Abstract
Infection with the human immunodeficiency virus (HIV) is associated with a progressive decrease in CD4 T-cell number and a consequent impairment in host immune defenses. Analysis of T cells from patients infected with HIV, or of T cells infected in vitro with HIV, demonstrates a significant fraction of both infected and uninfected cells dying by apoptosis. The many mechanisms that contribute to HIV-associated lymphocyte apoptosis include chronic immunologic activation; gp120/160 ligation of the CD4 receptor; enhanced production of cytotoxic ligands or viral proteins by monocytes, macrophages, B cells, and CD8 T cells from HIV-infected patients that kill uninfected CD4 T cells; and direct infection of target cells by HIV, resulting in apoptosis. Although HIV infection results in T-cell apoptosis, under some circumstances HIV infection of resting T cells or macrophages does not result in apoptosis; this may be a critical step in the development of viral reservoirs. Recent therapies for HIV effectively reduce lymphoid and peripheral T-cell apoptosis, reduce viral replication, and enhance cellular immune competence; however, they do not alter viral reservoirs. Further understanding the regulation of apoptosis in HIV disease is required to develop novel immune-based therapies aimed at modifying HIV-induced apoptosis to the benefit of patients infected with HIV.
Introduction
Patients infected with the human immunodeficiency virus (HIV) experience a progressive decline in CD4 T-cell number, resulting in immunodeficiency and increased susceptibility to opportunistic infections and malignancies. Although CD4 T-cell production is impaired in patients infected with HIV,1there is now overwhelming evidence that the primary basis of T-cell depletion in patients infected with HIV is increased apoptosis of CD4 and CD8 T cells. Since it was first proposed as a potential mechanism of CD4 T-cell depletion in patients infected with HIV,2apoptosis and its dysregulation after HIV infection has become a major focus of research. Although apoptosis may result from the effects of continuous immune activation that occurs in HIV-infected patients, considerable data indicate that there are additional distinct mechanisms by which HIV (and HIV-specific proteins) enhances apoptosis. Importantly, only a minor fraction of apoptotic lymphocytes are physically infected by HIV, indicating that the enhanced apoptosis of lymphocytes seen in infected persons results from mechanism(s) other than direct infection. Thus, understanding of the mechanisms of HIV-associated lymphocyte apoptosis may lead to new and more effective therapies for HIV disease and acquired immunodeficiency syndrome.
Overview of HIV-associated lymphocyte apoptosis
Chronic uncontrolled infections provide continuous antigenic stimulation that causes persistent immune activation and consequent apoptosis. This is the mechanism by which infectious diseases, such as cytomegalovirus, cause enhanced apoptosis and lymphopenia. Chronic HIV infection provides a chronic immunologic stimulus; however, it may be unique in its ability to induce lymphocyte apoptosis through direct or indirect mechanism(s) that are distinct from immune activation alone. Although numerous pathogenic viruses have developed mechanisms to prevent apoptosis of host cells, no such antiapoptotic machinery is present in HIV. Indeed, HIV-encoded proteins may induce apoptosis of infected cells and uninfected cells (ie, paracrine death) through various mechanisms, some of which are defined; others are as yet unidentified (Table 1).
Proposed mechanisms of HIV-associated lymphocyte apoptosis
| Effector . | Proposed mechanism . | Target cell . |
|---|---|---|
| HIV Tat | Enhanced Fas sensitivity | Infected + |
| Enhanced Fas ligand production | uninfected cells | |
| HIV Nef | Activation | Infected + |
| Enhanced FasL production | uninfected cells | |
| ? Binding to unidentified receptor | ||
| HIV vpr | Cell cycle arrest | Infected + |
| Direct effect on mitochondrial permeability | uninfected cells | |
| HIV protease | Cleavage of host structural proteins | Infected cells |
| Activation-induced cell death | HIV-associated activation | Uninfected cells |
| Increased TRAIL/APO-2L, FasL, or both | ||
| gp 120/160 | Inappropriate activation after CD4 ligation | Uninfected cells |
| Enhanced Fas susceptibility/FasL production | ||
| ? Nonapoptotic death by CXCR4 | ||
| Autologous cell–mediated killing | Enhanced production of cytotoxic ligands by HIV-infected cells | Uninfected cells |
| Effector . | Proposed mechanism . | Target cell . |
|---|---|---|
| HIV Tat | Enhanced Fas sensitivity | Infected + |
| Enhanced Fas ligand production | uninfected cells | |
| HIV Nef | Activation | Infected + |
| Enhanced FasL production | uninfected cells | |
| ? Binding to unidentified receptor | ||
| HIV vpr | Cell cycle arrest | Infected + |
| Direct effect on mitochondrial permeability | uninfected cells | |
| HIV protease | Cleavage of host structural proteins | Infected cells |
| Activation-induced cell death | HIV-associated activation | Uninfected cells |
| Increased TRAIL/APO-2L, FasL, or both | ||
| gp 120/160 | Inappropriate activation after CD4 ligation | Uninfected cells |
| Enhanced Fas susceptibility/FasL production | ||
| ? Nonapoptotic death by CXCR4 | ||
| Autologous cell–mediated killing | Enhanced production of cytotoxic ligands by HIV-infected cells | Uninfected cells |
Overview of the regulation of apoptosis
Apoptosis regulatory proteins
Many elements influence whether a cell will undergo apoptosis6 (Figure 1). Four cellular receptors induce apoptosis after ligation; they are the Fas receptor,7 p55 tumor necrosis factor (TNF) receptor,8 and TRAIL/APO 2-L (TNF-related apoptosis-inducing ligand) receptors 1 and 2.9 Fas Ligand (FasL), TNF, and TRAIL/APO 2-L, respectively, bind these receptors to initiate apoptosis. In the case of FasL and TNF, membrane-associated proteins may be cleaved by the action of matrix metalloproteases to release soluble ligands that maintain their biologic activity.10-12 It is unknown whether TRAIL/APO 2-L exists as a soluble molecule. Ligation of these death receptors recruits the adaptor proteins FADD (Fas-associated death domain)13-15TRADD (TNF receptor-associated death domain), or both,16,17 which sequentially activate a family of cysteine proteases that cleave at aspartate residues (cysteine-dependent, aspartate-specific protease), or caspases. Caspases are synthesized as inactive zymogens and become activated after proteolytic removal of a terminal prodomain.18-20 Fourteen mammalian caspase family members have been identified, each with varying involvements in the regulation of apoptosis. For example, caspase 8 (FLICE)21-23 and caspase 3 (CPP32)24-26 are involved in apoptosis mediated by Fas, p55 TNF receptor, and TRAIL/APO 2-L receptor ligation. Activated caspases catalyze the cleavage of other caspases, which, in turn, activate various cellular proteases and endonucleases that cleave host cell structural and regulatory proteins and host nuclear DNA,27 ultimately causing the cell to undergo the morphologic and biochemical changes that are characteristic of apoptosis.28
In addition to receptor-mediated apoptosis, other stimuli (eg, chemotherapy, ultraviolet radiation, and ionizing radiation) induce changes in mitochondria that include opening of the permeability transition pore and loss of mitochondrial inner transmembrane potential, which allows the release of apoptosis regulatory proteins (including cytochrome c, Apaf-1, and caspase 9)29-32 that initiate further caspase activation, ultimately leading to apoptosis. Although classical Fas-induced apoptosis (see above) involves direct caspase activation without mitochondrial involvement (type 1), in certain cell types Fas-induced apoptosis may also require mitochondrial activation (type 2).7
Antiapoptosis regulatory molecules
In addition to the proteins involved in mediating apoptosis described above, other proteins act to inhibit apoptosis. One such family of regulatory proteins is cellular FLICE-like inhibitory protein (c-FLIP), which inhibits apoptosis by binding to FADD and thus prevents the activation of caspase 8.33,34 The inhibitor of apoptosis proteins (IAP) family, including HIAP, XIAP, and others, acts by inhibiting the activation of caspase 3 and possibly other caspases.35-37
Bcl2 and related family members,38,39 including BclXS, BclXL, Bad, and Bax, influence apoptosis by regulating the intracellular signals that induce apoptosis. Some family members (Bcl2) are antiapoptotic, whereas others (Bax) are proapoptotic. Cells that contain a predominance of proapoptotic Bcl2 family molecules promote apoptosis, and cells with a predominance of antiapoptotic Bcl2 family proteins are relatively apoptosis resistant. Bcl2 consistently blocks apoptosis induced by anticancer and nitric oxide,41 and these effects may result from the inhibition of calcineurin activation,40,42,43 NFAT activation,40 or transcription of Fas ligand.40 Conversely, reports on the effects of Bcl2 on Fas-induced apoptosis are conflicting: Bcl2 may variably inhibit44 or not inhibit45Fas-induced death. Because members of the Bcl2 family are principally localized within mitochondria, their influence may be greatest in forms of apoptosis that are associated with mitochondrial activation. Thus, Bcl2 overexpression may not inhibit death receptor–initiated apoptosis in cells with a type 1 (mitochondria-independent) Fas pathway, but it may block Fas-initiated death in type 2 (mitochondria-dependent) cells.46
Physiologic T-cell apoptosis
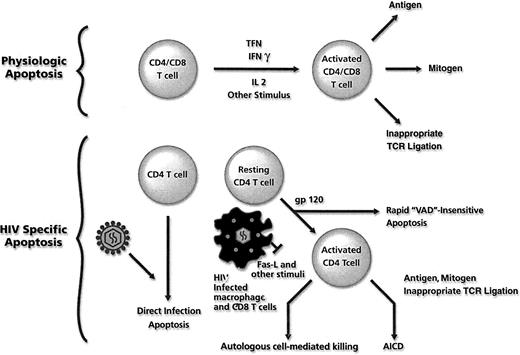
Healthy subjects orchestrate a physiologic immune response to a foreign antigen by T-cell activation and proliferation. If this T-cell proliferative response were not regulated, each encounter with a foreign antigen would lead to unending T-cell expansion. Down-regulation of T-cell proliferation occurs by an apoptotic program that is initiated after activation47(Figure 2, top). After T-cell activation, c-FLIP expression is reduced, and the cells become susceptible to Fas ligation and to caspase 8–mediated apoptosis.33 Exposure to a second activation stimulus (eg, CD3 stimulation in the absence of CD28 costimulation) promotes de novo production of FasL, leading to both autocrine and paracrine Fas/FasL-mediated T-cell apoptosis.48-52 It is important to note that not all physiologic T-cell apoptosis is regulated solely by Fas/FasL interactions; Fas-deficient cells maintain T-cell receptor (CD3)–induced apoptosis that is inhibited by TNF antagonists.53 54
T-cell apoptosis.
Mechanisms of physiologic T-cell apoptosis (top) and mechanisms of increased T-cell apoptosis associated with HIV infection (bottom). VAD refers to the pan-caspase inhibitor 2-VAD-Fmk.
T-cell apoptosis.
Mechanisms of physiologic T-cell apoptosis (top) and mechanisms of increased T-cell apoptosis associated with HIV infection (bottom). VAD refers to the pan-caspase inhibitor 2-VAD-Fmk.
Measurement of apoptosis
As noted, apoptosis is characterized by distinct morphologic and biochemical changes, including chromatin condensation, shrinkage of the cytoplasm, membrane blebbing, and formation of apoptotic bodies. Apoptosis is a complex and sequential process, and, as such, some assays detect changes that occur early, whereas other assays detect later events. The most common assays used in the detection of apoptosis are listed in Table 255-99; many have been used to evaluate apoptosis in patients infected with HIV. In a direct comparison of the relative benefits of these assays for use in the evaluation of apoptosis of HIV-infected patients, TUNEL staining was the most specific and therefore may be the most accurate assay to use in this patient population.100
Assays of apoptosis and their relationship to events of apoptosis
| Event . | Assays . | Detection . |
|---|---|---|
| Changes in nuclear morphology: | DNA stains (DAPI) | Microscopy |
| Chromatin condensation, segmentation, | ||
| and formation of apoptotic bodies | ||
| Changes in membrane permeability | Vital dyes (PI) | Microscopy |
| Permeable DNA stains: (DAPI, Hoechst 33258) | Flow cytometry with simultaneous size determination | |
| Changes in membrane composition: | Annexin V binding | Flow cytometry |
| Externalization of phosphatidylserine | Confocal and epifluorescence microscopy | |
| Cleavage of nuclear proteins | Poly ADP ribose polymerase | Western blot |
| Mitochondrial function and integrity | ||
| Changes in permeability transition (ΔΨm) | Vital dyes (DiOC6, JC-1) | Flow cytometry |
| Accessibility to mitochondrial antigens | Apo 2,7 antibody | Flow cytometry |
| Release of cytochrome-c | Anti–cytochrome-c antibody | Flow cytometry, Western blot |
| Production of free radicals | DPPP/dihydroethidium | Flow cytometry |
| Caspase activation | ||
| Detection of caspase cleavage product | Known caspase substrates; PARP, caspase 3, caspase 8, DNA-PK, PK-C | Western blot |
| Detection of active caspase | Anti–activated caspase 3 antibody | Western blot |
| Detection of caspase activity | Cleavage of fluorescent or colorimetric substrate(s) | Fluorometer, plate reader |
| DNA degradation | ||
| Large fragments | DNA stains (EtBr, SYBR green) | Pulse-field gel electrophoresis |
| DNA stains (EtBr) | Comet | |
| Radioactivity (C14) | Detection of radio-labeled DNA by filter binding | |
| Small fragments | DNA stains (EtBr) | Agarose gel electrophoresis (DNA ladder) |
| Radioactivity (C14) | Detection of radio-labeled DNA by filter binding | |
| Sub-G1 peak detection | DNA stains (PI, Hoechst) | Flow cytometry |
| Detection of DNA strand breaks | Terminal dUTP nick end labeling (TUNEL) | In situ hybridization |
| Flow cytometry | ||
| Ligation-mediated polymerase chain reaction | Agarose or polyacrylamide gel electrophoresis |
| Event . | Assays . | Detection . |
|---|---|---|
| Changes in nuclear morphology: | DNA stains (DAPI) | Microscopy |
| Chromatin condensation, segmentation, | ||
| and formation of apoptotic bodies | ||
| Changes in membrane permeability | Vital dyes (PI) | Microscopy |
| Permeable DNA stains: (DAPI, Hoechst 33258) | Flow cytometry with simultaneous size determination | |
| Changes in membrane composition: | Annexin V binding | Flow cytometry |
| Externalization of phosphatidylserine | Confocal and epifluorescence microscopy | |
| Cleavage of nuclear proteins | Poly ADP ribose polymerase | Western blot |
| Mitochondrial function and integrity | ||
| Changes in permeability transition (ΔΨm) | Vital dyes (DiOC6, JC-1) | Flow cytometry |
| Accessibility to mitochondrial antigens | Apo 2,7 antibody | Flow cytometry |
| Release of cytochrome-c | Anti–cytochrome-c antibody | Flow cytometry, Western blot |
| Production of free radicals | DPPP/dihydroethidium | Flow cytometry |
| Caspase activation | ||
| Detection of caspase cleavage product | Known caspase substrates; PARP, caspase 3, caspase 8, DNA-PK, PK-C | Western blot |
| Detection of active caspase | Anti–activated caspase 3 antibody | Western blot |
| Detection of caspase activity | Cleavage of fluorescent or colorimetric substrate(s) | Fluorometer, plate reader |
| DNA degradation | ||
| Large fragments | DNA stains (EtBr, SYBR green) | Pulse-field gel electrophoresis |
| DNA stains (EtBr) | Comet | |
| Radioactivity (C14) | Detection of radio-labeled DNA by filter binding | |
| Small fragments | DNA stains (EtBr) | Agarose gel electrophoresis (DNA ladder) |
| Radioactivity (C14) | Detection of radio-labeled DNA by filter binding | |
| Sub-G1 peak detection | DNA stains (PI, Hoechst) | Flow cytometry |
| Detection of DNA strand breaks | Terminal dUTP nick end labeling (TUNEL) | In situ hybridization |
| Flow cytometry | ||
| Ligation-mediated polymerase chain reaction | Agarose or polyacrylamide gel electrophoresis |
HIV-mediated alterations in molecules that regulate the apoptotic process
Cells obtained from HIV-infected patients and cells infected with HIV in vitro show changes in the regulation of Fas and Fas ligand (reviewed in101). Acute HIV infection of the promonocytic cell line U937 is associated with viral replication-dependent apoptosis102 that is characterized by the increased membrane expression of Fas102 and FasL,102 by the down-regulation of antiapoptotic proteins Bcl2 and BclXL,103,104 and by a concomitant increase in proapoptotic BclXS and Bax.103,104 The hypothesis that Fas/FasL interactions may be responsible for HIV-induced apoptosis is supported by the observation that soluble Fas receptor decoys block HIV-associated death in U937 cells.102 This is in marked contrast to the effects of acute HIV infection of T-cell lines, which is Fas independent despite increased Fas expression.105-108 Interestingly, though T cells from HIV-infected patients have altered expression of Bcl2, the expression of Bax, BclXL, and BclXS does not differ from that of uninfected controls.109
T cells from HIV-infected patients exhibit both increased Fas receptor expression and enhanced susceptibility to Fas-mediated death.110-117 FasL is elevated in peripheral blood mononuclear cells (which contain monocytes)114,118,119from HIV-infected patients, and the plasma level of soluble FasL is increased in HIV-positive patients and correlates with HIV RNA burden.120 The demonstrated increases in Fas expression, Fas susceptibility, and Fas ligand expression suggest that these molecules may be important in some forms (see below) of HIV-induced cell death, though direct T-cell killing is independent of Fas.105-108
Intracellular levels of c-FLIP in resting cells from HIV-negative patients decrease after activation, resulting in enhanced sensitivity to Fas-mediated apoptosis.33 This observation, coupled with observations that apoptosis in patients infected with HIV occurs in activated CD45 RO+, HLA-DR+, CD28− cells,121-124 suggests that decreased c-FLIP expression may be responsible for the enhanced susceptibility of cells from these patients to apoptosis. However, c-FLIP expression in bulk peripheral blood lymphocytes (PBL) or in purified CD4 or CD8 T cells from HIV-infected patients does not differ from that of HIV-negative patients.125 It remains possible that defined cellular subsets may have reduced levels of c-FLIP that are missed in bulk analysis.
The regulation of TNF, TNF receptors, or both is fundamentally altered in HIV-infected patients.126-129 Both cognate receptors for TNF, p75 TNFR and p55 TNFR,130 are expressed in a variety of cell types. However, only ligation of the p55 TNF receptor leads to apoptosis.16,17,53,131,132 Elevated serum TNF levels are seen in symptomatic HIV-infected patients126-129 but not in asymptomatic patients.133,134 Furthermore, (1) HIV infection of lymphocytes or monocytes results in TNF production,135,136and (2) TNF activates the transcription factor NFkB, which, in turn, activates HIV transcription,137,138 initiating an autocrine loop that results in high levels of TNF production and increased levels of HIV transcription. In addition, elevated serum levels of soluble p75 TNFR are predictive of HIV disease progression, independent of other immunologic or virologic prognostic markers.139 Although little is known of the ability of TNF to induce apoptosis in HIV-infected cells, HIV-infected macrophage-mediated killing of uninfected CD4 T-cell blasts (see below) can be partially reduced by the administration of soluble TNFR decoys,40 and TNF may contribute to apoptosis induced by gp120-mediated cross-linking of CD4138 (see below). The potential role of TNF as a mediator of HIV disease has prompted trials of anti-TNF therapy to retard HIV disease progression. Thalidomide reduces TNF secretion,141 and pentoxifylline reduces TNF mRNA half-life.138 However, clinical trials with each of these agents have consistently failed to show improvement in either immunologic or virologic outcomes.142-144 Other studies using soluble TNF antagonists have had similarly disappointing results.145
There is also relatively little information concerning the potential role of TRAIL/APO 2-L in apoptosis in HIV-infected patients. Current data suggest that TRAIL/APO 2-L can bind to 1 of 5 receptors, TRAIL/APO 2-L-R1, TRAIL/APO 2-L-R2, TRAIL/APO 2-L-R3, TRAIL/APO 2-L-R4,146 and osteoprotegerin.147Binding of TRAIL/APO 2-L to TRAIL/APO 2-L-R1 or R2 transduces apoptotic signals, whereas binding to TRAIL/APO 2-L-R3 or TRAIL/APO 2-L-R4 does not. The effects of TRAIL/APO 2-L binding to osteoprotegerin are unknown. Although it has been suggested that the relative expression of TRAIL/APO 2-L-R3 and TRAIL/APO 2-L-R4 to TRAIL/APO 2-L-R1 and TRAIL/APO 2-L-R2 influences susceptibility to TRAIL/APO 2-L–mediated killing,148-150 recent studies do not support this hypothesis. Rather, intracellular levels of c-FLIP may correlate with the sensitivity or resistance to TRAIL/APO 2-L–induced apoptosis in target cells.151 152
Although no studies to date have evaluated the relative expression of TRAIL/APO 2-L receptor(s) or TRAIL/APO 2-L expression in patients infected with HIV, it has been observed that (in contrast to cells from HIV-uninfected patients) cells from HIV-infected patients are susceptible to TRAIL/APO 2-L–mediated killing.153 This finding, together with the fact that activation-induced cell death in patients with HIV infection may be partially inhibited using antagonistic TRAIL/APO 2-L–specific antibodies,154suggests that TRAIL/APO 2-L and TRAIL/APO 2-L receptor dysfunction may contribute to HIV pathogenesis.
Apoptosis of uninfected and infected T cells induced by HIV proteins
HIV infection is associated with enhanced apoptosis in CD4 T cells infected by HIV and in uninfected T cells. In this section we review proposed mechanisms of CD4 T-cell apoptosis, focusing on whether the proposed mechanisms affect infected cells, uninfected cells, or both.
Gp120-induced apoptosis
Gp120 is an HIV viral envelope glycoprotein that can bind to and cross-link the CD4 receptor and the chemokine coreceptors. Cross-linking of CD4 T cells by gp120 causes the induction of enhanced susceptibility to Fas-mediated killing.155 In previously activated cells, gp120 cross-linking results in apoptosis156 (possibly mediated by IFN-γ, TNF, or both157), down-regulation of Bcl-2 expression,158 and activation of caspase 3.159 The apoptotic response to gp 120 is almost completely inhibited by soluble CD4 and by anti-gp120 antibodies.160 Further evidence for the specificity of this interaction is provided by the observation that a point mutation in the V3 loop of gp120 inhibits the induction of apoptosis in CD4 T cells.161 Finally, this interaction must also involve CD4 signaling because deletion or mutation of the intracytoplasmic portion of CD4 also abrogates the apoptotic response.162 163
Most of the experiments involving gp120-induced apoptosis evaluate apoptosis that occurs after several days. However, recent reports164-166 show that gp120 cross-linking of CD4 and CXCR4 chemokine receptor results in nonapoptotic death within several hours of stimulation by a mechanism that appears to be independent of p56LCK,164 g-protein–coupled signaling,166Fas, or TNF receptors.165 The administration of CXCR4 antagonists blocks this apoptosis response to the HIV envelope.167
It is appealing to invoke gp120 as a responsible mechanism for CD4 T-cell death in patients infected with HIV because it doesnot depend on the infection of all cells that become apoptotic and it does not require the presence of viable virions. Circulating immune complexes and replication-incompetent viruses that contain gp120 can induce death in a similar manner.168-170
Apoptosis induced by other HIV proteins
Transfection experiments demonstrate that the ectopic expression of HIV Tat induces apoptosis. Further, gp120/160-deleted HIV maintains its ability to induce infected cell apoptosis, potentially because of the Tat-directed up-regulation of caspase 8171 or because of Fas ligand.172 Importantly, Tat has also been implicated as an inducer of apoptosis in uninfected T cells, potentially by Fas-dependent mechanisms, superoxide dismutase inhibition, or activation of cyclin-dependent kinases.173-175 The ability of Tat to induce uninfected cell death has also been demonstrated in vitro for neurons, lymphocytes, and CD4 T-cell lines. Its clinical relevance is suggested by observations that Tat is readily secreted by infected cells176 and cellular or humoral immunity to Tat may have protective effects against HIV disease progression.177
Because Nef is essential for viral pathogenicity, HIV-encoded Nef has been suggested as a potential mediator of apoptosis.178This proposal is supported by the following findings: (1) human infection with naturally occurring Nef deletion mutants leads to less rapid CD4 T-cell depletion (compared to strains with Nef),179,180 though the differences may be related to the decreased efficiency of viral replication181-184; (2) Nef synergistically enhances the activating effects of T-cell receptor ligation,185-187 though this enhancement may be stimulus dependent187-191; (3) Nef-expressing T cells coexpress FasL,192 as do infected T cells from SIV-infected macaques but not T cells from macaques infected with similar strains of SIV that contain mutations within the Nef gene193 (the mechanism(s) by which Nef results in activation and FasL production remain unclear, yet mutational analysis indicates that the carboxy terminus of the CD4 receptor associates with both Nef and p56LCK194); lastly, (4) Nef may exert an apoptotic effect on uninfected CD4 T cells by binding to unidentified receptor(s),195 resulting in Fas-independent death.196 In this regard, Nef may induce apoptosis of infected and uninfected cells.
HIV-encoded vpr also has the ability to induce apoptosis through transfection and exogenous treatment. Proposed mechanisms include the induction of G2/M cell cycle arrest197,198 and a direct effect on mitochondrial permeability.199 Vpr also influences viral LTR transcription,200,201 cellular activation, and differentiation,202,203 suggesting a role in the development of HIV reservoirs. The seeming paradox of inducing apoptosis while promoting viral reservoirs is elucidated by data that vpr may, in certain situations, inhibit apoptosis.204-206The observation that virion-associated vpr acts as an immediate early viral protein to induce apoptosis207 is inconsistent with the apparent requirement that viral replication must occur before the onset of infected T-cell apoptosis. In addition, the finding that direct HIV-induced T-cell apoptosis occurs in all phases of the cell cycle107 brings into question the role of vpr in direct infection apoptosis. Vpr is more likely to be involved in regulation of latency, control of replication, and resistance to antiretroviral agents.208
Knowledge that HIV-encoded protease is a cytotoxic protein that leads to apoptosis in human and bacterial cells after transfection209-212 has been exploited as a method of screening compounds for potential HIV protease inhibitory activity.213 However, the relevance of HIV protease to HIV-infected T-cell death in vitro and in vivo is unknown. HIV protease expression (by Western blotting) correlates with the presence of apoptosis in vitro and in vivo.214 Further studies demonstrate that HIV protease directly cleaves caspase 8214 and modifies cellular susceptibility to apoptosis by virtue of proteolytic degradation of the antiapoptotic protein Bcl2.215 Together these findings indicate that HIV protease may also play a role in the death of HIV-infected T cells. There are no data to suggest that HIV protease may influence the death of uninfected cells.
Indirect mechanisms of HIV-associated apoptosis
In addition to apoptosis induced directly by HIV proteins, HIV infection may induce T-cell apoptosis through indirect mechanisms, including activation-induced cell death and autologous infected cell–mediated killing. The indirect mechanisms of T-cell death mediate the deaths principally of uninfected T cells (Figure 2).
Activation-induced cell death
T cells obtained from HIV-infected patients undergo spontaneous apoptosis at a greater rate than cells from HIV-seronegative subjects.111,216-219 Furthermore, the ex vivo activation of CD4 T cells from HIV-infected patients (using a variety of stimuli) consistently enhances apoptosis compared with cells from uninfected subjects.111,122,124,217-219 This phenomenon, termed activation-induced cell death (AICD), occurs only in cells that have been previously activated,49,52 and it may represent the in vitro model of the effects of repeated antigenic stimulation.49,52,220,221 Naive peripheral blood T cells from HIV-negative patients, when stimulated through the T-cell receptor, undergo proliferation, cytokine secretion,222-226 and the development of susceptibility to apoptosis induced by Fas ligation.48,52,220,221 Subsequent stimulation results in AICD by the de novo production of FasL, which mediates autocrine and paracrine apoptosis.48,52,220,221Both in vivo and in vitro, HIV infection is associated with an activated T-cell phenotype,227-231 increased expression of Fas, enhanced susceptibility to Fas-mediated killing,111,115,116,174,232,233 and increased T-cell–expressed FasL after T-cell receptor stimulation,114,234 suggesting a role for Fas/FasL in HIV-associated AICD. Findings that retinoic acid inhibits FasL expression and resultant apoptosis in vitro221 and that retinoic acid therapy in HIV-infected patients reduces CD4 T-cell depletion235 support a causal role for Fas/FasL interactions in T-cell death induced by HIV.
Elevated levels of apoptosis are seen after mitogenic stimulation or TCR cross-linking of PBL from HIV-seropositive patients.124,154,216-218,227,236,237 The molecular signals responsible for apoptosis in these patients are unclear, but the administration of Fas, TRAIL/APO 2-L, or TNF antagonists reduces AICD in cells from patients infected with HIV,154 237suggesting that all 3 signals—Fas, TNF, and TRAIL/APO 2-L—may be involved.
Autologous infected cell–mediated killing
Macrophages,102,140monocytes,213,238,239 peripheral blood mononuclear cells,240 CD4 T cells,241 and CD8 T cells242 derived from HIV-infected patients may induce the death of uninfected CD4 T lymphocytes. Autologous infected cell–mediated killing may involve gp120 interactions (see below), the Fas/FasL system, or both. Macrophages express basal levels of FasL that are significantly up-regulated after infection with HIV,102 and monocytes from HIV-infected patients have significantly increased FasL expression compared with monocytes from HIV-negative controls.243 HIV-infected macrophages (and, to a lesser extent, uninfected macrophages) have been shown to kill Fas-sensitive T-cell targets102 in a major histocompatibility complex–unrestricted and Fas/TNF-dependent manner.140 Macrophage-mediated killing appears to be selective for uninfected T cells,244 as opposed to the mechanisms involved in infected T-cell death described above. Macrophage-mediated CD4 T-cell apoptosis has implications in vivo because levels of tissue apoptosis directly correlate with levels of macrophage-associated FasL.245 Thus, FasL may be the mediator of uninfected CD4 T-cell death by monocytes, macrophages,213,239 and CD8 T cells.242 246
CD8 T-cell apoptosis
Although levels of CD8 T-cell apoptosis are consistently elevated in patients infected with HIV (whether this occurs spontaneously, in response to activation stimuli [AICD] or after coincubation with autologous infected cells102,122-124,154,216,227,240,244,247-249), the CD8 T-cell count is not significantly reduced in these patients. This apparent paradox may be resolved by observations in SIV-infected primates receiving total body irradiation, in which it was observed that CD8 T-cell recovery significantly precedes the recovery of CD4 T cells.250 A similar delay in CD4 repopulation is also seen in humans receiving high-dose chemotherapy.251,252 These data have several potential interpretations, yet they demonstrate that CD8 T-cell rebound occurs earlier than CD4 T-cell rebound after PBL depletion. In HIV-infected patients, it may therefore be expected that if rates of CD4 and CD8 T-cell loss were equal, the steady state CD8 number may be greater than the CD4 number because of the quicker recovery times. Further, HIV-associated apoptosis may lead to greater absolute numbers of CD4 T-cell apoptosis than CD8 T-cell apoptosis, because direct infection and gp120-mediated apoptosis selectively target cells that express CD4, whereas gp120 does not bind to (and thus cross-link) CD8. Nonetheless, it has recently been proposed that macrophage-associated gp120 may mediate CD8 T-cell apoptosis through interaction with CXCR4.253 Alternative potential mechanisms may also be involved (see below).
The fact that CD8 T cells from patients with HIV infection are more activated than are similar cells from HIV-uninfected persons227,254-257 suggests that the enhanced state of susceptibility to apoptosis is present in CD8 and in CD4 T cells and that CD8 T cells would be expected to die by apoptosis after exposure to another activation stimulus or with a preformed apoptosis-inducing ligand (eg, macrophage-associated FasL140). Furthermore, CD8 T cells express the CD4 receptor after activation, thereby rendering them susceptible to direct infection by the virus.258 259 In addition, the enhanced expression of CD4 antigen on CD8 T cells would be expected to render these double-positive cells more susceptible to the effects of gp120 cross-linking and subsequent apoptosis. Despite the several possible pathways that may be responsible for CD8 T-cell apoptosis in HIV-infected patients, chronic antigenic stimulation most likely contributes to CD8 T-cell apoptosis. The relative role of direct infection leading to CD8 T cell death remains untested.
Associations of apoptosis with HIV disease progression and response to therapy
Clinical studies in patients infected with HIV measure spontaneous apoptosis, Fas ligation-induced apoptosis, and apoptosis occurring in response to mitogenic activation or TCR cross-linking. In relation to the various mechanisms of apoptosis outlined above, spontaneous apoptosis may reflect infected cell apoptosis or gp120-induced apoptosis; Fas-induced apoptosis may reflect autologous cell-mediated killing of uninfected bystander cells or AICD; apoptosis in response to mitogen or CD3 ligation reflects AICD. In studies in which tissue apoptosis has been measured,260-262 few apoptotic cells are found to be physically infected by virus,260suggesting that tissue apoptosis reflects the killing of uninfected cells by gp120-induced or autologous cell-mediated killing of uninfected cells.
The magnitude of apoptosis observed in HIV-infected patients correlates well with the stage of HIV disease in longitudinal and cross-sectional analyses.263-265 Spontaneous apoptosis is greater in HIV-infected patients with progressive disease than in uninfected patients.266,267 In addition, spontaneous apoptosis in patients with long-term nonprogressive HIV infection are similar to those of HIV-negative patients.268 Thus, the rate of apoptosis correlates inversely with CD4 T-cell depletion. Because recent advances in HIV therapy have resulted in sustained increases in CD4 T cell number, if enhanced apoptosis causes CD4 T-cell depletion then apoptosis must decrease during therapy.
Numerous studies have shown that apoptosis in lymph nodes, rectal mucosa, and PBL subsets from patients infected with HIV decreases dramatically in response to protease inhibitor-based HIV treatment.125,269-274 This effect is seen for spontaneous apoptosis, apoptosis in response to T-cell receptor ligation, apoptosis in response to mitogenic stimulation, and apoptosis in response to Fas receptor ligation.125,271,272,274 The decrease in apoptosis is rapid and is seen as early as 4 days after protease inhibitor therapy is initiated125; it occurs in all patients within 14 days.271-274 Because the decrease precedes significant changes in viral replication, it has been suggested that protease inhibitors may be antiapoptotic,275 276 possibly by virtue of inhibiting the activity of effector proteases involved in apoptosis.
Effects of cytokines on HIV-associated apoptosis
One hallmark of infection with HIV is progressive T-helper-cell dysfunction. As HIV disease progresses, the balance of Th1 cytokines (IL-2 and IFN-γ) that enhance cellular immunity eventually shift to a Th2 cytokine profile (IL-4, IL-5, IL-6, and IL-10) that promotes humoral responses. The suggestion that helper cell dysfunction is central to the pathogenesis of HIV infection277 is supported by observations278-280 that the Th1-promoting cytokine IL-12, or the use of antagonistic antibodies specific for the Th2 cytokines IL-4 and IL-10, restores T-cell proliferative responses to recall antigens in HIV-infected patients. Because of the pervasive effects of cytokines in modulating apoptosis and apoptosis susceptibility, cytokine-based therapy may result in changes in apoptosis. Indeed, it has been reported that resistance to apoptosis in HIV and SIV infection is associated with a predominance of a Th1 phenotype,281 arguing that chronic immune activation and a Th2 shift may promote apoptosis. Consistent with this hypothesis, spontaneous apoptosis in cells from HIV-infected patients is blocked by the administration of IL-12, IFN-γ, anti–IL-4, anti–IL-10, and antilymphotoxin, but not by anti–IL-12 therapy.282Furthermore, IL-12 protects against the enhanced sensitivity to Fas-mediated apoptosis and enhanced sensitivity to AICD seen in HIV-infected patients.219
Apoptosis in patients with HIV infection is modulated by exogenous cytokines or cytokine antagonists that promote a Th1 helper cell phenotype and by cytokines that promote T-cell proliferation. IL-2 therapy in patients infected with HIV results in increased CD4 T-cell numbers unrelated to decreases in viral replication. Thus, IL-2 may modulate CD4 T-cell survival directly, possibly through an antiapoptotic mechanism, a hypothesis supported by in vitro studies in which clinically relevant concentrations of IL-2 significantly reduce spontaneous apoptosis in CD4+ T cells from HIV-infected patients but not from HIV-uninfected patients.283
IL-15 is a T-cell growth factor whose effects include T-cell proliferation, enhanced cytotoxicity of T cells and natural killer cells, B-cell proliferation, and immunoglobulin secretion.284 The effects of IL-15 on T cells are related to its ability to bind to a trimeric receptor consisting of the IL-15Rα subunit and the shared IL-2Rβ and IL-2Rγ subunits. Thus, many physiologic effects of IL-15 parallel those of IL-2. In addition, the incubation of peripheral blood mononuclear cells from HIV-infected patients with IL-15 results in enhanced production of the Th1 cytokine IFN-γ,285 CD8 T-cell activation, increased numbers of CD8 T cells,286 enhanced lymphoproliferative responses,287 and decreased spontaneous T-cell apoptosis,288 possibly mediated by increases in Bcl-2 expression.288 It is significant that although IL-2 increases HIV replication, IL-15 does not share this effect.287 288 Finally, IL-16 may have therapeutic implications for HIV-associated apoptosis.
IL-16 is a chemoattractant289 that inhibits lymphocyte activation290 and may also inhibit HIV replication.291 Possibly because of its antiproliferative effects, IL-16 treatment in vitro decreases levels of anti-CD3– or anti-Fas–induced apoptosis in lymphocytes from HIV-infected patients.292 However, the inhibitory effects of IL-16 on apoptosis are not seen in the context of spontaneous apoptosis.292
T-cell regeneration in response to therapy
The institution of highly active antiretroviral therapy (HAART) has witnessed a major impact on immune reconstitution: sustained increases in numbers of circulating CD4 T cells associated with a rapid drop in plasma viral RNA levels. The mechanisms proposed to explain the increase in numbers of CD4 T cells include cellular redistribution from lymphoid tissue,293 cellular proliferation of the peripheral T-cell pool,294 new T-cell synthesis from a thymic source,295,296 and reduced levels of apoptosis (see above). We have previously demonstrated that HAART therapy rapidly reduces apoptosis in lymphoid tissue273 and significantly decreases apoptosis in PBL.125,273 The decrease in apoptosis occurs before significant changes on plasma viral RNA levels and when patients are receiving only the protease inhibitor component of the HAART regimen.125 This finding has led to the proposal that protease inhibitors have an effect on immune reconstitution that is independent of their ability to suppress HIV replication.275,276,297 In vitro therapy with protease inhibitors has been shown to reduce the expression of selected caspases in treated cells and to reduce the rate of caspase 3 activation.275,297 Additional evidence for an indirect protease inhibitor effect comes from studies that demonstrate sustained CD4 rises in patients who experience virologic failure298-301 and who are receiving protease inhibitor-containing HAART regimens.
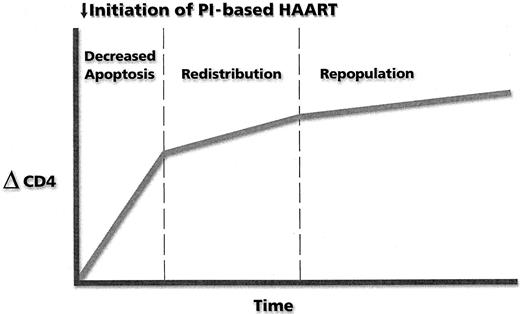
The early rise (2 weeks) in CD4 cells attributable to a reduction in apoptosis appears to be followed by a phase of CD4 cell increase due to cellular redistribution and proliferation of predominately memory CD4 T cells302-306 (Figure 3). A possible third phase consists mainly of new T-cell synthesis characterized by cells with a naive phenotype.307 This third phase of T-cell regeneration is characterized by the presence of circular DNA elements formed after the rearrangement of possible T-cell receptor alleles, thereby indicating that these are newly produced T cells that have matured in the thymus.295 296
Kinetics of change in CD4 T-cell number after the initiation of protease inhibitor (PI)–based HAART.
Kinetics of change in CD4 T-cell number after the initiation of protease inhibitor (PI)–based HAART.
Alteration of apoptosis as a therapeutic approach in HIV infection
If CD4 T-cell depletion in HIV infection results from enhanced apoptosis, then the prevention of apoptosis might be expected to modify the course of HIV disease. In vitro studies using apoptosis inhibitors (with no intrinsic antiviral properties) on PBL from HIV-infected patients cause increased viral production and increased cell survival.308 These findings suggest that non-apoptotic–infected cells serve as viral reservoirs and that it is unlikely that phenotypic and functional abnormalities of infected cells will be reversed by merely inhibiting apoptosis. Thus, blocking apoptosis alone fails to meet 2 objectives of effective HIV therapy: it does not decrease viral replication or decrease viral reservoirs, and it does not increase cellular immune competence.
The main obstacle to viral eradication in HIV-infected patients (reviewed in Chun and Fauci309) is the presence of chronically infected latent reservoir cells, such as macrophages, and latently infected CD4 T lymphocytes.310-313 In these cellular populations, HIV infection is not associated with apoptosis but with a chronic productively infected phenotype. Indeed, latently infected CD4 T cells have a markedly prolonged half-life (estimated at 6 months), which limits the probability that viral reservoirs can be eliminated by interference in viral replication alone.314In fact, recent estimates based on the half-life of latently infected cells suggest that 60 years of viral suppression would be required to eliminate viral reservoirs.311 A possible way to achieve viral eradication is to target infected macrophages and latently infected CD4 T cells to undergo apoptosis after infection. Along these lines, it has recently been proposed that treatment with a pro–caspase 3 analogue, which contains an HIV protease–specific sequence in its prodomain, may cause apoptosis of all infected cells.315Additional research is required to evaluate the clinical usefulness of this and other approaches designed to enhance the apoptosis of cells that normally function as reservoirs for HIV. The concept of enhancing HIV-associated apoptosis is, however, a potentially significant step forward in attempts to modify apoptosis for the benefit of patients infected with HIV. It further underscores the need for continued efforts to understand the regulation of apoptosis induced by HIV infection.
Acknowledgments
The authors thank Ms A. Carisse for expert secretarial assistance and the entire Badley laboratory staff and Dr B. W. D. Badley for helpful discussions and manuscript review.
Supported by grants from the Medical Research Council of Canada (HOP-36047) and the Doris Duke Foundation (T98026), the AIDS Program Committee of Ontario, and the Ontario HIV Treatment Network.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andrew D. Badley, Division of Infectious Diseases, Ottawa Hospital Research Institute, 501 Smyth Rd, Ottawa, Ontario K1H 8L6, Canada; e-mail: abadley@ottawahospital.on.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal