Abstract
The effect of interferon γ (IFN-γ) and interleukin 6 (IL-6) on infection of macrophages with human immunodeficiency virus type 1 (HIV-1) was investigated. By using a polymerase chain reaction–based viral entry assay and viral infectivity assay, it was demonstrated that IL-6 and IFN-γ augmented susceptibility of monocyte-derived macrophages (MDMs) to infection with T-cell tropic CXCR4-utilizing (X4) HIV-1 strains. Consistent with this finding, IFN-γ and IL-6 augmented fusion of MDMs with T-tropic envelope-expressing cells. The enhanced fusion of cytokine-treated MDMs with T-tropic envelopes was inhibited by the CXCR4 ligand, SDF-1, and by T22 peptide. IFN-γ and IL-6 did not affect expression of surface CXCR4 or SDF-1–induced Ca++ flux in MDMs. In contrast to the effect of IFN-γ on the infection of MDMs with X4 strains, IFN-γ inhibited viral entry and productive infection of MDMs with macrophage-tropic (M-tropic) HIV-1. Consistent with this finding, IFN-γ induced a decrease in fusion with M-tropic envelopes that correlated with a modest reduction in surface CCR5 and CD4 on MDMs. It was further demonstrated that macrophage inflammatory protein (MIP)-1α and MIP-β secreted by cytokine-treated MDMs augmented their fusion with T-tropic–expressing cells and inhibited their fusion with M-tropic envelope-expressing cells. These data indicate that proinflammatory cytokines, which are produced during opportunistic infections or sexually transmitted diseases, may predispose macrophages to infection with X4 strains that, in turn, could accelerate disease progression.
Introduction
Macrophages and CD4+ T cells are the major targets of infection with the human immunodeficiency virus type 1 (HIV-1). Most primary viral isolates can infect both cell types.1,2 However, cytotropism of the predominant viral isolates varies during disease progression. Macrophage (M)-tropic CCR5-utilizing (R5) viruses have been shown to establish the primary infection during vertical and sexual transmission.3 4These viruses are usually isolated during clinical latency, are slow to replicate, do not form syncytia, and do not infect CD4+T-cell lines. The development of acquired immunodeficiency syndrome (AIDS) is frequently associated with the emergence of fast-replicating viral strains that form syncytia and can infect both primary CD4+ T cells and CD4+ T-cell lines (T-tropic). The mechanisms underlying the emergence of T-tropic viral strains during disease progression are of major interest in HIV research.
HIV infection and replication are also affected by the cytokine network. Importantly, cytokines may exert multiple effects on viral entry and reverse transcription as well as on proviral reactivation.5 In addition to tumor necrosis factor α (TNF-α) and interleukin (IL)-2, IL-6 was shown to promote viral replication in both acutely infected macrophages and in a chronically infected promonocytic cell line.6 Interferon γ (IFN-γ) belongs to a group of cytokines that exert pleiotropic effects on HIV-1 infection. Exposure of infected macrophages to IFN-γ leads to a reduction in viral replication.7,8 Alternatively, exposure of macrophages or a promonocytic cell line to IFN-γ prior to infection resulted in subsequent enhancement of viral production.9 Elevated levels of plasma IFN-γ and IL-6 were detected in patients with HIV-1 in the absence of concurrent opportunistic infections,10-12 and a high number of IFN-γ–producing cells were detected in lymph node biopsies of HIV patients with persistent generalized lymphoadenopathy.13
In persons infected with HIV-1, the progressive decline of cell-mediated immunity results in a higher susceptibility to opportunistic infections (OIs). Visceral leishmaniasis (VL) and tuberculosis (TB) are among the most common opportunistic infections in individuals with AIDS.14,15 Epidemiological data demonstrated that infection with TB or VL facilitates HIV disease progression in HIV-1–infected patients.16,17 Infections with either Leishmania or Mycobacterium tuberculosis are associated with an enhanced production of proinflammatory cytokines (IFN-γ, IL-6, TNF-α, IL-1).17-19 A positive correlation was observed between the levels of proinflammatory cytokines and HIV viremia in HIV-1/VL coinfected patients.17 In addition, pleural fluid from patients with TB (containing proinflammatory cytokines) was shown to increase HIV replication in vitro.20 These and other opportunistic pathogens may alter the immunologic homeostasis of the host by inducing the pattern of cytokines beneficial for virus propagation and thus could accelerate the course of HIV. Therefore, it is of interest to investigate the mechanism of cytokine-induced acceleration of HIV disease.
The chemokine receptors CCR5 and CXCR4 are the principal cell surface molecules that together with CD4 allow target cell entry by M-tropic (R5) and T-tropic (X4) HIV strains, respectively. CCR5 is expressed at high levels by differentiated macrophages21-23 and is a critical factor for HIV pathogenesis, because individuals deficient in CCR5 show high levels of resistance to HIV-1 infection.24,25 The role of CXCR4 in the infection of macrophages with T-tropic strains is controversial. Low levels of CXCR4 were detected on differentiated macrophages,21,26,27 and CXCR4 was shown to support infection of macrophages with primary isolates27,28 but not with syncytia-inducing T-cell line–adapted viral strains.27,29,30 Other studies demonstrated that T-cell line–adapted viral strains can enter macrophages using CXCR4, but viral replication was restricted at a postentry level.31,32 A recent study33 from our laboratory demonstrated differences in the biochemical properties of CXCR4 molecules on monocytes and macrophages that correlated with the reduced ability of macrophages to form syncytia with X4 envelope-expressing cells compared with monocytes.
Macrophages play a crucial role in the establishment of primary HIV infection and serve as a reservoir of HIV during opportunistic infections.34 The cytokine milieu created during the onset of an OI or as a result of local inflammation may affect HIV infection of macrophages by providing a more favorable environment for emerging T-tropic viruses. Therefore, we studied the effect of IFN-γ and IL-6 on the susceptibility of macrophages to infection with X4 and R5 viruses and on the expression and function of CXCR4 and CCR5 in these cells.
Materials and methods
Generation of monocyte-derived macrophages and cytokine treatment
Elutriated monocytes and differentiated macrophages were 100% CD3−, more than 85% CD14+, and more than 95% HLA-DR+ as determined by flow cytometry. Monocyte-derived macrophages (MDMs) were derived from elutriated monocytes in 5- to 7-day cultures in DMEM supplemented with recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; 1000 U/mL; Immunex Corp, Seattle, WA) and 10% fresh “pooled” human serum (heat inactivated).
Six-day MDMs were incubated with IFN-γ (200 U/mL; BioSource, Camarillo, CA) or with IL-6 (20 ng/mL; BioSource) for the last 24 or 48 hours of culture (optimal time was determined in preliminary studies). In some experiments, conditioned medium (CM) from 6-day MDMs cultured with cytokines for the last 24 hours was collected.
In some experiments, a mixture of anti–macrophage inflammatory protein (MIP)-1α and anti–MIP-1β antibodies (2 μg/mL of each; R&D Systems, Minneapolis, MN) were added either to the cultures of MDMs along with cytokines for the last 24 hours or to the CM derived from cytokine-treated MDMs.
In some experiments, cycloheximide (Cx; 5 ng/mL) and 1-(5-isoquinolinesulfonyl)-2-methyl-piperazine (H7; 100 μmol/L; both from Sigma, St. Louis, MO) were added to the cultures of MDMs 1 hour prior to addition of the cytokines.
Infection of MDMs with X4 and R5 HIV-1
HIV-1LAI, HIV-1NL4-3, and HIV-1Ba-L viruses used in our experiments were propagated in the laboratory of Dr Keith Peden (CBER, FDA, Bethesda, MD). Although the HIV-1LAI virus derived from the molecular clone pLAI is an X4 virus and replicates in all T-cell lines,35 it is not a T-cell line–adapted virus, because it was only propagated in peripheral blood mononuclear cells (PBMCs) prior to cloning and not in T-cell lines.35,36 The virus derived from pNL4-3, which was cloned from HIV-1LAI and HIV-1NY-5after passage in CEM cells,37 is considered a T-cell line–adapted X4 virus.
Infection of MDMs and polymerase chain reaction (PCR) analysis were done as described.33 In brief, for the PCR assay, 1 million 6-day MDMs untreated or pretreated with cytokines were infected with the following strains (multiplicity of infection [MOI]): NL4-3 (0.1), LAI (0.4), Ba-L (0.02), and ADA (0.01). After 48 hours, cells were harvested and counted. DNA lysates (the equivalent of 1 × 104 cells) were amplified by PCR with gag-specific primers (SK 38/39), and the products were hybridized to a32P end-labeled SK19 probe.38 The signals on the autoradiographs were compared with those from simultaneously amplified DNA from serially diluted ACH-2 T cells, which contain one provirus per cell. Video images of the film were taken with the GDS 5000 system (UVP Inc, San Gabriel, CA), and densitometry was performed with NIH Image software.
To test for productive HIV-1 infection, MDMs, untreated or treated with cytokines, were plated at 200 × 103 cells/mL in triplicate in a 48-well plate. MDMs were infected with LAI or with Ba-L at the same MOI as for PCR for 4 hours. In some experiments, macrophages were infected with the following X4 primary viral isolates: 2166, 2314, 1954, and 1650 (all obtained from the NIH AIDS Research and Reference Program). Infections with primary isolates were performed at an MOI of 0.1 TCID50. Unbound virus was removed after 18 hours of incubation by washes with phosphate-buffered saline. HIV-1 infection was assessed by measuring the amount of soluble p24 in the supernatant.
Flow cytometry
MDMs were incubated with 20% “pooled” human serum for 30 minutes to block Fc receptors. The following monoclonal antibodies were used: biotinylated 12G5 (anti-CXCR4), FITC-conjugated 2D7 (anti-CCR5; both from Pharmingen, San Diego, CA), FITC-conjugated anti-CD4 (Becton Dickinson, San Jose, CA), and streptavidin-conjugated TRI-color reagent (TC, Caltag Laboratories, South San Francisco, CA). The biotinylated mouse immunoglobulin G2a (IgG2a) and FITC-conjugated mouse IgG2a isotype controls were purchased from Pharmingen. Flow cytometry was performed on FACScan (Becton Dickinson) and analyzed with the use of Cell Quest Software. Data are presented as the delta mean fluorescent channel (ΔMFC) and were calculated by subtracting the control MFC values from the experimental values.
Ca++ flux measurements
MDMs (107 cells) were loaded with 2 μmol FURA2/am (Molecular Probes, Eugene, OR) in 1 mL of phosphate-buffered saline for 30 to 60 minutes at 37°C and washed twice in HBSS (BioWhittaker, Walkersville, MD). SDF-1 (100 nmol; PeproTech, Rocky Hill, NJ) was added at the indicated times to 106 cells in 2 mL of HBSS in a continuously stirred cuvette at 37°C in an MS-III fluorometer (Photon Technology, Inc, South Brunswick, NJ). The relative ratio of fluorescence emitted at 510 nm following sequential excitation at 340 and 380 nm was recorded every 200 milliseconds.38
CC chemokine enzyme-linked immunosorbent assay
CM from 6-day MDMs either untreated or treated with cytokines was collected and analyzed with the use of MIP-1α, MIP-1β, and RANTES ELISA (enzyme-linked immunosorbent assay) kits according to the manufacturer's instructions (Endogen, Inc, Woburn, MA).
HIV Env-dependent cell fusion assay
CD4−12E1 cells were infected with recombinant vaccinia viruses encoding envelope from JR-FL (M-tropic) strain at 10 plaque-forming units/cell. Macrophages were mixed with TF228 cells expressing IIIB/BH8 envelope39 or with 12E1 cells expressing JR-FL envelope at a 1:1 ratio (105 cells each) and cocultured (in duplicates) for 18 hours. Cell-fusion activity was quantified by counting syncytia. Where indicated, SDF-1α, RANTES (1 μg/mL), or synthetic peptide T22 (2 μmol),40generously provided by Dr Sam Hwang, were added to macrophage cultures for 1 hour at 37°C before the addition of Env-expressing cells.
In some experiments, CM from the cultures of untreated or cytokine-treated MDMs were collected and incubated with a mixture of anti–MIP-1α and anti–MIP-1β antibodies for 1 hour at room temperature. In control samples, recombinant MIP-1β and MIP-1α proteins were incubated with the same antibodies. Recombinant chemokines, CM from untreated MDMs or from cytokine-treated MDMs (either alone or preincubated with anti-CC chemokine antibodies), were added to the macrophages from the same donor for 1 hour prior to the addition of the effector cells. Syncytia formation was scored after 18 hours.
Statistical analysis
Analysis was performed with the use of JMP statistical software (ver.3.2.2, SAS Institute Inc, Cary, NC). Underlying distributions of syncytia counts and ΔMFC values for CXCR4, CCR5, and CD4 were examined, and cytokine treatment groups were then compared by analysis of variance. Syncytia counts showed a log normal distribution so that log transformation was used.
Results
IFN-γ and IL-6 induce an increase in the fusion of MDMs with T-tropic envelope-expressing cells
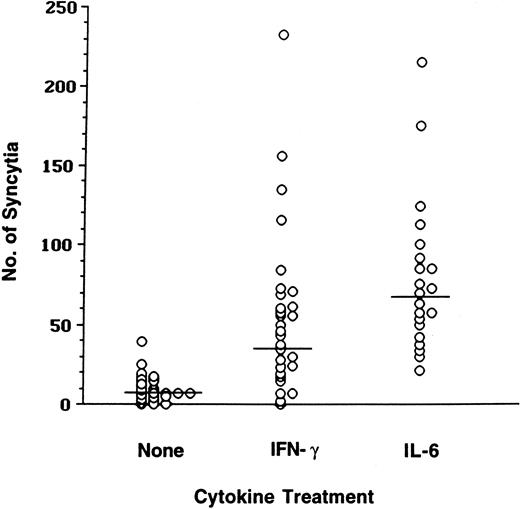
Unlike monocytes, macrophages have been shown to form limited numbers of syncytia with X4-tropic envelope-expressing cells.33,38,41,42 To determine whether proinflammatory cytokines affect the ability of macrophages to fuse with X4 envelope-expressing cells, macrophages were generated from elutriated monocytes in a 6-day culture in the presence of GM-CSF.33In our preliminary studies, differentiated MDMs were treated with a panel of cytokines (IFN-γ, TNF-α, IL-6, and IL-10) previously shown to regulate viral replication43 and mixed with cells expressing a prototypic T-tropic IIIB envelope (data not shown). Because only IFN-γ and IL-6 demonstrated reproducible enhancement in syncytia numbers, further studies were performed using these cytokines. MDMs were generated from elutriated monocytes of healthy donors, incubated with IFN-γ or IL-6, and subsequently mixed (1:1 ratio) with X4 envelope-expressing target cells. Syncytia numbers were quantified in these cultures and analyzed using t tests of log-transformed counts. Low syncytia numbers were scored in the cultures of untreated macrophages (mean count 7; Figure1). In contrast, IFN-γ and IL-6 induced an increase in the fusion of MDMs with cells expressing IIIB envelope; mean syncytia counts were 35 and 67, respectively. Although there was high variability in the amount of fusion of primary macrophages derived from healthy individuals, the statistical analysis of data from 30 separate experiments demonstrated that the increase in fusion of macrophages with IIIB envelope-expressing cells induced by IFN-γ or by IL-6 was highly significant (P < .0001; Figure 1).
Fusion of MDMs with T-tropic envelope-expressing cells is increased in the presence of IFN-γ or IL-6.
MDMs cultured in the absence or in the presence of IFN-γ or IL-6 were mixed with the effector TF228 cells expressing IIIB (BH8) envelope. Syncytia were scored after 18 hours.
Fusion of MDMs with T-tropic envelope-expressing cells is increased in the presence of IFN-γ or IL-6.
MDMs cultured in the absence or in the presence of IFN-γ or IL-6 were mixed with the effector TF228 cells expressing IIIB (BH8) envelope. Syncytia were scored after 18 hours.
Infection of MDMs with X4 HIV-1 is enhanced by IFN-γ and IL-6
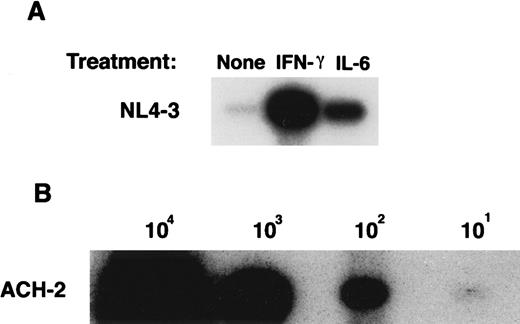
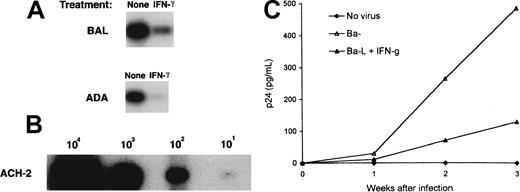
To determine whether an increase in fusion of cytokine-treated MDMs with T-tropic envelope-expressing cells correlates with an enhancement of X4 HIV-1 viral entry, a PCR-based analysis of viral DNA at 48 hours postinfection was performed (Figure2A). MDMs were incubated with either IL-6 or with IFN-γ, washed to remove cytokines, and were infected with HIV-1NL4-3. When DNA was extracted from macrophages immediately after infection (time zero), no signal was detected, demonstrating that no residual viral DNA was present in the virus stock preparations (data not shown). In parallel, DNA from serially diluted ACH-2 cells was PCR amplified (Figure 2B) and used to generate a standard curve. Low levels of viral complimentary DNA were detected in NL4-3–infected macrophages in the absence of cytokines (Figure 2A). In contrast, treatment of macrophages with IL-6 or with IFN-γ induced 76-fold and 198-fold increases in the number of cells containing NL4-3 viral DNA, respectively (Figure 2A). Similar data were obtained when macrophages were infected with HIV-1LAI (data not shown).
Effect of IFN-γ and IL-6 on the susceptibility of macrophages to HIV-1NL4-3 entry.
(A) Six-day MDMs untreated or treated with IFN-γ (for the last 24 hours) or with IL-6 (for the last 48 hours) were infected with NL4-3 for 48 hours. DNA was extracted from infected and uninfected MDMs and amplified by PCR using gag-specific primers. (B) DNA from serial dilutions of ACH-2 cells (containing one proviral copy/cell) was amplified by PCR as an internal standard control. Data represent 3 experiments.
Effect of IFN-γ and IL-6 on the susceptibility of macrophages to HIV-1NL4-3 entry.
(A) Six-day MDMs untreated or treated with IFN-γ (for the last 24 hours) or with IL-6 (for the last 48 hours) were infected with NL4-3 for 48 hours. DNA was extracted from infected and uninfected MDMs and amplified by PCR using gag-specific primers. (B) DNA from serial dilutions of ACH-2 cells (containing one proviral copy/cell) was amplified by PCR as an internal standard control. Data represent 3 experiments.
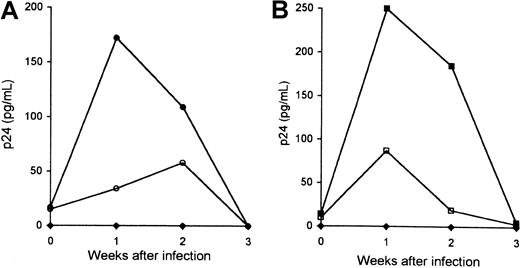
To determine whether an increase in the early production of viral DNA in cytokine-treated MDMs results in an increased viral replication, 6-day MDMs were incubated with cytokines as described above, washed to remove cytokines, and were infected with HIV-1LAI (Figure3). Infection of untreated macrophages with HIV-1LAI led to a low-level production of p24. In contrast, production of p24 by macrophages treated with IFN-γ was 5-fold to 2-fold higher than in control cultures by week 1 and 2, respectively (Figure 3A). Production of p24 by macrophages infected with LAI was also increased in IL-6–treated MDMs compared with untreated MDMs: 3-fold and 10-fold increase by week 1 and 2, respectively (Figure 3B). A similar increase in p24 production in NL4-3–infected MDMs was observed in the presence of IFN-γ and IL-6 (data not shown). Together our data suggest that both IFN-γ and IL-6 promote an increase in the efficiency of X4-tropic viral entry that results in an increased productive infection of MDMs with X4-tropic HIV-1.
Kinetics of in vitro productive infection of macrophages with T-tropic HIV-1.
Six-day MDMs cultured in the absence of cytokines (○, ■) or in the presence of IFN-γ (●) (A) or IL-6 (▪) (B) were infected with HIVLAI. Infected MDMs were cultured in the presence of cytokines. Viral replication was monitored by p24 measurements. Control cultures included uninfected MDMs (♦). Data represent 3 experiments performed with macrophages derived from different individuals.
Kinetics of in vitro productive infection of macrophages with T-tropic HIV-1.
Six-day MDMs cultured in the absence of cytokines (○, ■) or in the presence of IFN-γ (●) (A) or IL-6 (▪) (B) were infected with HIVLAI. Infected MDMs were cultured in the presence of cytokines. Viral replication was monitored by p24 measurements. Control cultures included uninfected MDMs (♦). Data represent 3 experiments performed with macrophages derived from different individuals.
Role of CXCR4 in IFN-γ– and IL-6–mediated increase in the fusion of MDMs with X4-tropic envelopes
To determine whether the fusion of cytokine-treated MDMs with the X4-tropic envelopes was CXCR4 dependent, syncytium formation was quantified in the absence or in the presence of the CXCR4 agonist, SDF-1α, or in the absence or in the presence of CXCR4 antagonist, synthetic peptide, T2240 (Table1). Low numbers of syncytia were scored in the cultures of untreated macrophages, and the addition of IFN-γ or IL-6 directly to the fusion assay did not increase fusion (data not shown). Pretreatment of MDMs with IFN-γ induced an 8-fold and a 4-fold increase of fusion in 2 separate experiments. Similarly, pretreatment of MDMs with IL-6 induced a 12-fold to 25-fold increase of fusion with X4-tropic envelopes in 4 independent experiments. In both cases, the observed fusion with T-tropic envelope-expressing cells was completely blocked by SDF-1α or by T22 but not by RANTES or the control peptide (data not shown). These data demonstrate the involvement of CXCR4 in the enhanced macrophage fusion with X4-tropic envelopes (Table 1).
IL-6– and IFN-γ–induced increase in the fusion of macrophages with T-tropic envelope-expressing cells is CXCR4 dependent
| Experiment . | Cytokine* . | Inhibitor† . | No. of syncytia with IIIB envelope‡ . |
|---|---|---|---|
| 1 | None | None | 5 ± 1 |
| IFN-γ | None | 40 ± 6 | |
| IFN-γ | SDF-1α | 5 ± 4 | |
| IFN-γ | RANTES | 45 ± 8 | |
| 2 | None | None | 17 ± 1 |
| IFN-γ | None | 60 ± 6 | |
| IFN-γ | SDF-1α | 4 ± 2 | |
| IFN-γ | T22 | 0 | |
| IFN-γ | RANTES | 72 ± 9 | |
| 3 | None | None | 6 ± 1 |
| IL-6 | None | 75 ± 20 | |
| IL-6 | SDF-1α | 0 | |
| IL-6 | RANTES | 87 ± 4 | |
| 4 | None | None | 1 ± 1 |
| IL-6 | None | 57 ± 12 | |
| IL-6 | SDF-1α | 3 ± 1 | |
| IL-6 | T22 | 0 | |
| IL-6 | RANTES | 49 ± 4 |
| Experiment . | Cytokine* . | Inhibitor† . | No. of syncytia with IIIB envelope‡ . |
|---|---|---|---|
| 1 | None | None | 5 ± 1 |
| IFN-γ | None | 40 ± 6 | |
| IFN-γ | SDF-1α | 5 ± 4 | |
| IFN-γ | RANTES | 45 ± 8 | |
| 2 | None | None | 17 ± 1 |
| IFN-γ | None | 60 ± 6 | |
| IFN-γ | SDF-1α | 4 ± 2 | |
| IFN-γ | T22 | 0 | |
| IFN-γ | RANTES | 72 ± 9 | |
| 3 | None | None | 6 ± 1 |
| IL-6 | None | 75 ± 20 | |
| IL-6 | SDF-1α | 0 | |
| IL-6 | RANTES | 87 ± 4 | |
| 4 | None | None | 1 ± 1 |
| IL-6 | None | 57 ± 12 | |
| IL-6 | SDF-1α | 3 ± 1 | |
| IL-6 | T22 | 0 | |
| IL-6 | RANTES | 49 ± 4 |
IL-6 indicates interleukin 6; IFN-γ, interferon γ.
Cytokines were added for the last 24 hours IFN-γ at 200 U/mL) or for the last 48 hr IL-6 at 20 ng/mL) of the 6-day monocyte-derived macrophage (MDM) culture.
Chemokines (1 μg/mL) or T22 peptide (2 μmol) were added to MDMs 1 hour prior to addition of Env-expressing cells.
Mean syncytia counts are shown for duplicate cultures.
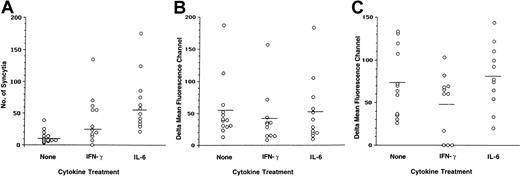
To determine whether there was a correlation between enhanced fusion of cytokine-treated MDMs with X4-tropic envelopes and CXCR4 surface expression, flow cytometry was performed on the MDMs that were used in the fusion assays (Figure 4). Statistical analysis of data derived from 12 separate experiments confirmed that the differences in the numbers of syncytia formed by untreated and IFN-γ– or IL-6–treated MDMs were significant (P values of .0372 and <.0001, respectively; Figure 4A). A considerable degree of variability in the levels of surface CXCR4 and CD4 was observed in MDMs obtained from different individuals (Figure 4B-C). When MDMs were treated with IFN-γ, a minor reduction in the CXCR4 surface expression was observed compared with untreated MDMs (P = .063; Figure 4B). Nonparametric analysis (Spearman correlation) demonstrated that the negative correlation between log syncytia values and CXCR4 ΔMFC values in IFN-γ–treated MDMs was not significant (data not shown). No significant differences in the levels of CXCR4 were induced by IL-6 in the MDMs (P = .24; Figure 4). IFN-γ was previously shown to induce reduction in the surface CD4 levels on macrophages.8 We also observed an IFN-γ–induced decrease in the density of surface CD4 in some donors, from a mean ΔMFC of 73 in untreated MDMs compared with a mean ΔMFC 47 in IFN-γ–treated MDMs (P = .0472; Figure 4C). At the same time, IL-6 did not alter the CD4 levels on MDMs (P = .246; Figure 4C). These data suggest that fusion of cytokine-treated MDMs with X4-tropic envelopes depends on CXCR4 but could not be attributed to an increase in the level of surface CXCR4 expression.
IFN-γ and IL-6 induce an increase in fusion of MDM with T-tropic envelopes in the absence of an increase in CXCR4 surface expression.
(A) MDMs untreated or treated with cytokines were cocultured with IIIB-envelope–expressing cells (TF228). Syncytia were scored after 18 hours. Aliquots of MDMs used for the fusion assay were stained with anti-CXCR4 (12G5) (B) and with anti-CD4 monoclonal antibodies (C). The ΔMFCs were calculated by subtracting the isotope control MFC from the experimental values. Results from 12 separate experiments are presented.
IFN-γ and IL-6 induce an increase in fusion of MDM with T-tropic envelopes in the absence of an increase in CXCR4 surface expression.
(A) MDMs untreated or treated with cytokines were cocultured with IIIB-envelope–expressing cells (TF228). Syncytia were scored after 18 hours. Aliquots of MDMs used for the fusion assay were stained with anti-CXCR4 (12G5) (B) and with anti-CD4 monoclonal antibodies (C). The ΔMFCs were calculated by subtracting the isotope control MFC from the experimental values. Results from 12 separate experiments are presented.
SDF-1–induced Ca++ flux in cytokine-treated macrophages
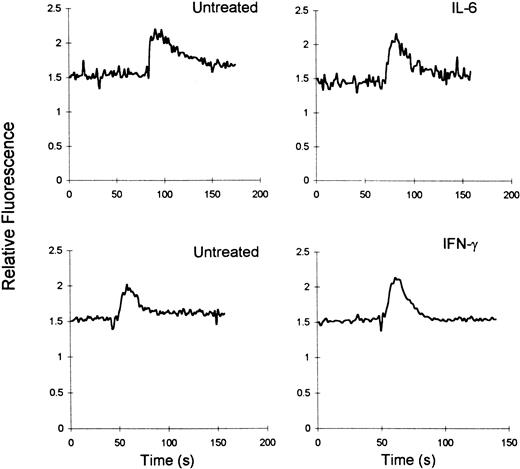
Because both IFN-γ and IL-6 induced an increase in the fusion of MDMs with X4-tropic envelopes in the absence of an increase in CXCR4 expression, it was of interest to determine whether IFN-γ or IL-6 induced conformational changes in CXCR4 in macrophages that resulted in an improved ligand binding. As was reported in our previous study, MDMs mobilized Ca++ in response to SDF-1α.33However, no differences in the magnitude of Ca++mobilization in response to SDF-1 were observed between untreated and IFN-γ– or IL-6–treated MDMs (Figure5). These findings suggest that these cytokines do not affect the ability of CXCR4 to bind to its ligand.
IFN-γ and IL-6 do not alter SDF-1–induced Ca++ flux in MDMs.
Six-day MDMs untreated or treated with cytokines were loaded with Fura 2, and Ca++-dependent fluorescent changes in response to SDF-1α (100 nmol) were recorded. Results are representative of 5 and 6 experiments for IFN-γ and IL-6, respectively.
IFN-γ and IL-6 do not alter SDF-1–induced Ca++ flux in MDMs.
Six-day MDMs untreated or treated with cytokines were loaded with Fura 2, and Ca++-dependent fluorescent changes in response to SDF-1α (100 nmol) were recorded. Results are representative of 5 and 6 experiments for IFN-γ and IL-6, respectively.
Effect of Cx and of H7 on the IFN-γ- and IL-6-induced fusion of MDMs with T-tropic envelopes
The signaling pathways initiated by IL-6 and by IFN-γ include activation of Stat1/3 proteins.44,45 It was important to determine whether the IFN-γ– and IL-6–mediated fusion of MDMs with X4-tropic envelopes is associated with Stat1/3 activation. To test this hypothesis, MDMs were pretreated with an inhibitor of Stat1/3 activation, the serine-threonine kinase inhibitor, H7,46-48 prior to and during cytokine treatment; these cells were then mixed with IIIB envelope-expressing cells (Table2). Addition of IL-6 or IFN-γ induced an increase in mean syncytia counts of MDMs from 7 ± 4 to 72 ± 9 and from 14 ± 1 to 61 ± 13, respectively. However, when macrophages were incubated with H7, the cytokine-induced increase in IIIB envelope fusion was inhibited (Table 2). In addition, Cx, a protein synthesis inhibitor, was also an effective inhibitor of the cytokine-induced fusion of MDMs with T-tropic envelopes (Table 2). Neither Cx nor H7 had any effect on the fusion of MDMs in the absence of cytokine treatment. These data suggest that the mechanisms (including the initial signal transduction steps) by which IFN-γ and IL-6 increase the X4 fusion potential of MDM may involve serine-threonine phosphorylation and protein synthesis.
H7 and cycloheximide inhibit IL-6– and IFN-γ–induced fusion of macrophages with T-tropic envelope-expressing cells
| Cytokine* . | Inhibitor† . | No. of syncytia† . |
|---|---|---|
| None | None | 7 ± 4 |
| IL-6 | None | 72 ± 9 |
| IL-6 | Cx | 6 ± 2 |
| IL-6 | H7 | 9 ± 1 |
| None | Cx | 8 ± 4 |
| None | H7 | 3 ± 0 |
| None | None | 14 ± 1 |
| IFN-γ | None | 61 ± 13 |
| IFN-γ | Cx | 13 ± 1 |
| IFN-γ | H7 | 12 ± 2 |
| None | Cx | 11 ± 1 |
| None | H7 | 9 ± 1 |
| Cytokine* . | Inhibitor† . | No. of syncytia† . |
|---|---|---|
| None | None | 7 ± 4 |
| IL-6 | None | 72 ± 9 |
| IL-6 | Cx | 6 ± 2 |
| IL-6 | H7 | 9 ± 1 |
| None | Cx | 8 ± 4 |
| None | H7 | 3 ± 0 |
| None | None | 14 ± 1 |
| IFN-γ | None | 61 ± 13 |
| IFN-γ | Cx | 13 ± 1 |
| IFN-γ | H7 | 12 ± 2 |
| None | Cx | 11 ± 1 |
| None | H7 | 9 ± 1 |
IL-6 indicates interleukin 6; IFN-γ, interferon γ.
Cytokines were added for the last 24 hours of the monocyte-derived macrophage culture.
Cycloheximide (Cx) at 5 ng/mL and 1-(5-isoquinolinesulfonyl)-2-methyl-piperazine (H7) at 100 μmol were added to MDMs 1 hour prior to cytokines.
Syncytia were scored after 18 hours of co-culture of the macrophages (untreated or pretreated with cytokines) and TF228 cells expressing IIIB envelope.
IFN-γ down-regulates HIV-1Ba-L replication and HIV-1Ba-L viral entry in macrophages
Because IFN-γ and IL-6 augmented X4-tropic viral entry and replication MDMs, it was of interest to determine the effect of the same cytokines on infection of MDMs with R5-tropic strains. MDMs were infected with HIV-1Ba-L and with HIV-1ADA, and cell lysates were subjected to HIV-1 DNA PCR after 48 hours. IFN-γ induced a 3-fold and a 14-fold decrease in the number of macrophages containing Ba-L and ADA proviral DNA, respectively (Figure6). To confirm that the reduced viral entry correlated with a reduction in viral production, HIV-1Ba-L–infected MDMs were cultured in the absence or in the presence of IFN-γ, and aliquots of the culture medium were assayed for p24 (Figure 6C). In agreement with the observed reduction in HIV-1Ba-L viral DNA, an inhibition of virus replication was observed in MDM cultures in the presence of IFN-γ (Figure 6C). No effects were seen in IL-6–treated MDM cultures (data not shown).
Effect of IFN-γ on the in vitro infection of macrophages with M-tropic HIV-1.
(A) PCR-based analyses of viral DNA from MDMs infected with M-tropic HIV-1. MDMs untreated or treated with IFN-γ were infected with Ba-L or with ADA for 48 hours. DNA lysates were amplified with gag-specific primers as described in Figure 2. (B) DNA from a serial dilution of ACH-2 cells (containing one proviral copy/cell) was amplified by PCR as internal control. (C) MDMs untreated (▵) or treated with IFN-γ (▴) were infected with Ba-L for 48 hours. Infected MDMs were washed and cultured in the presence of IFN-γ. At indicated time points, the aliquots of the culture medium were assayed for p24. Control cultures included uninfected MDMs (♦). Data shown for 1 experiment are representative of 3 experiments performed with macrophages derived from different individuals.
Effect of IFN-γ on the in vitro infection of macrophages with M-tropic HIV-1.
(A) PCR-based analyses of viral DNA from MDMs infected with M-tropic HIV-1. MDMs untreated or treated with IFN-γ were infected with Ba-L or with ADA for 48 hours. DNA lysates were amplified with gag-specific primers as described in Figure 2. (B) DNA from a serial dilution of ACH-2 cells (containing one proviral copy/cell) was amplified by PCR as internal control. (C) MDMs untreated (▵) or treated with IFN-γ (▴) were infected with Ba-L for 48 hours. Infected MDMs were washed and cultured in the presence of IFN-γ. At indicated time points, the aliquots of the culture medium were assayed for p24. Control cultures included uninfected MDMs (♦). Data shown for 1 experiment are representative of 3 experiments performed with macrophages derived from different individuals.
IFN-γ induces a reduction in the cell-to-cell fusion of macrophages with M-tropic envelope-expressing cells and a reduction in surface CCR5 density
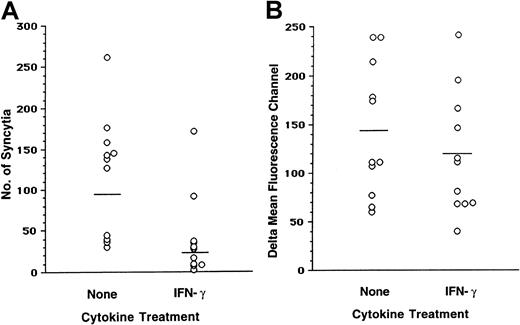
The reduction in R5-tropic virus replication and in the R5-tropic viral entry observed in the presence of IFN-γ could be mediated in part by down-modulation of CD48 and/or CCR5 expression. To investigate these possibilities, the fusion assay was performed in parallel with surface staining. MDMs formed high numbers of syncytia with 12E1 cells infected with recombinant vaccinia virus expressing the JR-FL envelope (Figure7A). A reduction in fusion with the JR-FL envelope was observed in MDM cultures pretreated with IFN-γ. Similar results were observed when other R5-tropic envelopes (ADA, Ba-L) were used (data not shown). Staining with the CCR5-specific 2D7 monoclonal antibody revealed a considerable degree of variability in the CCR5 expression among macrophages derived from 11 individuals. Treatment of MDMs with IFN-γ resulted in a modest reduction of surface CCR5 (Figure 7A-B). Analysis of data from 11 separate experiments revealed that inhibition of fusion of MDMs with M-tropic envelopes induced by IFN-γ was highly significant (P = .0006), whereas the reduction in surface CCR5 levels was moderate but statistically significant (P = .022; Figure 7B).
IFN-γ induced decrease in the fusion of MDM with M-tropic envelopes and reduction in the surface CCR5.
(A) Fusion was set up between MDMs cultured in the absence or in the presence of IFN-γ and effector 12E1 cells infected with recombinant vaccinia virus expressing M-tropic envelope (vCB28, JR-FL). Syncytia were scored after 18 hours. (B) The same MDMs were stained with anti-CCR5 2D7 monoclonal antibody. The ΔMFC was calculated as described in Figure 4. The results represent 11 separate experiments.
IFN-γ induced decrease in the fusion of MDM with M-tropic envelopes and reduction in the surface CCR5.
(A) Fusion was set up between MDMs cultured in the absence or in the presence of IFN-γ and effector 12E1 cells infected with recombinant vaccinia virus expressing M-tropic envelope (vCB28, JR-FL). Syncytia were scored after 18 hours. (B) The same MDMs were stained with anti-CCR5 2D7 monoclonal antibody. The ΔMFC was calculated as described in Figure 4. The results represent 11 separate experiments.
Effect of IL-6-treatment on the infection of macrophages with X4 primary viral isolates
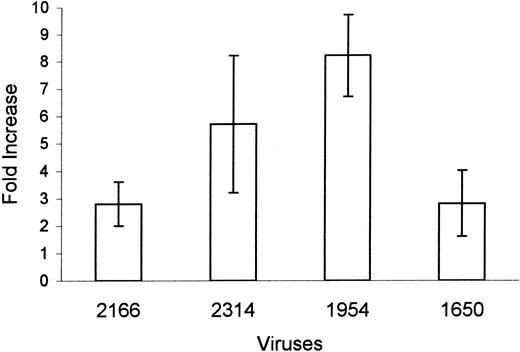
To determine whether cytokines can alter susceptibility of macrophages to primary viral isolates, 4 X4 primary isolates were used for the infection of MDMs that were untreated or treated with IL-6 (Figure 8). The p24 production by untreated MDMs was low, ranging from 0.1 ± 0.05 to 0.4 ± 0.1 ng/mL, depending on the virus. Addition of IL-6 to the macrophage cultures induced a 2-fold to a 12-fold increase in p24 production by macrophages infected with X4 primary isolates (Figure 8). These data suggest that proinflammatory cytokines can increase the susceptibility of macrophages to the infection with multiple primary X4 viruses.
IL-6 induced an increase in productive infection of MDM with primary viral isolates.
Six-day MDMs cultured in the absence or in the presence of IL-6 were infected with X4 HIV-1 primary viral isolates: 2166 (clade E), 2314 (clade BF), 1954 (clade D), and 1650 (clade A). Infected MDMs were cultured in the presence of cytokines. Viral replication was monitored by p24 measurements. Data represent fold increase ± SEM in p24 production (day 17) from the duplicate cultures of IL-6–treated macrophages compared with p24 production in infected macrophages in the absence of cytokine.
IL-6 induced an increase in productive infection of MDM with primary viral isolates.
Six-day MDMs cultured in the absence or in the presence of IL-6 were infected with X4 HIV-1 primary viral isolates: 2166 (clade E), 2314 (clade BF), 1954 (clade D), and 1650 (clade A). Infected MDMs were cultured in the presence of cytokines. Viral replication was monitored by p24 measurements. Data represent fold increase ± SEM in p24 production (day 17) from the duplicate cultures of IL-6–treated macrophages compared with p24 production in infected macrophages in the absence of cytokine.
Cytokine-treated MDMs secrete β-chemokines that alter fusion of macrophages with R5 and X4 envelope-expressing cells
To determine the mechanisms responsible for the effects of cytokine treatment on the fusion of MDMs with X4 and R5 envelope-expressing cells, we measured the levels of β-chemokines in the macrophage-conditioned medium. Production of MIP-1α and MIP-1β was significantly increased in IFN-γ–treated MDMs and to a lesser degree in IL-6–treated MDMs (Table 3). No RANTES was detected in the cultures of cytokine-treated MDMs (data not shown).
IFN-γ and IL-6 induce chemokine production in macrophages
| Treatment3-150 . | MIP-1α (pg/mL)3-151 . | MIP-1β (pg/mL)3-151 . |
|---|---|---|
| None | 143 ± 35 | 66 ± 3 |
| IFN-γ | 909 ± 37 | 1425 ± 12 |
| IL-6 | 647 ± 5 | 2176 ± 44 |
| Treatment3-150 . | MIP-1α (pg/mL)3-151 . | MIP-1β (pg/mL)3-151 . |
|---|---|---|
| None | 143 ± 35 | 66 ± 3 |
| IFN-γ | 909 ± 37 | 1425 ± 12 |
| IL-6 | 647 ± 5 | 2176 ± 44 |
IFN-γ indicates Interferon γ; IL-6, interleukin 6; MIP, macrophage inflammatory protein; MDMs, monocyte-derived macrophages.
MDMs were treated with cytokines for the last 24 hours of the culture.
Supernatants from the cultures of untreated MDMs or MDMs treated with cytokines were collected and analyzed by enzyme-linked immunosorbent assay in duplicates.
To determine whether β-chemokines secreted by macrophages can alter their fusion with R5 and X4 envelopes, macrophages were incubated with CM derived from the cytokine-treated macrophages. (Table4). Fusion of macrophages with JR-FL envelope-expressing cells was partially reduced by CM derived from the cytokine-treated macrophages (30%-40% reduction in 3 experiments) or by recombinant MIP-1α and MIP-1β at 10 and 1 ng/mL. In contrast, the same macrophages showed an increase in their ability to form syncytia with X4 envelope-expressing cells in the presence of either CM from cytokine-treated MDMs or in the presence of recombinant chemokines MIP-1α and MIP-1β. MCP3 had no effect on macrophage fusion (Table4). Importantly, both the negative effect of CM on the fusion with an R5 envelope and the positive effect of CM on the fusion with an X4 envelope were blocked by combination of anti–MIP-1α and anti–MIP-1β antibodies. Similarly, the effects of recombinant MIP-1α and MIP-1β on the fusion of macrophages with R5 and with X4 envelopes were also blocked in the presence of anti–MIP-1α and anti–MIP-1β antibodies. In an additional set of experiments, antibodies against β-chemokines were added along with cytokines for the last 24 hours of the macrophage culture (Table5). Both cytokines, IFN-γ and IL-6, induced an increase in fusion of MDMs with IIIB envelope-expressing cells. However, when a mixture of anti–MIP-1α and –MIP-1β antibodies was added together with cytokines to the macrophage cultures, no increase in fusion with T-tropic envelope was observed (Table 5).
Cell fusion activity of MDMs in the presence of conditioned media from cytokine-treated MDMs
| Treatment . | Antibodies4-151 . | No. of syncytia4-150 . | |
|---|---|---|---|
| IIIB . | JR-FL (% of control) . | ||
| None | None | 4 ± 1 | 228 ± 3 (100) |
| Control CM‡ | None | 3 ± 1 | 226 ± 9 (99) |
| IFN-γ CM | None | 34 ± 4 | 155 ± 12 (67) |
| IL-6 CM | None | 31 ± 3 | 162 ± 4 (71) |
| IFN-γ CM | a-MIP-1α; a-MIP-1β | 4 ± 1 | 261 ± 33 (114) |
| IL-6 CM | a-MIP-1α; a-MIP-1β | 8 ± 1 | 221 ± 14 (96) |
| MIP-1α; MIP-1β (10 ng/mL) | None | 56 ± 2 | 114 ± 18 (50) |
| MIP-1α; MIP-1β (1 ng/mL) | None | 37 ± 3 | 182 ± 11 (80) |
| MIP-1α; MIP-1β (10 ng/mL) | a-MIP-1α; a-MIP-1β | 2 ± 1 | 229 ± 20 (100) |
| MIP-1α; MIP-1β (1 ng/mL) | a-MIP-1α; a-MIP-1β | 1 ± 1 | 230 ± 6 (100) |
| MCP 3 | None | 2 ± 2 | 225 ± 21 (98) |
| Treatment . | Antibodies4-151 . | No. of syncytia4-150 . | |
|---|---|---|---|
| IIIB . | JR-FL (% of control) . | ||
| None | None | 4 ± 1 | 228 ± 3 (100) |
| Control CM‡ | None | 3 ± 1 | 226 ± 9 (99) |
| IFN-γ CM | None | 34 ± 4 | 155 ± 12 (67) |
| IL-6 CM | None | 31 ± 3 | 162 ± 4 (71) |
| IFN-γ CM | a-MIP-1α; a-MIP-1β | 4 ± 1 | 261 ± 33 (114) |
| IL-6 CM | a-MIP-1α; a-MIP-1β | 8 ± 1 | 221 ± 14 (96) |
| MIP-1α; MIP-1β (10 ng/mL) | None | 56 ± 2 | 114 ± 18 (50) |
| MIP-1α; MIP-1β (1 ng/mL) | None | 37 ± 3 | 182 ± 11 (80) |
| MIP-1α; MIP-1β (10 ng/mL) | a-MIP-1α; a-MIP-1β | 2 ± 1 | 229 ± 20 (100) |
| MIP-1α; MIP-1β (1 ng/mL) | a-MIP-1α; a-MIP-1β | 1 ± 1 | 230 ± 6 (100) |
| MCP 3 | None | 2 ± 2 | 225 ± 21 (98) |
MDMs indicate monocyte-derived macrophages; IFN-γ, interferon γ; CM, conditioned media; IL-6, interleukin 6; MIP, macrophage inflammatory protein.
Syncytia were scored after 18 hours of coculture of the macrophages and the effector cells: TF228 cells expressing IIIB envelope or 12E1 cells infected with recombinant vaccinia virus expressing R5 envelope (vCB28, JR-FL). Data represent 3 experiments.
Anti–MIP-1α and anti–MIP-1β antibodies were used at 2 μg/mL. Antibodies were incubated with the conditioned media or with MIP-1α and MIP-1β mixtures for 1 hour at room temperature.
CM were collected from the cultures of untreated MDMs (control CM) or from the cultures of cytokine-treated MDMs (IFN-γ CM and IL-6 CM) and were added to untreated autologous MDMs 1 hour prior to the addition of effector cells expressing X4 or R5 envelopes.
Antibodies against MIP-1α, MIP-1β chemokines inhibit fusion enhancement of MDMs with X4 envelope-expressing cells when added to the IFN-γ/IL-6–treated MDMs
| Treatment5-151 . | Antibodies5-152 . | No. of syncytia5-150 . | |
|---|---|---|---|
| IIIB . | JR-FL . | ||
| None | None | 9 ± 1 | 156 ± 1 |
| a-MIP-1α; a-MIP-1β | a-MIP-1α; a-MIP-1β | 8 ± 2 | 161 ± 20 |
| IFN-γ | None | 33 ± 3 | 37 ± 5 |
| IFN-γ | a-MIP-1α; a-MIP-1β | 5 ± 1 | 127 ± 17 |
| IL-6 | None | 32 ± 5 | 109 ± 9 |
| IL-6 | a-MIP-1α; a-MIP-1β | 2 ± 1 | 183 ± 6 |
| Treatment5-151 . | Antibodies5-152 . | No. of syncytia5-150 . | |
|---|---|---|---|
| IIIB . | JR-FL . | ||
| None | None | 9 ± 1 | 156 ± 1 |
| a-MIP-1α; a-MIP-1β | a-MIP-1α; a-MIP-1β | 8 ± 2 | 161 ± 20 |
| IFN-γ | None | 33 ± 3 | 37 ± 5 |
| IFN-γ | a-MIP-1α; a-MIP-1β | 5 ± 1 | 127 ± 17 |
| IL-6 | None | 32 ± 5 | 109 ± 9 |
| IL-6 | a-MIP-1α; a-MIP-1β | 2 ± 1 | 183 ± 6 |
MIP indicates macrophage inflammatory protein; MDMs, monocyte-derived macrophages; IFN-γ, interferon γ; IL-6, interleukin 6.
Syncytia were scored after 18 hours of coculture of the macrophages and the effector cells: TF228 cells expressing IIIB envelope or 12E1 cells infected with recombinant vaccinia virus expressing R5 envelope (vCB28, JR-FL). Data represent 2 experiments.
MDMs were treated with cytokines for the last 24 hours of the culture.
Anti–MIP-1α and anti–MIP-1β antibodies were used at 2 μg/mL. Antibodies were added to MDMs simultaneously with cytokines.
Discussion
In this study, we demonstrated that the proinflammatory cytokines IFN-γ and IL-6 exert a positive effect on the susceptibility of MDMs to infection with X4 HIV-1. This conclusion was supported by 3 types of experiments: IFN-γ and IL-6 induced an increase in X4 HIV-1 viral entry and productive infection of MDMs and promoted fusion of MDMs with X4 envelope-expressing cells. In contrast to the positive effect of IFN-γ on the susceptibility of MDMs to infection with X4 viral strains, an inhibitory effect of IFN-γ was observed on the R5 viral entry and R5 productive infection of MDM, and on fusion of macrophages with target cells expressing R5 envelopes. In addition, β-chemokines (MIP-1α and MIP-1β) were detected in the CM derived from IFN-γ– and IL-6–treated MDMs. This CM partially inhibited fusion of MDMs with R5 envelopes and enhanced fusion with X4 envelopes. Both effects of CM were inhibited in the presence of anti–MIP-1α/MIP-1β antibodies, suggesting that the cytokine-induced production of β-chemokines is responsible for the alterations in macrophage fusion potential.
The ability of differentiated macrophages to support infection with T-tropic HIV-1 has been a subject of intensive research over recent years. Earlier data demonstrated that MDMs are resistant to infection with T-cell line–tropic viruses. However, studies2,28reported that, under certain culture conditions, some primary syncytia-inducing viral isolates could infect MDMs. It is possible that different cell isolation and culture conditions for growing MDMs can affect the level of macrophage maturation, which was shown to determine their susceptibility to HIV-1 infection.49 Low levels of HIV-1NL4-3 entry and of HIV-1LAIentry (data not shown) into MDMs in the absence of cytokines were detected in our system in agreement with other reports.32IFN-γ and IL-6 induced a dramatic increase in HIV-1NL4-3viral entry into MDMs, which was followed by a modest increase in productive infection. The difference between the effect of cytokines on viral entry versus productive infection may be due to a partial postentry block in viral replication as was previously suggested.31 32 Importantly, we also observed an increase in p24 production by macrophages infected with X4 primary isolates in the presence of IL-6. Thus, our data strongly suggest that proinflammatory cytokines may play an important role in X4 virus infection of macrophages in vivo.
The observed increase in MDM fusion with T-tropic envelopes was specifically inhibited by the CXCR4 agonist, SDF-1α, as well as by the CXCR4 antagonist, T22 peptide, confirming the involvement of CXCR4 in the fusion. At the same time, our data demonstrated that neither IFN-γ nor IL-6 induced an increase in surface CXCR4 levels on MDMs. Moreover, no differences in CXCR4 messenger RNA were detected in untreated and cytokine-treated MDMs (data not shown). Thus, the enhanced fusion of cytokine-treated MDMs with T-tropic envelope-expressing cells that we observed is CXCR4 dependent but cannot be attributed to an increase in the surface CXCR4 levels. The ability of chemokine receptors to mediate fusion with HIV-1 envelopes was shown to be independent from their signaling function.50 Because no Ca++ flux in response to recombinant gp120 was detected in MDMs either untreated or treated with cytokines (data not shown), we measured SDF-1–induced Ca++ flux in cytokine-treated MDMs as a surrogate assay for the ability of CXCR4 to bind to its ligand. Because no differences were observed in the magnitude and duration of the response between untreated and cytokine-treated MDMs, it is unlikely that IFN-γ or IL-6 altered CXCR4 affinity to the ligand. Cytokine treatment of MDMs in the presence of inhibitors, Cx or H7, eliminated the cytokine-induced increase in fusion potential of MDMs but not in CXCR4 signaling or CXCR4 surface expression (data not shown). Although Cx and H7 both inhibited the cytokine-induced increase in macrophage fusion with X4 envelopes, it is most likely that these reagents interfere with different stages of signal transduction. As was demonstrated in several reports,46-48 H7 inhibits the early steps of Stat1/3 activation. Cx may be responsible for the inhibition of the downstream events (see below). According to our recently published data,33 there is a correlation between the ability of monocytes and MDMs to fuse with T-tropic envelope-expressing cells and the ratio between low- and high-molecular-weight species of the CXCR4 molecule on MDMs. In our current study, a modest reduction in the proportion of the high- versus low-molecular-weight CXCR4 species was observed in cytokine-treated MDMs (data not shown). Thus, it is possible that IFN-γ and IL-6 induce posttranslational modifications of CXCR4 molecules that enhance their fusion potential with T-tropic envelopes and/or their association with CD4 molecules without changing CXCR4-mediated signal transduction. Alternatively, proinflammatory cytokines may induce expression of other protein(s) that can modify the fusion potential of surface CCR5 and CXCR4.
In previous studies,9,24,33,39,41 we and others provided evidence that CD4, the primary receptor for HIV-1, has a natural affinity for coreceptors CCR5 and CXCR4 as demonstrated by coimmunoprecipitation of CD4 and coreceptors in monocytes and macrophages. We recently demonstrated that coexpression of CCR5 and CXCR4 in A2.01 T-cell line resulted in a significant inhibition of both fusion with X4 envelope-expressing cells and infection with X4 virus. This inhibition was reversed by antibodies against CCR5.51Thus, the susceptibility of cells with limited CD4 to infection with viral strains of different tropism may be influenced by competition between coreceptors for association with CD4. Because β-chemokines have been previously shown to enhance replication of X4 viruses in CD4+ T cells,52,53 we hypothesized that, in macrophages, cytokine-induced endogenous β-chemokines may augment fusion with X4 envelopes by interfering with the formation CCR5/CD4 complexes. Importantly, CM derived from cytokine-treated MDMs were almost as effective as recombinant chemokines in the enhancement of macrophage fusion with an X4 envelope. Because the effects of CM were blocked by antibodies against β-chemokines, it is very likely that cytokine-induced endogenous β-chemokines were responsible for increased infection of MDMs with X4 viruses in agreement with a previously published observation that neutralization of endogenous β-chemokines increases virus replication in PBMCs from HIV-infected individuals.54
The inhibitory effect of IFN-γ on M-tropic HIV-1 infection of MDMs was not unexpected. It has been reported that IFN-γ treatment can protect macrophages from M-tropic HIV infection.9 Thus, our data demonstrated a pleiotropic effect of IFN-γ on HIV-1 infection of MDMs: an increase in T-tropic virus infection (in spite of reduced surface CD4) and a decrease in M-tropic HIV-1 infection. Similar levels of β-chemokines were detected in CM derived from IFN-γ– and from IL-6–treated macrophages. However, only IFN-γ but not IL-6 induced down-regulation of the CD4 molecules and protected macrophages from infection with R5 viruses. Together these data suggest that β-chemokines alone are not sufficient to mediate long-term inhibition of macrophage infection with R5 viruses. The efficient inhibition may require concomitant reduction in CD4 and CCR5 levels. It is possible that β-chemokines either block or induce down-regulation of CCR5 on MDMs, thus increasing the availability of the limited CD4 for association with CXCR4.
TB and other OIs have been shown to contribute to the pathogenicity of HIV and to accelerate the course of HIV disease.16,17,55,56 The role of macrophages in accelerating the course of HIV disease was established by demonstrating in situ the presence of integrated T-tropic HIV-1 DNA as well as p24 production in macrophages of OI-positive but not OI-negative HIV-1–infected individuals.34 It has been also demonstrated that macrophages become susceptible to T-tropic HIV infection after exposure to the components of the bacterial cell wall.57 In agreement with these reports, our data support the model in which common opportunistic pathogens alter susceptibility of macrophages to HIV by triggering production of cytokines that may be favorable for the entry and replication of more pathogenic T-tropic viral strains. This model is supported by the high levels of proinflammatory cytokines detected in patients with OI.17-19,58 Importantly, although OIs often occur following a decline in peripheral CD4 cell counts to below 200,59 some infections (Leishmania, herpes simplex virus, M tuberculosis,Candida, etc.) can occur early in the course of the disease before the onset of AIDS.60 It is conceivable that proinflammatory cytokines secreted during these infections at the latent stage of the disease may increase susceptibility of MDMs to infection with T-tropic HIV, thus “opening the gates” for the emerging T-tropic viruses. In addition, proinflammatory cytokines were detected in samples obtained by cervicovaginal lavage from women with infections of the genital tract in the absence of HIV infection.61,62 Increased levels of proinflammatory cytokines were observed in cervicovaginal lavage samples obtained from HIV-1–infected women.61 62 Our data support the notion that sexually transmitted diseases may affect local replication of X4 viruses and may partially explain the higher risk of HIV disease acceleration observed in women with these underlying conditions.
Acknowledgments
We thank the staff of the Department of Transfusion Medicine at NIH for providing elutriated monocytes; Dr Keith Peden for viral stocks; Dr Lee Tiffany for technical assistance; and Dr Philip Murphy, Dr Keith Peden, and Dr Basil Golding for reviewing the manuscript.
Partially supported by a grant from the Office of Women's Health, Food and Drug Administration, and by a grant from the National Institutes of Health Intramural AIDS Targeted Antiviral Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marina Zaitseva, Division of Viral Products, Center for Biologics Evaluation and Research, Food and Drug Administration, Bldg 29B, Rm 3G21, 8800 Rockville Pike, Bethesda, MD 20892; e-mail zaitseva@cber.fda.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal