Abstract
The mechanism of enhanced presentation of ovalbumin (OVA) internalized as immunoglobulin A (IgA)–OVA via the IgA Fc receptor (FcαR) was analyzed by focusing on the role of the FcαR-associated γ chain. Comparison of B-cell transfectants expressing FcαR plus wild-type (WT) γ chain or γ chain in which the immunoreceptor tyrosine-based activation motif (ITAM) was altered by tyrosine mutation or substitution with the ITAM of FcγRIIA showed that signaling-competent ITAM was not required for endocytosis of IgA-OVA. However, antigen presentation was impaired by ITAM changes. Signaling-competent γ-chain ITAM appeared necessary for transport of ligated FcαR to a lamp-1+ late endocytic compartment for remodeling and/or activation of that compartment and also for efficient degradation of IgA complexes. Moreover, FcαR ligation also activated efficient processing of nonreceptor-targeted antigen. The results suggest that γ-chain signaling activates the antigen processing compartment.
Introduction
Immunoglobulin-A (IgA) synthesis exceeds that of any other isotype and is a prominent feature of immune responses at mucosal sites.1 Because these sites are under continuous challenge by environmental antigens and pathogens, IgA-antigen complexes are being constantly formed and processed for removal. IgA receptor (FcαR)–bearing phagocytic cells, in particular macrophages, are found at mucosal sites in humans and rodents2 3 and are thought to be involved in IgA complex clearance.
Monocytes and macrophages are also capable of presenting antigen to T cells in context of major histocompatability complex (MHC) class II.4 While these cells can take up antigen non-specifically, antigen presentation is dramatically increased when antigen is complexed with antibody. Manca et al5demonstrated that polyclonal antibody enhanced macrophage presentation of beta galactosidase by more than 100-fold and that FcγR mediated this phenomenon as shown by blocking of enhancement with aggregated IgG. More recently, antigen-conjugated anti-FcR monoclonal antibodies (mAbs) have been used to show that FcγRI on monocytes6mediate enhanced presentation of receptor-targeted antigen. The induction of greater responsiveness by FcR targeting of antigens may offer a new approach to vaccine construction. It would be of particular benefit to design vaccines that would increase mucosal immunity. Macrophages at mucosal surfaces are strategically located antigen-presenting cells (APCs) to which vaccines could be delivered, and macrophage FcαR has potential as a receptor to which vaccines might be targeted.
It is not known whether the FcR plays an active role in enhanced processing, based on its ability to signal, or whether it merely allows capture of greater amounts of antigen. Several of the FcRs, including FcεRI, FcγRI, and FcαR, are associated with the FcR γ-chain homodimer (γ chain) which contains an immunoreceptor tyrosine-based activation motif (ITAM) and transduces signaling initiated by FcR aggregation.7 FcαR has a particularly strong association with γ chain7 due to interaction of a positively charged arginine in the transmembrane region with a negatively charged aspartic acid in the γ-chain transmembrane domain. By contrast to γ-chain–associated FcR, FcγRIIA and FcγRIIC are single-chain FcRs with one cytoplasmic domain ITAM.8 We have explored the possibility that the γ-chain ITAM plays a role in the processing of FcαR-targeted antigen, which is ultimately important for antigen presentation. To study the influence of γ-chain ITAM, we constructed altered γ chains, one in which the tyrosines were replaced by phenylalanines and a second in which the ITAM was replaced with the ITAM of FcγRIIA. B cells lacking endogenous FcR and γ-chain were used as model APCs after transfection with FcαR plus WT or altered γ chains. Our findings indicate that the γ-chain ITAM plays a role in mediating the processing of FcαR-targeted antigen, which correlates with the ability of the γ chain to signal.
Materials and methods
FcαR/γ-chain constructs
We used the pCAV vector containing the human FcαR complementary DNA (cDNA)9 (gift from Dr C. Maliszewski, Immunex, Seattle, WA) and cells of the A20 IIA1.6 B cell line, which is surface IgG+ and surface IgM− and FcR−.10 These cells were cotransfected with pCAV/FcαR cDNA and pNUT/γ-chain cDNA constructs by electroporation using a Bio-Rad electroporator (Bio-Rad Laboratories, Richmond, CA) at 250 V and 960 μF. The pNUT vector allows selection using methotrexate. Cells were maintained as bulk cultures and enriched for cells with FcαR expression by positive selection using the anti-FcαR IgG1 mAb and anti-mIgG1 magnetic beads (Dynal AS, Oslo, Norway.)
For the altered γ-chain constructs, the IIA ITAM γ chain represents a chimeric molecule in which the last 22 residues of the murine FcR γ-chain cytoplasmic domain were replaced by 29 residues of FcγRIIA. The Y→ F γ chain represents a mutant molecule in which the tyrosine residues at positions 65 and 76 within the ITAM of the γ chain were replaced by phenylalanine. The altered γ-chain constructs have been described previously.11
B-cell transfectant and T-cell culture
Transfectants expressing FcαR and γ chain were cultured in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal bovine serum (FBS), 50 μg/mL gentamycin, 2 mM L-glutamine, 1 mM sodium pyruvate, and 5 μM methotrexate. Cells from the OVA-specific T-cell hybridoma DO-11-10 (gift from Dr P. Marrack, National Jewish Medical and Research Center, Denver, CO) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with nonessential amino acids (Gibco BRL Life Technologies, Grand Island, NY), 1 mM sodium pyruvate, 2 mM L-glutamine, 0.75 mg/mL dextrose, 0.85 mg/mL sodium bicarbonate, 50 μg/mL gentamycin, and 10% FBS. The interleukin-2 (IL-2)–dependent T cell line HT-2 was cultured in the same medium supplemented with 5 U/mL IL-2.
Antigen presentation with B-cell transfectants
For studies on FcαR-mediated antigen uptake, OVA (Worthington Biochemical, Lakewood, NJ) was derivatized with NIP (nitro-iodophenol caproate-o-succinimide) (Genosys, The Woodland, TX) to give an average of 3 NIP haptens per OVA. For derivatization, NIP was dissolved in dimethyl formamide and added to OVA dissolved in borate-buffered saline (pH 8.3). After 1.5 hours, the mixture was dialyzed into phosphate-buffered saline (PBS) (pH 7.4). Soluble IgA-OVA complexes were made by mixing chimeric human IgA2 anti-NIP (gift from Drs R. Jefferis and D. M. Goodall, University of Birmingham, Edgebaston, England) with NIP-derivatized OVA at a molar ratio of 3:1. Studies on nontargeted antigen uptake used nonderivatized OVA. We cultured IIA1.6 cells in duplicate with IgA anti-NIP/NIP-OVA complexes (IgA-OVA) or OVA alone and OVA-specific DO-11-10 T cells at a 4:1 ratio for 20 hours, after which supernates were removed and frozen. Antigen presentation was measured by assaying the ability of serial dilutions of supernate to promote survival of the IL-2–dependent T cell line HT-2. The results are expressed as mean and spread of duplicates in representative experiments. In this well-documented assay system, titers that differ by 4-fold or more are considered significantly different.12
Flow cytometry of cell surface markers
The levels of MHC class II, FcαR, and costimulatory molecules on the IIA1.6 transfectants were measured by direct or indirect immunofluorescence staining and flow cytometry in comparison to isotype-matched negative control antibodies. FcαR was detected using My43, a FcαR-specific IgM mAb produced in our laboratory,13 and fluorescein isothiocyanate (FITC) anti-mIgM (Caltag Laboratories, South San Francisco, CA). I-Ad was detected using FITC-labeled M5 (gift from Dr R. Noelle, Dartmouth Medical School, Lebanon, NH). Costimulatory molecules were measured with commercially prepared antibodies (PharMingen, San Diego, CA): ICAM-1 using phycoerythrin (PE)-labeled 3E2, B7-1 using PE-labeled 16-10A1, and B7-2 using FITC-labeled GL-1.
RNA isolation and reverse transcriptase–polymerase chain reaction
Total cellular RNA was isolated from sheared cells using Trizol (Gibco), and 2 μg RNA from each preparation was transcribed into DNA. We performed γ-chain polymerase chain reaction (PCR) using 2 γ-chain–specific primers encompassing the transmembrane region of γ chain. The sequence of the 5′ oligomer primer was 5′-CAG CCG TGA TCT TGT TC-3′, and the sequence of the 3′ oligomer primer was 5′-CTC ACG GCT GGC TAT AGC-3′. After a 2-minute denaturing step the PCR was performed for 25 cycles (94°C, 52°C, and 70°C for 15 seconds each) with a 5-minute final extension at 70°C. Aliquots of 10 μL from each reaction were analyzed by agarose gel electrophoresis.
Endocytosis
Transfectants were incubated with a naturally polymeric human IgA myeloma protein for 1.5 hours on ice. The cells were then washed, and one aliquot was kept on ice. The remaining cells were incubated at 37°C in culture medium and transferred into ice-cold medium at the indicated times. All cells were subsequently stained with FITC antihuman IgA (Jackson Immunoresearch Laboratories, West Grove, PA). Negative control cells were identically treated but did not receive IgA. The amount of surface-bound IgA was measured by flow cytometry.
Catabolism studies
For studies of IgA catabolism by Western blot analysis, transfected B cells were incubated with polymeric human IgA myeloma protein at the concentrations stated. At the indicated times, cells were pelleted, subjected to 2 PBS washes, and lysed in 1% NP-40 containing a protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot using HRP antihuman IgA (Jackson Immunoresearch) and enhanced chemiluminescence (ECL) (Amersham Life Sciences, Arlington, IL) were used to detect IgA digestion products.
Catabolism of iodine 125 (125I) antimouse IgM μ chain (ICN Pharmaceuticals, Irvine, CA) was examined by first coating 2 × 106 transfected B cells on ice with the IgM anti-FcαR mAb My43. After 30 minutes the cells were washed and then incubated for 30 minutes on ice with 0.1 μg125I antimouse IgM μ chain (ICN) plus 2 μg nonlabeled antimouse μ chain (Jackson Immunoresearch). Cells were then washed and incubated at 37°C for 8 hours, after which they were pelleted, and the supernatants were removed. The amount of fully degraded125I anti-IgM in the cell supernatant was measured by the addition of trichloroacetic acid (TCA) to 5% by volume followed by a 10-minute incubation and centrifugation at 12 000g for 30 minutes. The TCA-soluble fraction was then counted. The total cell-associated counts were measured in duplicate cell aliquots harvested at the zero time point.
Tyrosine phosphorylation
Tyrosine phosphorylation was assessed after cross-linking FcαR on the transfectants with My43 for 15 minutes. Following one wash, anti-mIgM (Jackson Immunoresearch) was added. After 2 minutes at 37°C the cells were transferred into ice-cold lysis buffer containing 4 mM Na3 VO4 (sodium vanadate), 20 mM NaF (sodium fluoride), and 1% NP-40. Whole-cell lysates were analyzed by SDS-PAGE and transferred to nitrocellulose membranes. Equal loading and transfer of the samples to the membranes were ascertained by ponceau red staining. Membranes were stained for 5 minutes in 0.5% ponceau red/1% acetic acid followed by distilled water rinsing to remove unbound stain. The membranes were destained with 3 PBS washes of 5 minutes each. Tyrosine-phosphorylated proteins were then detected by Western blot using HRP-labeled antiphosphotyrosine (Upstate Biotechnology, Waltham, MA) and ECL.
Confocal microscopy
For FcαR/lamp-1 costaining, cells were first incubated with A77, an IgG1 anti-FcαR mouse mAb. This was followed by FITC antimouse IgG1 (Jackson Immunoresearch) for 10 minutes at 4°C, then washing. The cells were then warmed to 37°C and incubated for 30 minutes, fixed with 3% paraformaldehyde/3% sucrose, and permeabilized with 0.05% saponin as described.14 Cells were then incubated for one hour at ambient temperature with the anti–lamp-1 mAb ID4B (gift from Andrea Sant, University of Chicago, Chicago, IL), washed, and stained with anti-rat IgG Cy3 (Jackson Immunoresearch). Confocal sections of approximately 0.75-1 μm were acquired using a Zeiss 410 confocal microscope and displayed by pseudo-coloring using LSM software (both from Zeiss, Oberkochen, Germany.)
Results
Targeted and nontargeted antigen presentation
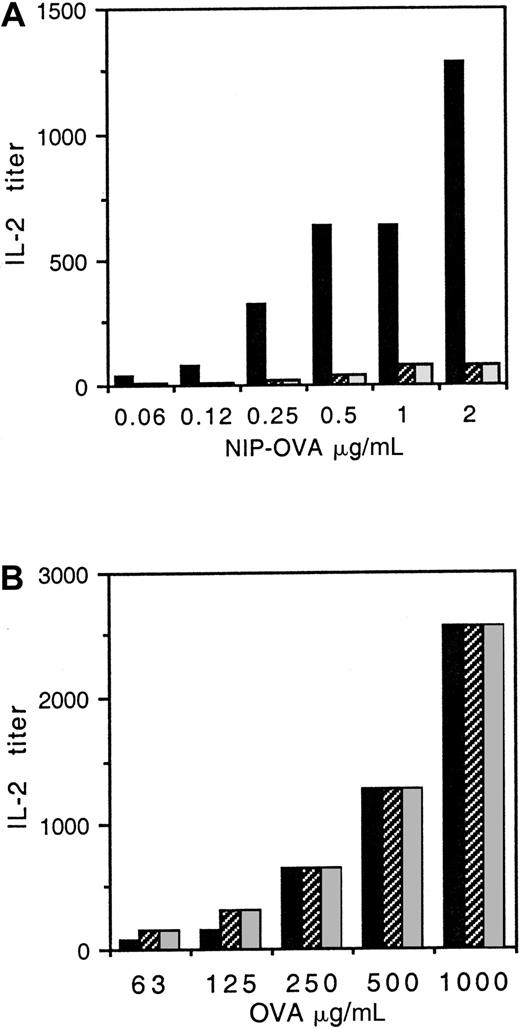
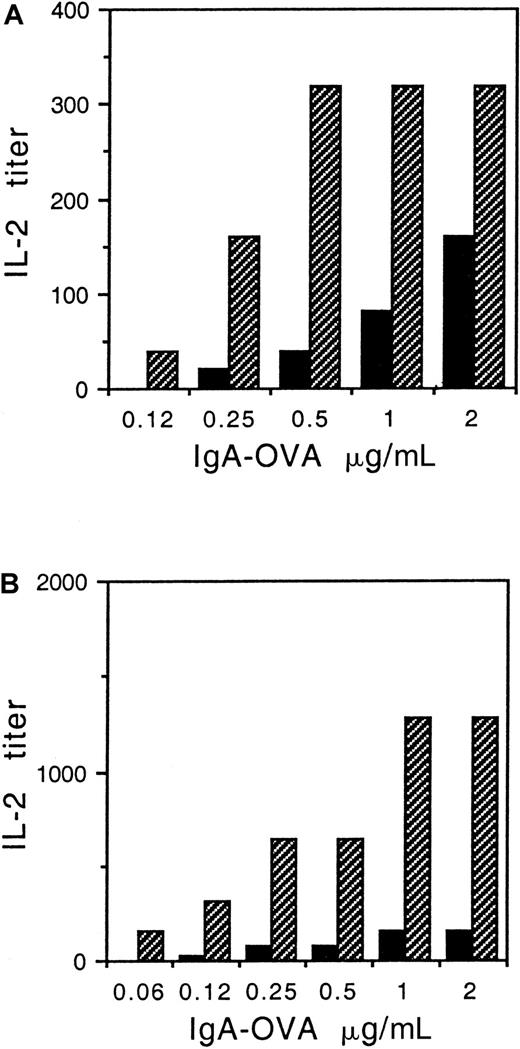
While monocytes express FcαR and are capable of antigen presentation, the endogenous expression of γ chain by monocytes precludes a detailed investigation of the role of γ chain in FcαR-enhanced antigen presentation. Whether FcαR makes an active contribution to antigen processing or serves only to capture IgA-coated antigen at the APC surface is the focus of the present study in which we examined the role of the FcαR-associated γ chain and in particular the γ-chain ITAM. This motif is required for receptor signaling, however tyrosine motifs also play a role in FcR endocytosis, a necessary first step in antigen processing.15 To study the γ-chain ITAM, IIA1.6 B cells were stably cotransfected with FcαR plus WT or altered γ chain and compared for ability to present IgA-OVA to DO-11-10 T cells. The results in Figure1A show that the FcαR + WT γ transfectant presented IgA-OVA more effectively than either the transfectant with the IIA ITAM mutant γ chain or the Y→ F γ chain, in that 10-fold less IgA-ag was required by the FcαR + WT γ transfectant to achieve 40 units of IL-2 production. In the absence of antigen, IL-2 production was undetectable. In addition, nontransfected IIA1.6 cells were completely lacking in ability to present IgA-OVA at these concentrations (data not shown.)
Presentation of IgA-OVA by FcαR/γ-chain transfectants is diminished in cells with altered γ chain.
(A) IIA1.6 B cells were cotransfected with FcαR and either WT γ chain (indicated with black bars), γ chain with IIA ITAM (indicated with cross-hatched bars), or γ chain with Y→ F mutation (indicated with gray bars). Transfectants and DO-11-10 OVA-specific T cells were incubated with various concentrations of NIP-haptenated OVA (NIP-OVA) opsonized with an IgA anti-NIP antibody. IL-2 secretion by the T cells was quantified as a measure of antigen presentation, as described in “Materials and methods.” (B) Nontargeted OVA is presented equally by WT or altered γ-chain transfectants. FcαR transfectants with WT γ chain (indicated with black bars), IIA ITAM γ chain (indicated with cross-hatched bars), or Y→ F γ chain (indicated with gray bars) were incubated together with DO-11-10 T cells and OVA at the concentrations indicated, and IL-2 secretion was measured as given in panel A. Similar results were obtained in 3 separate experiments.
Presentation of IgA-OVA by FcαR/γ-chain transfectants is diminished in cells with altered γ chain.
(A) IIA1.6 B cells were cotransfected with FcαR and either WT γ chain (indicated with black bars), γ chain with IIA ITAM (indicated with cross-hatched bars), or γ chain with Y→ F mutation (indicated with gray bars). Transfectants and DO-11-10 OVA-specific T cells were incubated with various concentrations of NIP-haptenated OVA (NIP-OVA) opsonized with an IgA anti-NIP antibody. IL-2 secretion by the T cells was quantified as a measure of antigen presentation, as described in “Materials and methods.” (B) Nontargeted OVA is presented equally by WT or altered γ-chain transfectants. FcαR transfectants with WT γ chain (indicated with black bars), IIA ITAM γ chain (indicated with cross-hatched bars), or Y→ F γ chain (indicated with gray bars) were incubated together with DO-11-10 T cells and OVA at the concentrations indicated, and IL-2 secretion was measured as given in panel A. Similar results were obtained in 3 separate experiments.
To determine that the transfectants with altered γ chain did not have an overall defect in antigen presentation, we also examined their ability to present nontargeted OVA. Transfectants with WT or altered γ chains were equally capable of presenting nontargeted OVA (Figure1B). In the absence of IgA opsonization, approximately 250-fold more OVA was required to achieve 1280 units IL-2 production by WT γ-chain transfectants (Figure 1B), demonstrating that FcαR targeting of OVA enhances antigen presentation.
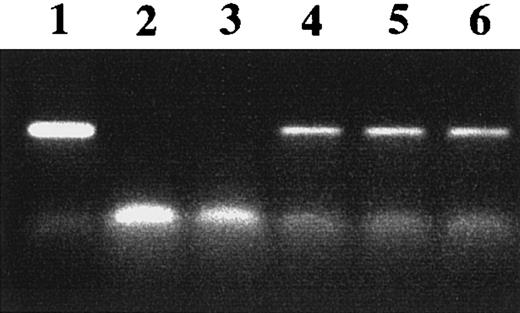
The transfectants expressed similar levels of FcαR (Table1), and the level of class II MHC did not account for the difference in antigen presentation because the WT γ-chain transfectants expressed less class II than the altered γ-chain transfectants. Expression of ICAM-1 and B7-1 was somewhat lower in transfectants with altered γ chain compared to the WT γ-chain transfectant. B7-2 was expressed at a very low level on the WT γ-chain transfectant and was absent from the other transfectants. However, blocking studies with anti–B7-1 or anti–B7-2 demonstrated that neither of these costimulatory molecules was necessary for presentation to DO-11-10 cells by IIA1.6 B cells (data not shown.) This is consistent with previous reports showing that activation of T-cell hybridomas is not dependent on costimulation.16 Figure 2shows that the transfectants expressed equivalent levels of γ-chain transcripts detected by reverse transcriptase (RT)-PCR. When PCR was performed in the absence of RT, the band corresponding to the γ-chain PCR product was not obtained, demonstrating that the RNA preparations did not contain any DNA contamination (data not shown.)
Expression of receptors, MHC class II, and costimulatory molecules on IIA1.6 transfectants
| . | FcαR cotransfected with . | ||
|---|---|---|---|
| WT γ chain . | IIA ITAM γ chain . | Y → F γ chain . | |
| FcαR | 81 ± 11 | 84 ± 10 | 76 ± 16 |
| IAd | 110 ± 11 | 139 ± 3 | 224 ± 19 |
| B7-1 | 14 ± 5 | 18 ± 3 | 5.5 ± 3 |
| B7-2 | 15 ± 2 | 0 | 0 |
| ICAM-1 | 959 ± 127 | 609 ± 187 | 573 ± 47 |
| BCR | 187 ± 11 | 221 ± 45 | 167 ± 54 |
| . | FcαR cotransfected with . | ||
|---|---|---|---|
| WT γ chain . | IIA ITAM γ chain . | Y → F γ chain . | |
| FcαR | 81 ± 11 | 84 ± 10 | 76 ± 16 |
| IAd | 110 ± 11 | 139 ± 3 | 224 ± 19 |
| B7-1 | 14 ± 5 | 18 ± 3 | 5.5 ± 3 |
| B7-2 | 15 ± 2 | 0 | 0 |
| ICAM-1 | 959 ± 127 | 609 ± 187 | 573 ± 47 |
| BCR | 187 ± 11 | 221 ± 45 | 167 ± 54 |
IIA1.6 cells expressing FcαR and either WT γ chain, γ chain with IIA ITAM, or γ chain with Y → F mutation were stained for surface expression of FcαR; class II MHC (IAd); costimulatory molecules B7-1, B7-1, and ICAM-1, and surface IgG (BCR), as described in “Materials and methods.” Results are expressed as the mean fluorescence intensity (MFI) of 10 000 cells minus MFI of the isotype control. The results show the mean and SD of 3 separate experiments.
Transcription of γ chain in transfected cells.
Total cellular RNA was isolated from cells cotransfected with FcαR and WT or altered γ-chain cDNA. The presence of γ-chain message was detected by RT-PCR using γ-chain–specific primers, as described in “Materials and methods.” The γ-chain–specific PCR product was detected as shown in lane 1 (γ-chain cDNA+ control), lane 4 (FcαR + WT γ chain), lane 5 (FcαR + IIA ITAM γ chain), and lane 6 (FcαR + Y → F γ chain). The γ-chain–specific product was not detected when RNA was not added (lane 2) or with parent IIA1.6 cells (lane 3).
Transcription of γ chain in transfected cells.
Total cellular RNA was isolated from cells cotransfected with FcαR and WT or altered γ-chain cDNA. The presence of γ-chain message was detected by RT-PCR using γ-chain–specific primers, as described in “Materials and methods.” The γ-chain–specific PCR product was detected as shown in lane 1 (γ-chain cDNA+ control), lane 4 (FcαR + WT γ chain), lane 5 (FcαR + IIA ITAM γ chain), and lane 6 (FcαR + Y → F γ chain). The γ-chain–specific product was not detected when RNA was not added (lane 2) or with parent IIA1.6 cells (lane 3).
Endocytosis and catabolism of IgA aggregates
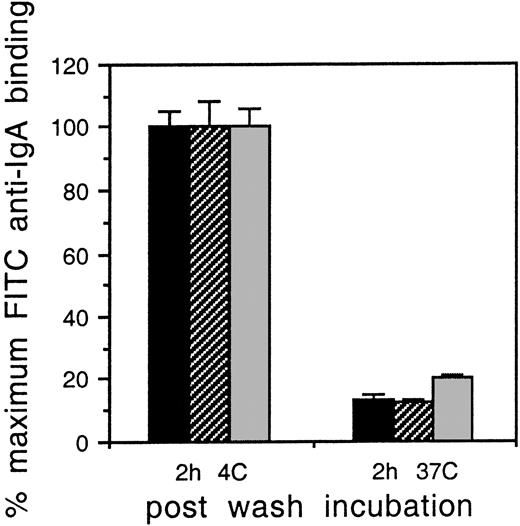
The ITAM of the γ chain is known to be required for phagocytosis,17 thus it was possible that differences in internalization of the IgA-OVA complexes accounted for the disparate levels of IgA-OVA presentation. We therefore examined the ability of the transfectants to endocytose polymeric IgA. Cells were coated with IgA, washed, and incubated at 4°C or 37°C for 1 hour, after which they were stained with FITC anti-IgA to measure surface-bound IgA. Figure 3 shows that incubation at 37°C reduced surface IgA by 63% in FcαR/WT γ chain, 64% in FcαR/IIA ITAM γ chain, and 66% in FcαR/Y → F γ chain compared to cells held at 4°C in otherwise identical conditions. The results, representative of 3 individual experiments, demonstrate that the transfectants had equal capacity for FcαR-mediated endocytosis.
Endocytosis of multimeric IgA by FcαR/γ-chain transfectants is not diminished in cells with altered γ chain.
IIA1.6 cells expressing FcαR and either WT γ chain (indicated by black bars), γ chain with IIA ITAM (indicated by cross-hatched bars), or γ chain with Y → F mutation (indicated by gray bars) were incubated with a polymeric human myeloma IgA at 4°C. After 1.5 hours, aliquots were removed, washed, and incubated at 37°C for the time period indicated. All cells were then washed and stained to detect surface-bound IgA. The results are expressed as the percentage of MFI of samples held at 4°C for 2 hours. The results are shown as the mean and SD of triplicate experiments.
Endocytosis of multimeric IgA by FcαR/γ-chain transfectants is not diminished in cells with altered γ chain.
IIA1.6 cells expressing FcαR and either WT γ chain (indicated by black bars), γ chain with IIA ITAM (indicated by cross-hatched bars), or γ chain with Y → F mutation (indicated by gray bars) were incubated with a polymeric human myeloma IgA at 4°C. After 1.5 hours, aliquots were removed, washed, and incubated at 37°C for the time period indicated. All cells were then washed and stained to detect surface-bound IgA. The results are expressed as the percentage of MFI of samples held at 4°C for 2 hours. The results are shown as the mean and SD of triplicate experiments.
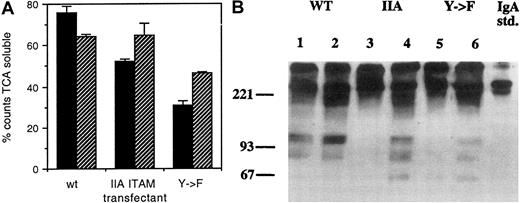
Thus, IgA complexes appeared to enter the endocytic pathway equally in the transfectants, raising the possibility that postendocytic processing differences might account for the disparity in presentation of IgA-OVA. Therefore, we examined the catabolism of 2 FcαR ligands: (1) 125I-labeled anti-IgM μ chain bound to the anti-FcαR IgM mAb (My43) and (2) polymeric IgA. Cells were coated at 4°C with My43 plus 125I-labeled anti-IgM and incubated for 8 hours at 37°C (Figure 4A). Catabolism was measured by the appearance of TCA-soluble counts in the supernatant from cells that had internalized My43 plus125I-labeled anti-IgM. In cells with WT γ chain, 75.9% of the total cell-associated counts were in the TCA soluble fraction, whereas in the cells with IIA ITAM or Y → F γ chains, this fraction contained 52.5% and 30.6% of total counts, respectively.
Catabolism of 125I-labeled anti-mIgM μ chain is diminished in transfectants with altered γ chain, but it is increased by BCR ligation.
(A) FcαR on IIA1.6 cells expressing FcαR and either WT γ chain (wt), γ chain with IIA ITAM (IIA ITAM), or γ chain with Y → F mutation (Y → F) were ligated at 4°C with the anti-FcαR mAb My43 followed by 125I-labeled antimouse IgM μ chain. The cells were then incubated at 37°C without further treatment or with additional cross-linking of BCR with antimouse IgG. After an 8-hour incubation at 37°C, supernates were harvested, and TCA-soluble supernate counts were measured as described in “Materials and methods.” The solid bars represent the amount of TCA-soluble counts released in the absence of BCR cross-linking. The hatched bars represent the amount of TCA-soluble counts released when BCR was cross-linked. Error bars indicate range of duplicate samples. The results are representative of 3 experiments. (B) IgA catabolism is defective in altered γ-chain transfectants but is restored by BCR ligation. FcαR transfectants with WT γ chain (WT, lane 1), γ chain with IIA ITAM (IIA, lane 2), or γ chain with Y → F mutation (Y → F, lane 3) were incubated with 50 μg/mL human myeloma IgA. After 4 hours the cells were washed and lysed, and intracellular IgA and IgA catabolism products were detected by SDS-PAGE and anti-IgA Western blot. Addition of 10 μg/mL anti-MIgG to the incubation mixture did not affect IgA catabolism by the WT γ-chain transfectant (lane 2), but the addition altered IgA catabolism by the IIA ITAM (lane 4) and Y → F (lane 6) γ-chain transfectants. Three separate experiments yielded the same result.
Catabolism of 125I-labeled anti-mIgM μ chain is diminished in transfectants with altered γ chain, but it is increased by BCR ligation.
(A) FcαR on IIA1.6 cells expressing FcαR and either WT γ chain (wt), γ chain with IIA ITAM (IIA ITAM), or γ chain with Y → F mutation (Y → F) were ligated at 4°C with the anti-FcαR mAb My43 followed by 125I-labeled antimouse IgM μ chain. The cells were then incubated at 37°C without further treatment or with additional cross-linking of BCR with antimouse IgG. After an 8-hour incubation at 37°C, supernates were harvested, and TCA-soluble supernate counts were measured as described in “Materials and methods.” The solid bars represent the amount of TCA-soluble counts released in the absence of BCR cross-linking. The hatched bars represent the amount of TCA-soluble counts released when BCR was cross-linked. Error bars indicate range of duplicate samples. The results are representative of 3 experiments. (B) IgA catabolism is defective in altered γ-chain transfectants but is restored by BCR ligation. FcαR transfectants with WT γ chain (WT, lane 1), γ chain with IIA ITAM (IIA, lane 2), or γ chain with Y → F mutation (Y → F, lane 3) were incubated with 50 μg/mL human myeloma IgA. After 4 hours the cells were washed and lysed, and intracellular IgA and IgA catabolism products were detected by SDS-PAGE and anti-IgA Western blot. Addition of 10 μg/mL anti-MIgG to the incubation mixture did not affect IgA catabolism by the WT γ-chain transfectant (lane 2), but the addition altered IgA catabolism by the IIA ITAM (lane 4) and Y → F (lane 6) γ-chain transfectants. Three separate experiments yielded the same result.
While measurement of release of TCA-soluble radio-label is a good measure of overall ligand degradation, it gives no information on the processing of peptides derived from the ligand. We reasoned that analysis of catabolism by Western blotting, which detects epitopes formed by the protein structure, might be a useful adjunct to measurement of TCA-soluble counts for catabolism studies. Figure 4B shows the results of an experiment (representative of 3) in which the cells were allowed to ingest polymeric IgA for 4 hours at 37°C, after which they were lysed and analyzed by SDS-PAGE and Western blot with HRP anti-IgA. In Figure 4B the FcαR/WT γ-chain lysate (lane 1) shows bands corresponding to catabolism products of IgA at approximately 80 and 100 kd. These bands are markedly reduced in lanes 3 and 5, which contain lysates of FcαR/IIA ITAM γ chain and Y → F γ chain, respectively. Incubation of these transfectants with greater IgA concentrations or for longer time periods did not restore the diminished IgA catabolism in these cells (data not shown.) Lanes 2, 4, and 6 of Figure 4B show the effect of simultaneous ligation of BCR. The appearance of bands corresponding to catabolism products in lanes 4 and 6 are discussed in detail later in this section.
We also compared the ability of the different transfectants to catabolize the BCR ligand, goat anti-mIgG. The transfectants showed equal degradation of this ligand (data not shown.) Thus, by 2 different assay methods, the impaired ability to catabolize FcαR ligands appeared specific for FcαR with altered γ chains.
Intracellular localization of FcαR ligand
A recent study on intracellular morphological changes during internalization of ligated BCR showed that the BCR signals the reorganization of late endosomes into a complex of acidified, MHC class II–rich, lamp-1+ large vesicles.18 Ligated BCR rapidly translocated to this vesicle complex, which had all the characteristics of a MHC class II peptide loading compartment (MIIC).18 Furthermore, formation of this structure was dependent on tyrosine kinase and PKC activity following BCR ligation. We examined the FcαR/γ-chain transfectants to determine whether FcαR ligation and its attendant γ-chain signaling produced a similar morphological change and transport of ligated FcαR to lamp-1+ vesicle clusters. We also addressed whether FcαR with altered γ chain was capable of inducing MIIC formation.
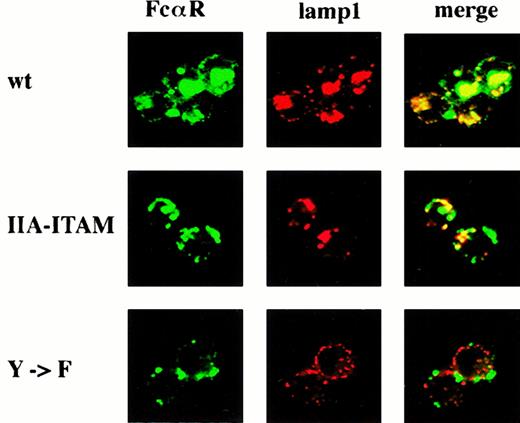
Figure 5 shows the intracellular location of anti-FcαR IgG1 mAb A77 after 30 minutes of ligand internalization. FcαR was stained with FITC anti-mIgG1 and the cells then counterstained with anti–lamp-1 visualized with Cy3-labeled secondary antibody. In the FcαR/WT γ-chain transfectant, almost all of the anti-FcαR mAbs colocalized with lamp-1 in large vesicles or vesicle clusters, which appeared yellow by the combination of the green fluorescent anti-FcαR and the red-fluorescent anti–lamp-1. A different pattern was observed in the transfectant with IIA ITAM γ chain. Lamp-1 colocalized with a proportion of the FcαR ligand in small vesicles, and the cluster of large vesicles was not apparent. Even less FcαR/lamp-1 colocalization was observed in the transfectant with Y → F γ chain, and again the formation of the vesicle cluster was not evident. When BCR was cross-linked, large lamp-1+vesicles were formed in all of the transfectants (data not shown.) Thus, there was no endogenous defect in the ability of any of the transfectants to form this structure with appropriate stimulation.
Colocalization of FcαR with lamp-1 in clustered vesicles occurs in transfectants with WT γ chain but not in those with altered γ chain.
Transfectants expressing FcαR and either WT γ chain (WT gamma), γ chain with IIA ITAM (IIA ITAM), or γ chain with Y → F mutation (Y → F) were treated with anti-FcαR IgG1 mAb and FITC anti-mIgG1 (green) at 37°C, after which they were fixed, permeabilized, and counterstained with rat anti–lamp-1 and Cy3 antirat IgG (red). Yellow staining denotes areas of colocalized FcαR and lamp-1.
Colocalization of FcαR with lamp-1 in clustered vesicles occurs in transfectants with WT γ chain but not in those with altered γ chain.
Transfectants expressing FcαR and either WT γ chain (WT gamma), γ chain with IIA ITAM (IIA ITAM), or γ chain with Y → F mutation (Y → F) were treated with anti-FcαR IgG1 mAb and FITC anti-mIgG1 (green) at 37°C, after which they were fixed, permeabilized, and counterstained with rat anti–lamp-1 and Cy3 antirat IgG (red). Yellow staining denotes areas of colocalized FcαR and lamp-1.
Signaling in response to FcαR cross-linking
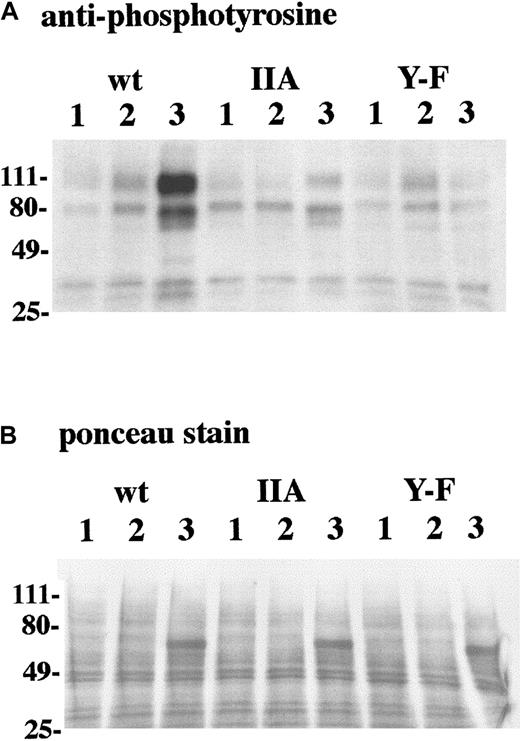
The γ-chain homodimer associates with FcR, such as FcγRI, FcγRIIIA, and FcαR, which lack signaling motifs in their cytoplasmic domain. The cytoplasmic domain of γ chain contains an ITAM, enabling these FcRs to transduce signals.17 19 Our data demonstrate that FcαR cotransfection with γ chains containing altered ITAM regions produces diminished ability to catabolize IgA and to present IgA-OVA. This led us to investigate whether FcαR signaling was also defective in these cells. Following FcαR ligation for 2 minutes, tyrosine-phosphorylated proteins were observed at approximately 100, 80, and 70 kd in lysates of the transfectant with WT γ chain (Figure 6A). Similar phosphorylated proteins of lower intensity were obtained after FcαR ligation of the transfectant with IIA ITAM γ chain. These bands were almost undetectable in lysates of the transfectant with Y → F γ chain. The same nitrocellulose membrane was stained with ponceau red, a sensitive protein stain (Figure 6B). This staining demonstrated that phosphorylation differences could not be attributed to unequal amounts of lysate between lanes. The heavy band at approximately 70 kd in lane 3 of the WT, IIA ITAM, and Y → F samples was μ chain–derived from the anti-FcαR mAb My43 used for activation. These results were reproduced in 3 independent experiments. Longer incubations before lysis did not result in appearance of phosphorylated proteins in the transfectants with altered γ chain (data not shown.) In contrast to FcαR, the amount of phosphorylation in response to BCR cross-linking was the same in all transfectants (data not shown.) These results indicated that the transfectants with altered γ chains, which were less capable of IgA catabolism, FcαR/lamp-1 colocalization, and IgA-OVA presentation, were also deficient in FcαR-mediated signaling.
Tyrosine phosphorylation after FcαR cross-linking is reduced in transfectants with altered γ chain.
(A) Transfectants expressing FcαR and either WT γ chain (WT), γ chain with IIA ITAM (IIA), or γ chain with Y → F mutation (Y → F) were treated as follows: (1) medium, (2) anti-mIgM, and (3) anti-FcαR IgM mAb + anti-IgM. Tyrosine phosphorylation in whole-cell lysate was detected by SDS-PAGE and Western blot analysis with antiphosphotyrosine. Three separate experiments yielded similar results. (B) Prior to immunoblotting with antiphosphotyrosine, the same nitrocellulose membrane shown in panel A was stained with ponceau red to ascertain equivalent loading. Details of the above are described in “Materials and methods.”
Tyrosine phosphorylation after FcαR cross-linking is reduced in transfectants with altered γ chain.
(A) Transfectants expressing FcαR and either WT γ chain (WT), γ chain with IIA ITAM (IIA), or γ chain with Y → F mutation (Y → F) were treated as follows: (1) medium, (2) anti-mIgM, and (3) anti-FcαR IgM mAb + anti-IgM. Tyrosine phosphorylation in whole-cell lysate was detected by SDS-PAGE and Western blot analysis with antiphosphotyrosine. Three separate experiments yielded similar results. (B) Prior to immunoblotting with antiphosphotyrosine, the same nitrocellulose membrane shown in panel A was stained with ponceau red to ascertain equivalent loading. Details of the above are described in “Materials and methods.”
Augmentation of IgA-OVA presentation and IgA catabolism by BCR cross-linking
A previous study by Casten and Pierce20 demonstrated that B-cell presentation of nonspecifically internalized antigen was augmented by ligating the BCR with anti-Ig. These findings suggested that signaling could augment the processing of an antigen that was not physically associated with the signaling receptor. We were interested in the possibility that BCR signaling might augment the defective presentation of IgA-OVA associated with FcαR/IIA ITAM and FcαR/Y → F γ-chain receptor complexes. Our rationale was that if signaling were to play a role in driving antigen processing, then BCR signaling might compensate for the defective signaling of FcαR in these transfectants. Indeed, we observed that presentation of FcαR-targeted OVA was enhanced in the IIA ITAM and Y → F γ-chain transfectants by treating the transfected cells with antimouse IgG at a concentration (10 μg/mL) that promoted BCR signaling (Figure7A,B).
Defective presentation of IgA-OVA is restored by BCR ligation.
IIA1.6 cells transfected with (A) FcαR + IIA ITAM γ chain or (B) FcαR + Y→F mutant γ chain were incubated with DO-11-10 T cells and increasing concentrations of IgA-complexed NIP-OVA (NIP-OVA) with (indicated by cross-hatched bars) or without (indicated by black bars) ligation of BCR with 10 μg/mL anti-mIgG. IL-2 secretion by the T cells, a measure of antigen presentation, was assayed as described in “Materials and methods.” Similar results were obtained in 3 separate experiments.
Defective presentation of IgA-OVA is restored by BCR ligation.
IIA1.6 cells transfected with (A) FcαR + IIA ITAM γ chain or (B) FcαR + Y→F mutant γ chain were incubated with DO-11-10 T cells and increasing concentrations of IgA-complexed NIP-OVA (NIP-OVA) with (indicated by cross-hatched bars) or without (indicated by black bars) ligation of BCR with 10 μg/mL anti-mIgG. IL-2 secretion by the T cells, a measure of antigen presentation, was assayed as described in “Materials and methods.” Similar results were obtained in 3 separate experiments.
We also examined the effect of BCR ligation on the ability of the FcαR transfectants with altered γ chain to catabolize FcαR ligands. Figure 4A shows that release of TCA-soluble counts was diminished by BCR ligation in the FcαR/WT γ-chain transfectant. Conversely, BCR ligation increased the release of TCA-soluble counts in transfectants with altered γ chain. The data show a trend of augmentation of ligand catabolism by BCR cross-linking in cells with altered γ chain, although statistical values cannot be assigned to duplicate observations. Figure 4B shows that lysates of FcαR/WT γ-chain cells contained bands of approximately 80 and 100 kd irrespective of whether BCR had been cross-linked during incubation (lanes 1 and 2, from left). In contrast, these bands were undetectable in cells with IIA ITAM or Y→ F γ chains (Figure 4B, lanes 3 and 5, from left), but they were detected in samples treated with anti-IgG (lanes 4 and 6, from left). Thus, by 2 assay methods, BCR signaling appeared to reverse the deficiency in catabolism of FcαR ligand in the transfectants with altered γ chains. This correlates with the observation that BCR cross-linking enhanced the presentation of IgA-OVA in the transfectants with altered γ chain.
Augmentation of nontargeted OVA presentation by BCR and FcαR
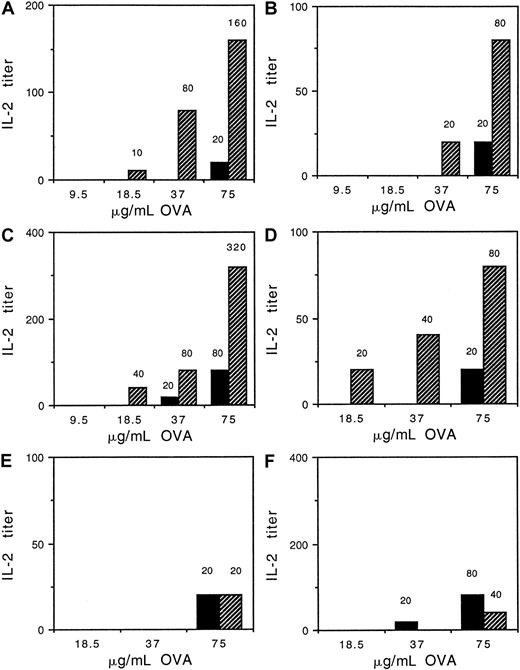
These results indicated that BCR could augment presentation of antigen without its association with BCR. This suggested that other signaling receptors, such as FcαR, might increase presentation of OVA taken up nonspecifically. To test this idea we compared the signaling-competent BCR and FcαR/WT γ chain with signaling-defective FcαR/IIA ITAM and FcαR/Y→ F γ-chain transfectants for ability to augment the presentation of low levels of nontargeted OVA. At concentrations of OVA that gave suboptimal levels of antigen presentation, cross-linking of BCR with anti-IgG enhanced presentation in all transfectants irrespective of whether they had WT or altered γ chain (Figure 8A-C). In contrast, when FcαR was cross-linked with My43 (mIgM) and anti-IgM, presentation of suboptimal amounts of OVA was enhanced only in the transfectant with WT γ chain (Figure 8D). FcαR cross-linking did not enhance OVA presentation in transfectants with IIA ITAM or Y→ F γ chain (Figure 8E,F, respectively). It should be noted that IIA1.6 cells do not express surface IgM. These results were reproduced in 3 independent experiments.
Presentation of nontargeted OVA is enhanced by BCR and FcαR/WT γ-chain cross-linking but not by cross-linking of FcαR with altered γ chain.
IIA1.6 cells expressing FcαR and either (A) WT γ chain, (B) γ chain with IIA ITAM, or (C) γ chain with Y → F mutation were incubated with increasing concentrations of OVA with (indicated by cross-hatched bars) or without (indicated by black bars) 10 μg/mL antimouse IgG in the presence of DO-11-10 T cells. Transfectants with FcαR and either (D) WT γ chain, (E) γ chain with IIA ITAM, or (F) γ chain with Y → F mutation were also incubated with OVA with (indicated by cross-hatched bars) or without (indicated by black bars) 1:5 diluted My43 anti-FcαR IgM hybridoma supernatant and 10 μg/mL anti– mIgM in the presence of DO-11-10 T cells. Supernatants were harvested and assayed for IL-2, a measure of antigen presentation, as described in “Materials and methods.” Numbers above histogram bars denote IL-2 titers. A difference in titer of 4-fold or more is significant. Similar results were obtained in 3 separate experiments.
Presentation of nontargeted OVA is enhanced by BCR and FcαR/WT γ-chain cross-linking but not by cross-linking of FcαR with altered γ chain.
IIA1.6 cells expressing FcαR and either (A) WT γ chain, (B) γ chain with IIA ITAM, or (C) γ chain with Y → F mutation were incubated with increasing concentrations of OVA with (indicated by cross-hatched bars) or without (indicated by black bars) 10 μg/mL antimouse IgG in the presence of DO-11-10 T cells. Transfectants with FcαR and either (D) WT γ chain, (E) γ chain with IIA ITAM, or (F) γ chain with Y → F mutation were also incubated with OVA with (indicated by cross-hatched bars) or without (indicated by black bars) 1:5 diluted My43 anti-FcαR IgM hybridoma supernatant and 10 μg/mL anti– mIgM in the presence of DO-11-10 T cells. Supernatants were harvested and assayed for IL-2, a measure of antigen presentation, as described in “Materials and methods.” Numbers above histogram bars denote IL-2 titers. A difference in titer of 4-fold or more is significant. Similar results were obtained in 3 separate experiments.
It has been reported that IIA1.6 cells are capable of secreting IL-2 in response to certain receptor cross-linking treatments.12We did not observe IL-2 secretion by any of our transfectants in response to either BCR or FcαR cross-linking alone when using the same conditions under which we observed enhanced presentation of nonreceptor-targeted OVA. In particular, FcαR cross-linking failed to elicit IL-2 production by the FcαR + WT γ-chain transfectant in 5 independent experiments.
Discussion
It is known that Fc receptors mediate the enhanced presentation of IgG-complexed antigen; however, the intracellular mechanisms leading to the enhancement of presentation are not fully understood.21Two cellular processes involved in presentation are the targeting of antigen to processing compartments by FcR motifs and the intracellular trafficking of antigen. We hypothesize that FcαR mediates enhanced presentation of IgA-OVA because signals generated by the associated γ chain reconfigure these processes in the APC.
FcαR-mediated presentation was diminished by alteration of the γ chain; however, there was no loss of endocytosis, thereby indicating that differences in antigen presentation stem from events beyond internalization. Diminished presentation by the Y → F γ-chain transfectant indicates a role for ITAM in enhanced presentation. Previous studies on a chimeric IgG receptor bearing a γ-chain cytoplasmic domain also showed a link between ITAM and presentation22; however, this receptor consisted of a single chain with the cytoplasmic domain of γ chain. Mutation of either tyrosine in the γ-chain ITAM abrogated endocytosis and thus presentation of IgG-antigen, which suggests that the tyrosine residues are important for internalization in absence of a receptor α chain. In contrast, the receptor complex FcαR/Y → F γ chain, in which the tyrosine mutant γ chain is paired with FcαR α chain, supported normal endocytosis. This suggests that internalization can be supported by the FcαR cytoplasmic domain. The FcαR/γ-chain interaction is unusual among γ-chain–associated FcR because it is of high affinity and not disrupted under conditions that dissociate other FcR from γ chains.7 Mutation of the transmembrane arginine in FcαR completely abolishes γ-chain association,23 suggesting that transmembrane interaction alone drives FcαR /γ-chain association and that the cytoplasmic tail of γ chain plays no role in the association.
Cells transfected with FcαR and WT γ chain supported greater FcαR-mediated catabolism and presentation than cells transfected with FcαR and IIA ITAM γ chain. These findings corroborate previous observations11 that a chimeric FcγRIIA with the γ-chain cytoplasmic domain promoted greater presentation of IgG antigen than WT FcγRIIA. Our findings are further supported by the report that chimeric FcγRI with the cytoplasmic domain of γ chain mediated lysosomal delivery and catabolism of ligand, whereas chimeric FcγRI with the cytoplasmic domain of FcγRIIA did not.24 This functional disparity was attributed to signaling differences. The γ-chain and FcγRIIA ITAMs differ structurally in that there are 12 intervening residues between YXXL sequences in the FcγRIIA ITAM compared to 7 residues in the γ chain. It is likely that this forms the basis for signaling differences.
We observed a correlation between the ability of FcαR/γ chain to signal and to enhance IgA-OVA presentation. The FcαR/WT γ-chain transfectant demonstrated good signaling and antigen presentation, whereas in the FcαR/IIA ITAM γ-chain transfectant, both functions were poor. In the FcαR/Y → F γ-chain transfectant, signaling was undetectable and presentation was also poor. Our observations correlate with previous studies showing that FcαR signaling was diminished by γ-chain tyrosine mutation.25 The association between FcαR signaling and enhanced antigen presentation is consistent with reports linking BCR signaling to augmented antigen processing26 and with the finding that protein kinase inhibitors blocked BCR-enhanced processing.27 Our data suggest that receptor signaling is necessary to induce the processing compartment. Signaling may also assist trafficking of the receptor-antigen complex to this site, which is consistent with a report28 that kinase inhibitors decreased passage of BCR-internalized antigens to the class II loading compartment.
We observed inability of altered γ-chain transfectants to catabolize IgA aggregates, suggesting that signaling may impact processing. Endocytosed material is degraded during trafficking through vesicles of the endocytic pathway.29 Antigen first enters early endosomes that are mildly acidic and whose primary function is sorting. Most degradation occurs in late endosomes and in lysosomes, which contain hydrolytic enzymes. Cross-linking of FcRs, which otherwise recycle from early endosomes to the cell surface, promoted their targeting to lysosomes.30 The reduced catabolism of FcαR ligands in the altered γ-chain transfectants indicates that ligand failed to enter the site of optimal degradation. Transport into the optimal catabolic environment may be hindered by lack of appropriate downstream phosphorylation or by incorrect receptor configuration.
Confocal microscopy showed differences in the distribution of internalized FcαR and its colocalization with the late endosome/lysosomal marker lamp-1. In the WT γ-chain transfectant, the majority of FcαR appeared in a large perinuclear cluster of vesicles that were positive for lamp-1. By contrast, although FcαR and lamp-1 colocalization was also seen in clustered small vesicles in the FcαR/IIA ITAM γ chain, a large proportion of FcαR remained in discrete vesicles that were lamp-1−. In the Y → F γ-chain mutant, minor amounts of colocalization of lamp-1 and FcαR were seen in small vesicles, but again, much of the FcαR appeared to be separate from lamp-1. A previous report18 showed that ligation of BCR resulted in its rapid internalization into clustered vesicles that were lamp-1+ and MHC class II+and of acidic pH. This vesicle cluster was characterized as a class II peptide-loading compartment or MIIC. These authors concluded that the lamp-1+ aggregates are the major site for ligand catabolism and class II loading. Correspondingly, in the WT γ-chain transfectant we observed that vesicle aggregation correlated with efficient FcαR ligand catabolism and enhanced presentation. Transfectants with altered γ chains failed to induce lamp-1 vesicle aggregates, which correlated with their inability to present IgA-OVA or catabolize ligand efficiently.
A role of signaling in receptor-enhanced antigen presentation was further suggested by our observation that signals associated with enhanced antigen presentation can be provided in trans to the receptor that internalizes the antigen. BCR cross-linking elevated the presentation of IgA-OVA by transfectants with altered γ chains. This appeared to occur through an effect on antigen processing because BCR cross-linking in the altered γ-chain transfectants caused an increase in the catabolism of FcαR ligands in these cells. A model in which γ-chain structure only serves to target FcαR-associated antigen to the processing compartment appears insufficient to explain these data. Additionally, we observed that in the signaling-competent WT γ-chain transfectant, FcαR cross-linking enhanced presentation of nontargeted OVA, whereas in the signaling-deficient transfectants, FcαR cross-linking was without effect. This further demonstrates an association between signaling and increased processing activity.
In summary, we propose that the FcαR /γ-chain complex serves to do more than capture IgA-antigen complexes. It appears that γ chain of FcαR links receptors to a particular endocytic route and metabolic fate. Cross-linking of the receptor by the IgA complex promotes signaling that leads to formation of lamp-1+ vesicle clusters into which ligated FcαRs are transported, resulting in increased degradation of captured antigen. FcαR and BCR signaling also have amplifying effects on the processing of antigen that is not physically bound to the receptor.
Supported by grants AI 22816, GM52736, and AI35306 from the National Institutes of Health, Bethesda, MD, and by grant 901-12-214 from NWO (Netherlands Organization for Scientific Research), Den Haag, The Netherlands.
Submitted April 4, 2000; accepted August 25, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Li Shen, Department of Microbiology, Dartmouth Medical School, Dartmouth-Hitchcock Medical Center, 1 Medical Center Dr, Lebanon, NH 03756; e-mail: lilian.shen@dartmouth.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal