Abstract
Human T-cell lymphotropic virus type I (HTLV-I)–associated adult T-cell leukemia/lymphoma (ATL) is a malignancy of mature activated T cells resistant to conventional chemotherapy. The viral transactivator protein Tax plays a critical role in HTLV-I–induced transformation and apoptosis resistance by inducing IκB-α degradation, resulting in the activation of the NF-κBpathway. In these HTLV-I–transformed cells, arsenic trioxide (As) and interferon (IFN)-α synergize to induce cell cycle arrest and apoptosis. We demonstrate that cell death induction is only partly dependent upon caspase activation and is not associated with modulation of bcl-2, bax, or p53 expression. However, combined As and IFN induce the degradation of Tax, associated with an up-regulation of IκB-α resulting in a sharp decrease in RelA DNA binding nuclear factor (NF)-κB complexes because of the cytoplasmic retention of RelA. Taken the role of Tax in HTLV-I–induced transformation, its down-regulation probably accounts for cell death induction through inactivation of the NF-κB pathway. Such specific targeting of the viral oncoprotein by As-IFN treatment, reminiscent of As targeting of promyelocytic leukemia/retinoic acid receptor-α in acute promyelocytic leukemia, provides strong rational for combined As-IFN therapy in ATL patients.
Introduction
Adult T-cell leukemia/lymphoma (ATL) is an aggressive malignancy of mature activated CD4+ T cells associated with the human T-cell lymphotropic virus type I (HTLV-I) infection.1 The exact mechanism of HTLV-I–induced tumorogenesis is not fully elucidated, although HTLV-I infection appears to represent an early event in a multistep oncogenic process.2 HTLV-I transactivator protein Tax activates several major cellular transcription factor pathways, such as nuclear factor (NF)-κB/c-Rel, cAMP response element binding protein (CREB)/AP-1 transcription factor (ATF), and serum response factor (SRF),3 which mediate viral transformation. Tax target genes include interleukin (IL)-2 and the IL-2α receptor (IL-2R), which initiate and sustain an autocrine pathway of T-cell activation. Disruption of this autocrine loop using anti–IL-2R antibodies arrests leukemic cell growth and induces clinical remissions in patients with ATL.4
NF-κB is a complex transcription factor involved in the regulation of lymphoid genes, among which are IL-2 and IL-2R.5Homodimers or heterodimers of RelA (p65), p50, c-Rel, p52, and RelB bind to the consensus κB site and activate or repress transcription. Activation of NF-κB involves the dissociation of the RelA subunit from inhibitory proteins, mainly IκB-α, and its translocation from the cytoplasm to the nucleus.6 RelA then dimerizes with the p50 or c-Rel, binds DNA, and activates target genes. Release of RelA from IκB-α is the result of IκB-α degradation by the proteasome following its phosphorylation by specific IκB kinases (IKK).6 NF-κB possesses a significant antiapoptotic role that is only associated with RelA-containing complexes.7,8 In contrast, c-Rel– or p50-containing complexes possess proapoptotic properties.8 9
Tax is a powerful activator of the NF-κB pathway10-12 through an ill-understood activation of the IKK resulting in the degradation of IκB-α13,14 and, hence, the nuclear translocation of RelA.14,15 Tax is also found to stabilize NF-κB complexes binding to DNA.16Upon acute HTLV-I infection, dramatic changes in the NF-κB composition are observed, with a shift toward RelA-containing complexes and target gene activation.13 Later, during chronic HTLV-I infection, NF-κB complexes are more heterogeneous, containing significant amounts of RelA, p50, c-Rel, RelB, and p52.13,17-19 Nevertheless, Tax-expressing cells have been shown to resist apoptosis triggered by a wide variety of stimuli.20
ATL remains of poor prognosis mainly because of its resistance to conventional as well as high-dose chemotherapy, possibly due to Tax-induced apoptosis resistance.21,22 The antiviral treatment with the combination of zidovudine and interferon (IFN) has been documented to result in a high response rate in ATL patients and significantly improves survival.23-25 However, most patients eventually relapse. We have recently shown that the combination of IFN and arsenic trioxide (As) is highly synergistic in the induction of cell cycle arrest and apoptosis in HTLV-I–transformed cells and in fresh ATL cells ex vivo.26 In this study we investigate the mechanisms by which As-IFN triggers apoptosis. Our data demonstrate that the combination of As-IFN sharply down-regulates Tax expression, resulting in loss of nuclear RelA-containing NF-κB dimers, providing a plausible molecular mechanism for cell death induction.
Materials and methods
Cells
HuT-102, MT-2, and C91-PL cells (gift from A. Gessain, Pasteur Institute, Paris) are ATL-derived HTLV-I–infected CD4+ T cell lines that constitutively express HTLV-I virus. HTLV-I− CD4+ T-cell lines CEM and MOLT-4 were used as controls. Cells were grown in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS) and antibiotics (Gibco-BRL, Gaithersburg, MD). Cell growth was assessed by the incorporation of3H-thymidine, cell count (hemocytometer), and trypan blue dye exclusion protocols. Cell concentration of 1 × 105cells/mL was chosen for seeding for all experiments.
After informed consent, peripheral blood mononuclear cells were extracted from diluted venous blood from a patient with acute ATL by Ficoll-Hypaque centrifugation (Lymphoprep, Nyegaard, Oslo, Norway). Cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FCS, antibiotics, and 100-U/mL recombinant human IL-2.
Reagents and drugs
Recombinant IFN (Hoffman-La Roche, Basel, Switzerland), As, and actinomycin D (Sigma Chemical Co, St Louis, MO) were prepared as previously described.26 The in vitro dose of 1 μmol/L of As corresponds to the pharmacologic level in vivo. IFN was used at 100 U/mL or 1000 U/mL. The general caspase inhibitor zVAD (Bachem Bioscience, King of Prussia, PA) was dissolved in dimethyl sulfoxide and used at the concentration of 50 μmol/L. The proteasome inhibitor zLLL (Biomol Research Laboratories, Plymouth Meeting, PA) was used at a final concentration of 50 μmol/L. Test drugs and their vehicles were added at the initiation of culture, and cell proliferation was assessed by incorporation of3H-thymidine, while the induction of apoptosis was assessed by the TUNEL assay as previously described.26
Rabbit polyclonal antisera to IκB-α, IκB-βbcl-2, bax, and NF-κB subunits RelA (C20), p50 (NLS), c-Rel, and p52 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody to p53 was purchased from Upstate Biotechnology (Lake Placid, NY). Mouse monoclonal antibody to Tax was obtained from the National Institutes of Health AIDS Research and Reagent Program and was used in Western blot and immunofluorescence analyses. Rabbit anti–glutathion S-transferase (GST)-Tax fusion protein polyclonal serum was used in immunofluorescence analyses.
Western blot analysis
Approximately 107 cells from various treatments were solubilized at 4°C in lysis buffer (0.125-mol/L Tris-HCl, pH 6.8; 2% sodium dodecyl sulfate [SDS], 2.5% β-mercaptoethanol; and 10% glycerol). The samples were loaded onto a 12% SDS-polyacrylamide gel, subjected to electrophoresis, and transferred to nitrocellulose membrane. After blocking of the membrane in 5% skimmed milk and 0.05% Tween 20 in triethanolamine-buffered saline, the blots were incubated with specific antibodies against bcl-2, bax, p53, IκB-α IκB-β, or Tax. After several washes, the protein bands were visualized using chemiluminescence (Amersham, Buckinghamshire, England).
Immunofluorescence
Cells from different treatments were fixed in 70% ice-cold ethanol and processed for indirect immunofluorescence. Background binding of the antibodies was reduced by a preincubation with normal goat serum (10% in phosphate-buffered saline [PBS]). Cells were then incubated with primary antibodies for 2 hours at room temperature, washed in PBS, and incubated with the secondary antibodies. After several washes, the specific binding and cellular distribution of the antigens was assessed by fluorescence microscopy (LSM 410, Zeiss, Germany).
Northern blot analysis
Total cellular RNA was extracted by the guanidium isothiocyanate-phenol-chloroform method. A total of 20 μg of RNA was separated on a 1% agarose gel and transferred to nitrocellulose membrane. The filter was hybridized with the HTLV-I full genomic probe (pMT-2) (gift from A. Gessain).
Electrophoretic mobility shift assay
Nuclear extracts were prepared as described.27Briefly, cells were washed with ice-cold PBS after various treatments. Cells were collected by centrifugation, and the pellet was lysed by rapid freezing in dry ice and ethanol and thawed by resuspension in 70 μL of ice-cold buffer containing 10-mmol/L HEPES, 10-mmol/L KCl, 1.5-mmol/L MgCl2, and 1-mmol/L dithiothreitol (DTT). The nuclei were centrifuged in a microcentrifuge at 3500 rpm for 10 minutes at 4°C and suspended in 15μL of buffer containing 20-mmol/L HEPES, 400-mmol/L NaCl, 1.5-mmol/L MgCl2, 0.2-mmol/L ethylenediaminetetraacetic acid (EDTA), 1-mmol/L DTT, 0.5-mmol/L phenylmethylsulfonyl fluoride (PMSF), and 25% (vol/vol) glycerol. The nuclei were then extracted by gentle mixing for 30 minutes at 4°C, followed by centrifugation at 14 000 rpm in a microcentrifuge for 20 minutes at 4°C. The supernatant was diluted with 30 μL of buffer consisting of 20-mmol/L HEPES, 50-mmol/L KCl, 20% (vol/vol) glycerol, 0.2-mmol/L EDTA, 1-mmol/L DTT, and 0.5-mmol/L PMSF and stored at −70°C. Protein concentration was determined using the colorimetric dye binding assay (BioRad, Hertfordshire, England).
NF-κB or retinoid X receptors (RXR) consensus oligonucleotide (Santa Cruz, CA) was end-labeled with γ-32P adenosine triphosphate using T4 polynucleotide kinase. Hybridization reaction was performed using 10 to 20 μg of nuclear extract, 1 μg of poly(dIdC), 1 μg of poly(dN6) as nonspecific competitor, and 10 μg of bovine serum albumin in 20-mmol/L HEPES, 50-mmol/L KCl, 1-mmol/L EDTA, and 5-mmol/L DTT. This reaction was diluted with water before the labeled probe was added. The mixture was incubated for 30 minutes and then stopped by adding 6 μL of 15% Ficoll. The reaction mixture (20 μL of each sample) was electrophoresed on a 5% nondenaturing polyacrylamide gel, dried at 80°C under vacuum for 2 hours on Whatman filter paper, and processed for autoradiography at −80°C overnight. Specific NF-κB or RXR bands were determined by competition experiments using a mutant oligonucleotide that has lost its ability to bind to the transcription factor. Subunit specificity was determined by using specific antibodies to the NF-κB components (anti-RelA, p50, c-Rel, and p52) in the incubation step, known to supershift NF-κB complexes.
Results
To investigate the mechanism of action of the combination of As and IFN on ATL cells, we used 3 different HTLV-I–transformed cell lines: HuT-102, MT-2, and C91-PL. Whereas HuT-102 cells are relatively sensitive to As, with a major synergy between As and IFN in the induction of cell cycle arrest and apoptosis,26 MT-2 cells are resistant to As alone but sensitive to the combination of As and IFN, although to a lesser extent than HuT-102 (not shown); however, C91-PL cells were extremely sensitive to As alone with a complete cell death with As-IFN (not shown).
As-IFN–induced apoptosis is partially caspase-independent
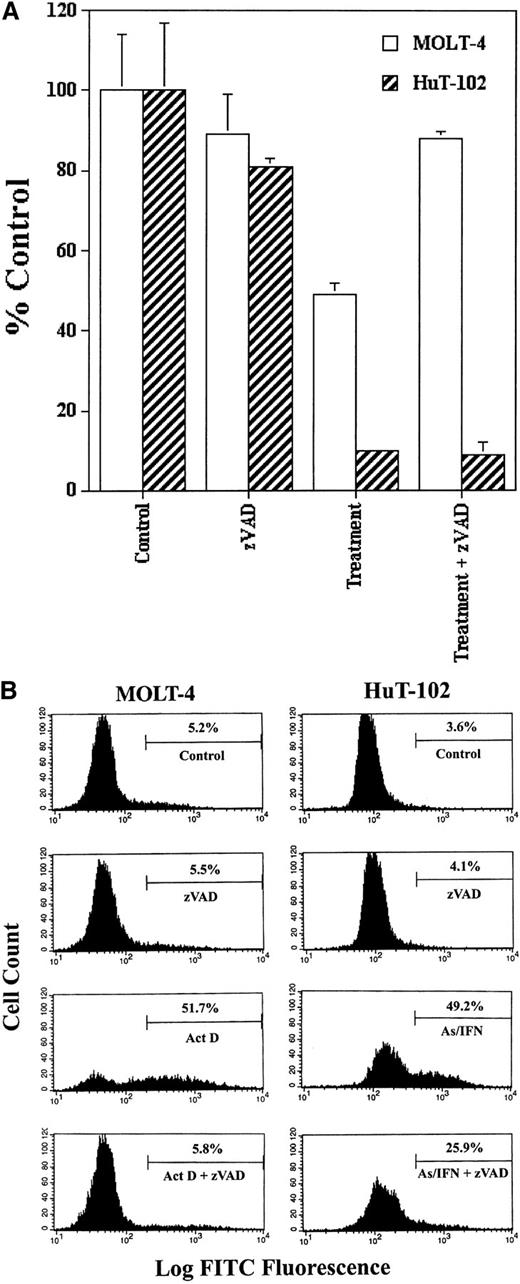
We tested the possible involvement of caspase activation on cell cycle arrest and apoptosis induced by As-IFN treatment in HuT-102 cells in the presence or absence of zVAD. Because HuT-102 cells are resistant to cytotoxic agents, actinomycin D–treated MOLT-4 cells were used as control of caspase activation and zVAD inhibition. The inhibition of cell growth of HuT-102 cells by As-IFN treatment was not reversed by zVAD, while the inhibition of cell growth of MOLT-4 cells by actinomycin D was totally blocked by zVAD (Figure1A). In the presence of zVAD the percentage of TUNEL+ HuT-102 cells treated by As-IFN only decreased from 50% to 25%, while the percentage of TUNEL+MOLT-4 cells treated with actinomycin D returned to the control levels (Figure 1B). The results obtained with C91-PL cells were similar to those obtained with HuT-102 (data not shown). These results demonstrate that As-IFN–triggered cell cycle arrest is caspase-independent, while cell death is only partially caspase-associated, similar to what has been observed in other cell systems.28
Caspase involvement in As-IFN induced cell growth inhibition and apoptosis.
(A) Inhibition of cell growth. Effects of caspase inhibitor zVAD (50 μmol/L) on the growth inhibition induced by As-IFN in HuT-102 cells. Thymidine incorporation is expressed as percentage ± SD of control and represents the mean of 3 independent experiments. Actinomycin D– (100 ng/mL) treated MOLT-4 cells serve as control of caspase-dependant apoptosis. (B) TUNEL analysis of MOLT-4 and HuT-102 cell lines. Histogram analysis was achieved by setting a region for control cells and defining the region for positive TUNEL to include 3% of the normal cells.
Caspase involvement in As-IFN induced cell growth inhibition and apoptosis.
(A) Inhibition of cell growth. Effects of caspase inhibitor zVAD (50 μmol/L) on the growth inhibition induced by As-IFN in HuT-102 cells. Thymidine incorporation is expressed as percentage ± SD of control and represents the mean of 3 independent experiments. Actinomycin D– (100 ng/mL) treated MOLT-4 cells serve as control of caspase-dependant apoptosis. (B) TUNEL analysis of MOLT-4 and HuT-102 cell lines. Histogram analysis was achieved by setting a region for control cells and defining the region for positive TUNEL to include 3% of the normal cells.
As and IFN treatment down-regulates Tax expression
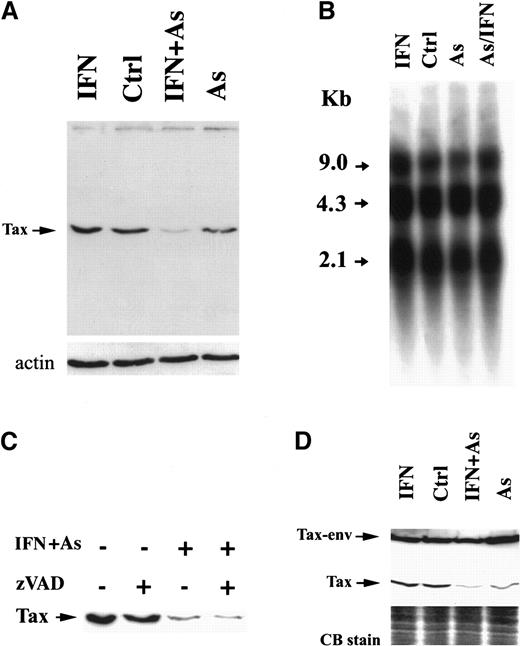
We examined the level of Tax expression by Western blot analysis using Tax-specific monoclonal antibodies. In HuT-102 cells, one specific band of 40 kd was recognized (Figure2A), which was absent from CEM and MOLT-4 cells (not shown). In MT-2 cells, 2 distinct protein bands were detected (Figure 2D), corresponding to Tax and a previously described Tax-env fusion protein.29,30 In HuT-102 cells, As and IFN induced a sharp decrease in Tax expression, visible after 24 hours of treatment (not shown) but complete after 48 hours (Figure 2A). Arsenic alone partially reduced Tax expression, while IFN did not (Figure 2A). Northern blot analysis of HTLV-I messenger RNA in HuT-102 cells treated by IFN, As, or both for 48 hours did not reveal any significant change in the levels of the 3 major viral transcripts (the full genomic RNA of 9 kilobases [kb], the single-spliced form of 4.3 kb, and the double-spliced form encoding Tax and Rex of 2.1 kb) (Figure 2B). Hence, As-IFN–induced down-regulation of Tax is a posttranscriptional event. Caspases were not responsible for Tax degradation, because zVAD did not reverse Tax degradation (Figure 2C). Because of the toxicity of proteasome inhibitors in these cells, zLLL could only be used for 6 hours and led to a minimal reversal of Tax degradation, precluding any conclusion on the involvement of this degradation pathway (data not shown). The expression of HTLV-I structural proteins was also unaffected by IFN, As, or As-IFN (data not shown). Thus, As-IFN treatment specifically targets the viral transactivator oncoprotein. Similarly, the exposure of MT-2 cells to As or As-IFN for 72 hours resulted in a sharp down-regulation of the 40-kd Tax without affecting the level of Tax-env fusion protein (Figure 2D). Note that, in these cells, apoptosis is only observed at 72 hours, consistent with the kinetics of Tax degradation. Again, expression of viral RNA and structural proteins was unaffected by As-IFN (data not shown).
Effects of IFN, As, and their combination on Tax protein levels.
Effects are shown in HuT-102 (A) and MT-2 (D) cells after 48 hours of exposure to these drugs. (B) The expression of HTLV-I RNA in HuT-102 cells treated with IFN, As, and their combination. (C) The effects of zVAD (50 μmol/L) on Tax expression. Equal protein loading was assessed by hybridization with antiactin antibodies (A) or by Coomassie blue staining (D).
Effects of IFN, As, and their combination on Tax protein levels.
Effects are shown in HuT-102 (A) and MT-2 (D) cells after 48 hours of exposure to these drugs. (B) The expression of HTLV-I RNA in HuT-102 cells treated with IFN, As, and their combination. (C) The effects of zVAD (50 μmol/L) on Tax expression. Equal protein loading was assessed by hybridization with antiactin antibodies (A) or by Coomassie blue staining (D).
To investigate whether As or As-IFN treatment modifies the intracellular distribution of Tax, immunofluorescence analysis was performed on HuT-102 using rabbit anti-Tax polyclonal serum or mouse anti-Tax monoclonal antibodies. As previously described,31 32 Tax had a diffuse nuclear staining excluding the nucleolus, with a few speckles together with a weak cytoplasmic distribution. In As-IFN–treated cells, the stain for Tax was sharply decreased, with no major change in its subcellular distribution (data not shown).
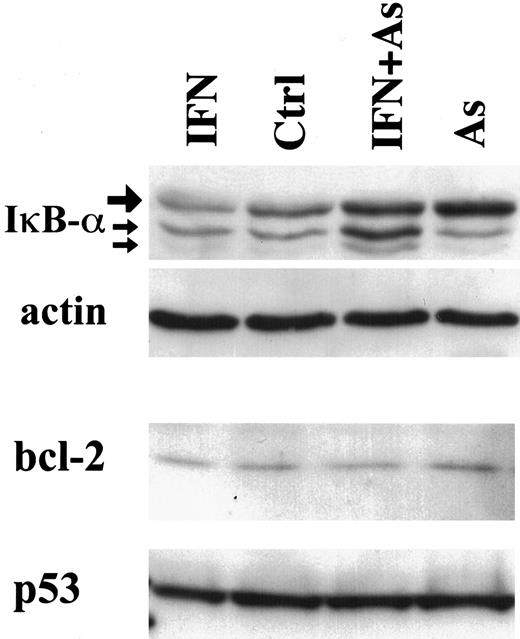
In contrast to Tax degradation, bcl-2, p53 (Figure3), or bax expression (data not shown) was not modified in cells treated with IFN, As, or their combination. Together with the stability of structural viral proteins, these observations strongly suggest that As-IFN treatment specifically targets the Tax oncoprotein, whose down-regulation could promote apoptosis rather than be its consequence.
Effects of IFN, As, and their combination on the expression of IκB-α, bcl-2, and p53 in HuT-102 cells treated for 48 hours with IFN and/or As.
For IκB-α expression, the upper band (large arrow) represents the 36-kd major IκB-α protein, also observed in CEM cells (not shown). The lower bands of 34 and 32 kd (small arrows) presumably represent N-terminal proteolytic forms of IκB-α 48 up-regulated by As-IFN treatment.
Effects of IFN, As, and their combination on the expression of IκB-α, bcl-2, and p53 in HuT-102 cells treated for 48 hours with IFN and/or As.
For IκB-α expression, the upper band (large arrow) represents the 36-kd major IκB-α protein, also observed in CEM cells (not shown). The lower bands of 34 and 32 kd (small arrows) presumably represent N-terminal proteolytic forms of IκB-α 48 up-regulated by As-IFN treatment.
As and IFN treatment up-regulates IκB-α and decreases RelA-containing DNA binding complexes
Tax has been shown to target IκB-α for degradation.13,14 Tax degradation would therefore be expected to diminish IκB-α proteolysis. Indeed, in HuT-102, MT-2, and C91-PL cells, Western blot analysis demonstrated that both As and As-IFN up-regulate IκB-α expression, consistent with their effects on Tax degradation (Figure 3, data not shown). In contrast, the level of IκB-α was not affected in HTLV-I–negative cells under any of these conditions (not shown). As previously described,33 34 IκB-β was undetectable in both HuT-102 and MT-2—treated or not (not shown).
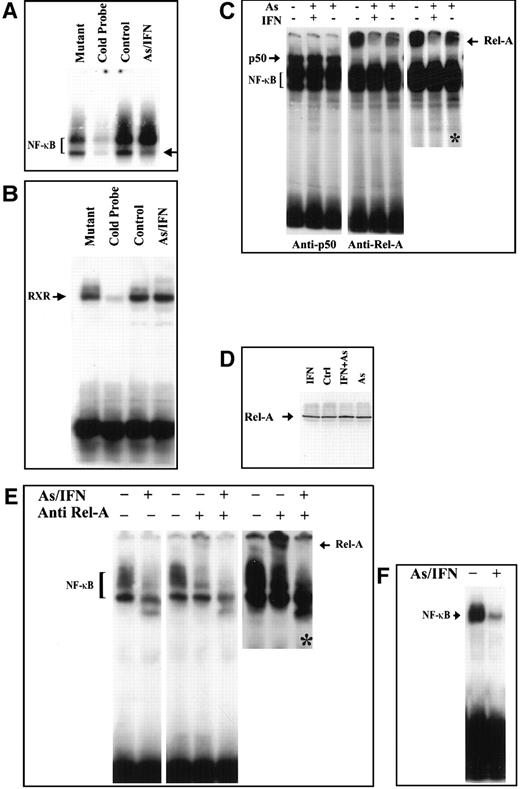
We then examined whether As, IFN, and their combination could modulate the NF-κB functional assembly by assessing DNA binding NF-κB complexes. We first confirmed the constitutive expression of NF-κB in HuT-102, MT-2, and C91-PL cells by electrophoretic mobility shift assay. Two positive complexes were detected by the radiolabeled NF-κB consensus oligonucleotide (Figure 4A and not shown). These complexes disappeared in the presence of excess of unlabeled NF-κB consensus oligonucleotide but not with excess unlabeled mutant oligonucleotide (Figure 4A), confirming their specificity. In HuT-102 and MT-2 cells, IFN exposure did not alter NF-κB complex formation, while the higher-mobility complex was slightly reduced upon As exposure but was drastically reduced by As-IFN combination (Figure 4 and not shown). In C91-PL cells, As-IFN resulted in a drastic reduction of the lower-mobility complex (Figure 4B). The specificity of the As-IFN effect on NF-κB complexes was confirmed by the fact that the DNA binding activity of an irrelevant transcription factor (RXR homodimer) was unaffected by As-IFN (Figure 4B).
Effects in an HuT-102 cell line.
Effects of a 48-hour treatment of IFN and As in an HuT-102 cell line on the activation of the transcription factors NF-κB (A) and RXR (B) assessed by electrophoretic mobility shift assay using consensus oligonucleotide probes for NF-κB and RXR, respectively. Note that As-IFN significantly diminishes 1 of the 2 NF-κB complexes (arrow in A). (C) NF-κB subunit specificity was determined by using antibodies to the NF-κB components p50 and p65/RelA, resulting in “supershift” (arrow). A longer exposure (*) of the RelA supershift is shown. (D) Effects of IFN, As, and their combination on the expression of p65/RelA protein. Combined As-IFN treatment dramatically decrease Rel-A–containing complexes without inducing Rel-A degradation. Effects of a 48-hour As-IFN treatment on NF-κB binding in C91-PL cell line (E) and fresh ATL leukemic cells (F). As above, subunit specificity was determined by “supershift” (arrow) and * indicates longer exposure.
Effects in an HuT-102 cell line.
Effects of a 48-hour treatment of IFN and As in an HuT-102 cell line on the activation of the transcription factors NF-κB (A) and RXR (B) assessed by electrophoretic mobility shift assay using consensus oligonucleotide probes for NF-κB and RXR, respectively. Note that As-IFN significantly diminishes 1 of the 2 NF-κB complexes (arrow in A). (C) NF-κB subunit specificity was determined by using antibodies to the NF-κB components p50 and p65/RelA, resulting in “supershift” (arrow). A longer exposure (*) of the RelA supershift is shown. (D) Effects of IFN, As, and their combination on the expression of p65/RelA protein. Combined As-IFN treatment dramatically decrease Rel-A–containing complexes without inducing Rel-A degradation. Effects of a 48-hour As-IFN treatment on NF-κB binding in C91-PL cell line (E) and fresh ATL leukemic cells (F). As above, subunit specificity was determined by “supershift” (arrow) and * indicates longer exposure.
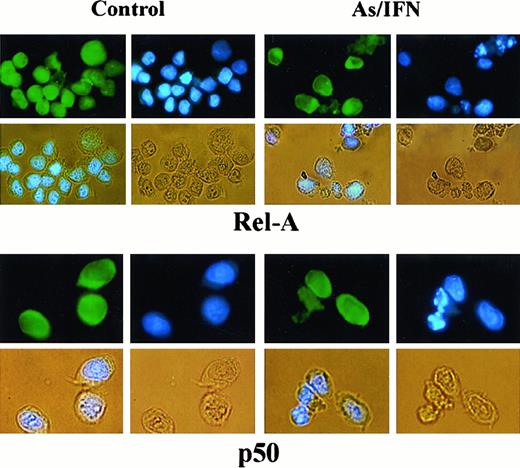
To determine the subunit composition of these NF-κB complexes, we used RelA-, c-Rel–, p50-, or p52-specific antibodies to induce a supershift. In HuT-102 cells, only RelA, p50, and c-Rel lead to supershifting of a fraction of the NF-κB complexes, consistent with the heterogeneous composition of the DNA binding species in these cells.13, 17-19 In C91-PL cells, RelA resulted in a complete supershift of the lower-mobility complex (Figure 4E). In contrast, HTLV-I− cells (CEM) showed only p50-p50 homodimers and no DNA-bound RelA, reflecting the absence of constitutive NF-κB activation. In HuT-102 cells, RelA-containing complexes were unaltered by IFN, decreased by As, but disappeared with As-IFN (Figure 4C). This dramatic decrease in RelA-containing complexes was not due to a decrease in RelA expression (Figure 4D). In contrast, complexes supershifted by anti-p50 (Figure 4C) or anti–c-Rel (not shown) antibodies were totally unaffected by As, IFN, or both. In C91-PL cells, RelA-containing complexes disappeared with As-IFN (Figure4E). Similarly, in HTLV-I–negative cells (CEM), the p50 homodimers were unaffected by As-IFN treatment (data not shown). This specific decrease of DNA-binding RelA-containing complexes is consistent with enhanced expression of IκB-α, which sequesters RelA to the cytoplasm. Indeed, using anti-RelA antibodies, a mixed homogeneous nuclear and cytoplasmic staining was observed in most untreated HuT-102 cells, while cells treated with As-IFN showed exclusively cytoplasmic staining (Figure 5). In contrast, p50 expression was predominantly cytoplasmic both in treated or untreated HuT-102 cells (Figure 5). As expected, As-IFN treatment generates apoptotic cells that do not exhibit RelA or p50 staining (Figure 5).
Effects of 48-hour As-IFN treatment on the subcellular distribution of the NF-κB subunits RelA or p50.
Effects were determined by immunofluorescence (green). Nuclear staining was performed by Hoechst (blue). Phase-contrast images are also shown (yellow). Treatment with As-IFN induces the cytoplasmic transfer of Rel-A but not p50.
Effects of 48-hour As-IFN treatment on the subcellular distribution of the NF-κB subunits RelA or p50.
Effects were determined by immunofluorescence (green). Nuclear staining was performed by Hoechst (blue). Phase-contrast images are also shown (yellow). Treatment with As-IFN induces the cytoplasmic transfer of Rel-A but not p50.
Fresh leukemic cells from a patient with ATL were then studied. As-IFN induced both cell cycle arrest and some apoptosis after 48 hours, despite the continuous presence of IL-2.26 Nuclear extracts of these cells showed a dramatic decrease in NF-κB binding (Figure 4F), previously shown to correspond to RelA-p50 heterodimers.19 The very low levels of Tax expression in primary ATL cells precluded analysis of Tax degradation by As-IFN. Hence, in this primary ATL sample, as in HuT-102 cells, As-IFN treatment ex vivo induces apoptosis associated with loss of RelA-containing NF-κB complexes.
Discussion
In this study we identify Tax as a molecular target of As-IFN treatment in ATL cells. Tax down-regulation probably accounts for the induction of apoptosis through its effects on NF-κB pathway and could represent a novel example of oncogene-targeted therapy of human leukemia.
We have demonstrated that Tax is down-regulated upon As exposure and that IFN, which has no effect on its own, sharply enhances As effects. This pattern of Tax degradation (or RelA complex disappearance)—with moderate effect of As, no effect of IFN, and a major effect of the combination—is identical to the pattern of ATL cell response in terms of growth arrest or apoptosis.26 Hence, Tax down-regulation could be responsible for the latter. It is most likely that Tax is degraded by an as yet unidentified pathway, although we cannot rule out a modulation in the efficiency of translation for Tax messenger RNA. However, the role of As to induce promyelocytic leukemia (PML)–RAR-α degradation in acute promyelocytic leukemia (APL) or PML degradation in non-APL cells35 does not favor this hypothesis. Moreover, As exposure was shown to induce the degradation of key regulatory proteins, including papillomavirus E2 and p53.36,37 In that respect, Tax binds CREB binding protein (CBP)/P300, which was recently demonstrated to be required for mdm2-dependent p53 catabolism.38 This IFN enhancement of As effects suggests that IFN is up-regulating a protein that facilitates the action of arsenic. Circumstantial evidence points to PML as a candidate for being this protein. PML is a primary target of IFN,39 is degraded in response to As exposure35 and, like Tax, binds CBP.40Moreover, some protein catabolism was suggested to take place in PML nuclear bodies.41 Although we have not been able to demonstrate Tax-PML colocalization, it is possible that a transient association of these 2 proteins would result in their catabolism upon As exposure.
Previous studies have shown that Tax is sufficient to transform T lymphocytes and induce bcl-2 and bcl-XL expression and apoptosis resistance.42-44 Hence, the down-regulation of Tax may be sufficient to sensitize ATL cells to apoptotic stimuli such as those triggered by IFN and As. NF-κB activation, one of the hallmarks of Tax transformation, is dramatically reduced, at least for its RelA-dependant part. The latter is most likely due to IκB-α up-regulation, although As alone up-regulates IκB-α as much as As-IFN does. Hence, some other factor is likely to account for the greater decrease in RelA-containing NFκB complexes with As-IFN compared with As alone. Previous studies clearly established the importance of NF-κB activation and RelA-containing complexes in both HTLV-I–induced transformation and resistance to apoptosis.45,46 RelA appears to be a major target of As-IFN combination, but the pathway triggered by As-IFN in these cells is still obscure, because the exact NF-κB target genes implicated in apoptosis control have not been identified. It is of interest that both cell cycle arrest and, at least in part, apoptosis induction are independent of caspase activation. PML was shown to trigger both cell cycle arrest and caspase-independent apoptosis28 in an As-enhanced manner. Hence, in addition to NF-κB down-regulation, the PML pathway may contribute to the effects of As-IFN in this model system.
Recent clinical studies have shown that IFN is well tolerated in ATL patients23-25 and that As treatment exhibits very little toxicity in APL patients.47 In fact, a recent phase II trial has shown that IFN and As combination is well tolerated by patients with ATL (O. Hermine, unpublished observation). In addition, some definite antileukemic effect of this combination has been observed, suggesting that ATL cells may similarly respond to As-IFN by apoptosis or by cell cycle arrest in vivo. Tax degradation by As-IFN could also provide a rational basis for the treatment of high-risk HTLV-I–infected carriers as well as tropical spastic paraparesis/HTLV-I–associated myelopathy patients. Such a treatment may dramatically reduce both viral load and infected lymphocyte proliferation. Should the ongoing trial in ATL patients confirm the clinical efficacy of this association, As-IFN would constitute, together with retinoic acid or As in APL, the second example of oncogene-targeted therapy of leukemia.
Acknowledgments
The authors thank Dr F. Homaidan for her critical reading of this manuscript. The expert assistance from the personnel of the Core Laboratory Facilities of the American University of Beirut and the photography department (R. Nancel) in Hôpital St Louis are greatly appreciated.
Supported by the American University of Beirut Medical Practice Plan, the Lebanese National Research Council, the CNRS, ARC, the Foundation St Louis, and the Programme Franco-Libanais Coopération pour l'Evaluation et le Développement de la Recherche.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hugues de Thé, CNRS UPR 9051, Hôpital St Louis 1 av. C. Vellefaux, 75475 Paris, Cedex 10, France; e-mail:dethe@chu-stlouis.fr; or Ali Bazarbachi, Department of Internal Medicine, Faculty of Medicine, American University of Beirut, PO Box 113-6044, Beirut, Lebanon; e-mail: bazarbac@aub.edu.lb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal