Abstract
It is now widely accepted that polymerase chain reaction (PCR) analysis of cutaneous T-cell clonality is of diagnostic value in cutaneous T-cell lymphomas (CTCLs) and most helpful in the diagnosis of mycosis fungoides (MF). However, the diagnostic and prognostic value of circulating clonal T cells remains unclear. We studied T-cell clonality in the peripheral blood (PB) and the cutaneous lesion, sampled at the same time, in 363 consecutively seen patients with a clinical suspicion of cutaneous lymphoma. Using a PCR technique providing a specific imprint of T-cell clones (PCRγ–denaturing gradient gel electrophoresis), we found that detection of identical circulating and cutaneous T-cell clones was associated with the diagnosis of CTCL (P < .001). Detection of circulating tumor cells in patients with MF was infrequent (12.5%), except in those with erythrodermic MF (42%; P = .003). Moreover, among the 46 patients who had identical circulating and cutaneous T-cell clones, 25 (56%) had erythroderma. The finding of a dominant clone in the PB but not in the skin was frequent, regardless of the clinicohistologic classification; it occurred in 30% of patients with CTCL, 41% with non-CTCL malignant infiltrates, and 34% with benign infiltrates. This pattern was significantly more frequent in patients over 60 years of age (P < .002), even in the CTCL group (P < .01). In conclusion, dominant T-cell clones detected in the PB of patients with MF by using a routine PCR technique are rarely tumoral and are more often related to age. A multicenter prospective study is under way to establish the prognostic value of circulating tumor cells.
Introduction
The recent development of molecular techniques for routine hospital work has led to the definition of new diagnostic and prognostic variables. For example, in a patient presenting with a clinical suspicion of cutaneous T-cell lymphoma (CTCL), it is now accepted that the identification by polymerase chain reaction (PCR) of a dominant T-cell clone in a cutaneous lesion supports the diagnosis of CTCL.1,2 Most reported PCR techniques identified a dominant cutaneous T-cell clone in 45% to 70% of cases of mycosis fungoides (MF).3-6 Previously, we reported that the presence of a dominant T-cell clone in the cutaneous infiltrate in patients with MF decreases the probability of remission induction after topical treatment, and we therefore proposed that this variable be included as a prognostic factor in clinical trials in such patients.7
The clinical extent of both cutaneous and extracutaneous lesions remains the main prognostic factor in MF.8 It has long been known that dissemination to the peripheral blood (PB) indicates a poor prognosis.9 However, the techniques that have been used are either not sensitive (cytogenetic analysis) or not specific (cytologic analysis and electron microscopy). Initial molecular studies used Southern blot analysis, which improved the specificity of disease-extension studies and confirmed the poor prognosis associated with circulating tumor cells.9-11 However, Southern blotting is laborious and expensive and is thus not suitable for routine hospital work. Analysis of T-cell clonality by PCR and migration of PCR products on a denaturing gradient gel, ie, PCR-γ–denaturing gradient gel electrophoresis (PCR-γ– DGGE),12-14 provides an imprint consisting of 1 or, more frequently, 2 bands specific for every T-cell clone. The imprint can be identified in a malignant lesion and then used to look for evidence of malignant disease at distant sites. Using PCR-γ–DGGE, we detected a circulating population of tumor cells in 42% of 37 patients selected for a histologically established diagnosis of CTCL (all histologic subtypes included).4
The aim of this study was to determine the frequency and diagnostic value of circulating tumor cells as detected by PCR-γ–DGGE in a prospective cohort of 363 patients presenting consecutively with a clinical suspicion of cutaneous lymphoma. On one hand, the presence in the PB of a T-cell clone identical to that in the skin was associated with the diagnosis of CTCL. This was the case in only 12.5% of patients with MF, and almost half of them had erythroderma. On the other hand, 30% of the patients with MF had a circulating clone that was not found in the skin. We considered the importance of these clones.
Patients, materials, and methods
Patients
From January 1, 1994, until June 30, 1997, a cutaneous biopsy specimen and a simultaneously obtained PB sample from 363 consecutively seen patients followed at Henri-Mondor Hospital and Avicenne Hospital were studied prospectively for T-cell clonality. Patients were included when cutaneous lymphoma was clinically considered in the differential diagnosis. Patients presenting with a clinically typical benign skin condition, such as psoriasis, eczema, or lichen planus, were not eligible for the study. Patients provided informed consent to molecular studies of their skin and blood samples. Forty of the patients, seen between January and August 1994, were included in previously reported studies assessing either the diagnostic or the prognostic value of cutaneous T-cell clonality.2,7 Staging in patients with MF was done according to the modified tumor-node-metastasis classification system for CTCLs.15,16
Histologic diagnosis
Patients were classified according to the histologic data from the skin lesion, which was analyzed for T-cell clonality. Patients with confirmed cutaneous lymphoma were placed in the following groups on the basis of the classification system of the European Organization for Research and Treatment of Cancer (EORTC)17: MF, Sézary syndrome (SS), pleomorphic T-cell lymphoma (PTCL), lymphomatoid papulosis (LyP), and cutaneous B-cell lymphomas (CBCL).
Patients with a histologically established diagnosis of non-CTCL disease were divided into 2 groups. The first group consisted of patients with well-defined benign dermatoses, such as contact dermatitis, drug-induced dermatoses, lichenoid reactions, benign follicular mucinosis, psoriasis, folliculitis, benign panniculitis, vasculitis, prurigo, sarcoidosis, Jessner-Kanof infiltration, discoid lupus erythematosus, granuloma annulare, pityriasis lichenoides, dermatophytosis, cutaneous lymphoid hyperplasia (ie, lymphocytoma), and benign tumors. The second group consisted of patients with non-CTCL malignant disease (non-CTCL MD).
In some patients with persistent plaques or erythroderma, MF or SS was clinically suspected but it was not possible to confirm the diagnosis on skin biopsy. The specimens from these patients were divided in 2 categories: uncertain MF and nonspecific lesions. Uncertain MF was defined as a linear subepidermal lymphocytic infiltrate with few Sézary cells and focal epidermotropism. Lesions were considered nonspecific if the lymphocytic infiltrates were patchy and perivascular and had neither Sézary cells nor epidermotropism.
T-cell receptor gene rearrangement analysis by GC-chain clamp multiplex PCR-γ–DGGE
DNA was extracted from frozen 4-mm punch skin biopsy samples or PB mononuclear cells by a standard procedure using proteinase K digestion and phenol-chloroform precipitation. T-cell receptor (TCR) γ-chain gene rearrangements were studied by using a GC-clamp multiplex PCR-γ–DGGE procedure as previously described.14 Briefly, 4 oligonucleotides matching the 4 Vγ segment families and 4 oligonucleotides matching the Jγ junction segments were used in a single 50-μL PCR reaction (multiplex PCR) in a thermal cycle (model 480 thermocycler; Perkin Elmer, Foster City, CA). To avoid contamination by the amplification products, deoxyuridine triphosphate nucleotides were substituted for deoxythymidine triphosphate in the amplification reaction mixture and samples were first subjected to uracil-DNA glycosylase activity. After 40 PCR cycles, 30 μL of amplified products were run on a 6.5% polyacrylamide gel containing a linearly increasing 10% to 60% denaturing gradient (DGGE). The use of oligonucleotides matching all Vγ and Jγ functional segments, combined with DGGE, allows creation of a migration profile specific for each T-cell clone.14Three positive controls were added in each series: the Jurkat cell line with a biallelic VIJI and VIVJI rearrangement, and 2 samples from patients with dominant T-cell clones with VIIJP/VIIJP and VIIIJP/VIIIJP1 rearrangements. The PCR results were considered positive (PCR-γ+) when a dominant T-cell clone was detectable and negative (PCR-γ−) when a polyclonal pattern of T-cell infiltration was observed. In the latter case, the presence of a smear on the gel ensured that T-cell DNA was present and amplified.
The sensitivity of this technique depends on the type of Vγ-Jγ rearrangement used by the malignant T-cell clone and the relative number of clonal and polyclonal T cells in the sample. We determined the sensitivity of the technique by diluting DNA from the Jurkat T-cell line in DNA extracted from normal skin,4 from reactive lymph nodes,14 and from PB (data not shown). We found the sensitivity to be 0.1% for the rare VIVJI rearrangement in a poor T-cell infiltrate and to be 1% and 5% for the frequent VIJI rearrangement diluted in PB mononuclear cells and in a dense reactive T-cell infiltrate, respectively. This means that 1 or 2 discrete bands are visible when a dominant T-cell clone consists of 0.1% to 5% (or more) skin cells4 14 and more than 1% of PB cells.
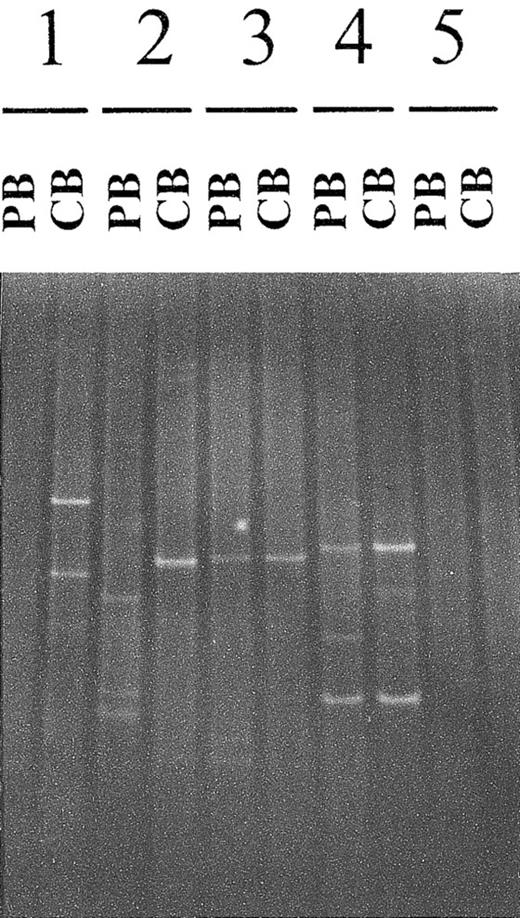
Because the patients with CTCL were assumed to have a primary cutaneous lymphoma, tumor cells in their skin were characterized by their PCR-γ–DGGE imprint. Therefore, circulating T cells were considered to be tumor cells when the PCR-γ imprint was the same in both the PB and skin specimen (Figure 1). The background remaining T-cell repertoire could be either polyclonal (smear on the gel) or oligoclonal (bands additional to the tumor-cell–specific bands).
Comparison of cutaneous and circulating T-cell clones detected by polymerase chain reaction γ–denaturing gradient gel electrophoresis.
For each patient, PCR-γ–DGGE was performed by using DNA extracted from a cutaneous biopsy (CB) sample and a peripheral blood (PB) sample obtained on the same day. The 2 PCR products were analyzed on the same gel, thereby allowing precise comparison of the dominant clonal populations. Four of the 5 patterns are shown: presence of a dominant T-cell clone in the CB sample but not in PB (patient 1); simultaneous presence of a dominant T-cell clone in the CB sample and PB, with the 2 clones being different (patient 2); identical T-cell clones in the CB sample and PB (patients 3 and 4); and no dominant clone in either the CB sample or PB (patient 5). An example of a positive PB sample and a negative CB sample is not shown.
Comparison of cutaneous and circulating T-cell clones detected by polymerase chain reaction γ–denaturing gradient gel electrophoresis.
For each patient, PCR-γ–DGGE was performed by using DNA extracted from a cutaneous biopsy (CB) sample and a peripheral blood (PB) sample obtained on the same day. The 2 PCR products were analyzed on the same gel, thereby allowing precise comparison of the dominant clonal populations. Four of the 5 patterns are shown: presence of a dominant T-cell clone in the CB sample but not in PB (patient 1); simultaneous presence of a dominant T-cell clone in the CB sample and PB, with the 2 clones being different (patient 2); identical T-cell clones in the CB sample and PB (patients 3 and 4); and no dominant clone in either the CB sample or PB (patient 5). An example of a positive PB sample and a negative CB sample is not shown.
Exceptionally, 2 dominant clones from 2 patients seemed to comigrate on the 10% to 60% DGGE gel, particularly when monoallelic rearrangements were amplified. However, the 2 rearrangements could be easily distinguished by loading the PCR products on a discriminating 30% to 50% denaturing gradient gel.
Statistical methods
Comparisons of categorical data were done by using the χ2 test or, when appropriate, the Fisher exact test.
Results
Patient characteristics
We studied a biopsy specimen from a skin lesion in 363 consecutively seen patients in whom a clinical diagnosis of cutaneous lymphoma was initially considered. The histologic diagnoses and the ages of the patients with those diagnoses are shown in Table1. There were 88 patients with MF, 22 with SS, 28 with PTCL, and 14 with LyP, for a total of 152 patients with CTCL. The benign dermatoses (n = 72) were 55 reactive dermatoses, 7 benign tumors, and 10 cases of cutaneous lymphoid hyperplasia. Non-CTCL MD was diagnosed in 27 patients (24 cases of CBCL, 1 of Merkel cell carcinoma, 1 of true histiocytic lymphoma, and 1 of myelomonocytic proliferation). The uncertain MF (n = 27) group and the nonspecific (n = 85) group included the patients in whom the diagnosis of MF could not be supported by the histologic data from the biopsy. These prospective findings confirm that a dominant monoclonal T-cell population is detected in cutaneous lesions of CTCL (all histologic subtypes) in 70% of cases4 and in 62.5% of patients with MF2 (Table 1). Also, we found that 17 of 72 patients in whom CTCL was initially considered in the differential diagnosis, despite benign histologic characteristics, had a dominant T-cell clone in the skin (Table 1).
Frequency of identical T-cell clones in the skin and peripheral blood, according to histologic diagnosis in 363 patients assessed by polymerase chain reaction-γ-denaturing gradient gel electrophoresis
| Diagnosis (no. of patients) . | Mean age (range) . | Skin positive for clone, no. (%) of patients . | PB positive for identical clone, no. (%) of patients . |
|---|---|---|---|
| MF (88) | 60 (17-94) | 55 (62.5) | 11 (12.5) |
| SS (22) | 65 (45-88) | 17 (77) | 15 (68) |
| PTCL (28) | 52 (14-91) | 26 (93) | 7 (25) |
| LyP (14) | 40 (10-79) | 8 (57) | 0 |
| All CTCL (152) | 57 (14-94) | 106 (70) | 33 (22) |
| Benign dermatoses (72) | 55 (7-93) | 17 (24)* | 2 (3) |
| Non-CTCL MD (27) | 67 (38-91) | 7 (26) | 0 |
| Uncertain MF (27) | 64 (19-87) | 6 (22) | 1 (4) |
| Nonspecific (85) | 61 (13-91) | 21 (25) | 10 (12) |
| All non-CTCL (211) | 60 (7-91) | 51 (24) | 13 (6) |
| Diagnosis (no. of patients) . | Mean age (range) . | Skin positive for clone, no. (%) of patients . | PB positive for identical clone, no. (%) of patients . |
|---|---|---|---|
| MF (88) | 60 (17-94) | 55 (62.5) | 11 (12.5) |
| SS (22) | 65 (45-88) | 17 (77) | 15 (68) |
| PTCL (28) | 52 (14-91) | 26 (93) | 7 (25) |
| LyP (14) | 40 (10-79) | 8 (57) | 0 |
| All CTCL (152) | 57 (14-94) | 106 (70) | 33 (22) |
| Benign dermatoses (72) | 55 (7-93) | 17 (24)* | 2 (3) |
| Non-CTCL MD (27) | 67 (38-91) | 7 (26) | 0 |
| Uncertain MF (27) | 64 (19-87) | 6 (22) | 1 (4) |
| Nonspecific (85) | 61 (13-91) | 21 (25) | 10 (12) |
| All non-CTCL (211) | 60 (7-91) | 51 (24) | 13 (6) |
PB indicates peripheral blood; MF, mycosis fungoides; SS, Sézary syndrome; PTCL, pleomorphic T-cell lymphoma; LyP, lymphomatoid papulosis; CTCL, cutaneous T-cell lymphomas, and MD, malignant disease.
The histologic diagnoses were benign follicular mucinosis (3 patients), contact dermatitis (3), benign panniculitis (3), pityriasis lichenoides (2), cutaneous lymphoid hyperplasia (2), Jessner-Kanof infiltration (1), drug-induced dermatoses (1), folliculitis (1), and pemphigoides (1).
Detection of an identical cutaneous and circulating T-cell clone is associated with the diagnosis of CTCL
The biopsy specimen of the skin lesion and a simultaneously obtained PB sample were analyzed for T-cell clonality. Use of PCR-γ–DGGE allowed the identity of 2 dominant clones detected in the 2 samples to be established. Among the 363 patients, 46 (13%) had the same dominant clone in the PB and skin (Table 1). Detection of an identical clone in the PB and skin occurred significantly more often in the 152 patients (22%) with histologically confirmed CTCL than in the 211 patients (6%) in the non-CTCL group (P < .001). This difference remained significant (P < .02) when the 22 patients with SS were not taken into account because a high proportion of them, as expected, had circulating tumor cells (Table 1).
Detection of circulating tumor cells is infrequent in patients with MF
Analysis of the 88 patients with MF (Table 1) showed that 55 had a dominant clone in the skin. Eleven of these 55 patients (20%)—ie, 12.5% of the 88 patients with MF—also had a circulating tumoral clone. When we examined the influence of disease stage on the finding of circulating tumor cells (Table2), we found that circulating tumor cells were infrequent in nonerythrodermic stages of MF; the proportions were 4%, 15%, and 0%, respectively, in patients with stage Ia, stage Ib, and stage IIa or IIb. Circulating tumor cells were detected more frequently in the 12 MF patients with erythroderma (stage III) (42%;P = .003).
Polymerase chain reaction-γ–denaturing gradient gel electrophoresis detection of circulating malignant T-cell clones in 88 patients with mycosis fungoides
| TNM stage (no. of patients) . | Skin positive for clone, no. (%) of patients . | PB positive for identical clone, no. (%) of patients* . |
|---|---|---|
| Ia (26) | 16 (61.5) | 1 (4) |
| Ib (34) | 21 (62) | 5 (15) |
| IIa (6) | 3 (50) | 0 |
| IIb (3) | 3 (100) | 0 |
| III (12) | 7 (58) | 5 (42) |
| nd (7) | 5 (71) | 0 |
| TNM stage (no. of patients) . | Skin positive for clone, no. (%) of patients . | PB positive for identical clone, no. (%) of patients* . |
|---|---|---|
| Ia (26) | 16 (61.5) | 1 (4) |
| Ib (34) | 21 (62) | 5 (15) |
| IIa (6) | 3 (50) | 0 |
| IIb (3) | 3 (100) | 0 |
| III (12) | 7 (58) | 5 (42) |
| nd (7) | 5 (71) | 0 |
TNM indicates tumor-node-metastasis; PB, peripheral blood; nd, not determined.
The T-cell clone detected in the PB was identical to the clone in the mycosis fungoides skin lesion and was therefore considered malignant.
Most patients with identical circulating and cutaneous T-cell clones had erythroderma
Remarkably, among the 46 patients who had the same dominant clone in the PB and skin, 26 (57%) had erythroderma at presentation. As expected, the 15 patients with SS were among these 26, but there were also 11 patients who did not have SS (35%): 5 had MF and 6 had non-CTCL skin lesions (Table 3).
Clinical characteristics of the 46 patients with identical skin and peripheral blood T-cell clones
| Patient . | Age, y . | Skin lesion . | Histologic diagnosis . |
|---|---|---|---|
| 1 | 68 | Erythroderma | SS |
| 2 | 67 | Erythroderma | SS |
| 3 | 79 | Erythroderma | SS |
| 4 | 73 | Erythroderma | SS |
| 5 | 46 | Erythroderma | SS |
| 6 | 57 | Erythroderma | SS |
| 7 | 74 | Erythroderma | SS |
| 8 | 59 | Erythroderma | SS |
| 9 | 62 | Erythroderma | SS |
| 10 | 59 | Erythroderma | SS |
| 11 | 84 | Erythroderma | SS |
| 12 | 58 | Erythroderma | SS |
| 13 | 46 | Erythroderma | SS |
| 14 | 45 | Erythroderma | SS |
| 15 | 84 | Erythroderma | SS |
| 16 | 56 | Erythroderma | MF |
| 17 | 63 | Erythroderma | MF |
| 18 | 75 | Erythroderma | MF |
| 19 | 64 | Erythroderma | MF |
| 20 | 85 | Erythroderma | MF |
| 21 | 54 | Erythroderma | Uncertain MF |
| 22 | 54 | Erythroderma | Nonspecific |
| 23 | 81 | Erythroderma | Nonspecific |
| 24 | 63 | Erythroderma | Contact dermatitis |
| 25 | 80 | Erythroderma | Nonspecific |
| 26 | 67 | Erythroderma | Uncertain MF |
| 27 | 63 | Patches/plaques | MF |
| 28 | 67 | Patches/plaques | MF |
| 29 | 70 | Patches/plaques | MF |
| 30 | 94 | Patches/plaques | MF |
| 31 | 52 | Patches/plaques | MF |
| 32 | 33 | Patches/plaques | MF |
| 33 | 88 | Tumor | PTCL |
| 34 | 38 | Tumor | PTCL |
| 35 | 48 | Tumor | PTCL |
| 36 | 50 | Tumor | PTCL |
| 37 | 64 | Tumor | PTCL |
| 38 | 22 | Tumor | PTCL |
| 39 | 63 | Tumor | PTCL |
| 40 | 67 | Maculopapules | Nonspecific |
| 41 | 85 | Patches | Nonspecific |
| 42 | 57 | Patches | Nonspecific |
| 43 | 50 | Papulonodule | Nonspecific |
| 44 | 30 | Patches | Nonspecific |
| 45 | 91 | Papule | Nonspecific |
| 46 | 59 | Papule | Jessner-Kanof |
| Patient . | Age, y . | Skin lesion . | Histologic diagnosis . |
|---|---|---|---|
| 1 | 68 | Erythroderma | SS |
| 2 | 67 | Erythroderma | SS |
| 3 | 79 | Erythroderma | SS |
| 4 | 73 | Erythroderma | SS |
| 5 | 46 | Erythroderma | SS |
| 6 | 57 | Erythroderma | SS |
| 7 | 74 | Erythroderma | SS |
| 8 | 59 | Erythroderma | SS |
| 9 | 62 | Erythroderma | SS |
| 10 | 59 | Erythroderma | SS |
| 11 | 84 | Erythroderma | SS |
| 12 | 58 | Erythroderma | SS |
| 13 | 46 | Erythroderma | SS |
| 14 | 45 | Erythroderma | SS |
| 15 | 84 | Erythroderma | SS |
| 16 | 56 | Erythroderma | MF |
| 17 | 63 | Erythroderma | MF |
| 18 | 75 | Erythroderma | MF |
| 19 | 64 | Erythroderma | MF |
| 20 | 85 | Erythroderma | MF |
| 21 | 54 | Erythroderma | Uncertain MF |
| 22 | 54 | Erythroderma | Nonspecific |
| 23 | 81 | Erythroderma | Nonspecific |
| 24 | 63 | Erythroderma | Contact dermatitis |
| 25 | 80 | Erythroderma | Nonspecific |
| 26 | 67 | Erythroderma | Uncertain MF |
| 27 | 63 | Patches/plaques | MF |
| 28 | 67 | Patches/plaques | MF |
| 29 | 70 | Patches/plaques | MF |
| 30 | 94 | Patches/plaques | MF |
| 31 | 52 | Patches/plaques | MF |
| 32 | 33 | Patches/plaques | MF |
| 33 | 88 | Tumor | PTCL |
| 34 | 38 | Tumor | PTCL |
| 35 | 48 | Tumor | PTCL |
| 36 | 50 | Tumor | PTCL |
| 37 | 64 | Tumor | PTCL |
| 38 | 22 | Tumor | PTCL |
| 39 | 63 | Tumor | PTCL |
| 40 | 67 | Maculopapules | Nonspecific |
| 41 | 85 | Patches | Nonspecific |
| 42 | 57 | Patches | Nonspecific |
| 43 | 50 | Papulonodule | Nonspecific |
| 44 | 30 | Patches | Nonspecific |
| 45 | 91 | Papule | Nonspecific |
| 46 | 59 | Papule | Jessner-Kanof |
SS indicates Sézary syndrome; MF, mycosis fungoides; PTCL, pleomorphic T-cell lymphoma.
The high frequency of circulating T-cell clones not detected in the skin is similar in all histologic groups
The frequency of circulating dominant T-cell clones that were not detected in the skin was assessed in 2 ways. Thus, the patients in whom the circulating clone was different from the dominant cutaneous clone were considered separately from the patients who did not have a dominant cutaneous clone and in whom, therefore, the meaning of the dominant circulating clonal population remained uncertain. Overall, such clones were detected in 33% of patients with MF and there were no differences (P = .99) among the 8 clinicohistologic groups analyzed in the frequency of a circulating clone that was not found in the skin (Table4).
Frequency of dominant T-cell clones in the peripheral blood that were not detected in the skin in 363 patients
| Diagnosis (no. of patients) . | Skin positive for different clone, no. of patients4-150 . | Skin negative for clone, no. of patients4-151 . | Total no. (%) of patients . |
|---|---|---|---|
| MF (88) | 16 | 13 | 29 (33) |
| SS (22) | 1 | 4 | 5 (23) |
| PTCL (28) | 8 | 1 | 9 (32) |
| LyP (14) | 1 | 1 | 2 (14) |
| Total CTCL (152) | 26 | 19 | 45 (30) |
| Uncertain MF (27) | 3 | 7 | 10 (37) |
| Nonspecific (85) | 4 | 25 | 29 (34) |
| Benign dermatoses (72) | 6 | 18 | 24 (33) |
| Non-CTCL MD (27) | 3 | 8 | 11 (41) |
| Total non-CTCL (211) | 16 | 58 | 74 (35) |
| Diagnosis (no. of patients) . | Skin positive for different clone, no. of patients4-150 . | Skin negative for clone, no. of patients4-151 . | Total no. (%) of patients . |
|---|---|---|---|
| MF (88) | 16 | 13 | 29 (33) |
| SS (22) | 1 | 4 | 5 (23) |
| PTCL (28) | 8 | 1 | 9 (32) |
| LyP (14) | 1 | 1 | 2 (14) |
| Total CTCL (152) | 26 | 19 | 45 (30) |
| Uncertain MF (27) | 3 | 7 | 10 (37) |
| Nonspecific (85) | 4 | 25 | 29 (34) |
| Benign dermatoses (72) | 6 | 18 | 24 (33) |
| Non-CTCL MD (27) | 3 | 8 | 11 (41) |
| Total non-CTCL (211) | 16 | 58 | 74 (35) |
MF indicates mycosis fungoides; SS, Sézary syndrome; PTCL, pleomorphic T-cell lymphoma; LyP, lymphomatoid papulosis; CTCL, cutaneous T-cell lymphoma.
The T-cell clones in peripheral blood were different from those in skin.
There were no detectable T-cell clones in the skin.
Circulating T-cell clones not detected in the skin were more frequent in patients older than 60 years
The frequency of a circulating T-cell clone that was not detected in the skin was significantly greater in patients aged 60 years or older (76/194) than in patients under 60 years of age (38/169;P = .002; Table 5). This age-related difference remained significant when only the 152 patients with CTCL were analyzed (P < .01). In contrast, detection in the PB of a T-cell clone identical to that in the skin, which was associated with a diagnosis of CTCL, was not influenced by the age of the patient (P > .5).
Relation between frequency of dominant T-cell clones in the peripheral blood and patient age
| T-cell clones . | Age < 60 y (n = 169), no. (%) of patients . | Age > 60 y (n = 194), no. (%) of patients . | P value on χ2 test . |
|---|---|---|---|
| Detected in PB but not in skin | 38 (22) | 76 (39) | .002 |
| Identical in PB and skin | 20 (12) | 26 (13) | .8 |
| T-cell clones . | Age < 60 y (n = 169), no. (%) of patients . | Age > 60 y (n = 194), no. (%) of patients . | P value on χ2 test . |
|---|---|---|---|
| Detected in PB but not in skin | 38 (22) | 76 (39) | .002 |
| Identical in PB and skin | 20 (12) | 26 (13) | .8 |
PB indicates peripheral blood.
Discussion
We conducted a prospective study comparing T-cell clonality in the skin lesion and a simultaneously obtained PB sample in 363 consecutively seen patients in whom cutaneous lymphoma was clinically considered in the differential diagnosis. On histologic assessment, 152 of these patients were found to have CTCL and 88 of these 152 had MF. The study confirms that 70% of patients with CTCL and 62% of patients with histologically proven MF have a dominant T-cell clone in the cutaneous lesion that is detectable by PCR-γ–DGGE.2
At first, it may appear that the 24% positive samples found in the group considered to have non-CTCL on the basis of histologic analysis represent a high percentage. However, previous studies using molecular techniques found dominant T-cell clones in patients with various benign dermatoses, eg, in 22% of patients with cutaneous lymphoid hyperplasia,13 14% with contact dermatitis,18 14% with drug-induced dermatoses,19 14% with pseudolymphoma,5 and in individual cases of pityriasis lichenoides20 and benign follicular mucinosis.21 To compare the results in our non-CTCL group with those in true control groups in previous reports (namely, patients with ascertained clinicopathological diagnoses),3-6,13 the final diagnosis was considered at the end of the study. The final diagnoses, based on clinical presentation and outcome, repeated biopsy evaluations, and biologic data and available for 325 patients, were 182 cases of CTCL, 14 of large-plaque parapsoriasis, 26 of unclassified cutaneous inflammation, and 103 of non-CTCL. In the 103 patients with non-CTCL, 18% still had a detectable T-cell clone in the skin. It remains possible that CTCL would have developed in these patients during a longer follow-up period. Wood et al13 suggested that “clonal dermatitis” is a subgroup of histologically nonspecific dermatitis in which patients are at increased risk of development of CTCL. This is consistent with the fact that 30 patients in our study were finally reclassified as having CTCL and half of these patients had a detectable T-cell clone in the skin lesion. The current findings further illustrate that a dominant T-cell clone detectable in the skin supports a diagnosis of CTCL.
Because PCR-γ–DGGE allows T-cell clones in different samples to be compared without having to sequence the PCR products,14 we could prospectively analyze simultaneously obtained skin and blood samples in 363 patients. Detection of a dominant circulating clone identical to that in the skin was more frequent (22%) in the histologically defined CTCL group than in the non-CTCL group (6%). The difference was even greater when the final diagnosis was considered: 41 of 182 patients (22%) with CTCL and 1 of 103 (< 1%) with non-CTCL had identical clones in the PB and skin (P < .003). Our results show that the presence of a circulating T-cell clone identical to the dominant T-cell clone in the skin provides an additional criterion for CTCL diagnosis with respect to the analysis of cutaneous clonality alone. Because of the low frequency of identical clones in patients with histologically defined non-CTCL (6%) in our prospective study, the PB analysis is most useful when the histologic diagnosis is uncertain or nonspecific and PCR analysis of the cutaneous biopsy specimen yields positive results.
In the group of 88 patients with MF, detection of circulating tumoral cells was rare (12.5% of cases). This detection rate is similar to that obtained by Southern blot analysis,9 a technique with specificity and sensitivity in PB assessments similar to that of the PCR-γ–DGGE method used here. Our results differ from those of Muche et al,22 who found a circulating clonal population in 45% of 40 patients with MF by using a more sensitive PCR temperature-gradient gel electrophoresis technique (0.1%). These authors did not provide a T-cell-clone–specific imprint, and the identity of T-cell clones in the PB and skin was assessed by sequencing in 6 of 6 patients studied. It is of note that the study included pre-established cases of MF and the inclusion criteria did not clarify how the patients with MF were selected for further evaluation by sequencing. Our results remain compatible with the presence of a low level of circulating tumor cells, demonstrated in all cases studied by a highly sensitive (10−5) and clone-specific technique.23 Finally, PCR-γ–DGGE is useful for clinical purposes because it allows identification of 2 groups of patients with MF—those with and those without a circulating tumoral clone—who potentially have different prognoses.
It is of note that more than half (57%) of the patients with a circulating clonal population identical to the dominant cutaneous T-cell clone presented with erythroderma. Although this was expected for patients with SS (who, by definition, have circulating tumor cells and erythroderma), among the group of patients with MF, the frequency of a circulating tumoral population was significantly greater in those with the erythrodermic forms (42%; P = .003). Remarkably, in the non-CTCL group, 6 of 13 patients with an identical circulating and cutaneous clone had erythroderma. When the final diagnosis in these patients was considered, 4 of them did in fact have CTCL (3 patients with SS and 1 with erythrodermic MF) and 2 continued to be considered to have unclassified cutaneous inflammation. This is in keeping with the finding that nonspecific histologic findings are compatible with the diagnosis of CTCL.24,25
The nosologic distinction between erythrodermic MF and SS is not currently clear. In the EORTC classification,17erythrodermic MF is not defined; rather, EORTC recommends reserving the label of MF for classic disease with progression through the patch, plaque, and tumor stages. SS is characterized by the triad of erythroderma, generalized lymphadenopathy, and the presence of tumoral T cells (Sézary cells) in the skin, lymph nodes, and PB. Recent reviews of MF and SS8,26 did not categorize erythrodermic MF as a separate entity, although the distinction between the 2 conditions appears to have prognostic importance. Thus, lymph node involvement and the presence of more than 5% Sézary cells, which distinguish SS from erythrodermic MF, were 2 factors indicative of a poor prognosis in a series of 106 patients with erythroderma and a histologic diagnosis of epidermotropic cutaneous lymphoma.27 In another series, the absence of circulating tumor cells was a good prognostic factor for overall survival in patients with erythrodermic CTCL treated by total skin irradiation.28 Biologically, SS is defined by the number of circulating Sézary cells (> 15% of leukocytes in this study). However, there is no consensus regarding how these cells should be identified and the percentage required to make the diagnosis. None of the current techniques (cytologic analysis, electron microscopy, or immunophenotyping) are specific for a tumoral population. The monoclonal nature of the Sézary cell has been demonstrated in studies using molecular techniques; however, most of these studies also found a small percentage of patients with SS who did not have a detectable circulating monoclonal population on either Southern blot analysis10,29,30 or PCR.31 32
Altogether, on one hand, nearly 50% of patients with erythrodermic MF have circulating tumor cells and, on the other hand, a small subgroup of patients with SS do not have a detectable clone. The prognostic value of a circulating tumoral population detected by PCR-γ–DGGE in patients with erythrodermic CTCL, regardless of their classification as having erythrodermic MF or SS, is currently being analyzed.
In addition to circulating tumoral clones, a dominant T-cell clone in the PB not found in the skin was detected in 30% of patients with CTCL. The importance of these circulating clones remains unknown, although 3 hypotheses can be considered. First, in MF, some clones could correspond to genuine tumor cells not detected in the skin because the cutaneous clone is diluted in a reactive infiltrate. However, this situation must be rare because the frequency of circulating dominant clones (30%) was the same in the group of 72 patients with documented benign disease. Moreover, among the patients with MF who had a dominant T-cell clone in PB that was not detectable in skin lesions, none subsequently had lesions positive for the circulating clone. Second, these circulating clones could be reactive to the cutaneous tumor, as suggested recently for small-plaque parapsoriasis.33 Our results do not support this hypothesis, however, because a similar frequency (between 30% and 40%) of circulating T-cell clones not detected in the skin was found in all clinicopathologic categories studied. Third, these circulating dominant clones might be related to the clonal T-cell expansions in elderly patients, which were originally characterized in the CD8+ CD28− T-cell subset34 and then in the CD4+ T-cell subset as well.35 In support of this hypothesis, the frequency of a circulating T-cell clone not found in the skin was significantly higher in the patients older than 60 years (P = .002). Also, this was the case when patients with CTCL were considered (P < .01). These circulating dominant populations of unknown meaning were stable during follow-up (data not shown) and, so far, none of these clones have evolved into a malignant population.
In conclusion, our prospective study of PB T-cell clonality in patients with a clinical suspicion of cutaneous lymphoma showed that detection of a dominant clone in the PB identical to that in the skin provides additional evidence for the diagnosis of CTCL with respect to the demonstration of T-cell clonality in the skin alone. This study found that half of the patients with an identical T-cell clone in the PB and skin had erythroderma. The prognostic value of circulating tumor cells detected by PCR-γ–DGGE in patients with CTCL is currently being studied. We also observed that circulating clones not found in the skin are frequently detected (> 30%) in patients with CTCL as well as in those with non-CTCL. This finding has no value in the diagnosis of CTCL, but the meaning of this type of clone remains to be established.
Acknowledgment
We thank Samir Agrawal for rereading the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marie-Hélène Delfau-Larue, Service d'Immunologie Biologique, Hôpital Henri-Mondor, 51 av du Marechal de Lattre de Tassigny, 94010 Créteil, France; e-mail:marie-helene.delfau@hmn.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal