Abstract
The primary immunologic barrier to overcome before clinical xenotransplantation can be successful is rejection mediated by preformed natural antibodies in the host, directed toward a single carbohydrate epitope Galα1-3Galβ1-4GlcNAc-R (αGal) present on porcine tissue, encoded for by the enzyme glucosyltransferase UDP galactose:β-D-galactosyl-1,4-N-acetyl-D-glucosaminide α(1-3)galactosyltransferase (EC 2.4.1.151) or simply αGT. Although we have shown previously that a gene therapy approach could be used to prevent production of natural antibodies specific for αGal, the ability to induce and maintain tolerance after rigorous antigen challenge would be required if similar approaches are to be used clinically. Here, we demonstrate in αGT knockout mice (GT0 mice), which, like humans, contain in their serum antibodies that bind αGal, that the efficient transduction and expression of a retrovirally transduced αGT gene in bone marrow–derived cells induces stable long-term tolerance to the αGal epitope. GT0 mice reconstituted with αGT-transduced bone marrow cells were unable to produce antibodies that bind αGal after extensive immunization with pig cells. Furthermore, using ELISPOT assays, we were unable to detect the presence of B cells that produce αGal reactive antibodies after immunization, suggesting that such B cells were eliminated from the immunologic repertoire after gene therapy. Interestingly, after tolerance to αGal is induced by gene therapy, the antiporcine non-αGal humoral response changes from a predominantly IgM to an IgG response. This suggests that once the natural antibody barrier is eliminated by the induction of tolerance, the antipig response changes to a typical T-cell–dependent response involving isotype switching. Thus, gene therapy approaches may be used to overcome immunologic responses leading to xenograft rejection, and similar gene therapy approaches could be used to overcome autoimmunity.
Introduction
Shortages of human organs for transplantation have led to research into the possibility of using nonhuman species as organ donors. Pigs are now regarded as the most likely species to serve as donors for clinical xenotransplantation.1,2 However, rejection of pig tissues and organs, mediated by the host's immune system, remains a major barrier to successful xenotransplantation. The primary immunologic hurdle to overcome is rejection mediated by antibodies in the host that recognize antigens present on xenogeneic tissues. Because these antibodies are produced in the host without the need for immunization, they are referred to as xenoreactive natural antibodies (XNAs). In the pig-to-primate discordant combination, at least 80% to 90% of antipig XNAs recognize a single carbohydrate antigen present on pig tissue, Galα1-3Galβ1-4GlcNAc-R, hereafter referred to as αGal.3-8 The specificities of the other antibodies remain uncertain.9
Binding of human or primate XNAs to αGal expressed on vascular endothelium within pig organs initiates activation of the complement system, which in turn induces rapid destruction of endothelial cells and loss of vascular integrity culminating in hyperacute rejection.10 In the absence of complement, αGal XNA is still the major cause of delayed xenograft rejection or acute vascular rejection.11 These antibodies have also been implicated in chronic rejection of tissue grafts.12 Although several approaches have been developed to prevent hyperacute rejection mediated by αGal XNAs, delayed rejection caused by these antibodies remains a formidable challenge.13 14 Because it is not yet possible to generate αGal-deficient pigs, other approaches must be developed to overcome αGal XNA-mediated rejection.
The αGal epitope is synthesized by the addition of a terminal galactosyl group to a pre-existing galactose residue linked to an N-acetyl-glucosaminyl structure and is catalyzed by the glucosyltransferase UDP galactose: β-D-galactosyl-1,4-N-acetyl-D-glucosaminide α(1-3)galactosyltransferase (E.C. 2.4.1.151), or αGT. All placental mammals, except humans, apes, and Old World monkeys, express a functional αGT enzyme and αGal epitopes on most tissues, including vascular endothelium.15 These animals are immunologically tolerant to αGal because their immune system develops in an environment that recognizes this antigen as “self.” Because animals that express a functional αGT enzyme are immunologically tolerant to αGal, they do not produce antibodies that bind the αGal epitope. In contrast, humans, apes, and Old World monkeys carry a nonfunctional αGT gene, the function of which appears to have been lost during evolution approximately 30 million years ago.16 These species do not recognize αGal structures as self and consequently produce αGal XNAs. Mutant mice, generated by gene targeting in embryonic stem cells, which lack a functional αGT gene (GT0 mice) produce αGal reactive natural antibodies as do humans.17-19 These mice have therefore been used as a model to examine methods that will prevent production of αGal reactive antibodies.
It has been known for many years that tolerance across allogeneic and concordant xenogeneic species barriers can be achieved by inducing a state of mixed lymphohematopoietic chimerism after bone marrow transplantation. Indeed, mixed chimerism induced by bone marrow transplantation has been shown to induce tolerance to the αGal epitope in GT0 mice.20 However, there are several issues that currently make this approach impractical for inducing tolerance to the αGal epitope or other pig antigens in primates, including difficulty in establishing porcine bone marrow engraftment and long-term lymphohematopoiesis in primates.21 We have recently shown that reconstitution of lethally irradiated GT0 mice with autologous bone marrow cells expressing a retrovirally transduced αGT gene inhibits the production of αGal reactive natural antibody.22 Thus, the genetic modification of bone marrow hematopoietic progenitors using retroviral delivery systems can be used to prevent the production of natural antibodies by inducing molecular chimerism. However, for this approach to be clinically useful in a xenotransplantation setting, specific tolerance to the αGal epitope after subsequent rigorous antigen challenge must be achieved. Here, we demonstrate that, by using retroviral gene therapy, it is possible to attain levels of αGal expression on bone marrow–derived cells sufficient to induce tolerance to this epitope after repeated immunization with potent xenogeneic αGal-expressing cells.
Materials and methods
Mice
The derivation of GT0 mice has been described previously.17 The breeding of GT0 mice for these studies is described by Bracy et al.22 All experiments were conducted using mice between 6 and 8 weeks of age. C57BL/6SnJ mice were used as GT+ controls and were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in autoclaved microisolator conditions and maintained on irradiated feed and autoclaved acidified drinking water.
Retrovirus
Construction of viral producer lines to generate LGTA7 and NEOr (NB5) viruses is described by Bracy et al.22
Bone marrow harvest and transduction
Bone marrow cells were transduced as described previously.23 Briefly, bone marrow cells from mice treated 7 days previously with 5-fluorouracil (5-FU) (150 mg/kg) were plated on 70% to 80% confluent virus producer cell lines at a density of 6 to 10 × 105 cells per milliliter in Dulbecco minimum essential medium containing 15% lot-tested fetal calf serum, 100 ng/mL human interleukin (IL)-6 (R&D Systems, Minneapolis, MN), 100 ng/mL recombinant mouse stem cell factor (SCF) (BioSource International, Camarillo, CA), 50 ng/mL recombinant mouse thrombopoietin (TPO) (R&D Systems), 50 ng/mL recombinant mouse Flt-3 ligand (R&D Systems), and 8 μg/mL Polybrene (Sigma Chemical Co, St Louis, MO). Cultures were supplemented with cell-free viral supernatants with a titer of 1 to 5 × 105 viral particles per milliliter. All transductions were performed at 37°C with 5% CO2 for 72 hours. Nonadherent cells were harvested and replated for an additional 2 hours in tissue culture dishes to allow adherence of contaminating producer cells.
Bone marrow transplantation
Lethally irradiated (10.25 Gy) GT0 mice were reconstituted with 2 to 3 × 106 transduced bone marrow cells as described by Bracy et al.22 Mice were bled weekly beginning at 5 weeks after bone marrow transplantation to collect blood cells and serum samples for analyses. To analyze colony forming units in the spleen (CFU-s), groups of lethally irradiated mice were reconstituted with limiting numbers of bone marrow cells (1-5 × 104) and killed 12 days later to collect spleen colonies. DNA was isolated from colonies as described24 and analyzed by polymerase chain reaction (PCR) using primers specific for porcine αGT (5′-TTACCACGAAGAAGAAGACGC forward primer; 5′-TACCACTGGAGCCTTCCATC reverse primer) to determine the percentage of colonies containing integrated virus. Primers specific for mouse β-actin (5′-AACCCCAAGGCCAACCGCGAGCCGATGACC forward primer; 5′-GGTGATGACCTGGCCGTCAGGCAGCTCGTA reverse primer) were used as controls.
Immunization
Mice were immunized intraperitoneally (ip) with 106irradiated porcine peripheral blood mononuclear cells (pPBMCs). Heparinized pig blood was collected from miniature swine from the Massachusetts General Hospital herd housed at the Tufts University School of Veterinary Medicine.25 Lymphocytes were purified as described previously.26
Adoptive transfer of transduced bone marrow cells into secondary and tertiary recipients
Mice reconstituted with either LGTA7- or NEOr-transduced bone marrow were killed at 18 weeks after bone marrow transplantation to collect bone marrow cells. Lethally irradiated (10.25 Gy) major histocompatibility complex (MHC)–matched GT0 mice were then reconstituted with 107 cells from mice reconstituted initially with either LGTA7- or NEOr-transduced bone marrow by tail vein injection.
FACS analyses
To examine expression of αGal epitopes on cells in the periphery of mice reconstituted with LGTA7-transduced bone marrow, the αGal-specific IB4 lectin from Bandeiraea simplicifolia(BS-I isolectin B4)27 was used. Single cell suspensions were prepared from blood or lymphoid tissues, washed in staining buffer (Hanks' balanced salt solution containing 25 mmol/L HEPES, 1% heat-inactivated normal rabbit serum, and 0.1% sodium azide), stained using saturating concentrations of FITC-labeled IB4 lectin, and analyzed by flow cytometry as described previously.22 To examine expression of αGal epitopes on various lymphoid lineages, cells were double- stained with FITC-labeled IB4 lectin and saturating concentrations of PE-conjugated monoclonal antibodies specific for macrophages (clone F4/80, Caltag, Burlington, CA), CD3 (clone CT-CD3), CD19 (clone 1D3, Pharmingen), NK1.1 (clone PK136, Biosource International), and B220 (clone RA3-6B2, PharMingen, San Diego, CA) and then analyzed by flow cytometry.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assays (ELISAs) were conducted as described previously.22 Briefly, ELISA plates (Corning, Corning, NY) were coated overnight at 4°C with either αGal-conjugated to bovine serum albumin (BSA) or lactosamine conjugated to BSA (Lac-BSA, V-Labs, Inc, Covington, LA) in carbonate buffer (pH 9.5) and then washed with PBS containing 0.05% Tween-20 (PBS-Tween). Lac-BSA shares all determinants with αGal-BSA, except for the terminal galactose structure, and serves as a specificity control. The wells were blocked with 1.0% BSA in PBS-Tween for 1 hour at room temperature and then washed. Serum samples were serially diluted in PBS-Tween, added to the plates and incubated for 1 hour at 37°C. The plates were then washed 5 times with 300 μL PBS-Tween and bound antibodies were detected using horseradish peroxidase (HRP)–conjugated goat antimouse IgM and IgG antibodies. The plates were incubated for 1 hour at 37°C and again washed 5 times with PBS-Tween. To develop the assays, 0.01 mg/mL o-phenylenediamine dihydrochloride in substrate buffer was then added for 20 minutes at room temperature. The reaction was terminated by adding NH2SO4 to each well and absorbency read at 492 nm. Background values obtained from Lac-BSA–coated plates were subtracted from those obtained using Gal-BSA–coated plates. Assays were performed in duplicate.
Serum analyses for antipig antibodies
10 μL of serum was incubated for 2 hours at 4°C with 5 × 105 pPBMCs, after which red blood cells were lysed in 1 mL FACS Lysing Solution (Becton Dickinson, Franklin Lakes, NJ), according to manufacturer's instructions. Samples were then washed in staining buffer and incubated with FITC-conjugated goat antimouse IgM or PE-conjugated goat antimouse IgG for 1 hour at 4°C. After a final wash, the cells were analyzed for the presence of antipig antibodies by flow cytometry.
ELISPOT assays
MultiScreen-HA plates (Millipore, Bedford, MA) were coated with 5 μg/mL of either αGal-BSA or Lac-BSA (to determine background) in PBS at 4°C overnight. The plates were then washed 3 times with PBS, allowing the plates to soak 5 minutes between each wash. The plates were blocked with IMDM media containing 0.4% BSA and penicillin and streptomycin for 2 hours at 37°C. The blocking medium was then removed and 5-fold serial dilutions (starting at 1 × 106cells per well) of spleen cells prepared in IMDM blocking media were added to the wells. The plates were incubated at 37°C with 5% CO2 overnight and then washed 3 times in PBS, followed by 3 additional washes in PBS-Tween. HRP-conjugated goat antimouse IgM was then added to each well and incubated for 2 hours at 4°C. The plates were washed 3 more times with PBS-Tween, followed by PBS, at which point the assays were developed by adding filtered chromogen substrate (3-amino-9-ethyl-carbazole) in acetate buffer (pH 5.0). Plates were incubated in the presence of chromogen substrate at room temperature for 20 minutes and the reaction terminated by washing the plate with water. Spots were enumerated using a dissecting microscope. In all assays, the number of background spots obtained on Lac-BSA–coated plates were subtracted from the number obtained on corresponding Gal-BSA–coated plates. All samples were plated in duplicate.
Results
Retroviral gene transfer conditions leading to improved expression of the αGal epitope
In our original report, the efficiency of bone marrow transduction and level of αGal expression obtained on bone marrow–derived cells was sufficient to prevent production of αGal reactive natural antibodies. However, we were only able to detect cells carrying the transduced αGT gene in reconstituted mice using PCR-based methods, indicating that the transduction levels achieved were low.22 In addition, although we were able to prevent production of natural antibodies using our initial transduction conditions, it became apparent in pilot studies that gene expression fell below our level of detection using PCR-based methods 25 weeks after reconstitution. Mice that had lost gene expression were observed to be hyporesponsive rather than tolerant to the αGal epitope after rigorous antigen challenge (J.B. and J.I., personal observation). Therefore, to test the feasibility of inducing life-long tolerance to the αGal epitope by genetic engineering of bone marrow, we first set out to improve our retroviral transduction conditions. To this end, we examined the effect of various cytokine combinations on our ability to improve transduction of 5-FU–treated bone marrow cells from GT0 mice with retroviruses carrying the porcine αGT gene.
Transduction of 5-FU–treated GT0 bone marrow cells with retroviruses carrying the porcine αGT gene (LGTA7 virus) in the presence of IL-6 (100 ng/mL), SCF (100 ng/mL), Flt-3L (50 ng/mL), and TPO (50 ng/mL) led to a dramatic increase in the percentage of bone marrow cells expressing αGal epitopes. Taking the average of 3 separate experiments, approximately 44.3% ± 13.2% of bone marrow cells expressed αGal epitopes on their surface immediately after transduction, as detected by flow cytometry after staining with FITC-labeled IB4 lectin. In contrast, we were unable to detect αGal epitope expression by flow cytometry on bone marrow cells after infection using our previous transduction conditions that included only IL-6 (100 ng/mL) and IL-3 (40 ng/mL) (not shown). Bone marrow cells from GT0 mice transduced with control retroviruses carrying only the neomycin resistance gene (NEOr) were always negative. The percentage of colonies containing the retrovirally transduced αGT gene in the spleens (CFU-s) of mice reconstituted with limiting numbers of bone marrow cells transduced in the presence of IL-6, SCF, Flt-3L, and TPO 12 days after reconstitution was approximately 100% in all animals analyzed, compared with approximately 78% when using IL-3 and IL-6 alone.22
Long-term expression of αGal epitopes on bone marrow–derived cells in vivo
To examine the effect of our improved transduction conditions on in vivo expression of the αGal epitope, lethally irradiated GT0 mice were reconstituted with MHC-matched GT0 bone marrow cells infected in the presence of IL-6, SCF, IL-3, and Flt-3L with either LGTA7 or NEOr. Bone marrow–derived cells expressing αGal epitopes on their surface were detectable in the blood of mice receiving LGTA-transduced bone marrow at the earliest time point examined (5 weeks) after bone marrow transplantation (Figure 1). The average percentage of blood cells expressing αGal epitopes at 5 weeks after bone marrow transplantation in mice receiving LGTA7-transduced bone marrow was 9.3% ± 1.9% (n = 14). As expected, bone marrow–derived cells expressing αGal epitopes were not detected in the blood of control mice reconstituted with NEOr-transduced bone marrow.
Expression of αGal epitopes in the blood of reconstituted mice.
Comparison of αGal epitope expression on blood cells from a wild-type GT+ mouse (A), a GT0 mouse reconstituted with NEOr-transduced bone marrow (B), or a GT0 mouse reconstituted with LGTA7-transduced bone marrow (C). Blood samples from reconstituted mice were analyzed 5 weeks after bone marrow transplantation. Shown is the pattern of CD19 (B-cell lineage) versus IB4 lectin (specific for αGal) staining gated on the total lymphocyte population. Expression of αGal epitopes was analyzed on CD19-positive cells because B-lineage cells, even in wild-type mice, express relatively high levels of αGal epitopes (J.B. and J.I., personal observation). Samples shown are from representative mice.
Expression of αGal epitopes in the blood of reconstituted mice.
Comparison of αGal epitope expression on blood cells from a wild-type GT+ mouse (A), a GT0 mouse reconstituted with NEOr-transduced bone marrow (B), or a GT0 mouse reconstituted with LGTA7-transduced bone marrow (C). Blood samples from reconstituted mice were analyzed 5 weeks after bone marrow transplantation. Shown is the pattern of CD19 (B-cell lineage) versus IB4 lectin (specific for αGal) staining gated on the total lymphocyte population. Expression of αGal epitopes was analyzed on CD19-positive cells because B-lineage cells, even in wild-type mice, express relatively high levels of αGal epitopes (J.B. and J.I., personal observation). Samples shown are from representative mice.
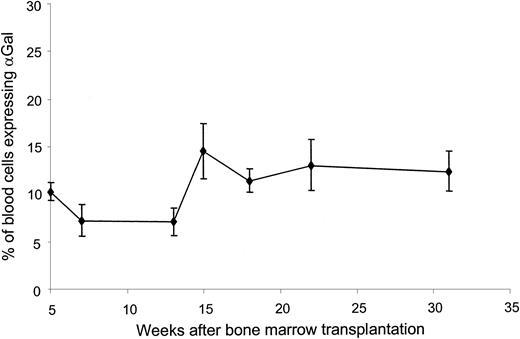
To examine long-term expression of αGal epitopes, blood cells were collected once a week over a 31-week period from mice reconstituted with either LGTA7- or NEOr-transduced bone marrow cells and analyzed by flow cytometry after staining with IB4 lectin. As shown in Figure 2, αGal positive cells were detectable in the blood of mice reconstituted with LGTA7-transduced bone marrow at all time points analyzed over the 31-week follow-up period. Importantly, the percentage of αGal expressing cells in the blood remained relatively stable over the period analyzed. At 18 weeks after reconstitution, mice from each group were killed and cells from bone marrow, spleen, thymus, and lymph nodes were analyzed for αGal expression by cell surface staining and flow cytometry. All lymphoid tissue harvested from mice reconstituted with LGTA7-transduced bone marrow contained cells expressing αGal epitopes (Figure3). Shown in Table1 is the lineage distributions of αGal expressing cells in various tissues. We were able to detect αGal-positive B cells (B220+), macrophages (F4/80), T cells (CD3+), and natural killer (NK) cells (NK1.1+) in mice reconstituted with LGTA7-transduced bone marrow (Table 1). Lymphoid tissues from control mice reconstituted with NEOr-transduced cells did not contain αGal-expressing cells (Figure 3).
Long-term expression of αGal epitopes in vivo.
Shown are the percentages of αGal-expressing blood cells as determined by staining with IB4 lectin, followed by flow cytometry in mice reconstituted with LGTA7-transduced bone marrow over a 31-week follow-up period. In all experiments, mice reconstituted with NEOr-transduced marrow were used as negative controls to set flow cytometry analysis gates. The values provided represent the mean and standard deviations from 2 combined experiments containing a total of 14 mice.
Long-term expression of αGal epitopes in vivo.
Shown are the percentages of αGal-expressing blood cells as determined by staining with IB4 lectin, followed by flow cytometry in mice reconstituted with LGTA7-transduced bone marrow over a 31-week follow-up period. In all experiments, mice reconstituted with NEOr-transduced marrow were used as negative controls to set flow cytometry analysis gates. The values provided represent the mean and standard deviations from 2 combined experiments containing a total of 14 mice.
Analysis of αGal epitope expression in lymphoid tissues.
Analysis of αGal expression in lymphoid tissues of mice reconstituted with either LGTA7- (column A) or NEOr- (column B) transduced bone marrow 18 weeks after bone marrow transplantation by staining with IB4 lectin, followed by flow cytometry. Flow cytometry analysis gates were set as described in Figure 2. Expression of αGal epitopes in lymphoid tissues of secondary mice reconstituted with either LGTA7- (column C) or NEOr- (column D) transduced bone marrow 22 weeks after secondary bone marrow transplantation. Shown are the results from representative mice.
Analysis of αGal epitope expression in lymphoid tissues.
Analysis of αGal expression in lymphoid tissues of mice reconstituted with either LGTA7- (column A) or NEOr- (column B) transduced bone marrow 18 weeks after bone marrow transplantation by staining with IB4 lectin, followed by flow cytometry. Flow cytometry analysis gates were set as described in Figure 2. Expression of αGal epitopes in lymphoid tissues of secondary mice reconstituted with either LGTA7- (column C) or NEOr- (column D) transduced bone marrow 22 weeks after secondary bone marrow transplantation. Shown are the results from representative mice.
Lineage distribution of αGal-expressing cells in reconstituted mice
| . | Spleen . | Lymph node . | Bone marrow . | Thymus . | Blood . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Lin +* . | % GT +† . | % Lin + . | % GT + . | % Lin + . | % GT + . | % Lin + . | % GT + . | % Lin + . | % GT + . | |
| Total | N/A | 48 ± 10 | N/A | 6 ± 2 | N/A | 44 ± 13 | N/A | 10 ± 4 | N/A | 12 ± 4 |
| B220 | 19 ± 2 | 61 ± 4 | 15 ± 4 | 18 ± 3 | 18 ± 4 | 50 ± 7 | N/A | N/A | 13 ± 3 | 52 ± 14 |
| F4/80 | 80 ± 5 | 4 ± 2 | 7 ± 4 | 3 ± 2 | 33 ± 4 | 47 ± 6 | 4 ± 3 | 22 ± 9 | 66 ± 10 | 10 ± 8 |
| CD3 | 13 ± 2 | 23 ± 3 | 7 ± 1 | 87 ± 2 | 12 ± 4 | 18 ± 6 | 5 ± 2 | 57 ± 10 | 8 ± 2 | 46 ± 15 |
| NK1.1 | 80 ± 8 | 4 ± 1 | 11 ± 2 | 6 ± 2 | 31 ± 3 | 35 ± 3 | 4 ± 1 | 58 ± 14 | 36 ± 9 | 11 ± 3 |
| . | Spleen . | Lymph node . | Bone marrow . | Thymus . | Blood . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Lin +* . | % GT +† . | % Lin + . | % GT + . | % Lin + . | % GT + . | % Lin + . | % GT + . | % Lin + . | % GT + . | |
| Total | N/A | 48 ± 10 | N/A | 6 ± 2 | N/A | 44 ± 13 | N/A | 10 ± 4 | N/A | 12 ± 4 |
| B220 | 19 ± 2 | 61 ± 4 | 15 ± 4 | 18 ± 3 | 18 ± 4 | 50 ± 7 | N/A | N/A | 13 ± 3 | 52 ± 14 |
| F4/80 | 80 ± 5 | 4 ± 2 | 7 ± 4 | 3 ± 2 | 33 ± 4 | 47 ± 6 | 4 ± 3 | 22 ± 9 | 66 ± 10 | 10 ± 8 |
| CD3 | 13 ± 2 | 23 ± 3 | 7 ± 1 | 87 ± 2 | 12 ± 4 | 18 ± 6 | 5 ± 2 | 57 ± 10 | 8 ± 2 | 46 ± 15 |
| NK1.1 | 80 ± 8 | 4 ± 1 | 11 ± 2 | 6 ± 2 | 31 ± 3 | 35 ± 3 | 4 ± 1 | 58 ± 14 | 36 ± 9 | 11 ± 3 |
N/A indicates not analyzed. Percentages shown represent the mean and standard deviation of quadruplicate samples from 3 mice analyzed at 18 weeks after bone marrow transplantation.
Percentage of lineage (Lin) marker positive cells that express αGa epitopes.
Percentage of αGal+ cells expressing each lineage marker.
Because our data indicated long-term expression of αGal epitopes in vivo on multiple lymphoid lineages, we hypothesized that relatively early hematopoietic progenitors may have been infected. To test this possibility, groups of mice reconstituted with LGTA7- or NEOr-transduced bone marrow cells were killed 18 weeks after bone marrow transplantation and the bone marrow cells harvested. The bone marrow cells were then used to reconstitute secondary lethally irradiated GT0 mice. Beginning at 5 weeks after bone marrow transplantation, secondary mice were bled and peripheral blood cells were analyzed by flow cytometry for αGal expression. All secondary mice reconstituted with bone marrow cells obtained from mice reconstituted with LGTA7-transduced bone marrow contained αGal-positive cells in their blood (5.75% ± 1.02%, n = 5), albeit at slightly lower levels than primary recipients, whereas blood cells from secondary mice reconstituted with bone marrow from mice receiving NEOr-transduced bone marrow stained negative (not shown). The percentage of αGal epitope expression on blood cells in mice receiving adoptively transferred bone marrow was stable for at least 22 weeks after transplantation, the latest time point analyzed. As shown in Figure 3, 22 weeks after reconstitution of secondary recipients, we were able to detect αGal-expressing cells in all lymphoid organs analyzed. At 22 weeks, the lineage distribution of αGal-expressing cells was similar to that observed in primary recipients analyzed at 18 weeks after bone marrow transplantation (not shown). Together, these data suggest that relatively early bone marrow progenitors were infected using the transduction conditions described.
Expression of αGal epitopes on retrovirally transduced bone marrow–derived cells induces stable long-term tolerance to αGal
To determine whether expression of αGal epitopes on bone marrow–derived cells prevents development of αGal reactive natural antibodies, mice reconstituted with either LGTA7- or control NEOr-infected bone marrow using our improved transduction conditions were bled and sera analyzed starting at 7 weeks after bone marrow transplantation for the presence of αGal reactive antibodies by ELISA. As expected, on the basis of our previously published work,22 although lethally irradiated GT0 mice reconstituted with NEOr-transduced bone marrow were able to produce αGal reactive antibodies, we were unable to detect the presence of αGal reactive antibodies in mice receiving LGTA7-transduced bone marrow for a 31-week follow-up period. In addition, although secondary mice reconstituted with bone marrow cells harvested from mice receiving NEOr-transduced bone marrow produced levels of αGal reactive serum antibodies similar to those observed in control GT0 mice, we were unable to detect the presence of αGal reactive antibodies in secondary recipients receiving bone marrow from LGTA7-transduced bone marrow (not shown).
To examine whether mice reconstituted with LGTA7-transduced bone marrow were tolerant to the αGal epitope, mice were immunized at weeks 13 and 15 (primary recipients) or weeks 19 and 21 (secondary recipients) after reconstitution with 106 irradiated pPBMCs that express high levels of αGal structures (J.I. and J.B., personal observation). Serum was collected and analyzed at 1-week intervals after each immunization for the presence of antibodies capable of binding αGal-BSA by ELISA. As shown in Figure4A, control mice reconstituted with NEOr-transduced bone marrow exhibited a significant increase in αGal reactive antibody titer after repeated immunizations with pPBMCs, similar to that observed in immunized GT0control mice that had not undergone a bone marrow transplant. Binding of serum antibodies to Lac-BSA, an antigen that shares all determinants with αGal-BSA, except for the terminal galactose, was examined in parallel to confirm that antibody binding to αGal-BSA was specific. In contrast, αGal reactive antibodies were undetectable in the serum of mice reconstituted with LGTA7-transduced bone marrow after immunization (Figure 4A), as well as immunized secondary recipients (not shown). Similar results were obtained in experiments in which mice were immunized and boosted twice with 107 irradiated pPBMCs (data not shown). After immunization of mice reconstituted with LGTA7, the percentage of blood cells expressing αGal epitopes was unchanged from the percentage before immunization (not shown). Thus, repeated immunization with cells expressing high levels of αGal was unable to stimulate production of αGal reactive antibodies, and did not affect expression of αGal epitopes in the periphery.
GT0 mice reconstituted with LGTA7-transduced bone marrow are tolerant to αGal.
Panel A: analysis of αGal reactive IgM serum antibodies in mice reconstituted with either LGTA7- (▪, n = 9), or NEOr-transduced bone marrow (●, n = 9) after immunization with pPBMCs by ELISA. Shown are the mean and standard deviation of combined data from 2 independent experiments. Control GT0 (○) and GT+ (▵) mice immunized at the same time that did not receive a bone marrow transplant are shown for comparison purposes. Panel B: analysis of splenic anti-Gal–producing B-cell frequency by ELISPOT. Shown are values obtained for immunized GT+ mice (░) and GT0 control mice (▨), and mice receiving LGTA7- (■), or NEOr-transduced bone marrow (▪) after immunization. Shown are the mean and standard deviation values for each group. All samples were assayed in duplicate.
GT0 mice reconstituted with LGTA7-transduced bone marrow are tolerant to αGal.
Panel A: analysis of αGal reactive IgM serum antibodies in mice reconstituted with either LGTA7- (▪, n = 9), or NEOr-transduced bone marrow (●, n = 9) after immunization with pPBMCs by ELISA. Shown are the mean and standard deviation of combined data from 2 independent experiments. Control GT0 (○) and GT+ (▵) mice immunized at the same time that did not receive a bone marrow transplant are shown for comparison purposes. Panel B: analysis of splenic anti-Gal–producing B-cell frequency by ELISPOT. Shown are values obtained for immunized GT+ mice (░) and GT0 control mice (▨), and mice receiving LGTA7- (■), or NEOr-transduced bone marrow (▪) after immunization. Shown are the mean and standard deviation values for each group. All samples were assayed in duplicate.
To confirm our ELISA results and rule out the possibility that αGal reactive antibodies were being absorbed onto cells expressing αGal epitopes in mice reconstituted with LGTA7-transduced bone marrow, ELISPOT assays were performed using splenocytes harvested from mice 3 weeks after the second immunization. Although we were able to detect the presence of splenic B cells producing αGal reactive antibodies in mice reconstituted with NEOr-transduced bone marrow, we were unable to detect B cells producing αGal reactive antibodies in the spleen of mice reconstituted with LGTA7 bone marrow (Figure 4B). Similar results were observed in secondary recipients of LGTA7-transduced bone marrow (not shown). These results, along with our ELISA data, strongly suggest that B cells producing αGal reactive antibodies are functionally eliminated from the repertoire of mice that express the retrovirally transduced αGT gene on bone marrow–derived cells.
Tolerance to αGal switches the antiporcine humoral response from IgM to IgG
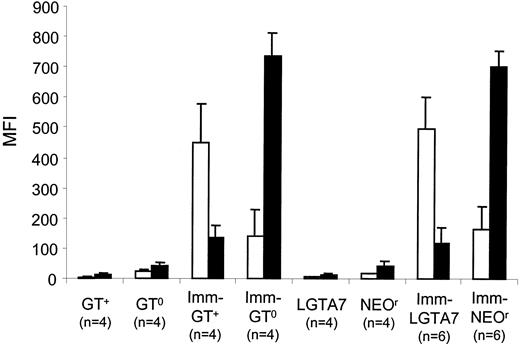
To determine how tolerance to αGal may affect the humoral response to other pig antigens, we examined the ability of serum antibodies from mice reconstituted with LGTA7- or NEOr-transduced bone marrow to bind porcine cells after immunization. Mice reconstituted with either LGTA7- or NEOr-transduced bone marrow exhibited a sharp increase in the level of pig-cell reactive antibodies after immunization with pig peripheral blood cells (Figure 5). Immunization with porcine peripheral blood cells in mice reconstituted with NEOr-transduced bone marrow elicited predominately an IgM response, which was similar to that observed in control GT0 mice. In contrast, mice reconstituted with LGTA7-transduced bone marrow elicited predominantly an IgG response after immunization, similar to that observed in wild-type mice (Figure5). These data show that mice reconstituted with LGTA7-transduced bone marrow, although tolerant to αGal, are capable of responding to pig antigens other than αGal. Therefore, the tolerance induced by gene therapy is specific.
Analysis of the antipig response in mice that are tolerant to αGal.
Shown are the median fluorescent intensities (MFI) obtained by indirect staining of pPBMCs with sera from either control GT+ or GT0 mice, as well as mice reconstituted with LGTA7- or NEOr-transduced bone marrow. Values for unimmunized and immunized (Imm) groups are shown. Antipig IgG (■) and IgM (▪) antibodies are shown. The values plotted represent the mean and standard deviation of MFI for each group.
Analysis of the antipig response in mice that are tolerant to αGal.
Shown are the median fluorescent intensities (MFI) obtained by indirect staining of pPBMCs with sera from either control GT+ or GT0 mice, as well as mice reconstituted with LGTA7- or NEOr-transduced bone marrow. Values for unimmunized and immunized (Imm) groups are shown. Antipig IgG (■) and IgM (▪) antibodies are shown. The values plotted represent the mean and standard deviation of MFI for each group.
Discussion
Although the most stable means of inducing tolerance relies on bone marrow transplantation to achieve a state of mixed hematopoietic cellular chimerism, the use of bone marrow transplantation to induce tolerance for the purpose of xenotransplantation is limited because of the severity of the host preparative regimen required to allow engraftment, the potential for graft-versus-host disease, and the difficulty in establishing engraftment of bone marrow across discordant xenogeneic barriers. We hypothesized that it may be possible to utilize gene therapy to inhibit production of αGal XNA by introducing a functional αGT gene via retroviral gene transfer into bone marrow cells. This approach allows for the transduced αGT gene to synthesize αGal epitopes that can be expressed on the surface of bone marrow–derived cells. The αGal-expressing cells could then tolerize B cells making αGal XNA. Importantly, by establishing molecular rather than cellular chimerism, difficulties associated with engraftment of pig bone marrow in primates for the purpose of inducing tolerance could be circumvented. Indeed, we have shown previously that this approach can be used to inhibit production of αGal reactive natural antibodies.22 However, the issue of whether this approach could be used in a setting involving rigorous antigen challenge, similar to what is encountered after transplantation, was not addressed. If a similar gene therapy approach is to be applicable to clinical xenotransplantation, stable tolerance resistant to rigorous antigen challenge must be demonstrated.
In our previous study, the conditions used during retroviral infection resulted in relatively low transduction efficiencies.22Indeed, as discussed previously, we were unable to detect the presence of αGal epitopes in the periphery of mice reconstituted with LGTA7-transduced bone marrow by flow cytometry and gene expression was lost late after reconstitution. Furthermore, after loss of gene expression and subsequent immunization, the mice were shown to be hyporesponsive rather than tolerant to αGal. We therefore reasoned that to induce tolerance, transduction conditions would have to be improved. To this end, we examined the effect of changing the cytokine cocktail used during infection of bone marrow on transduction efficiencies. (A detailed analysis of various transduction conditions is in preparation.) The addition of TPO, SCF, Flt-3L, and the elimination of IL-3 led to a substantial increase in the percentage of αGal expressing cells after transduction of 5-FU–treated bone marrow with LGTA7 virus. Furthermore, mice reconstituted with bone marrow cells transduced using these conditions exhibited detectable expression of αGal epitopes on bone marrow–derived cells for a least 31 weeks, at which point the experiments were terminated. Thus, the combined use of cytokines shown to affect early bone marrow cell progenitor survival and proliferation28 allowed us to significantly increase the frequency of cells expressing αGal epitopes, achieve long-term expression of αGal epitopes, and on the basis of reconstitution of secondary recipients, allowed for infection of early hematopoietic progenitors. We favor the hypothesis that the increase in the frequency of αGal-expressing cells was related to improved transduction. We can exclude the possibility of poor vector design or vector silencing, because the same vectors and packaging systems were used in this study and our previous work.22 Therefore, it seems likely that if the vector design was poor or the vector subject to silencing that we should have observed a similar phenomenon in this study. The fact that we were able to achieve long-term expression, even in secondary recipients, argues against these possibilities. Because we are able to demonstrate that approximately 40% of transduced bone marrow cell express αGal before transplantation into hosts, and that αGal expressing cells can be detected long-term after transplantation, it is clear that natural antibody present in the host before transplantation does not completely eliminate αGal-expressing cells in vivo, nor does infusion of αGal-expressing bone marrow lead to sensitization (J.B. and J.I., personal observation). Although natural antibodies may affect the ability of αGal-expressing cells to engraft, in our previous study it was clear that after transduction very few cells expressed αGal epitopes. Nevertheless, we were able to inhibit production of αGal reactive antibodies, suggesting that the presence of these antibodies did not overcome the effect of gene therapy.
On the basis of analysis of serum antibodies by ELISA, GT0mice reconstituted with LGTA7-transduced bone marrow using our improved transduction conditions failed to produce αGal reactive antibodies after immunization with porcine peripheral blood cells. In addition, we were unable to detect the presence of B cells that secrete αGal reactive antibodies in the spleens of immunized mice by ELISPOT. However, immunized mice were capable of producing antibodies that bind non-αGal pig antigens. Together, these data strongly suggest that the level of αGal expression achieved by gene therapy is sufficient to induce a specific immunologic tolerance to the αGal epitope that remains stable after rigorous antigen challenge. Thus, gene therapy approaches can be used to fundamentally reshape the immunologic repertoire.
After immunization with pig cells, mice reconstituted with NEOr-transduced bone marrow made predominantly an IgM antipig response, whereas mice reconstituted with LGTA7-transduced bone marrow made an IgG antipig response. This suggests that B cells producing αGal reactive natural antibodies, which are predominantly IgM isotype in GT0 mice, normally dominate the antipig response. When αGal reactive antibodies are eliminated, as observed in mice reconstituted with LGTA7-transduced bone marrow, the antipig response then coverts to a typical T-cell–dependent antibody response, consisting mainly of antibodies that have switched to an IgG isotype. These data are consistent with the observation that αGal reactive natural antibodies have been eliminated in these animals, and suggest that once these antibodies are eliminated after tolerance induction, B-cell responses to pig antigens appear that are more similar to classic antigen responses involving an isotype switch. We are currently examining the specificities of non-αGal antipig antibodies. Because it is well known that immunoglobulin isotype switching requires T cell help, preventing helper T-cell activation by blocking CD40-CD40L interactions or the administration of drugs such as cyclosporine A or FK506 may prevent the production of non-αGal antipig antibodies. Thus, T-cell immunosuppression may turn out to be a key component needed to sustain long-term xenograft survival. This possibility is currently being tested in the laboratory.
To our knowledge, this is the first example in which gene therapy has been successfully applied to induce stable B-cell tolerance to pre-existing natural antibodies without any additional immunosuppression. Although others have shown that gene therapy can be used to induce B-cell hyporesponsiveness,29-31 these studies have fallen short of demonstrating true tolerance. Although we are not certain why in other models hyporesponsiveness rather than tolerance was achieved, we would suggest that levels of transduction could play a role. Indeed, Schumacher et al29 reported very low transduction efficiencies. In this study, it was not possible to determine whether full B-cell tolerance was achieved because only production of complement fixing antibodies was examined at relatively early time points (3 months) after reconstitution with genetically modified cells. It is also possible that differences in the cell types expressing the retrovirally transduced genes could have played a role. For example, the bone marrow transduction conditions used by Kang et al31 included the growth factor IL-7, which is known to favor survival and proliferation of B-cell lineages. Interestingly, it has been reported that B cells can only tolerize virgin T cells.32 Because the antibody response studied by Kang et al31 is clearly T-cell dependent, it is possible that, because sublethally (400 rads) irradiated mice were used as recipients of genetically modified cells, memory T cells were not effectively tolerized, resulting in hyporesponsiveness. Lastly, it is possible that the ability to induce tolerance by gene therapy depends on the type of antigen being examined. On the basis of preliminary experiments, a large fraction, if not all, of the αGal reactive antibodies present in GT0 mice appear to be T-cell independent (J.I. and J.B., in preparation). Thus, it may be possible that eliminating B cells that produce T-cell–independent antibodies from the repertoire may be easier because the additional requirement to induce T-cell tolerance to the same antigen is not necessary.
The use of genetically modified autologous bone marrow to establish molecular chimerism eliminates many of the complications associated with bone marrow transplantation across species barriers to establish cellular chimerism for the purpose of inducing transplantation tolerance. Most important, establishing molecular chimerism results in the same stable and specific tolerance to αGal established after induction of mixed cellular chimerism.20 We suggest that the use of autologous marrow mitigates the potential for graft-versus-host disease and engraftment failure, and may permit the use of less toxic host-conditioning regimens. For these reasons our approach may be clinically applicable. Similar approaches may eventually be applicable for the induction of tolerance in immunologic disorders such as autoimmune disease.
Although we have been able to demonstrate relatively efficient transduction of murine bone marrow cells, efficient transduction of primate bone marrow progenitor cells has been much more difficult. Nevertheless, it has recently been reported that relatively efficient transduction and long-term gene expression is possible in primates.33,34 On the basis of our own preliminary studies, the transduction conditions used in this report also appear to increase the efficiency of baboon CD34+ cell transduction (J.I. and J.B., personal observation). Indeed, even low levels of chimerism have been associated with tolerance.35 36 We hypothesize that, if we can achieve even a relatively low—but detectable—level of αGal expressing cells in primates, it is likely that we will be able to eliminate B cells making αGal reactive antibodies.
Acknowledgments
We thank Drs David H. Sachs, David K. C. Cooper, and Shiv Pillai for critically reading the manuscript, and members of the Iacomini lab for helpful discussions.
Supported in part by National Institutes of Health grants AI44268 and AI43619 to J.I. J.L.B. is supported in part by National Institutes of Health Training grant T32 AI 07529.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John Iacomini, Transplantation Biology Research Center, Massachusetts General Hospital, 149 13th St, Boston, MA 02129; e-mail: iacomini@helix.mgh.harvard.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal