Abstract
Dendritic cells (DCs) are professional antigen-presenting cells that are highly effective adjuvants for immunizing against pathogens and tumor antigens. The potential merit of genetic approaches to loading DCs with antigens is to express high and sustained levels of proteins that can be subsequently processed and presented to T lymphocytes. Replication-defective oncoretroviruses are able to efficiently transduce CD34+ progenitor-derived DCs but not monocyte-derived DCs. Here, it is shown that efficient gene transfer is obtained using a human immunodeficiency virus-1–derived lentiviral vector deleted of all structural and accessory genes. Infection of immature DCs with the lentiviral vector at a multiplicity of infection of 20 resulted in stable gene expression in 30% to 40% of the matured DCs. Proviral DNA was detectable by Alu polymerase chain reaction for the lentiviral but not the oncoretroviral vector. Most importantly, it is demonstrated that lentivirus-transduced DCs were fully functional and effectively activated autologous HLA A2.1+ peripheral blood cytotoxic T lymphocytes (CTLs). DCs expressing lentiviral vector-encoded Flu peptide were at least as efficient as DCs pulsed with the same peptide in stimulating specific CTLs. The efficacy of the lentivirus-transduced DCs was further demonstrated by their ability to directly activate freshly harvested peripheral blood Flu-specific CTLs in the absence of CD4+ T-cell help and exogenous cytokines. The availability of a stable gene delivery system based on a multiply attenuated lentivirus that does not encode any viral protein and that allows sustained antigen presentation by DCs derived from blood monocytes will be very useful for the biologic investigation of DCs and the improvement of immunotherapeutic strategies involving DCs.
Introduction
Dendritic cells (DCs) are specialized antigen-presenting cells with a remarkable ability to stimulate naive T lymphocytes and generate memory T lymphocytes. Immature DCs effectively take up antigen and upon maturation up-regulate expression of a number of molecules critical for effective antigen presentation.1DCs are thus currently viewed as ideal adjuvants for immunizing against pathogens or tumor antigens.2 3 There are nonetheless a number of unanswered questions about how to best use DCs for these purposes. The source of DCs, their requirements for differentiation and activation, and approaches to load them with antigen are important biologic parameters that will determine the efficacy of DC-based immunization.
Genetic approaches to antigen loading, based on the transduction of DCs with complementary DNA or messenger RNA encoding tumor antigens, offer several advantages over pulsing with peptide or cellular extracts. A set of selected antigens can be expressed at high levels in the DCs; antigens can be processed into HLA-restricted peptides irrespective of the HLA type of each individual and without prior knowledge of the peptide best suited for that particular HLA; and antigen presentation should be more sustained if the antigen is expressed endogenously rather than pulsed. Stable gene transfer can be achieved with viral vectors that integrate in host cell chromosomes. We and others have successfully used oncoretroviral vectors based on murine leukemia viruses (MLVs) to genetically modify CD34+ DC progenitors from various sources (eg, bone marrow, cord blood, and cytokine-elicited peripheral blood).4-9 Because oncoretroviral vectors require cell division for efficient transduction,10-12 their efficacy is restricted to DC progenitor cells that proliferate before terminal differentiation. This approach is thus not applicable to DCs generated from peripheral blood mononuclear cells (PBMCs), a common and practical source of DCs, because they do not show any substantial proliferation.13-15 Lentiviruses, on the other hand, are able to stably integrate their provirus in certain nondividing cells.16,17 Wild-type human immunodeficiency virus (HIV)-1 in particular is capable of infecting blood-derived DCs in the absence of cell proliferation.13 Thus, lentivirus-mediated gene transfer could offer the unique opportunity to investigate the biologic activity of DCs differentiating from blood monocytes if efficient transduction can be accomplished without impairing DC biologic activity.
However, the safe use of recombinant lentivirus requires attenuated vectors containing a minimum of viral sequences. We have therefore investigated whether efficient gene transfer in human DCs derived from PBMCs could be obtained using an HIV-1–derived lentiviral vector deleted of gag, pol, env, tat, rev, nef,vif, vpu, and vpr. We compared the lentiviral vector to an MLV-based oncoretroviral vector, both pseudotyped with the vesicular stomatitis virus G protein (VSV-G) and used under the same conditions. The lentiviral vector mediated stable gene transduction of human monocyte-derived DCs, whereas the oncoretroviral vector did not. Most importantly, we show that transduction with the lentiviral vector did not impair DC function in terms of induction of allogeneic T-cell proliferation and of a peptide-specific cytolytic response. To further demonstrate that lentivirus-transduced DCs could efficiently present a defined antigen to autologous T lymphocytes, we compared DCs pulsed with the HLA A2.1-restricted Flu peptide to DCs transduced with a vector encoding the corresponding sequence. In this manner, the vector-encoded peptide did not require antigen processing to be presented and was not part of a larger protein that could encode additional T-cell epitopes including T-helper epitopes. Here we show that the lentivirus-transduced DCs stimulated CD8+ T cells thoroughly depleted of CD4+ T cells at least as efficiently as the peptide-pulsed DCs. Thus, the Flu-specific T-cell response was not dependent on CD4+ T-helper cells, demonstrating the potential of DCs transduced with a lentiviral vector to directly stimulate cyotoxic T lymphocytes.
Materials and methods
Lentiviral vector construction and production
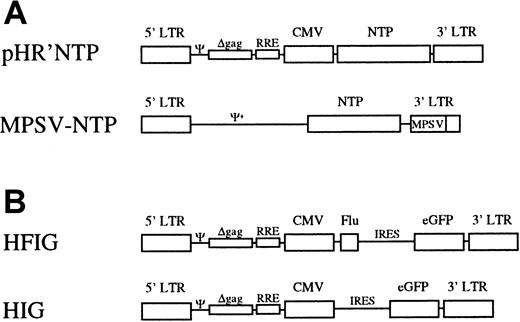
The lentiviral vector pHR'NTP was derived from pHR'CMVlacZ 18 by replacing theXbaI-XhoI fragment encoding the lacZ gene with the 0.8-kilobase (kb) XbaI-SalI fragment encoding the truncated human low-affinity nerve growth factor receptor, termed NTP.19 The vector HIG was constructed by inserting theBamHI-NcoI fragment containing the internal ribosomal entry site (IRES) of the encephalomyocarditis virus20 and the NcoI-XhoI fragment encoding the enhanced green fluorescence protein (eGFP; Clontech, Palo Alto, CA) into the BamHI and XhoI sites of pHR'CMVlacZ. The dicistronic vector HFIG derived from HIG by inserting the NcoI-EcoRI fragment containing the gene encoding the Flu peptide fused to the human CD8α leader sequence21 into the NcoI and EcoRI sites between the cytomegalovirus (CMV) promoter and the IRES element.
The lentiviral vector particles were produced by calcium phosphate transfection into 293T cells (American Type Culture Collection [ATCC], Rockville, MD) as previously described.22,23Briefly, 1 × 107 293T cells were seeded in 15-cm diameter dishes 24 hours prior to transfection in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Herndon, VA) with 10% heat-inactivated fetal calf serum (FCS; Hyclone, Logan, UT), penicillin (100 IU/mL), and streptomycin (100 μg/mL) in a 5% CO2incubator. A total of 58 μg plasmid DNA was used for the transfection of one dish: 12 μg pMD.G,24 24 μg packaging plasmid pCMVΔR8.925 and 22 μg vector plasmid. The precipitate was formed by adding the plasmids to a final volume of 630 μL of 1 mM Tris and 630 μL of 0.5 M CaCl2, mixing well. The DNA mix was then added dropwise to 630 μL of 2 × HEPES-buffered saline (281 mM NaCl, 100 mM HEPES, 1.5 mM Na2HPO4 [pH 7.07-7.12]) while vortexing. The precipitate was immediately added to the medium with gently swirling. The medium was replaced after 8 to 16 hours with fresh DMEM (20 mL) lacking FCS. The viral supernatants were collected 24 hours later, centrifuged for 10 minutes at 1200 rpm, and filtered through 0.45 μm low-protein binding filters (Millipore, Bedford, MA). The viral supernatants were concentrated 100 × by ultracentrifugation (50 000g, 1.5 hours, 4°C) as previously described,19 and the viral pellets were resuspended in DMEM or TNE (50 mM Tris, pH7.8; 130 mM NaCl; 1 mM ethylenediaminetetraacetic acid). The titer of the concentrated viral vectors was determined by infecting 1 × 105 HeLa cells in 6-well plates with serial dilutions of each vector in the presence of polybrene (Sigma, St. Louis, MO) at 8 μg/mL in a volume of 2 mL. Efficiency of transduction was determined by flow cytometry analysis (FACS) as previously described.19
Oncoretroviral vector construction and production
The oncoretroviral vector myeloproliferative sarcoma virus (MPSV)-NTP was generated by subcloning the NTP gene into an MFG-derived vector that contains a chimeric MLV/MPSV long terminal repeat (LTR).26,27 A high-titer producer clone was generated by transfection of gpg29 packaging cells24 with the MPSV-NTP vector DNA as described.28
Generation of dendritic cells
Peripheral blood was obtained from normal HLA A2.1+donors in heparinized tubes. HLA typing was performed by polymerase chain reaction (PCR) in the HLA laboratory at Memorial Sloan-Kettering Cancer Center (New York, NY). PBMCs were isolated by density gradient centrifugation on lymphocyte separation medium (Accurate Chemical & Scientific, Westbury, NY). DCs were generated as described.29,30 Briefly, the T-cell–depleted (ER−) population was prepared by rosetting with sheep red blood cells (Colorado Serum Company, Denver, CO) as described.14 A total of 2 × 106ER− cells per well were plated in 6-well plates. Granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex, Seattle, WA) and interleukin (IL)-4 (R & D Systems, Minneapolis, MN) were added at 1000 U/mL every second day for 7 days. Monocyte-conditioned medium was prepared by adding 50 × 106 ER− cells on Petri dishes coated with human gamma globulins (Sigma) at 10 mg/mL. Nonadherent cells were removed and the monocyte-conditioned medium, which was collected after 24 hours, was added (one half or one third of the final volume) to the cells for 4 days to get fully mature DCs.
T-cell purification and CD4 depletion of purified T cells
T cells were purified as described.21,31 Briefly, the T-cell–enriched (ER+) population was collected from the same donors. After lysis of the rosetted sheep red blood cells and 3 washes in phosphate-buffered saline with 2% FCS, B cells, natural killer cells, monocytes-macrophages, and activated T cells were depleted. This was accomplished by incubating cells with mouse immunoglobulin G (IgG) monoclonal antibodies directed against CD11b, CD16, and HLA-DP, -DQ, -DR (Pharmingen, San Diego, CA) at 1 μg per 1 × 106 cells for 30 minutes, followed by a panning on Petri dishes coated with goat antimouse IgG (Caltag, Burlingame, CA) as described.32 After 3 washes in phosphate-buffered saline with 2% FCS, the T cells were resuspended at a final concentration of 1 × 107 cells/mL. These resulting T cells were more than 99% pure (data not shown). For the experiments with CD4-depleted T cells, purified T cells were incubated with mouse IgG1 monoclonal antibody directed against CD4 (Leu-3a; Monoclonal Antibody Core Facility at Memorial Sloan-Kettering Cancer Center) at 1 μg per 1 × 106 cells for 30 minutes. Panning on Petri dishes was performed as described.32 CD4 depletion, after 3 or 4 rounds of panning, was more than 99%.
Culture conditions
DCs and T lymphocytes were maintained in RPMI 1640 (Mediatech) with 10% FCS. Penicillin at 100 U/mL and streptomycin at 100 μg/mL were added to all the cultures.
Viral transduction of dendritic cells
DCs were infected at a multiplicity of infection (MOI) of 2 to 20 in 6-well plates with 1 × 106 to 2 × 106 DCs per well in a volume of 2 mL per well. The cells were infected for 8 to 16 hours at 37°C in the presence of 4 μg/mL polybrene. GM-CSF and IL-4 were added at 1000 U/mL. After infection the DCs were washed and resuspended in fresh RPMI with 10% FCS and cytokines. Four days after infection, genomic DNA was extracted for Southern blot analysis.28
Alu PCR
Genomic DNA was extracted from the HeLa cells or DCs 4 days after exposure to the viral vectors pHR'NTP and MPSV-NTP. Junctions between viral and genomic sequences were amplifed by using oligonucleotides specific for the conserved sequences of the human Alu element (3′Alu: 5′-TGAGCCGAGATCGCGCCACTGCAC-3′) and for the vector-encoded NTP reporter gene (NTP3: 5′-TCCCTGGCCGTTGGATTACACGGTC-3′). The PCRs were carried out with 300 ng DNA in a volume of 50 μL. The cycling conditions were 94°C for 5 minutes, followed by 30 cycles of 94°C for 30 seconds, 66°C for 30 seconds, and 72° C for 5 minutes, with 10 minutes at 72°C for the final extension step. The PCR products were analyzed on 0.8% agarose gels, and Southern blot hybridization was performed using theXhoI-BamHI fragment of the NTP gene (448 bp) as a probe.
Flow cytometry analysis
The cell surface phenotype of DCs was analyzed using phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)–labeled monocloal antibodies against CD14, CD80, CD40, HLA-DR (Becton Dickinson, Mountain View, CA), and a monoclonal antibody against CD83 (Immunex, Marseille, France). To measure gene transfer, cells were stained with the anti-LNGFR antibody 20.4 (ATCC). Anti-CD83 antibody and 20.4 were detected by a phycoerythrin-labeled polyclonal goat antimouse antibody (Caltag, South San Francisco, CA).
Allogeneic and syngeneic mixed leukocyte reactions
Stimulatory cells (untransduced DCs, transduced DCs, or total PBMCs) were irradiated (1500 rad) and plated in triplicates in round-bottomed 96-well tissue culture plates at graded concentrations, each well containing 1 × 105 allogeneic or syngeneic T cells, in a total volume of 200 μL regular medium. PBMCs, T cells, and DCs were obtained as described. T cells were thoroughly depleted of B cells, natural killer cells, monocytes-macrophages, and preactivated T cells as described. Five or six days after the start of the cocultures, 37 × 102 Bq (1 μCi) of3H-thymidine (3H-TdR; New England Nuclear, Boston, MA) was added to each well for 12 hours. Incorporation of3H-TdR is given as the mean cpm ± SD of triplicates. Wells containing only stimulators or T cells always incorporated less than 100 cpm 3H-TdR.
Stimulation of specific cytotoxic T lymphocytes
Untransduced and HIG-transduced DCs were pulsed with the Flu peptide (10 μM) for 2 hours at room temperature in RPMI without serum. In vitro stimulation of the T cells was carried out at the ratio 10 T lymphocytes to 1 DC in 24-well plates, with 1 × 106T cells per well for 8 to 10 days in RPMI with 10% FCS. Ten days later the T cells were restimulated by adding DCs following the same procedure. Then, 20 IU/mL IL-2 (Chiron, St. Louis, MO) was added every third day.
Cytotoxicity assays
Standard chromium release assays were performed. As target cells, we used TAP-deficient HLA A2.1+ T2 cells (kind gift of Dr J. W. Young) loaded with the Flu or the MART-1 peptide (10 μM, 1 hour at room temperature, in RPMI without serum) before pulsing with 51Cr for 1 hour at 37°C. A total of 5000 T2 cells per well were used in 96 V-bottom plates at different effector-to-target cell (E:T) ratios for 4 hours. Specific51Cr release was calculated using the formula [(51Cr release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Peptide synthesis
The peptides were synthesized in the Peptide Synthesis Facility at Memorial Sloan-Kettering Cancer Center, resuspended in 50% (vol:vol) RPMI/dimethylsulfoxide (Sigma), and stored at −20°C. The following peptides were used in this study: the influenza matrix protein-derived peptide58-66 GILGFVFTL (Flu peptide); and the MART-1 protein-derived peptide27-35 AAGIGILTV (MART-1 peptide).33
Results
Recombinant lentiviruses efficiently transduce blood monocyte-derived DCs
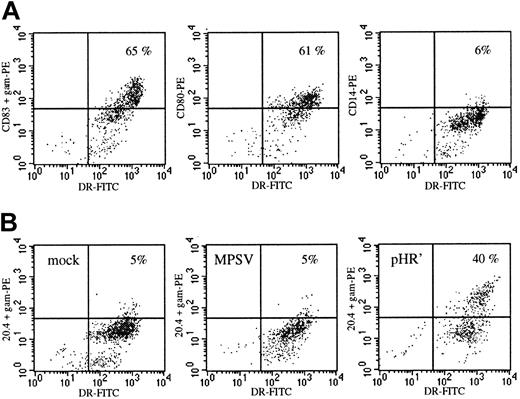
To compare lentivirus- and MLV-mediated gene transfer in human blood-derived DCs, cultured DCs were transduced under identical conditions using 2 vectors encoding an inactive cell surface marker termed NTP.20 NTP expression is easily and accurately monitored by FACS analysis, allowing enumeration of DC transduction on a single-cell basis. The HIV- and MLV-based vectors, respectively termed MPSV-NTP and pHR'NTP (Figure 1A), were pseudotyped with the VSV-G envelope providing both vectors with the same mode of cell entry via receptor-mediated endocytosis. Viral titers of both vectors were measured on HeLa cells, and viral stocks were set at a concentration of 3 × 107 IU/mL. DCs were generated by differentiation from blood monocytes with GM-CSF and IL-4. On day 8, monocyte-conditioned medium was added to the cultures to induce terminal maturation (Figure 2A). The DCs were infected on either day 3, 7 (immature DCs), or 12 (mature DCs). The MPSV-NTP or the pHR'NTP particles were admixed at an MOI ranging from 2 to 20. Infection with the lentiviral vector at MOI 20 on day 3 led to NTP expression in 35% of the DCs as shown in one representative experiment in Figure 2B. The highest levels of NTP expression were achieved at an MOI of 20 on day 3 or day 7 of the culture, when the DCs are immature (18%-35% NTP+, data not shown). At lower MOIs (2-4), 15% to 17% of the HLA-DR++ population was NTP+ (data not shown). In contrast, susceptibility of the DCs to lentiviral vector transduction was reduced considerably, although not abolished, when infection was carried out after the DCs have matured (5%-10% NTP+ at MOI 20 on day 12, data not shown), corroborating observations by others using a less attenuated lentiviral vector.34 The MLV vector, on the other hand, did not lead to significant transduction efficiencies (0%-3% NTP+) at any time during DC differentiation (Figure 2B and data not shown). Infection of DCs at MOI of 10 to 20 was toxic, reducing cell yields by 50% or more after several days (data not shown). In the functional studies, DCs were therefore infected at MOI 2 to 4 on day 7 to obtain undiminished yields of mature cells.
Schematic representation of the viral vectors.
(A) The lentiviral vector pHR'NTP expresses NTP under the transcriptional control of the CMV promoter and/or the HIV LTR. The MLV-vector, MPSV-NTP, contains a chimeric MPSV/MLV-LTR in which the U3 region of MLV was replaced by the U3 region of the myeloproliferative sarcoma.27 (B) In the bicistronic vector HFIG, the Flu peptide and the eGFP reporter gene are linked by the IRES of the encephalomyocarditis virus. In the vector HIG, the Flu peptide has been deleted. Ψ indicates packaging signal of HIV-1; Ψ+, extended packaging signal of MLV; Δgag, truncated gag gene containing an out-of-frame mutation; RRE, Rev-responsive element.
Schematic representation of the viral vectors.
(A) The lentiviral vector pHR'NTP expresses NTP under the transcriptional control of the CMV promoter and/or the HIV LTR. The MLV-vector, MPSV-NTP, contains a chimeric MPSV/MLV-LTR in which the U3 region of MLV was replaced by the U3 region of the myeloproliferative sarcoma.27 (B) In the bicistronic vector HFIG, the Flu peptide and the eGFP reporter gene are linked by the IRES of the encephalomyocarditis virus. In the vector HIG, the Flu peptide has been deleted. Ψ indicates packaging signal of HIV-1; Ψ+, extended packaging signal of MLV; Δgag, truncated gag gene containing an out-of-frame mutation; RRE, Rev-responsive element.
Maturation and transduction of monocyte-derived DCs.
(A) On day 18 the cells had the typical phenotype of mature DCs with high expression levels of HLA-DR, expression of CD80 and CD83, but loss of expression of the monocyte-macrophage marker CD14. (B) Transduction efficiency of DCs infected on day 3 with the oncoretroviral vector MPSV-NTP or the lentiviral vector pHR'NTP at an MOI of 20. On day 18 (15 days after infection) the cells were double stained with anti–HLA-DR and anti-LNGFR monoclonal (20.4) antibodies. NTP expression: y-axis; HLA-DR: x-axis. Data from 1 representative experiment of 3.
Maturation and transduction of monocyte-derived DCs.
(A) On day 18 the cells had the typical phenotype of mature DCs with high expression levels of HLA-DR, expression of CD80 and CD83, but loss of expression of the monocyte-macrophage marker CD14. (B) Transduction efficiency of DCs infected on day 3 with the oncoretroviral vector MPSV-NTP or the lentiviral vector pHR'NTP at an MOI of 20. On day 18 (15 days after infection) the cells were double stained with anti–HLA-DR and anti-LNGFR monoclonal (20.4) antibodies. NTP expression: y-axis; HLA-DR: x-axis. Data from 1 representative experiment of 3.
Stability of transgene expression in DCs
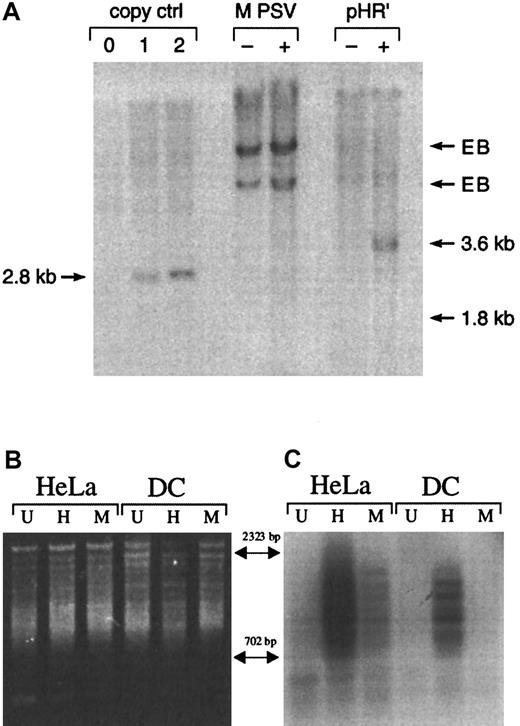
Expression of lentiviral vector-encoded NTP was maintained for at least 2 weeks, suggesting that intact reverse transcription of the viral RNA into DNA, transport of the preintegration complex into the nucleus, and proviral integration had occurred. To address whether viral DNA was present after viral transduction, Southern blot analysis was performed on genomic DNA from DCs infected with either pHR'NTP or MPSV-NTP. Three days after transduction, infection with the pHR'NTP vector system resulted in a detectable band of the predicted size (3.6 kb) representing integrated or episomal vector sequence (Figure3A). The predicted 1.8-kb band corresponding to the MPSV-NTP vector DNA was not detectable even after prolonged exposures, suggesting that the low level of NTP+cells measured by FACS analysis (0%-3%) corresponds to either very low transduction efficiency or pseudotransduction.19 We used Alu PCR to resolve whether transduction of DCs with the lentiviral vector leads to integration of the provirus.35 36Alu-repeats appear on average every 4 kb in the human genome and therefore allow PCR amplification of viral sequences integrated at random sites within the genome. Two primers were designed—one specific for the 3′ end of the consensus Alu sequence, the other specific for the NTP reporter gene of the viral vectors. Alu PCR carried out on genomic DNA from untranduced (U) and transduced (H, M) HeLa cells showed that both viral vectors gave the characteristic smear of NTP+ bands representing multiple integration sites (Figure3B,C). Alu PCR performed on genomic DNA from DCs resulted in an NTP+ smear only in case of transduction with the vector pHR'NTP (H), demonstrating that the lentiviral vector was capable of proviral integration in DCs in absence of proliferation whereas the MLV-based vector (M) was not (Figure 3B,C). The intensity of the signal detected by Southern blot hybridization with the NTP probe was proportional to the fraction of NTP+ cells detected by flow cytometry (HeLa, pHR'NTP: 50%; HeLa, MPSV-NTP: 5%; DC, pHR'NTP: 10%; data not shown).
Gene transfer in human monocyte-derived DCs with MPSV-NTP or pHR'NTP.
(A) Detection of vector sequence in DCs by Southern blot analysis. DCs were infected on day 4 at an MOI of 4 with the oncoretroviral vector MPSV-NTP (MPSV) or the lentiviral vector pHR'NTP (pHR'). Four days after infection genomic DNA was extracted and digested with eitherNheI (MPSV-NTP) or ScaI (pHR'NTP), which cut in the LTR. Southern blot analysis was performed using a radiolabeled NTP complementary DNA probe. The left 3 lanes represent copy controls obtained from MEL clones bearing 0, 1, or 2 copies of the MLV vector SFG-NTP digested with NheI. Position of the signals expected for the vector DNAs are indicated (copy control = 2.8 kb; MPSV = 1.8 kb; pHR' = 3.6 kb). EB indicates endogenous bands; −, untransduced; +, transduced. No band was observed in the MPSV+ lane, even after extended exposure (24 hours in Figure 3; 10 days for extended exposure). (B,C) Detection of integrated lentiviral vector DNA by Alu PCR. HeLa cells and DCs were either untreated (U) or infected with the HIV-based vector pHR'NTP (H) or the MLV-based vector MPSV-NTP (M). Proviral junctions were amplified using the primers 3′Alu and NTP3. PCR products were analyzed by gel electrophoresis (B) and Southern blot hybridization with an NTP-specific probe (C).
Gene transfer in human monocyte-derived DCs with MPSV-NTP or pHR'NTP.
(A) Detection of vector sequence in DCs by Southern blot analysis. DCs were infected on day 4 at an MOI of 4 with the oncoretroviral vector MPSV-NTP (MPSV) or the lentiviral vector pHR'NTP (pHR'). Four days after infection genomic DNA was extracted and digested with eitherNheI (MPSV-NTP) or ScaI (pHR'NTP), which cut in the LTR. Southern blot analysis was performed using a radiolabeled NTP complementary DNA probe. The left 3 lanes represent copy controls obtained from MEL clones bearing 0, 1, or 2 copies of the MLV vector SFG-NTP digested with NheI. Position of the signals expected for the vector DNAs are indicated (copy control = 2.8 kb; MPSV = 1.8 kb; pHR' = 3.6 kb). EB indicates endogenous bands; −, untransduced; +, transduced. No band was observed in the MPSV+ lane, even after extended exposure (24 hours in Figure 3; 10 days for extended exposure). (B,C) Detection of integrated lentiviral vector DNA by Alu PCR. HeLa cells and DCs were either untreated (U) or infected with the HIV-based vector pHR'NTP (H) or the MLV-based vector MPSV-NTP (M). Proviral junctions were amplified using the primers 3′Alu and NTP3. PCR products were analyzed by gel electrophoresis (B) and Southern blot hybridization with an NTP-specific probe (C).
Dendritic cells transduced with a multiply attenuated lentiviral vector retain normal allostimulatory functions and efficiently stimulate Flu-specific primary cytotoxic T cells
The function of lentivirus-transduced DCs was assessed in 2 ways: induction of an allogeneic proliferative T-cell response and stimulation of an antigen-specific autologous cytotoxic T-cell response. In the mixed leukocyte reactions, DCs were 100- to 300-fold more potent than DCs in inducing proliferation of allogeneic T cells (Figure 4), demonstrating the high allostimulatory potential expected from fully mature DCs.9Transduction of DCs neither reduced nor augmented the proliferative response of the same T cells when compared with untransduced DCs (Figure 4).
Transduction of DCs with multiply attenuated lentiviral vector does not alter the allostimulatory capacity of these DCs.
Untransduced DCs (DC.utr) or transduced DCs (in this case with the HIG vector, DC.HIG) were cultured in graded doses with allogeneic (allo) or syngeneic (syn) T cells. Incorporation of 3H-TdR was measured after 6 days of coculture. PBMCs were used as a control to assess DC potency. X-axis: number of stimulating cells per 105 T cells (logarithmic scale); y-axis: 3H-TdR incorporation by T cells (logarithmic scale).
Transduction of DCs with multiply attenuated lentiviral vector does not alter the allostimulatory capacity of these DCs.
Untransduced DCs (DC.utr) or transduced DCs (in this case with the HIG vector, DC.HIG) were cultured in graded doses with allogeneic (allo) or syngeneic (syn) T cells. Incorporation of 3H-TdR was measured after 6 days of coculture. PBMCs were used as a control to assess DC potency. X-axis: number of stimulating cells per 105 T cells (logarithmic scale); y-axis: 3H-TdR incorporation by T cells (logarithmic scale).
Transduced DCs and peptide-pulsed DCs were then compared in their ability to recall a specific CTL response using autologous peripheral blood T cells as the responder population. To avoid the use of a precursor protein that would require processing and eventually introduce additional epitopes including T-helper epitopes into the DC, we turned to an established model system based on the peptide58-66 GILGFVFTL derived from the influenza matrix protein, termed Flu peptide. The peptide is presented in the context of the A2.1 major histocompatibility complex (MHC) class I molecule and has previously been shown to stimulate T cells efficiently.37,38 It is well established that HLA A2.1-restricted Flu peptide–specific CTLs are capable to lyse with the same efficiency both Flu peptide–pulsed and influenza virus–infected lymphoid or monocytic target cells.39-44 Therefore, the use of HLA-A2.1+ T2 cells pulsed with the Flu peptide as target cells provides a convenient and reliable system to study a biologically relevant HLA-A2.1+-restricted CD8+response.
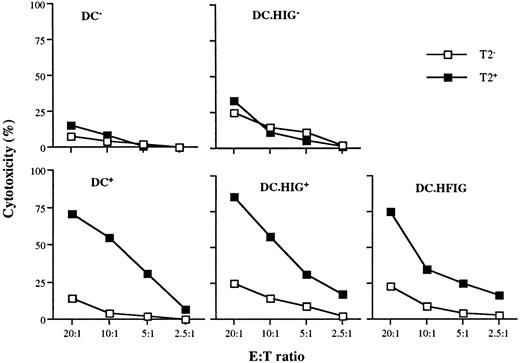
To generate the lentiviral vector HFIG, the Flu peptide coding sequence was fused to that of the CD8α leader to target the endoplasmic reticulum21 45 and cloned in place of the NTP complementary DNA in pHR'NTP (Figure 1B). The HIG vector lacks the Flu peptide and served as a negative control. Immature DCs were infected on day 7 at an MOI of 2 to 4 with the lentiviral vectors HFIG or HIG. Expression of the reporter gene eGFP was evaluated on day 11 and showed that the DCs were transduced with an efficiency of 15% to 18%, consistent with the efficiency of the NTP gene transfer achieved with pHR'NTP (data not shown). DCs transduced with the HFIG vector were tested for their ablility to present the Flu peptide in the context of HLA A2.1 and induce a specific cytotoxic T-cell response. T-cell stimulation was achieved by incubating mature transduced or peptide-pulsed DCs with autologous T cells at a 1:10 stimulator-to-responder ratio. Untransduced DCs, either pulsed (DC+) or nonpulsed (DC−) with the Flu peptide, served as controls. The HFIG-transduced DCs (DC.HFIG) were able to activate a strong cytotoxic T-cell response against the Flu peptide, albeit with slightly lower efficiency than the peptide-pulsed, untransduced DCs (DC+) (Figure5). The high potential of the genetically modified DCs is underlined by the fact that only a fraction of the DCs was transduced by the HFIG vector (about 15%-17% of the DCs in these experiments). DCs transduced with the control vector HIG pulsed with the Flu peptide (DC.HIG+) were comparable to untransduced DCs in their ability to stimulate Flu-specific T cells, indicating that lentivirus-mediated transduction did not perturb DC function.
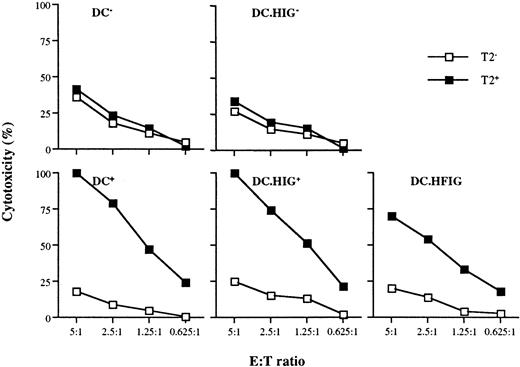
Flu-specific CTL activation by DCs transduced with the lentiviral vector HFIG.
DCs were infected with HIG or HFIG or left uninfected. The ability of HFIG-transduced DCs to activate Flu-specific CTLs (DC.HFIG) was compared with untransduced or HIG-transduced DCs either nonpulsed (DC−, DC.HIG−) or pulsed (DC+, DC.HIG+) with the Flu peptide. CTL assays were performed after incubation of the DCs with autologous T cells for 8 to 10 days. T2 cells pulsed with the Flu peptide (T2+) or with the irrelevant MART-1 peptide (T2−) were used as targets. Data are shown for 1 of 3 independent experiments.
Flu-specific CTL activation by DCs transduced with the lentiviral vector HFIG.
DCs were infected with HIG or HFIG or left uninfected. The ability of HFIG-transduced DCs to activate Flu-specific CTLs (DC.HFIG) was compared with untransduced or HIG-transduced DCs either nonpulsed (DC−, DC.HIG−) or pulsed (DC+, DC.HIG+) with the Flu peptide. CTL assays were performed after incubation of the DCs with autologous T cells for 8 to 10 days. T2 cells pulsed with the Flu peptide (T2+) or with the irrelevant MART-1 peptide (T2−) were used as targets. Data are shown for 1 of 3 independent experiments.
Lentivirus-transduced DCs effectively restimulate primed CTLs
To increase the specific response against the Flu peptide, the T cells were restimulated for another 8 days with newly generated DCs from the same donor. As expected, the restimulation with either transduced or peptide-pulsed DCs led to a strong increase of the Flu-specific response (Figure 6). The primary induction resulted in 75% to 80% Flu-specific cytotoxicity at E:T ratios of 20:1, whereas the restimulation of the T cells led to generation of CTLs with at least 4-fold higher activity (75%-100% cytotoxicity at the 5:1 E:T ratio). Thus, lentivirus-transduced DCs were able to efficiently restimulate the cytotoxic lymphocytes without loss of antigen specificity.
Restimulation of Flu-specific CTLs with monocyte-derived DCs.
Ten days after primary induction the T cells in each group (as for Figure 5) were restimulated with newly generated untransduced DCs (DC−, DC+), HIG (DC.HIG−, DC.HIG+), or HFIG (DC.HFIG)-transduced DCs.
Restimulation of Flu-specific CTLs with monocyte-derived DCs.
Ten days after primary induction the T cells in each group (as for Figure 5) were restimulated with newly generated untransduced DCs (DC−, DC+), HIG (DC.HIG−, DC.HIG+), or HFIG (DC.HFIG)-transduced DCs.
Lentivirus-transduced DCs do not require CD4+ T cell help to stimulate Flu-specific CTLs
To further assess the potential of DCs transduced with the lentiviral vector, we investigated whether the stimulation of Flu-specific CTLs in vitro required CD4+ T-cell help. T cells were therefore depleted of CD4+ T cells to high purity (< 0.2% CD4+, Figure7A,B) and stimulated with DCs as described above, in the absence of any exogenous cytokine. DCs transduced with the HFIG vector (DC.HFIG) were compared with Flu peptide–pulsed untransduced (DC+) and HIG-transduced DCs (DC.HIG+). As shown in Figure 7C, DCs+ were able to stimulate Flu-specific T cells in the absence of CD4+ T-cell help and without addition of any exogenous cytokine. Interestingly, DC.HIG+ stimulated specific CTLs the same way as untransduced DCs. With DC.HFIG, the specific response was comparable or even stronger (3- to 5-fold higher in Figure 7C, right panel). These results obtained with CD4+-depleted peripheral blood T cells further demonstrate that lentiviral transduction does not alter DC function and enables DCs to efficiently present a specific MHC class I peptide to cytotoxic T cells.
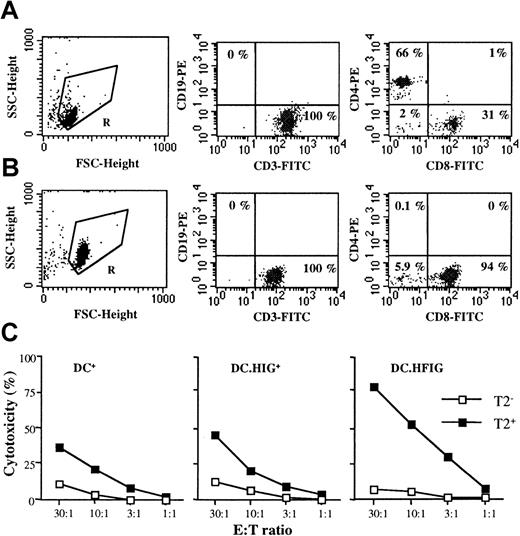
Transduced DCs induce a specific cytotoxic response against the Flu peptide in the absence of CD4+ T cells and in the absence of exogenous cytokines.
(A,B) Purified HLA A2.1+ T cells before and after depletion of CD4+ T cells. (A) T cells (gate R, left panel) were purified as described in “Materials and methods” and stained with anti-CD3 and anti-CD19 antibodies (middle panel) or anti-CD8 and anti-CD4 antibodies (right panel). (B) Depletion of CD4+ T cells was accomplished as described in “Materials and methods.” After depletion, T cells (gate R, left panel) were stained with the same antibodies (middle and right panels). Background staining given by mouse isotype IgG controls was always lower than 0.25% and subtracted. (C) HLA A2.1+ DCs were cultured with autologous CD4-depleted T cells for 8 to 10 days in the absence of exogenous cytokines. CTL assays were performed on T2 cells as described in Figures 5 and 6. The following populations of DCs were compared: DCs pulsed with the Flu peptide (DC+), DCs transduced with the HIG vector and pulsed with the Flu peptide (DC.HIG+), and DCs transduced with the HFIG vector (DC.HFIG). Without CD4 depletion and using the same absolute number of T cells at the start of the cocultures, cytotoxic activity was comparable to that shown in Figure 5(data not shown).
Transduced DCs induce a specific cytotoxic response against the Flu peptide in the absence of CD4+ T cells and in the absence of exogenous cytokines.
(A,B) Purified HLA A2.1+ T cells before and after depletion of CD4+ T cells. (A) T cells (gate R, left panel) were purified as described in “Materials and methods” and stained with anti-CD3 and anti-CD19 antibodies (middle panel) or anti-CD8 and anti-CD4 antibodies (right panel). (B) Depletion of CD4+ T cells was accomplished as described in “Materials and methods.” After depletion, T cells (gate R, left panel) were stained with the same antibodies (middle and right panels). Background staining given by mouse isotype IgG controls was always lower than 0.25% and subtracted. (C) HLA A2.1+ DCs were cultured with autologous CD4-depleted T cells for 8 to 10 days in the absence of exogenous cytokines. CTL assays were performed on T2 cells as described in Figures 5 and 6. The following populations of DCs were compared: DCs pulsed with the Flu peptide (DC+), DCs transduced with the HIG vector and pulsed with the Flu peptide (DC.HIG+), and DCs transduced with the HFIG vector (DC.HFIG). Without CD4 depletion and using the same absolute number of T cells at the start of the cocultures, cytotoxic activity was comparable to that shown in Figure 5(data not shown).
Discussion
A variety of strategies to generate and transduce DCs are currently under investigation. Oncoretroviral vectors derived from MLV have been previously used for stable gene delivery into CD34+ DC progenitors purified from bone marrow, cord blood, or cytokine-elicited peripheral blood stem cells.4-7,9This approach is not applicable to DCs generated from blood monocytes because these cells show little or no cell proliferation, thus precluding oncoretoviral integration as shown here. Lentiviruses, on the other hand, have proven to be extremely efficient at infecting various nondividing cell types.16,47 Recombinant vectors derived from HIV-1 have successfully been used for in vivo or in vitro transduction of terminally differentiated cells such as neurons, hepatocytes, retinocytes, and macrophages.18,25 47-51
To improve the safety of lentiviral-mediated gene transfer, 1 or more of the 4 accessory genes, vpr, vpu,vif, and nef, which contribute to viral pathogenicity, have been removed in the second generation of lentiviral vectors.25,52 Here, we show that a multiply attenuated vector system based on HIV-1 is efficient in transducing DCs differentiating from blood monocyte precursors. The presence of integrated proviral DNA and detectable expression of the reporter gene up to 2 weeks postinfection demonstrate that gene transfer into DCs is stable. The 4 accessory genes, vpr, vpu,vif and nef, have been removed and are therefore not necessary for stable gene transfer into human DCs. In certain cell types, however, absence of accessory functions can diminish infectivity of wild-type HIV-1 and replication-defective recombinants. Studies on HIV-1 infection of macrophages show that the Vpr protein plays a crucial role in the nuclear import of the preintegration complex.25,53,54 For lentiviral vector-mediated gene delivery into neurons and growth arrested cell lines, Vpr turns out to be completely dispensable, whereas gene delivery into macrophages and hepatocytes is considerably impaired when vpr is deleted.25,49 Interestingly, transduction efficiency with the lentiviral vector was higher in immature DCs than later in the differentiation process. Down-regulation of a viral receptor as a cause for reduced cellular entry in more mature cells is unlikely because infection was mediated by the VSV-G glycoprotein substituted for the HIV-1 envelope. This finding corroborates observations on the infection by wild-type HIV-1 and is consistent with a block in reverse transcription in mature DCs.13 On the other hand, the failure of the MLV-derived vector to efficiently transduce DCs at any time during the culture likely reflects another limitation. Indeed, the oncoretrovirus, which was VSV-G–pseudotyped and admixed at the same MOI as the lentivirus, likely underwent reverse transcription, as did the lentivirus, but then failed to integrate, perhaps because of its inability to translocate to the nucleus.10
Most importantly, we found that lentivirus-transduced DCs were fully functional and could be used to stimulate specific cytotoxic T cells. First, genetic modification of DCs with a lentiviral vector did not alter the function of mature DCs. Indeed, untransduced and transduced DCs showed comparable stimulatory capacities in the allogeneic mixed leukocyte reaction. Furthermore, DCs transduced with a control vector (HIG) stimulated Flu-specific CTLs with an equal efficiency as untransduced DCs when pulsed with the relevant peptide under the same conditions. Stimulation of Flu-specific CTLs achieved in the absence of CD4+ T-cell help was comparable with either untransduced or control vector-transduced peptide-pulsed DCs. Second, DCs modified with the vector encoding the Flu peptide induced a strong specific T-cell response. Importantly, in vitro stimulation with CD8+ T cells thoroughly depleted of CD4+ T cells showed that Flu-specific CD8+ T cells were also activated in the absence of CD4+ helper T cells and without exogenous cytokine. This observation is consistent with direct activation of CTLs by fully mature DCs of human31,32,55 and mouse56-59 origin. Taken together, our data demonstrate that the efficiency of lentivirus-transduced DCs in stimulating specific CTLs in vitro does not depend on CD4+ T-cell help and that CD4-independent CTL activation did not result from lentivirus activation of DCs.60,61 In the absence of CD4+cells, however, the cytolytic activity was consistently reduced (2- to 4-fold), underscoring the role of CD4+ T cells in sustaining CTL activation under such experimental conditions. Importantly, although CD4+ T cells are dispensable for the activation of CTLs in vitro, T-cell help may be essential to sustain CTL activity and induce immunologic memory against tumor antigens in vivo.62
The level of Flu-specific cytolytic activity induced by transduced DCs was at least as good as that obtained with pulsed DCs. This demonstrates the highly efficient presentation of the lentivirus-encoded MHC class I–restricted peptide by the transduced DCs. Importantly, a second round of stimulation with transduced DCs further increased Flu-specific activity. Interestingly, background cytotoxicity was consistently higher in the cultures containing CD4+ T cells. Thus, induction of this background activity appears to depend on the recruitment of CD4+ T cells, implying presentation of MHC class II peptides possibly provided by media62 63 or virion components.
A number of clinical trials currently use monocyte-derived DCs as a convenient way to generate DC-based “vaccines” for cancer immunotherapy.3 Antigen presentation is usually achieved by pulsing the DCs with peptide, whole protein, or apoptopic cells. Oncoretroviral vectors can be used to transduce proliferating CD34+ progenitor-derived DCs9 but not monocyte-derived DCs as shown here. Other vector systems have been used to transduce blood-derived DCs, including those based on adenovirus,64 herpesvirus,65 or poxvirus.66-68 One distinguishing feature of recombinant lentiviruses is that efficient and stable gene delivery is achieved with a vector that does not itself encode any immunogenic proteins of viral origin. The efficient generation of immunocompetent lentivirus-transduced DCs derived from blood cells provides a valuable alternative to approaches based on oncoretroviral-mediated gene transfer in CD34+ progenitors. This will allow us to compare genetically modified monocyte- and CD34+-derived DCs and to investigate functionally distinct subsets of DCs.2 69-71 The availability of a stable gene delivery system based on a multiply attenuated lentivirus that does not encode any viral protein and allows efficient and sustained antigen presentation by DCs derived from blood monocytes may ultimately be useful for the improvement of immunotherapies.
Acknowledgments
We thank Dr D. Trono for providing us with the plasmids PHR'CMVLacZ and pCMVΔR8.9 and C. May for providing us with the pHR'NTP plasmid. We thank Drs A. Krause, M. C. Gong, and I. Rivière for reviewing the manuscript.
Supported by National Institutes of Health grants PO1 CA-59350, RO1 HL-56712, PO1 CA-65930, and the Memorial Sloan-Kettering Cancer Center DeWitt-Wallace Fund.
J.D. and J.-B.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michel Sadelain, Box 182, Department of Human Genetics, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021; e-mail: m-sadelain@ski.mskcc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal