Abstract
Expansion of primary solid tumors and their malignant dissemination are angiogenesis-dependent. Vascular endothelial growth factor (VEGF) is the key factor playing a pivotal role in solid tumor-induced angiogenesis. Recent studies indicate that angiogenesis may also be involved in the pathogenesis of certain hemic malignancies, including B-cell chronic lymphocytic leukemia (B-CLL). Mechanisms underlying angiogenesis in B-CLL and the role of VEGF in this process are incompletely understood. In this study, it was examined whether angiogenically functional VEGF is produced by B-CLL cells. Immunohistochemical staining with antibodies against VEGF and CD34, an endothelial cell marker, demonstrated the presence of VEGF protein and abundant blood vessels in infiltrated lymphoreticular tissues. Low levels of VEGF were detected by ELISA in the culture media of unstimulated cells; this was enhanced up to 7-fold by hypoxic stimulation. SDS-PAGE and Western blot analysis of the concentrated culture media showed 2 isoforms of VEGF protein with molecular weights of 28 and 42 kd, respectively. RNA hybridization showed that these cells expressed VEGF mRNA. Reverse transcription–polymerase chain reaction, combined with nucleotide sequence analysis, revealed that the predominantly expressed isoforms were VEGF121 and VEGF165. Moreover, 3H-thymidine incorporation and an in vivo angiogenic assay demonstrated that the VEGF produced by CLL cells can induce angiogenesis by stimulating endothelial cell proliferation. In conclusion, this study shows that B-CLL cells produce VEGF and demonstrates the angiogenic effects of this growth factor, which may be relevant for the tissue phase of the disease.
Introduction
Angiogenesis is the process in which new microvessels form from preexisting ones.1 It has been well established that the expansion and dissemination of primary solid tumors are angiogenesis-dependent2,3 and that vascular endothelial growth factor (VEGF) is one of the most potent factors involved in this process.4 Various solid tumors, including breast, lung, colon, and prostate carcinomas, secrete VEGF,5-8 and capillaries are found clustered along VEGF-producing tumor cells.9 Tumor angiogenesis and subsequent tumor growth are inhibited in vivo by antibodies directed against VEGF,10 by soluble VEGF receptors,11and by expression of dominant-negative VEGF receptors.12These properties have made the study of VEGF function important for the understanding of the angiogenesis associated with solid tumors.
Recent studies have suggested that angiogenesis may also be involved in the pathogenesis of certain hemic malignancies. A study13 of 88 patients with B-cell non-Hodgkin lymphoma showed an increase in microvessel density in lymph nodes that correlated with the severity of the disease. Another14 of childhood acute lymphocytic leukemia revealed an increased microvessel density in the patient's bone marrow compared with normal tissue. Increases in bone marrow microvessel density were also observed in a series of patients diagnosed with multiple myeloma and hairy-cell leukemia.15 16
B-cell chronic lymphocytic leukemia (B-CLL), the most common adult leukemia in Western Europe and North America, is characterized by a persistent lymphocytosis in the peripheral blood and by progressive infiltration of lymphoreticular tissue by malignant cells.17 In fact, increased vessel density has been observed in bone marrow from patients with B-CLL.18 The current study explores whether angiogenesis takes place in the expanding secondary lymphoid tissues of the disease and the role of VEGF in this process.
Although VEGF protein has been detected in serum from patients with B-CLL,19 whether the CLL cells themselves make VEGF protein remains controversial. By using Northern blot analysis and quantitative reverse transcription–polymerase chain reaction (RT-PCR) techniques, one group20 detected VEGF mRNA in the peripheral lymphocytes of 6 patients with B-CLL. However, another group21 could not detect any VEGF mRNA in B-CLL cells using an in situ hybridization technique.
In the current study, we show that B-CLL cells express VEGF mRNA and produce VEGF protein. Our functional studies reveal that the VEGF produced by B-CLL cells stimulates endothelial cell proliferation and in vivo angiogenesis. Furthermore, VEGF secretion by B-CLL cells was enhanced by hypoxic stimulation, a fundamental stimulus for neovascularization in tumor microenvironments.22
Patients, materials, and methods
Patients
All patients had typical CLL as defined morphologically (prolymphocytes less than 10%) and immunophenotypically (CD19+, CD5+, CD23+, and weak light-chain–restricted surface immunoglobulin). Clinical details are shown in Table 1. The table shows that the patients constituted a mixed group with regard to stage and treatment. Approximately half had early-stage disease (Rai, 0 and 1; Binet, A), and only 2 patients (CLL17, CLL18) displayed a clearly progressive course. Three of the patients (CLL11, CLL15, CLL16) had newly diagnosed disease and therefore the progressiveness of their cases is as yet unknown. All materials were obtained with informed consent.
Patient characteristics
| Patient . | Stage at diagnosis . | Stage at study . | Prior treatment . | ||
|---|---|---|---|---|---|
| Binet . | Rai . | Binet . | Rai . | ||
| CLL1 | A | I | A | 0 | CLB + pred |
| CLL2 | C | IV | C | IV | CLB + pred; flu |
| CLL3 | A | 0 | A | 0 | CLB |
| CLL4 | A | I | A | I | CLB + pred |
| CLL5 | A | I | A | I | CLB + pred |
| CLL6 | C | IV | C | IV | CLB; CHOP; etoposide |
| CLL7 | C | IV | C | IV | CLB; cyclo + pred |
| CLL8 | A | I | A | I | None |
| CLL9 | A | 0 | A | 0 | CLB; flu |
| CLL10 | C | III | C | III | None |
| CLL11 | A | 0 | A | 0 | None |
| CLL12 | B | II | B | II | None |
| CLL13 | A | 0 | A | 0 | None |
| CLL14 | A | I | A | I | None |
| CLL15 | C | III | C | III | None |
| CLL16 | A | 0 | A | 0 | None |
| *CLL17 | B | II | C | IV | CLB; CHOP; flu |
| *CLL18 | A | I | B | II | CLB + flu |
| †CLL19 | A | I | A | I | None |
| ‡CLL20 | B | II | B | II | pred; CLB + pred; cyclo + vincristine + pred |
| ‡CLL21 | A | I | A | I | CLB + pred |
| Patient . | Stage at diagnosis . | Stage at study . | Prior treatment . | ||
|---|---|---|---|---|---|
| Binet . | Rai . | Binet . | Rai . | ||
| CLL1 | A | I | A | 0 | CLB + pred |
| CLL2 | C | IV | C | IV | CLB + pred; flu |
| CLL3 | A | 0 | A | 0 | CLB |
| CLL4 | A | I | A | I | CLB + pred |
| CLL5 | A | I | A | I | CLB + pred |
| CLL6 | C | IV | C | IV | CLB; CHOP; etoposide |
| CLL7 | C | IV | C | IV | CLB; cyclo + pred |
| CLL8 | A | I | A | I | None |
| CLL9 | A | 0 | A | 0 | CLB; flu |
| CLL10 | C | III | C | III | None |
| CLL11 | A | 0 | A | 0 | None |
| CLL12 | B | II | B | II | None |
| CLL13 | A | 0 | A | 0 | None |
| CLL14 | A | I | A | I | None |
| CLL15 | C | III | C | III | None |
| CLL16 | A | 0 | A | 0 | None |
| *CLL17 | B | II | C | IV | CLB; CHOP; flu |
| *CLL18 | A | I | B | II | CLB + flu |
| †CLL19 | A | I | A | I | None |
| ‡CLL20 | B | II | B | II | pred; CLB + pred; cyclo + vincristine + pred |
| ‡CLL21 | A | I | A | I | CLB + pred |
CLB indicates chlorambucil; CHOP, cyclophosphamide + doxorubicin + vincristine + predniolone; pred, prednisolone; flu, fludarabine; cyclo, cyclophosphamide.
Both PB cells and node were studied.
Only node examined.
Only spleen examined.
Cell preparation and culture
B-CLL cells and hypoxia treatment.
Peripheral blood (PB) from 18 patients with high count (white blood cell count greater than 100 × 109/L) was drawn into heparin-containing tubes (20 U/mL). PB lymphocytes were isolated by Ficoll-Hypaque (Lymphoprep, Oslo, Norway) density-gradient centrifugation and cryopreserved.
T cells and monocytes were always less than 5%, and, of the remaining cells, more than 95% were CD5+ CLL cells. To ensure that cryopreservation did not affect VEGF production, in 3 patients both fresh and cryopreseved cells were studied. In all cases, unstimulated and stimulated VEGF production by fresh and cryopreserved cells was fully comparable. To recover cells from liquid nitrogen, they were rapidly thawed in a 37°C water bath and slowly resuspended in 10 mL QBSF-51 serum-free medium (Sigma-Aldrich, Dorset, UK). After centrifugation (1600 rpm for 5 minutes), the cell pellets were resuspended in the same medium before use.
For hypoxic stimulation, B-CLL cells were cultured for 24 hours in a specially designed air-tight chamber (Billups-Rothenberg, Del Mar, CA) that was prewarmed at 37°C overnight. The chamber, constructed with inflow and outflow valves, was infused with a preanalyzed air mixture containing 5% CO2/95% N2 (BOC, Surrey, UK) to achieve the desired oxygen level.23 To determine their viability, the cells were incubated with 5 μg/mL propidium iodide for 20 minutes at 4°C and then analyzed by flow cytometry. Propidium iodide stains dead cells but not live cells.
Human umbilical vein endothelial cells.
Umbilical cords were generously provided through the maternity unit of Liverpool Women's Hospital (Liverpool, UK). Human umbilical vein endothelial cells (HUVECs) were detached with trypsin using a published protocol.24 Cells were cultured in 199 medium (Gibco Life Technologies, Paisley, UK) supplemented with 20% fetal calf serum, 1 pg/mL epidermal growth factor, 5 U/mL heparin, 100 U/mL penicillin, and 100 μg/mL streptomycin. HUVECs were used at passage 3.
Preparation of conditioned medium.
Unless stated otherwise, the conditioned medium (CM) was the culture supernatant harvested from B-CLL cells. The cells were cultured in QBSF-51 at a density of 2 × 106/well in 24-well plates for 24 hours under either normoxic or hypoxic conditions. CM was centrifuged at 1600 rpm for 10 minutes and stored at −20°C until assayed. To test whether CLL-cell–derived CM contained angiogenic factor(s), the CM harvested from the cells stimulated with phorbol 12-myristate 13-acetate (PMA, 100 ng/mL) was concentrated by ammonium sulfate precipitation and a Centricon (Millipore, Hertfordshire, UK) spin column before assay. As a control, QBSF-51 medium not exposed to cells (referred as the control medium) was concentrated in a similar fashion.
Tissues
CLL nodes (n = 3) were diagnostic samples taken from the neck; 2 of the 3 nodes were from patients (CLL17, CLL18) whose PB CLL cells were also studied for VEGF production. Clinical details of all the patients from whom this material was obtained are given in Table 1. Splenectomy material (CLL20, CLL21) had been removed for treatment of autoimmune hemolytic anemia secondary to CLL. Normal nodes (n = 3) were obtained from axillary clearances for breast cancer and were macroscopically and microscopically normal. “Normal” spleen was tissue removed for the treatment of idiopathic thrombocytopenic purpura (n = 2) or because of surgical trauma during laparotomy (n = 1). All tissues were formalin-fixed and paraffin-embedded.
Immunostaining
CD34 and VEGF immunohistochemistry.
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded tissues. Briefly, sections were deparaffinized, rehydrated, and boiled in 10 mmol/L sodium citrate (pH 6.0) for 10 minutes. Nonspecific antibody-binding sites were blocked by incubating the sections in 10% bovine serum albumin in TBS (20 mmol/L Tris/HCl [pH 7.6], 137 mmol/L NaCl) for 10 minutes at room temperature (RT). For the detection of VEGF, sections were incubated overnight at 4°C with either rabbit anti-hVEGF antibody (1:200 dilution in TBS) or with nonspecific rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). For blood vessel staining, sections were incubated overnight at 25°C with mouse anti-hCD34 mAb (Serotec, Oxford, UK; 1:10 dilution in TBS) or with nonspecific mouse IgG (Becton Dickinson, UK). The slides were then incubated with goat antirabbit IgG (for VEGF Ab) or rabbit antimouse IgG (for CD34 mAb) conjugated to biotin, followed by incubation with ExtrAvidin alkaline phosphatase (both 30 minutes at RT). Color was developed by incubating slides with Fast Red TR/Naphthol AS-MX phosphate (Sigma) at RT for 20 minutes. Slides were counterstained with hematoxylin.
The finding of 3 or more contiguous CD34+ cells was considered as a microvessel.25 Such microvessels were counted in 40 high-power (40×) fields. The fields were chosen at random in CLL nodes. In normal nodes, 20 follicular and 20 interfollicular fields in different areas of cortex were scored. Vessel scores were expressed as the mean ± SD per field.
Immunocytochemical staining for VEGF.
B-CLL cells (8 × 104) cultured for 24 hours with and without PMA (100 ng/mL) were cytocentrifuged at 300 rpm for 3 minutes using a Cytospin 2 (Shandon, Pittsburgh, PA) and fixed at 4°C with ice-cold acetone for 3 minutes. Fixed slides were washed with phosphate-buffered saline (PBS) and incubated with 3% H2O2 in PBS for one hour at RT. Cells were then incubated with polyclonal rabbit anti-hVEGF antibody (10 μg/mL diluted with 1% BSA/PBS) or with nonspecific rabbit IgG (Santa Cruz Biotechnology) for one hour at RT, followed by incubation with goat antirabbit IgG conjugated to biotin and with ExtrAvidin–horseradish peroxidase (both for 30 minutes at RT). Color was developed by incubating the slides with 3-amino-9-ethylcarbazol at RT for 3 minutes. Slides were counterstained with hematoxylin.
Enzyme-linked immunosorbent assay.
The CM (200 μL) harvested from CLL cells cultured under hypoxic or normoxic conditions was tested for the presence of soluble VEGF protein by a commercial human VEGF immunoassay kit (Quantikine) according to the manufacturer's protocol (R & D Systems, Abingdon, UK). With this method, we found that the minimum detectable dose of VEGF contained in CM was typically less than 5.0 pg/mL.
Western blot analysis.
The concentrated CM collected from PMA-stimulated cells was fractionated by electrophoresis on 10% SDS polyacrylamide gels under nonreducing conditions and electrotransferred to nitrocellulose membranes. After blocking with 5% nonfat powdered milk in TBS containing 0.1% Tween-20 (TBS-T), the membranes were incubated for one hour at RT with anti-hVEGF mAb (2 μg/mL; R & D Systems). After washing with TBS-T, the membranes were incubated for one hour at RT with horseradish peroxidase–conjugated goat antimouse IgG (Affiniti, Exeter). After an extensive rinsing with TBS-T, immunoreactive protein bands were visualized with a chemiluminescence-based procedure using the ECL detection kit according to the instructions of the manufacturer (Amersham, Little Chalfont, UK).
RNA isolation and reverse transcription–polymerase chain reaction.
Total RNA was extracted from CLL cells using an RNeasy kit (Qiagen, Hilden, Germany). Single-strand DNA was synthesized from 1 μg total RNA using the First-Strand cDNA Synthesis Kit for RT-PCR (Boehringer Mannheim, Mannheim, Germany). Amplification of the cDNA by PCR was performed using the following primers: oligo 1, 5′-TCGGGCCTCCGAAACCATGC-3′; and oligo 2, 5′-CCTGGTGAGAGATCTGGTTC-3′ (30 cycles, annealing at 56°C). To normalize the product, the actin gene was also amplified using actin primers: oligo 3, 5′-CGCTGCGCTGGTCGTCGACA-3′; and oligo 4, 5′-GTCACGCACGATTTCCCGCT-3′ (30 cycles, annealing at 60°C). The amplified PCR products were subjected to electrophoresis and visualized by ethidium bromide staining. To confirm that the sequences of the products amplified by the PCR reaction represented different VEGF isoforms, the PCR products were cloned into pBlueScript plasmid (Stratagene, Cambridge, UK), and their sequences were determined by nucleotide sequence analysis using a DNA Sequencing kit (Amersham).
Slot-blot hybridization.
Total RNA (2 μg) extracted from unstimulated cells was loaded directly onto a nylon membrane using a slot-blot apparatus (Bio-Rad, Hercules, CA). Membranes were UV cross-linked and subsequently hybridized with a VEGF cDNA probe labeled with α-32P]-deoxycytidine triphosphate using a random-primed DNA labeling kit (Boehringer Mannheim). For control of the RNA loading of each lane, blots were rehybridized with a radiolabeled glyceraldehyde 3-phosphate dehydrogenase cDNA probe (Clontech Laboratory, Hampshire, UK). Autoradiography was performed at −70°C using Kodak XAR-5 film (Sigma). The intensity of each band appearing on the film was scanned and analyzed with Phoretix 1D-advanced version 3.1 software.
Proliferation assay.
HUVECS were plated at a density of 2 × 104/well in 24-well gelatin-coated plates and grown to approximately 90% confluence in the growth medium. The following day, the medium was removed from the wells, and the cells were washed twice with PBS and incubated for another 24 hours in 199 medium containing 5% fetal calf serum without heparin or EGF. Then the medium was replaced with the fresh medium containing 10% (vol/vol) concentrated CM derived from CLL cells or rhVEGF 165 protein (R & D Systems). In addition, the CM was preincubated for 1 hour at 22°C with a neutralizing anti-hVEGF MoAb (1 μg/mL, R & D Systems) before addition to the cultures. After 48 hours, the cells were incubated for 3 hours with3H-thymidine (0.5 μCi/well), the medium was aspirated, and the cells were washed twice with ice-cold PBS. Ice-cold 5% TCA was added to each well (0.5 mL/well), and the plates were placed at 4°C. After overnight incubation, TCA was aspirated, and cell monolayers were washed once with TCA and twice with 95% ethanol. To each dried well, 0.2 mol/L NaOH (0.5 mL/well) was then added to dissolve TCA-insoluble materials (37°C, 2 hours with gentle shaking). The samples (0.3 mL) were counted to determine the amount of 3H-thymidine taken up by the cells using a Tri-CARB Liquid Scintillation Analyzer (Packard, England).
Chick chorioallantoic membrane assay.
The chick chorioallantoic membrane (CAM) assay26 was used to determine whether the CLL cell–derived CM had angiogenic activity. To expose CAM, a window was created in the shells of 10-day-old fertilized chicken eggs. Filter paper disks soaked in test CM were placed on exposed CAMs. Disks soaked in rhVEGF 121 (10 ng in 10 μL; R & D Systems) or in control medium were used as positive and negative controls, respectively. The embryos were incubated at 37°C in a humidified egg incubator. CAMs were analyzed after 72 hours using a stereomicroscope. The density of branching blood vessels infiltrating under the disks was scored as follows: 0, negative; 0.5, change in vessel architecture but not directed to the point of sample application; 1, partial spoke-wheel (one third of the circumference exhibits directional angiogenesis); 2, spoke-wheel; 3, strong and full spoke-wheel. For photography, the membranes were fixed in situ with ice-cold 4% paraformaldehyde–PBS that was injected both from above and below the membrane. Membranes were excised, placed on a fresh microscope slide, and photographed under a Leitz binocular dissecting microscope and indirect fiberoptic illumination. Statistical analysis was performed using the Mann-Whitney U test.
Results
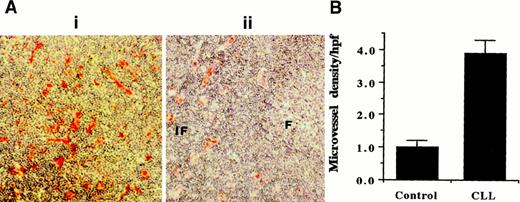
High blood vessel density is observed in lymph nodes infiltrated by B-CLL cells. Blood vessel density in lymph nodes from patients with B-CLL (n = 3) and healthy control donors (n = 3) was examined by using CD34 as an endothelial cell marker. In CLL, the normal architecture of the enlarged nodes is completely effaced by infiltrating CLL cells. Figure 1, panel Ai shows representative CD34 staining of a CLL node, indicating that vascular development accompanies the malignant cell infiltration and node enlargement. In contrast, in normal node (Figure 1Aii), only the interfollicular areas are vascularized, whereas follicular B-cell areas display little vascularization. This is quantitatively illustrated in Figure 1, panel B. These results indicate that, as proposed before for CLL bone marrow,18 angiogenesis may be required to supply oxygen and nutrients to the enlarged nodes often seen in CLL patients with tissue disease.
Vessel density analysis using CD34 mAb staining.
(Ai) Representative CD34 staining of CLL node. (Aii) CD34 staining of normal node where interfollicular (IF) areas are vascularized, and follicular (F) areas display very little vascularization. Original magnification, × 10. (B) Quantitative analysis shows the higher vessel density in CLL nodes (n = 3) compared with follicular B-cell areas of normal nodes (n = 3).
Vessel density analysis using CD34 mAb staining.
(Ai) Representative CD34 staining of CLL node. (Aii) CD34 staining of normal node where interfollicular (IF) areas are vascularized, and follicular (F) areas display very little vascularization. Original magnification, × 10. (B) Quantitative analysis shows the higher vessel density in CLL nodes (n = 3) compared with follicular B-cell areas of normal nodes (n = 3).
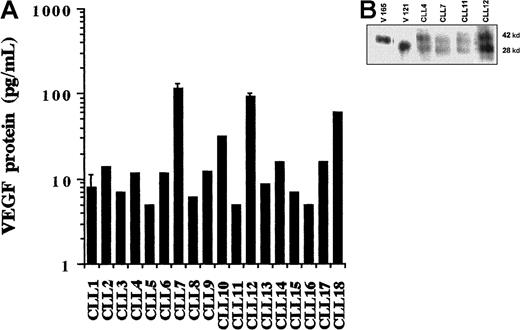
Both circulating and tissue-phase CLL cells produce VEGF protein. In view of the importance of tumor cell–derived VEGF in solid tumor angiogenesis,27 we next investigated the possible production of VEGF protein by B-CLL cells. After 24-hour incubation at 37°C, the CM was collected and examined for VEGF protein using an enzyme-linked immunosorbent assay (ELISA). Secreted VEGF was detected in all cases as demonstrated in Figure 2, panel A. This result shows that all tested B-CLL cells are capable of constitutively synthesizing and secreting variable amounts of VEGF (5-116 pg/8 × 106 cells/mL). Because of the relatively small number of patients studied and because of their heterogeneity with regard to disease stage and duration, no attempt was made to correlate levels of VEGF production with clinical parameters.
Detection of VEGF protein.
(A) ELISA measurement of secreted VEGF. Levels of VEGF in the CM collected from unstimulated CLL cells were measured using a commercial ELISA kit. The amount of VEGF protein in the samples was calculated using a reference curve established from serial dilutions of rhVEGF protein. Individual histograms represent the means of duplicates. Error bars are standard deviations calculated from 6 measurements in 3 separate experiments using the cells of 3 different patients. (B) Western blot analysis of secreted VEGF. CM was collected from cultures of the PMA-stimulated cells and subsequently concentrated by 10-fold. Each sample (100 μL) was loaded onto a 10% polyacrylamide gel and electrophoresed under nonreducing conditions. Separated proteins were transferred to a nitrocellulose membrane, and VEGF protein was detected with anti-hVEGF mAb and visualized by enhanced chemiluminescence.
Detection of VEGF protein.
(A) ELISA measurement of secreted VEGF. Levels of VEGF in the CM collected from unstimulated CLL cells were measured using a commercial ELISA kit. The amount of VEGF protein in the samples was calculated using a reference curve established from serial dilutions of rhVEGF protein. Individual histograms represent the means of duplicates. Error bars are standard deviations calculated from 6 measurements in 3 separate experiments using the cells of 3 different patients. (B) Western blot analysis of secreted VEGF. CM was collected from cultures of the PMA-stimulated cells and subsequently concentrated by 10-fold. Each sample (100 μL) was loaded onto a 10% polyacrylamide gel and electrophoresed under nonreducing conditions. Separated proteins were transferred to a nitrocellulose membrane, and VEGF protein was detected with anti-hVEGF mAb and visualized by enhanced chemiluminescence.
Because PMA is known to stimulate VEGF production in other cell types,28 we examined whether basal VEGF secretion by CLL cells could be increased by phorbol ester stimulation. PMA increased secreted VEGF levels from 49 ± 59 pg/mL to 266 ± 110 pg/mL (n = 7). Therefore, PMA stimulation was used to obtain enough CLL-cell–derived VEGF to be readily detectable by Western blot analysis (after 10-fold concentration). As shown in Figure 2, panel B, Western blotting using an anti-hVEGF mAb revealed 2 bands with molecular weights of 28 and 42 kd. These are the only known secreted isoforms of the cytokine encoded by the VEGF121 and 165 mRNA species demonstrated below (by RT-PCR) in unstimulated CLL cells.
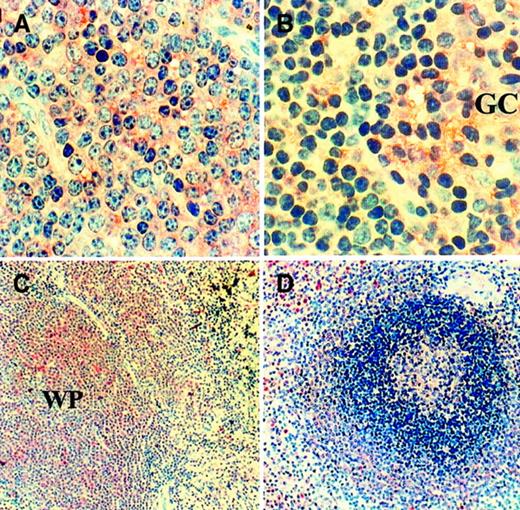
We then tested whether CLL cells that have infiltrated lymphoreticular tissues produce VEGF protein. Immunohistochemical staining (Figure3) showed that CLL cells infiltrating lymph node (A) and spleen (C) are positive for VEGF protein. CLL cells were uniformly positive, and the intensity of staining was weaker than that of any neutrophils present but stronger than that of the unstimulated PB CLL cells as described below. Figure 3 also shows positive staining of B-cell areas of normal node (B) and spleen (D).
Immunohistochemical staining of VEGF protein.
The presence of VEGF protein in CLL nodes (n = 3; CLL17 shown) and spleen (n = 2; CLL20 shown), together with corresponding normal tissues (n = 3), was examined using anti-VEGF antibody staining. Infiltrating cells in CLL lymph node (A) are all positive. Many cells in normal node (B) are also positive, particularly those in the germinal center (GC) of the follicles. (Original magnification, × 40.) CLL cells expanding the white pulp (WP) of the spleen (C) are also uniformly positive. In contrast, in normal spleen (D), the different layers of the white pulp show various degrees of positivity that was stronger in the marginal zone and follicle centers. (Original magnification, × 10.)
Immunohistochemical staining of VEGF protein.
The presence of VEGF protein in CLL nodes (n = 3; CLL17 shown) and spleen (n = 2; CLL20 shown), together with corresponding normal tissues (n = 3), was examined using anti-VEGF antibody staining. Infiltrating cells in CLL lymph node (A) are all positive. Many cells in normal node (B) are also positive, particularly those in the germinal center (GC) of the follicles. (Original magnification, × 40.) CLL cells expanding the white pulp (WP) of the spleen (C) are also uniformly positive. In contrast, in normal spleen (D), the different layers of the white pulp show various degrees of positivity that was stronger in the marginal zone and follicle centers. (Original magnification, × 10.)
VEGF production in CLL samples is not due to contamination by non-CLL cells. It has been shown that many hemic-cell types (eg, T lymphocytes,29 monocytes,30 and platelets31) contain VEGF protein. Therefore, it was important to verify that the observed production of VEGF protein in CLL samples was not due to contamination with other cell types. To address this, we used immunocytochemical staining to examine the VEGF protein positivity of the cells in the CLL samples used. The purity of CLL cells in all samples was greater than 95%. The staining showed that all unstimulated cells stained uniformly weakly for VEGF protein and that no minority population of strongly positive cells was observed. After stimulation with PMA, all CLL cells showed a substantial increase in VEGF positivity. These results (data not shown) indicated that the VEGF protein detected in the CLL cell cultures was produced by the CLL cells themselves.
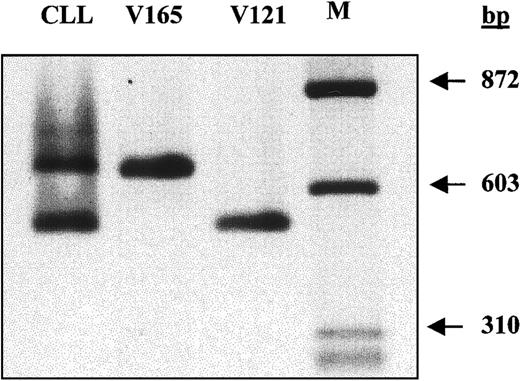
B-CLL cells express 2 smaller VEGF transcripts. There are 5 different VEGF mRNA isoforms generated from the VEGF gene by alternative splicing mechanisms.32-34 To investigate whether isoforms additional to the 2 detected by Western blotting are expressed in unstimulated CLL cells, both RT-PCR and nucleotide sequence analysis were performed. As shown in Figure4, only 2 bands were amplified from CLL cells, and they were identical in size to control VEGF 121 and 165 cDNA. Furthermore, nucleotide sequence analysis confirmed that the top band was VEGF 165 and the bottom band was VEGF 121 (data not shown).
Analysis of VEGF mRNA isoforms by RT-PCR.
First-strand cDNA was transcribed from 1 μg total RNA extracted from unstimulated cells. One tenth of the synthesized cDNA was amplified by PCR using primers recognizing all possible VEGF isoforms. VEGF 165 and VEGF 121 cDNAs were included in the PCR reaction as the positive controls.
Analysis of VEGF mRNA isoforms by RT-PCR.
First-strand cDNA was transcribed from 1 μg total RNA extracted from unstimulated cells. One tenth of the synthesized cDNA was amplified by PCR using primers recognizing all possible VEGF isoforms. VEGF 165 and VEGF 121 cDNAs were included in the PCR reaction as the positive controls.
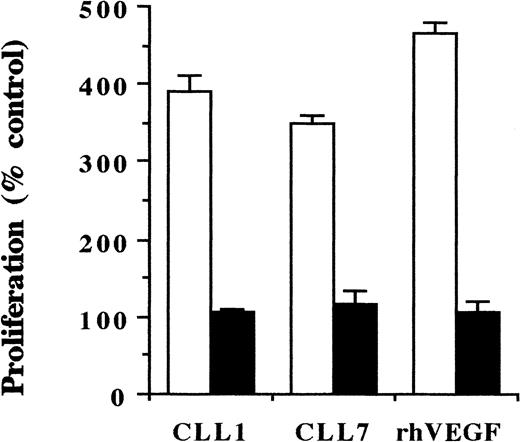
VEGF produced by CLL cells stimulates proliferation of endothelial cells. To examine whether the VEGF secreted by CLL cells stimulates endothelial cell proliferation, HUVECS were incubated for 48 hours with CM from CLL cell cultures, and cell proliferation was assessed by3H-thymidine uptake. As shown in Figure5, CLL-cell–derived CM increased endothelial cell proliferation by 3- to 4-fold. The addition of an anti-hVEGF neutralizing mAb completely blocked this growth-stimulatory effect. This demonstrates that the mitogenic effect of CLL-cell–derived CM is principally mediated by VEGF.
Proliferation of endothelial cells in response to CLL-cell–derived CM.
HUVECS were cultured in the presence of CM derived from the PMA-stimulated cells of 2 CLL patients (CLL1, CLL7), using rhVEGF protein as a positive control (■). In the same experiment, the CM was preincubated with a neutralizing anti-hVEGF mAb for 1 hour at RT before addition to endothelial cell cultures (▪). After 48 hours, proliferation was assessed by 3H-thymidine incorporation. Proliferation of cells cultured in the medium without CM was considered as 100%; blocking anti-hVEGF mAb had no effect on this proliferation.
Proliferation of endothelial cells in response to CLL-cell–derived CM.
HUVECS were cultured in the presence of CM derived from the PMA-stimulated cells of 2 CLL patients (CLL1, CLL7), using rhVEGF protein as a positive control (■). In the same experiment, the CM was preincubated with a neutralizing anti-hVEGF mAb for 1 hour at RT before addition to endothelial cell cultures (▪). After 48 hours, proliferation was assessed by 3H-thymidine incorporation. Proliferation of cells cultured in the medium without CM was considered as 100%; blocking anti-hVEGF mAb had no effect on this proliferation.
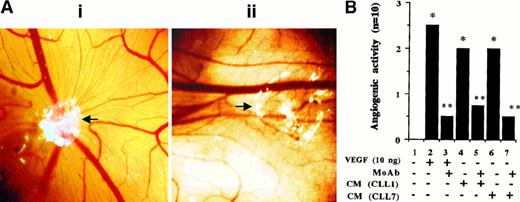
VEGF produced by CLL cells enhances angiogenesis. To test whether CLL-cell–secreted VEGF affects in vivo angiogenesis, the CAM assay was used with angiogenesis by rhVEGF 121 as a positive control. Results were assessed visually (Figure6A) and measured semiquantitatively (Figure 6B), as described in “Materials and methods.” The rhVEGF 121 induced a strong angiogenic response that was neutralized by an anti-hVEGF neutralizing antibody. CLL-cell–derived CM induced moderate angiogenesis that was also prevented by the neutralizing anti-hVEGF antibody. This indicates that CLL-cell–derived CM does have angiogenic activity and that VEGF contained in these supernatants is the major angiogenic factor.
Angiogenic responses of CAM to CM derived from CLL cells.
(A) Representative angiogenic response and its inhibition by a blocking anti-hVEGF mAb. The white area represents the site of application of filters containing either CM (collected from the PMA-stimulated cells) alone (i) or the same CM preincubated with anti-hVEGF MoAb for 2 hours at RT (ii). Microvessels radiating from the site of sample application in (i) are not formed in the presence of the blocking mAb (ii). (Original magnification, × 8.) (B) The semiquantitative data obtained by using rhVEGF and CM collected from PMA-stimulated cells from 2 patients. The grading of the angiogenic response is described in “Materials and methods.” *P < .05 vs control andP < .05 vs sample without the neutralizing antibody (Mann-Whitney U test). Each bar represents the score of 10 CAM used in 2 separate assays with similar results.
Angiogenic responses of CAM to CM derived from CLL cells.
(A) Representative angiogenic response and its inhibition by a blocking anti-hVEGF mAb. The white area represents the site of application of filters containing either CM (collected from the PMA-stimulated cells) alone (i) or the same CM preincubated with anti-hVEGF MoAb for 2 hours at RT (ii). Microvessels radiating from the site of sample application in (i) are not formed in the presence of the blocking mAb (ii). (Original magnification, × 8.) (B) The semiquantitative data obtained by using rhVEGF and CM collected from PMA-stimulated cells from 2 patients. The grading of the angiogenic response is described in “Materials and methods.” *P < .05 vs control andP < .05 vs sample without the neutralizing antibody (Mann-Whitney U test). Each bar represents the score of 10 CAM used in 2 separate assays with similar results.
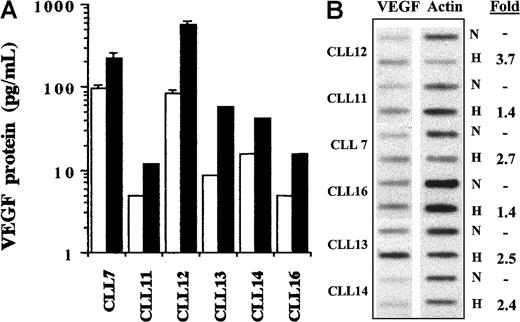
Hypoxia enhances the levels of VEGF mRNA and protein in CLL cells. One known mechanism of VEGF gene regulation is the low oxygen tension (hypoxia) often detected in rapidly expanding tissues.22An increase in VEGF production and resultant angiogenesis in response to hypoxia are presumably required to allow adequate metabolic support of expanding tissues, including infiltrated CLL lymphoreticular tissues. Because CLL cells are the principle cells in these organs, we investigated whether they respond to hypoxia by up-regulating their levels of VEGF mRNA and protein. After 24 hours, the percentage of viable cells cultured under normoxic and hypoxic conditions was 79% ± 6% and 86% ± 3%, respectively (n = 3; viability determined by propidium iodide staining and FACS analysis). The CM collected from the cultured cells was subjected to ELISA assay. Results demonstrated that hypoxia elevated VEGF protein levels to 7-fold in all patients with CLL (n = 6; Figure 7A). In addition, hypoxia also increased the level of VEGF mRNA in each sample as determined by slot-blot hybridization (Figure 7B).
VEGF production by CLL cells in response to hypoxic stimulation.
(A) CLL cells from 6 patients were cultured under normoxic and hypoxic conditions. After 24 hours, CM was collected and subjected to ELISA. Higher levels of VEGF protein were detected in the cells cultured under hypoxic conditions (▪) than in those grown under normoxic conditions (■). Histograms represent the mean of duplicates. Error bars are standard deviations calculated from 6 measurements in 3 separate experiments using the cells of 2 patients. (B) Cells from the same patients used for ELISA were also subjected to cellular VEGF mRNA determination by slot-blot hybridization. Hypoxia (H) compared with normoxia (N) increased VEGF mRNA expression in all cases. The bands were measured densitometrically, and the sample loading was determined by parallel measurement of the actin mRNA in each sample.
VEGF production by CLL cells in response to hypoxic stimulation.
(A) CLL cells from 6 patients were cultured under normoxic and hypoxic conditions. After 24 hours, CM was collected and subjected to ELISA. Higher levels of VEGF protein were detected in the cells cultured under hypoxic conditions (▪) than in those grown under normoxic conditions (■). Histograms represent the mean of duplicates. Error bars are standard deviations calculated from 6 measurements in 3 separate experiments using the cells of 2 patients. (B) Cells from the same patients used for ELISA were also subjected to cellular VEGF mRNA determination by slot-blot hybridization. Hypoxia (H) compared with normoxia (N) increased VEGF mRNA expression in all cases. The bands were measured densitometrically, and the sample loading was determined by parallel measurement of the actin mRNA in each sample.
Discussion
During the course of their illness, many patients with CLL display prominent lymphoreticular tissue enlargement in addition to blood and bone marrow involvement. The accumulation of malignant cells and the consequent expansion of secondary lymphoid tissues demand an increased blood supply. Thus, angiogenesis may be required for the metabolic support of the expanding tissues. In fact, higher blood vessel density has been observed in CLL than in normal bone marrow.18Here, we demonstrate that neovascularization also takes place in CLL nodes and that VEGF produced by the CLL cells themselves is likely to be the principle angiogenic factor.
Our immunohistochemical studies showed that CLL nodes, in which the normal architecture is completely disrupted by the infiltrating malignant cells, are uniformly vascularized. This indicates that new blood vessels are formed as the node expands. The vessel density in the CLL nodes was comparable to that observed in the interfollicular areas of normal nodes, whereas the follicular B-cell areas of normal nodes displayed a very low vessel density. This is likely to reflect the fact that most follicular B cells undergo apoptosis and that the remaining antigen-selected cells migrate out of the follicle. In contrast, the chronic nature of CLL node enlargement indicates the more permanent nature of the infiltrate requiring neovascularization. Because the overwhelming majority of cells in CLL nodes are malignant B cells, we postulated that the angiogenic stimulus may be provided by the CLL cells themselves.
In considering possible angiogenic factors, we chose to focus on VEGF. VEGF is a multifunctional protein that, on binding to its receptors on endothelial cells, affects vascular permeability,35-37cell proliferation,38,39 migration,40 and survival,41,42 all of which are required for angiogenesis. Among the variety of cytokines with angiogenic or endothelial cell–activating properties, or both, VEGF is considered the most predominant, direct, and selective.5-12,43,44 Therefore, we first examined the possible production of VEGF protein by B-CLL cells. We found, by ELISA and immunocytochemical staining, that B-CLL cells already constitutively produce measurable amounts of VEGF under in vitro culture conditions and that this production was increased by PMA stimulation. Moreover, because low oxygen tension is often detected in expanding tissues22 and enlargement of lymph node infiltrated by CLL cells is often clinically observed in patients with tissue disease, we have examined whether hypoxia up-regulates VEGF expression by PB CLL cells. Our observation revealed that the rate of VEGF secretion in these cells was indeed increased up to 7-fold in response to hypoxic stimulation. The in vivo relevance of these observations was supported by our demonstration of abundant VEGF protein associated with CLL cells within highly infiltrated lymph node and spleen.
Five human VEGF mRNA species encoding VEGF isoforms of 121-, 145-, 165-, 189- and, 206-aa peptides are produced by alternative splicing of the VEGF pre-mRNA.32-34 An important biologic property that distinguishes the different VEGF isoforms is their heparin- and heparan-sulfate–binding ability. VEGF 121 lacks the amino acids encoded by exons 6 and 7 of the VEGF gene32 and does not bind to heparin or extracellular matrix.45 VEGF 165 contains the addition of a 44 aa-long peptide encoded by exon 7 of the VEGF gene and shows weak affinity to heparin and heparan sulfate.45 Thus, these 2 isoforms are generally considered as the secreted cytokine. Our RT-PCR studies showed that VEGF 121 and VEGF 165 are the predominant isoforms expressed in unstimulated B-CLL cells cultured in vitro; they encode the 28- and 42-kd secreted forms of the cytokine that we detected by Western blotting. Therefore, we focused our investigations on the effects on endothelial cells of the secreted VEGF found in CLL-cell culture supernatants. The quantitative in vitro proliferation assay showed that CLL-cell–derived supernatants stimulated HUVEC proliferation and blocking by a specific mAb showed that the VEGF contained in the supernatant was the major mitogenic factor. However, these results do not necessarily mean that the supernatant would have the potential to stimulate in vivo angiogenesis because the latter is a complex process involving multiple in vivo steps.3 For this reason, we also used the CAM assay to demonstrate the angiogenic effect of the CLL-cell–derived supernatants in vivo. The results of both assays implicate CLL-cell–secreted VEGF in angiogenesis within lymphoreticular tissues such as node.
It is known that a wide range of malignant cells produce VEGF.46 Although the role of this cytokine in the angiogenesis-dependent growth of solid tumors is well established,47 its importance for the survival of different types of leukemic cells is less clear. In CLL, high blood vessel density in bone marrow has recently been noted and related to high levels of bFGF.18 Very recently, VEGF has been measured in the serum of patients with CLL48 and has also been demonstrated in the cells themselves.49 In these studies, low cellular and high serum levels of VEGF were related to an adverse prognosis.48 49
In conclusion, we demonstrate here, using several different techniques, that CLL cells produce and secrete VEGF. We also establish that the predominantly expressed mRNA isoforms are VEGF 121 and VEGF 165, which encode the secreted 28- and 42-kd protein isoforms of the cytokine. Furthermore, we show that VEGF is produced by both circulating and tissue-phase CLL cells, and we provide direct evidence of the angiogenic effects of CLL-cell–derived VEGF. We propose that this angiogenic effect is likely to be important for the expansion of lymphoreticular tissues in CLL. However, because VEGF has multiple biologic effects and because we have shown that CLL cells express VEGF receptors (unpublished data), the cytokine may also have important autocrine effects on different aspects of CLL-cell behavior, such as transmigration from blood to tissues and prolonged CLL-cell survival.
Acknowledgments
The authors thank Dr Roy Bicknell and ZENECA Pharmaceuticals for providing the VEGF 121 and VEGF 165 cDNAs and Peter Baker for the scanning analysis.
Supported in part by the Leukemia Research Fund and the North West Cancer Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Haijuan Chen, Department of Haematology, University of Liverpool, PO Box 147, Liverpool L69 3BX, United Kingdom; e-mail: hjchen@liverpool.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal