 108111In-Plat in a volume of 200 μL. After 90 minutes (time necessary for111In-Plat to equilibrate with the splenic pool) the animals were injected with Ab, and 24 hours later the distribution of radioactivity was analyzed in peripheral blood, spleen, liver, lungs, kidneys, and bone marrow. As shown in Figure4, a highly significant decrease of labeled platelets in circulation was detected in Ab-treated mice. On the other hand, in the mice treated with lip-clod, most of the platelets remained in circulation. The liver was largely the principal organ responsible for platelet sequestration in Ab-treated mice, whereas the radioactivity in bone marrow, lung, and kidneys was negligible and not different from control animals (not shown). However, the treatment with lip-clod significantly blocked the liver uptake. The splenic uptake of platelets was similar in all experimental groups, but significantly different from control. This result indicates that the splenic uptake of platelets in both lip-clod and lip-clod plus Ab-treated groups cannot be attributable to antibodies. The cause of this effect appears to be the nonspecific splenic uptake of platelets induced by lip-clod,39 which might also explain the transient thrombocytopenic state after treatment.
Effect of lip-clod in the organ uptake of111In-oxine labeled platelets.
Four groups of 6 BALB/c mice were injected intravenously with 200μL of saline, lip-PBS or, lip-clod. Twenty-four hours later, 6 × 108111In-Plat in a volume of 200 μL was administered. After 90 minutes all the groups were injected with 25 μL of Ab. The distribution of radioactivity in peripheral blood, spleen, and liver after 24 hours of treatment is shown. Bars represent mean ± SEM. *P < .001 and#P < .01, significantly different from control group, Bonferroni test (2-tailed).
Effect of lip-clod in the organ uptake of111In-oxine labeled platelets.
Four groups of 6 BALB/c mice were injected intravenously with 200μL of saline, lip-PBS or, lip-clod. Twenty-four hours later, 6 × 108111In-Plat in a volume of 200 μL was administered. After 90 minutes all the groups were injected with 25 μL of Ab. The distribution of radioactivity in peripheral blood, spleen, and liver after 24 hours of treatment is shown. Bars represent mean ± SEM. *P < .001 and#P < .01, significantly different from control group, Bonferroni test (2-tailed).
It is known that in patients with ITP the platelet destruction occurs in the liver and spleen but in most of them the predominant site of destruction is the spleen.40 However, we did not observe a shift from the liver to the spleen uptake by using different concentrations of Ab (1:15, 1:45, and 1:135) as described by Veerhuis and coworkers.41 Similar results were obtained in 2 additional experiments using different lots of lip-clod (not shown).
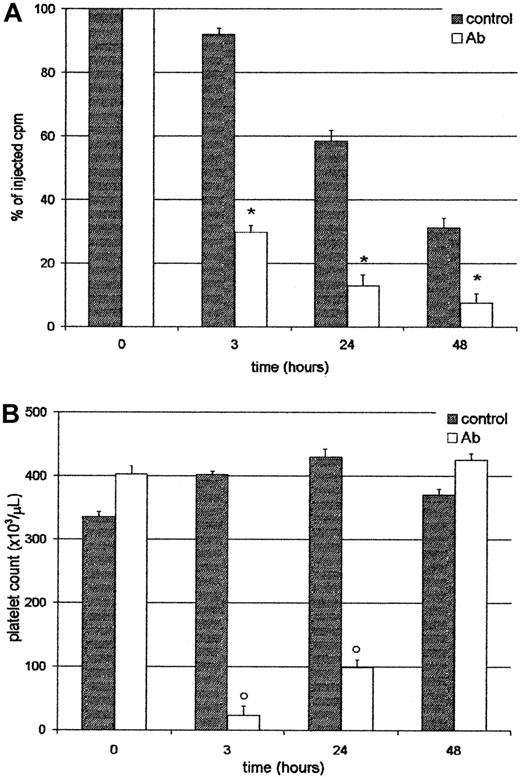
Platelets sequestered by the liver did not return to circulation
We analyzed whether platelets entrapped in the liver were able to return to the circulation. For this purpose, mice were injected with111In-Plat, and 90 minutes later with saline or Ab. As shown in Figure 5, at 3 and 24 hours after treatment, both the radioactivity in circulation and platelet counts were very low in the Ab-treated group. However, at 48 hours, although the level of radioactivity was similar to that found at 24 hours, the platelet count reached normal values. These results indicate that trapped labeled platelets do not return to circulation and that new cells might be derived from the platelet storage pool or new thrombocytogenesis. These data were confirmed by analysis of excised organs at 48 hours, where most of the radioactivity was still found in the liver (not shown). Moreover, at this time the 59.8% ± 2.6%, n = 4, of the residual radioactivity in circulation in Ab-treated animals was found free in the serum, indicating that platelets had been destroyed. The platelet survival of this group was 0.39 ± 0.1 days, n = 4. On the other hand, in the control group 94.2% ± 0.7%, n = 4, of the radioactivity was associated with platelets. The progressive decrease in the levels of radioactivity observed in the control group (without Ab) can be attributed to the senescence of platelets, because the platelet survival of BALB/c in our colony was 3.5 ± 0.3 days, n = 4.
Platelets sequestered by the liver do not return to circulation.
Two groups of 4 BALB/c mice were injected intravenously with111In-Plat and 90 minutes later with saline (control) or Ab. At 3, 24, and 48 hours after Ab administration the animals were bled and both the radioactivity (panel A) and peripheral platelet count (panel B) were determined. Values represent mean ± SEM. *P < .001 and #P < .01, significantly different from injected cpm to control groups at t = 3, 24, and 48 hours. °P < .001, significantly different from platelet count of control groups at t = 3 and 24 hours. Bonferroni test (2-tailed).
Platelets sequestered by the liver do not return to circulation.
Two groups of 4 BALB/c mice were injected intravenously with111In-Plat and 90 minutes later with saline (control) or Ab. At 3, 24, and 48 hours after Ab administration the animals were bled and both the radioactivity (panel A) and peripheral platelet count (panel B) were determined. Values represent mean ± SEM. *P < .001 and #P < .01, significantly different from injected cpm to control groups at t = 3, 24, and 48 hours. °P < .001, significantly different from platelet count of control groups at t = 3 and 24 hours. Bonferroni test (2-tailed).
Bleeding time and platelet aggregation in mice treated with lip-PBS and lip-clod
The bleeding time test is a good in vivo functional assay used for evaluating the arrest of hemorrhage by platelet plug formation. Then, we analyzed the bleeding time in control, lip-PBS, lip-clod, lip-clod plus Ab, and Ab-treated animals. The bleeding times in lip-clod treated animals, either with or without Ab, were not different from controls and lip-PBS–treated mice (< 2 minutes, n = 7), demonstrating that the hemostasis was well controlled in these animals. However, animals injected with Ab show a longer bleeding time (range, 4-6.5 minutes; n = 6).
Maximal platelet aggregation induced by ADP (50 μmol/L), collagen (20 μg/mL), or arachidonic acid (1 nmol/L) was reduced in lip-PBS to 60% of control, in lip-clod to 50% of control, and in lip-clod plus Ab-treated mice to 90% of control. Saline- and lip-PBS–treated mice in presence of Ab had less than 20 000 platelets per milliliter; therefore it was not possible to evaluate platelet aggregation in these groups. We also found a similar reduced platelet aggregation in the presence of lip-PBS or lip-clod added during the course of the test (not shown). These results indicated that liposomes can interfere with the normal platelet aggregation.
Discussion
Taking into account that the different second-line treatments for ITP are not devoid of several adverse or multiple side effects5 6 and the lack of consensus on how to approach the patients with refractory ITP, we investigated a new strategy for ITP treatment using a mouse model.
The significant, although moderate, decrease in platelet count observed in mice after lip-clod administration (48-72 hours) contrasts with the severe displacement of platelets from peripheral blood to the splenic cords observed by others.39 Although the mechanism of this effect is unknown, factors such as the clodronate concentration, the amount of liposomes injected, and the age and weight of the mice might be potential causes of these differences. A most accurate standardization will be necessary, because, for instance, the significant reduction of about 20% in the weight of spleen described in lip-clod treated mice,42 was found not significant by the same group in other studies.39 On the other hand, in agreement with others, the injection of low amounts of lip-clod (dilution 1:8 and 1:32 from the original preparation) did not induce changes in the platelet count,39 whereas these concentrations of lip-clod exerted a clear blockade of platelet uptake (Figure 2).
The hematocrit and leukocyte count were not affected by lip-clod treatment, and as previously shown,39,42 signs of illness or weakness in the treated mice were not observed. Furthermore, the administration of lip-PBS did not modify either hematocrit or platelet and leukocyte counts. The lack of additional side effects in mice treated with lip-clod is in agreement with the nontoxic effect of lip-clod for other cells than macrophages.22,43,44Moreover, it has been demonstrated that clodronate does not escape from liposomes,23 and the drug released in the circulation from dead macrophages does not cross cell membranes in the opposite direction.23 24
Lip-clod not only inhibited the uptake of sensitized platelets by the liver, but also induced the recovery of peripheral platelets (from 50 000 to 200 000/μL) in Ab-induced thrombocytopenic animals as soon as 24 hours after lip-clod injection (Figure 3). These values were maintained at least for 48 hours, despite additional Ab injection. This is a crucial point for the experimental model because one of the goals of treatment should be a rapid increase in the platelet count to a hemostatically safe value.5,45 In a recent randomized trial carried out in children with acute ITP with platelet counts of less than 20 000/μL, the conventional treatments with IVIg, anti-D, or prednisone, took about 72 hours to reach a platelet count of 50 000/μL.46 An additional and important issue was that recovery of platelets in circulation was observed at 24 hours after lip-clod injection, time at which the partial thrombocytopenia induced by lip-clod was not yet observed. Thus, the rapid reconstitution of the platelet count counterbalanced the slight but significant thrombocytopenia induced by lip-clod. Moreover, the recovery of platelet count after Ab injection can be achieved, although to a lesser extent, with concentrations of lip-clod (1:8 and 1:32) that did not modify the platelet count.
Although in patients with ITP the platelet destruction occurs in the liver and spleen, the predominant site of destruction is the spleen.40 In our experimental conditions, however, the liver was almost the exclusive organ of platelet sequestration. This is in agreement with previous reports in which the hepatic uptake of platelets was greater than the splenic uptake in dog47 and mouse48 models of ITP, suggesting that the site of accumulation of sensitized platelets may vary among species. Although it has been reported that the shift of uptake from liver to spleen depends on the number of IgG and C3 molecules on the cell surface,41 we had no success using different Ab concentration (dilutions 1:15, 1:45, and 1:135 from the original preparation), and therefore the inhibition of splenic uptake of IgG-sensitized red cells by lip-clod in mice25 could not be observed. On the other hand, as in human ITP,40 the liver sequestration of platelets in mice is not reversible (Figure 5), discarding any delayed traffic of platelets later returning to the circulation in a complement dependent rebound mechanism.46Moreover, this was followed by the destruction of platelets, because about 60% of the radioactivity in circulation was not cell associated, whereas in control animals most of the radioactivity was found in the platelets.
Although the platelet aggregation assay gives a rough estimation of the functionality of platelets, it is frequently used. We found less aggregation in platelets from lip-PBS, lip-clod, or lip-clod plus Ab-treated mice compared to control platelets. The cause of this effect is not known, although the partial platelet aggregation induced by ADP, collagen, or arachidonate in the presence of liposomes (added with the agonists), suggests a physical interference by these particles. In contrast, the bleeding time test, both in animals treated with lip-PBS or lip-clod, was within the normal range, demonstrating that hemostasis was well controlled in the animals studied. Although in most ITPs the bleeding time is prolonged,49 this parameter does not always correlate with the platelet count.49,50 However, this in vivo assay is used to determine the arrest of hemorrhage by platelet plug formation and it is considered a valuable screening test for platelet function.49 51
In this, our first attempt to investigate the management of experimentally induced ITP, we can summarize the potential advantages and disadvantages of lip-clod treatment. Among the potential advantages are (1) lip-clod rapidly restores the platelet count to hemostatic safe values; (2) lip-clod does not induce anemia nor leukopenia; (3) lip-clod is toxic to phagocytes but not to other cells22,43,44; (4) lip-clod does not induce changes in the bleeding time; and (5) lip-clod has a potential low risk of infections compared to plasma-derived products. Another important point is that relatively low concentrations of lip-clod (dilution 1:8 to 1:32; Figure2) are effective in the blockade of platelet clearance during the first 48 hours after injection. In addition, no effect induced by lip-clod treatments was observed on nonphagocytic spleen cells,52dendritic cells,23 and neutrophils.53
On the other hand, among the undesired effects we found the following: (1) Lip-clod exerts a moderate thrombocytopenia after 48 to 72 hours of injection. However, in agreement with others39 this effect is null with lip-clod 1:8 and 1:32, though they are still capable of inducing the RES blockade; (2) lip-clod interferes with platelet aggregation; (3) lip-clod induces a depletion of hepatic and splenic macrophages, although in a dose-dependent and reversible manner.23
Because lip-PBS are devoid of any visible side effects, confirming the inert nature of this neutral liposome,54 the possibility of using liposomes with different concentrations of clodronate, or other drugs,24 or lower amounts of lip-clod, encourages us in the development of this methodology. As previously suggested, the improvement of a controlled manipulation of macrophages,24as well as the evaluation of other hematologic parameters related to platelet functions, will be necessary in future experiments.
As far as we know this is the first report demonstrating that liposome-encapsulated drugs can be effective in the treatment of experimental ITP, and we think that because of the possibilities opened by this method further investigation would be worthwhile.
Acknowledgments
The authors thank Dr Susana Fink for reviewing the manuscript and Mr Antonio Morales for technical assistance.
Supported by Consejo Nacional de Investigaciones Cientı́ficas y Técnicas (CONICET), Agencia Nacional de Promoción Cientı́fica y Tecnológica, and Fundación Alberto J. Roemmers from Argentina.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martı́n A. Isturiz, Instituto de Investigaciones Hematológicas, Academia Nacional de Medicina, Pacheco de Melo 3081 (1425) Buenos Aires, Argentina; e-mail:isturiz@mail.retina.ar.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal