Abstract
The organization and expression of the BCL-6 gene in normal and neoplastic thymic T cells has not been fully determined. We examined 8 precursor T-cell lymphoblastic lymphomas (T-LBLs) and found significant BCL-6 expression in 4 cases. Three of the BCL-6+ cases expressed a common thymocyte phenotype (CD4+, CD8+), and one expressed a precursor thymocyte phenotype (CD4−, CD8−). In 6 cases evaluated, including those expressing BCL-6, molecular analyses demonstrated a germline configuration of the BCL-6 gene and a wild-type BCL-6 gene first exon-intron boundary region. We also evaluated 12 normal prenatal and postnatal thymuses for BCL-6 protein. BCL-6 was expressed by most cortical thymocytes and by scattered medullary thymocytes. BCL-6+ cortical and medullary thymocytes also expressed CD2, CD3, CD4, CD5, CD7, or CD8. We further analyzed the pattern of BCL-2 and BCL-XL expression and their coexpression with BCL-6 in normal thymus and T-LBL and compared it to that of follicle centers of reactive lymph nodes and follicular lymphoma. BCL-6+ cortical thymocytes coexpressed BCL-XLbut not BCL-2. All 4 BCL-6+ T-LBLs and 4 BCL-6− T-LBLs coexpressed BCL-2 and BCL-XL. Conceivably, T-LBLs may arise through clonal expansion of cortical thymocytes normally expressing the BCL-6 protein. The pattern of BCL-6, BCL-2, and BCL-XL expression in cortical thymocytes is highly reminiscent of germinal centers, and the abnormal coexpression of BCL-2, BCL-XL, and BCL-6 in T-LBL is analogous to coexpression in follicle center cell lymphomas, suggesting that coexpression of these anti-apoptotic genes may contribute to the pathogenesis of T-LBL.
Introduction
BCL-6 is a transcriptional repressor belonging to the POZ/zinc finger family of transcription factors that is implicated in normal lymphoid development and lymphomagenesis.1Recent in vitro studies suggest that BCL-6 may function as an anti-apoptotic molecule.2,3 The BCL-6 gene was identified because of its involvement in chromosomal translocations affecting band 3q27, which is a frequent break-point site in diffuse large B-cell lymphomas (DLBCLs).4-8 Rearrangements of this gene can be identified by Southern blot analysis in 30% to 45% of DLBCLs, in 6% to 10% of follicular lymphomas, and in 20% of acquired immunodeficiency syndrome (AIDS)-related non-Hodgkin's lymphomas (NHLs).9-12 Most rearrangement break points cluster within a 4-kilobase (kb) region spanning the BCL-6 promoter and first noncoding exon, resulting in the fusion of BCL-6 coding sequences (exons 2-10) to heterologous promoters from other chromosomes, leading to deregulated expression by the mechanism of promoter substitution.13
Subsequent analysis of BCL-6 has revealed the presence of point mutations and/or small deletions in 70% of DLBCLs, 45% of follicular lymphomas, and 58% of AIDS-associated NHLs.14,15 BCL-6 point mutations have also been identified in 43% of polymorphic post-transplantation lymphoproliferative disorders and 90% of post-transplantation lymphoproliferative disorders classified as NHL or multiple myeloma.16 These mutations occur within a 4-kb region spanning the first exon but tend to cluster in the 5′ region of the first intron and overlap with the major cluster of chromosomal break points. Analyses of both BCL-6 gene rearrangements and mutations have shown that structural alterations in the 5′ noncoding region of BCL-6 are present in virtually all cases of DLBCL and in most cases of follicular and AIDS-related NHLs.14,15 These findings imply that BCL-6 may contribute to B-cell lymphomagenesis, presumably by altering BCL-6 gene expression. BCL-6 expression was also reported in NHLs in the absence of any genetic alterations, suggesting the existence of other as yet unknown regulatory mechanisms resulting in its abnormal expression.17
BCL-6 protein expression has also been reported in CD30+T-cell anaplastic large cell lymphomas and in some neoplastic cells in angioimmunoblastic T-cell lymphomas,18 19 but structural alterations in the BCL-6 gene have not been reported in T-cell malignancies.
In normal lymphoid tissue, BCL-6 is preferentially expressed by germinal center B cells but not by immature B-cell precursors or differentiated plasma cells.17,20 Within the T-cell compartment, BCL-6 expression is found in CD4+ T cells within germinal centers and in scattered T cells in the perifollicular areas.17,21,22 BCL-6 also has been reported to be detectable in cortical thymocytes.17,21 23 However, no detailed immunohistochemical studies relating BCL-6 expression to discrete stages of thymocyte development have been undertaken. Similarly, the pattern of expression and genetic integrity of the BCL-6 gene in neoplastic thymic cells have not yet been fully characterized. Therefore, the goal of these studies was to assess the presence of structural alterations of the BCL-6 gene in precursor T-cell lymphoblastic lymphomas (T-LBLs) and analyze BCL-6 protein expression in these lymphomas as well as prenatal and postnatal human thymus to better understand its role in normal thymic ontogeny and possible implications for T-LBL lymphomagenesis. In addition, to gain additional insight into the possible role of other anti-apoptotic molecules in thymic lymphomagenesis, we analyzed the expression of BCL-2 and BCL-XL in T-LBLs and selected thymuses and determined their coexpression with BCL-6. Finally, we compared the expression of BCL-6, BCL-2, and BCL-XL in normal thymus and T-LBL to that of follicle centers of reactive lymph nodes and follicular lymphoma to determine whether T-LBL and follicular lymphoma share similarities in the abnormal pattern of their coexpression. Abnormal coexpression of BCL-6, BCL-2, and BCL-XL in T-LBLs as well as follicular lymphoma might imply a similar contribution of these anti-apoptotic genes in the pathogenesis of these 2 types of lymphoma.
Materials and methods
Samples
Eight T-LBLs and 12 normal human thymuses (14 weeks gestation to 13 years of age) were used for these studies. The cases of T-LBL were accessioned and processed in the Immunopathology Laboratory of New York-Presbyterian Hospital. Frozen tissue stored at −70°C from 7 T-LBLs and formalin-fixed, paraffin-embedded tissue from all cases were used in this study. The diagnosis of T-LBL was based on correlative morphologic, immunophenotypic, and genotypic analysis.24The fetal thymuses were obtained from surgical specimens of medically indicated abortions. The postnatal thymuses had been removed during cardiothoracic surgical procedures. In addition, formalin sections of 5 reactive lymph nodes and 6 cases of follicular lymphoma were used for study.
Immunohistochemistry
Immunohistochemical staining was performed using a TechMate 500 automated immunostainer (Ventana Medical Systems, Tucson, AZ). Both frozen tissue and formalin-fixed, paraffin-embedded tissue sections were used for immunophenotyping of the T-LBLs, while immunostaining was performed only on formalin-fixed tissue sections of the thymuses, reactive lymph nodes, and follicular lymphomas. Immunostaining on paraffin tissue sections was performed according to a modified MIP protocol (Ventana Medical Systems) using the ChemMate ABC Peroxidase Secondary Detection System (Ventana Medical Systems) or the Cap-Plus Peroxidase Detection System based on the labeled streptavidin-biotin method (Zymed Laboratory, South San Francisco, CA). Immunohistochemical analysis of frozen tissue sections was performed using the Ventana frozen tissue staining protocol (Ventana Medical Systems) and the ABC Vector Elite Peroxidase Detection System (Vector Laboratories, Burlingame, CA). Monoclonal and polyclonal antibodies to the following antigens were used for immunophenotyping and lineage assignment of the T-LBLs and thymuses: CD1a (010), CD19 (B4; Coulter-Immunotech, Miami, FL), CD2 (T11), polyclonal CD3, CD8 (C8/144B), CD20 (L26), CD30 (Ber-H2), CD45RO (UCHL1), Ki-67 (Ki-67), BCL-2 (124), BCL-6 (PG-B6; Dako Corp, Carpinteria, CA), CD3 (Leu4), CD4 (Leu-3a), CD5 (Leu1), CD7 (Leu9), CD38 (Leu17; BD Biosciences, San Jose, CA), CD2 (AB75), CD4 (1F6), CD5 (4C7), CD7 (CD7-272), CD10 (56C6), CD38 (AT13/5; Novocastra Laboratories, Ltd, Newcastle upon Tyne, United Kingdom), CD34 (clone QBEnd/10; BioGenex, San Ramon, CA), TdT (Supertechs, Bethesda, MD), TCRαβ (βF1), TCRγδ (TCRγδ; Endogen, Woburn, MA), Ki-67 (7B11), and BCL-XL(2H12; Zymed Laboratory). Prior to staining, frozen sections were fixed in acetone for 10 minutes. Paraffin tissue sections were pretreated in a pressure cooker using 10 mM citrate buffer, pH 6.0 (TdT, CD1a, CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD20, CD34, CD38, CD45RO, Ki-67, BCL-2, BCL-6, BCL-XL), in a microwave using 1 mM ethylenediaminetetraacetic acid (EDTA) buffer, pH 8.0 (CD30), or with 0.1% trypsin, pH 7.8, at 37°C for 10 minutes (TCRαβ).
Immunohistochemistry for BCL-6
Formalin-fixed, paraffin-embedded tissue sections of all T-LBL cases, as well as sections of prenatal and postnatal thymuses, were evaluated for BCL-6 expression using monoclonal antibody PG-B6 (Dako) and the Cap-Plus Peroxidase Detection System (Zymed Laboratory) according to the modified MIP protocol. Antigen retrieval was performed in a pressure cooker using 10 mM citrate buffer, pH 6.0. T-LBL cases were considered positive when more than 10% of the neoplastic cells showed nuclear staining, as previously described.18In addition, staining intensity and distribution of BCL-6–expressing neoplastic cells and thymocytes was also evaluated.
Two-color immunohistochemistry
To define the phenotypic characteristics of BCL-6–expressing tumor cells and thymocytes, all T-LBL cases expressing BCL-6 and selected thymuses were double stained for BCL-6 and CD1a, CD2, CD3, CD4, CD5, CD7, or CD8. In addition, selected cases of T-LBL expressing BCL-6 and selected thymuses, as well as reactive lymph nodes and cases of follicular lymphoma, were double stained for BCL-6 and BCL-2 or BCL-XL.
Paraffin tissue sections were stained for BCL-6 as described above, after which antigen retrieval was again performed in a pressure cooker with a 10 mM citrate buffer at pH 6. The expression of the second antigen was determined with the ChemMate Alkaline Phosphatase Detection System (Ventana Medical Systems) using the modified MIP protocol. The alkaline phosphatase reaction was developed by BT Red Reagent Substrate (Ventana Medical Systems) or Vector Blue Alkaline Phosphatase Substrate Kit III (Vector Laboratories).
Three-color immunohistochemistry
To evaluate the expression of BCL-6 by CD4+ and CD8+ cortical thymocytes, paraffin sections of selected thymuses were stained by 3-color immunohistochemistry for BCL-6, CD4, and CD8 using modified MIP protocol (Ventana Medical Systems). Before each round of staining, sections were retrieved in a pressure cooker using 10 mM citrate buffer, pH 6.0. BCL-6 protein was detected using LSAB2 Alkaline Phosphatase Kit (Dako). The alkaline phosphatase reaction was developed employing BT Red Alkaline Phosphatase Substrate (Ventana Medical Systems). CD4 antigen was detected using EnVision Peroxidase Mouse Detection System (Dako). Peroxidase reaction was developed employing Liquid DAB Substrate Chromogen System (Dako). CD8 antigen was detected with alkaline phosphatase antialkaline phosphatase (APAAP) method25 using APAAP mouse monoclonal and goat antimouse immunoglobulins (Dako). Alkaline phosphatase reaction was developed employing Vector Blue Alkaline Phosphatase Substrate Kit III (Vector Laboratories). Incubation times of the paraffin sections with reagents of each individual detection system were adjusted according to the manufacturers' protocols.
Southern blot hybridization analysis for BCL-6 gene rearrangements
Genomic DNA was extracted from frozen tissue blocks using a salting-out procedure.26 Five-microgram aliquots of genomic DNA were digested with BamHI and XbaI, respectively (Boehringer-Manheim, Indianapolis, IN), electrophoresed in 0.8% agarose gels, denatured with alkali, neutralized, and transferred to nitrocellulose filters according to Southern. The filters were hybridized to a 32P-labeled BCL-6 probe (Sac4.0) as previously described.4
Single-strand conformation polymorphism analysis
Five sets of primers, spanning a 741–base pair region that is altered in close to 70% of DLBCLs, were used for single-strand conformation polymorphism analysis. These sets have been designated E1.9 through E1.13 as follows: E1.9: 5′-GGGTTCTTAGAAGTGGTG-3′ and 5′-CAAAGCATTTGGCAAGAG-3′; E1.10: 5′-CTCTTGCCAAATGCTTTG-3′ and 5′-TAATTCCCCTCCTTCCTC-3′; E1.11: 5′-AGGAAGGAGGGGAATTAG-3′ and 5′-AAGCAGTTTGCAAGCGAG-3′; E1.12: 5′-TTCTCGCTT- GCAAACTGC-3′ and 5′-CACGATACTTCATCTCATC-3′; E1.13: 5′-GATGAGATGAAGTATCGTG-3′ and 5′-ACACTGAAAGGCATCGCA-3′. Polymerase chain reactions were performed with 100 ng genomic DNA, in the presence of 10 pmol of each primer, 25 μM deoxyribonucleoside triphosphate, 1 μCi [α-32P]deoxycytidine triphosphate (NEN; specific activity, III TBq/mmol), and 1.5 mM MgCl2. Thirty cycles of denaturation (94°C), annealing (56°C for E1.10, 58°C for E1.11, and 54°C for E1.10), and extension (72°C) were performed. The reaction mixture (2 μL) was diluted 1:25 in 0.1% sodium dodecyl sulfate, 10 mM EDTA, and further mixed 1:1 with a sequencing stop solution (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, and 0.05% xylene cyanol). Samples were heat-denatured and electrophoresed in a 6% acrylamide-TBE (Tris-borate-EDTA) gel containing 10% glycerol.
Results
Expression of BCL-6 in T-LBL
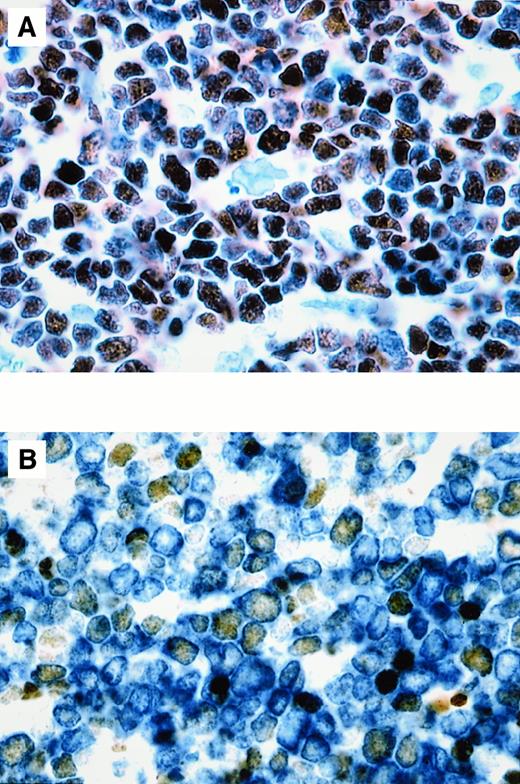
The immunophenotypic profiles of the 8 T-LBLs corresponded to the common or precursor thymocyte phenotype (Table1). Three T-LBLs were positive for both CD4 and CD8 (double-positive), 4 T-LBLs were CD4−, CD8− (double-negative), and one case was positive only for CD4 (immature single-positive; CD4+, CD8−, TCRαβ−, TCRγδ−). The BCL-6 protein was expressed in 4 of 8 (50%) T-LBLs analyzed. Three of these cases were double-positive T-LBL, corresponding to a common thymocyte phenotype, and one case was double-negative T-LBL, corresponding to a precursor thymocyte phenotype. BCL-6 protein was expressed by more than 90%, 80%, and 50% of malignant T lymphoblasts in 3 double-positive T-LBL cases, respectively (Figure 1A), and by 50% of malignant T lymphoblasts in the one double-negative T-LBL case (Figure1B). In all positive cases, anti–BCL-6 monoclonal antibody diffusely stained the nuclei of malignant T lymphoblasts with moderate to strong intensity. Two-color staining demonstrated that the malignant T lymphoblasts of all BCL-6+ T-LBLs expressed both BCL-6 and CD1a, CD2, CD3, CD5, CD7 (shown for CD3 in Figure 1B) and, in addition, in 3 double-positive T-LBLs, CD4 and CD8. All BCL-6+ T-LBLs were confirmed to have T-cell receptor (TCR) αβ or γ chain rearrangements by Southern blot, polymerase chain reaction analysis, or TCRβ protein expression by immunohistochemistry, except for the single BCL-6+, double-negative (CD4−, CD8−) T-LBL, which showed a germline configuration of the TCRβ and γ chain genes (data not shown). Three of 4 BCL-6− T-LBLs displayed a double-negative phenotype (CD4−, CD8−) of precursor thymocytes; one BCL-6− T-LBL displayed a phenotype of immature single-positive (CD4+, CD8−) precursor thymocytes.
Immunophenotypic characteristics of T-LBL
| Case No. Age, y/Sex . | BCL-6 . | CD1a . | CD2 . | CD3 . | CD4 . | CD5 . | CD7 . | CD8 . | CD10 . | CD19 . | CD20 . | CD30 . | CD34 . | CD38 . | CD45RO . | TCRαβ . | TCRγδ . | TdT . | Ki-67 . | BCL2 . | BCLXL . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | + | + | + | − | − | − | − | − | + | + | + | − | + | + | + | ± |
| 37/M | > 90% | cyt | 40% | > 90% | |||||||||||||||||
| 2 | + | + | + | + | + | + | + | + | − | − | − | − | − | + | + | + | − | + | + | + | ± |
| 17/M | 80% | cyt | 100% | > 90% | |||||||||||||||||
| 3 | + | + | + | + | + | + | + | + | − | − | − | − | − | + | + | + | − | + | + | + | ± |
| 36/M | > 50% | cyt | |||||||||||||||||||
| 4 | + | + | + | + | − | + | + | − | − | − | − | − | − | + | + | − | − | + | + | + | + |
| 7/F | 50% | 40% | cyt | 20% | > 90% | ||||||||||||||||
| 5 | − | + | + | + | − | + | + | − | − | − | − | − | − | ND | + | − | ND | + | + | + | + |
| 8/M | 40% | cyt | 90% | 70% | |||||||||||||||||
| 6 | − | + | − | + | − | + | + | − | − | − | − | − | − | + | − | − | ND | + | + | + | + |
| 7/F | 20% | cyt | 25% | 50% | > 80% | ||||||||||||||||
| 7 | − | + | + | + | − | + | + | − | − | − | − | − | + | + | − | − | − | + | + | + | ± |
| 19/F | cyt | 20% | > 90% | ||||||||||||||||||
| 8 | − | − | + | + | + | + | + | − | − | − | − | − | − | + | − | − | − | + | + | + | + |
| 51/M | cyt < 5% | 80% | > 80% |
| Case No. Age, y/Sex . | BCL-6 . | CD1a . | CD2 . | CD3 . | CD4 . | CD5 . | CD7 . | CD8 . | CD10 . | CD19 . | CD20 . | CD30 . | CD34 . | CD38 . | CD45RO . | TCRαβ . | TCRγδ . | TdT . | Ki-67 . | BCL2 . | BCLXL . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | + | + | + | − | − | − | − | − | + | + | + | − | + | + | + | ± |
| 37/M | > 90% | cyt | 40% | > 90% | |||||||||||||||||
| 2 | + | + | + | + | + | + | + | + | − | − | − | − | − | + | + | + | − | + | + | + | ± |
| 17/M | 80% | cyt | 100% | > 90% | |||||||||||||||||
| 3 | + | + | + | + | + | + | + | + | − | − | − | − | − | + | + | + | − | + | + | + | ± |
| 36/M | > 50% | cyt | |||||||||||||||||||
| 4 | + | + | + | + | − | + | + | − | − | − | − | − | − | + | + | − | − | + | + | + | + |
| 7/F | 50% | 40% | cyt | 20% | > 90% | ||||||||||||||||
| 5 | − | + | + | + | − | + | + | − | − | − | − | − | − | ND | + | − | ND | + | + | + | + |
| 8/M | 40% | cyt | 90% | 70% | |||||||||||||||||
| 6 | − | + | − | + | − | + | + | − | − | − | − | − | − | + | − | − | ND | + | + | + | + |
| 7/F | 20% | cyt | 25% | 50% | > 80% | ||||||||||||||||
| 7 | − | + | + | + | − | + | + | − | − | − | − | − | + | + | − | − | − | + | + | + | ± |
| 19/F | cyt | 20% | > 90% | ||||||||||||||||||
| 8 | − | − | + | + | + | + | + | − | − | − | − | − | − | + | − | − | − | + | + | + | + |
| 51/M | cyt < 5% | 80% | > 80% |
Expression of the corresponding antigens is expressed as positive (+), negative (−), and variable (±). For some antigens, the approximate percentage of positive cells within the tumor cell population is provided.
ND indicates not done; cyt, cytoplasmic staining.
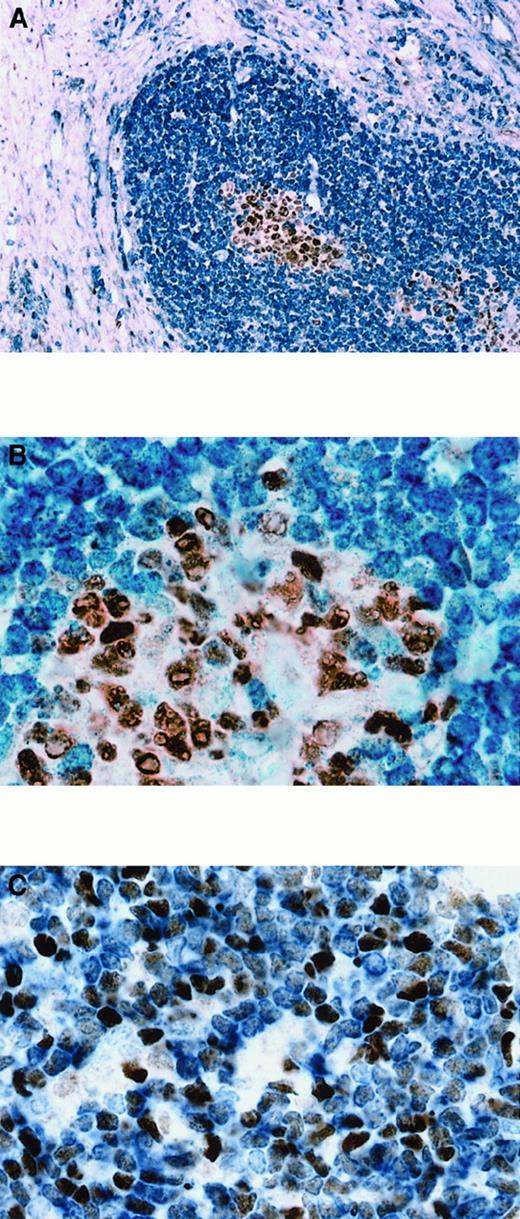
BCL-6 expression in T-LBL.
The percentage of BCL-6+ cells in T-LBL ranged from 50% to more than 90%. (A) Case No. 1. More than 90% of tumor cells, corresponding phenotypically to a double-positive (CD4+, CD8+) common thymocyte, show nuclear expression of BCL-6. (B) Double-staining for BCL-6 and CD3 in case No. 4, corresponding phenotypically to a double-negative (CD4−, CD8−) precursor thymocyte, shows that approximately 50% of the CD3+ malignant lymphoblasts (cytoplasmic, blue) express BCL-6 (nuclear, brown). Original magnification × 1000 (A), × 1000 (B).
BCL-6 expression in T-LBL.
The percentage of BCL-6+ cells in T-LBL ranged from 50% to more than 90%. (A) Case No. 1. More than 90% of tumor cells, corresponding phenotypically to a double-positive (CD4+, CD8+) common thymocyte, show nuclear expression of BCL-6. (B) Double-staining for BCL-6 and CD3 in case No. 4, corresponding phenotypically to a double-negative (CD4−, CD8−) precursor thymocyte, shows that approximately 50% of the CD3+ malignant lymphoblasts (cytoplasmic, blue) express BCL-6 (nuclear, brown). Original magnification × 1000 (A), × 1000 (B).
Expression of BCL-6 in normal thymus
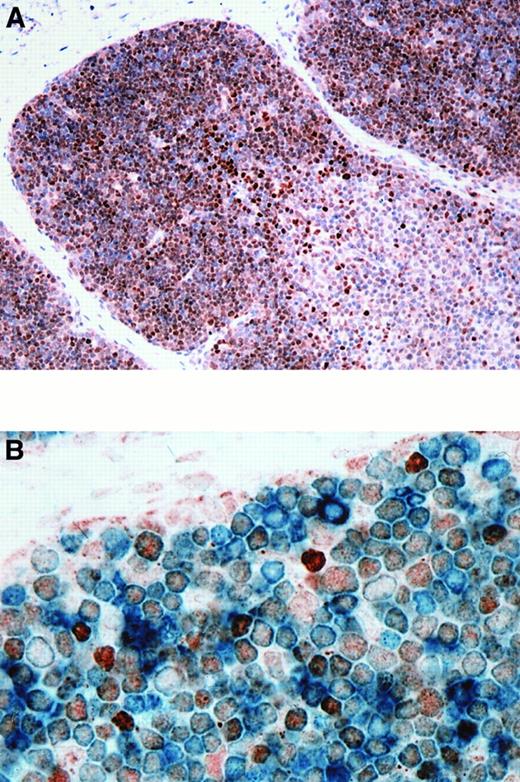
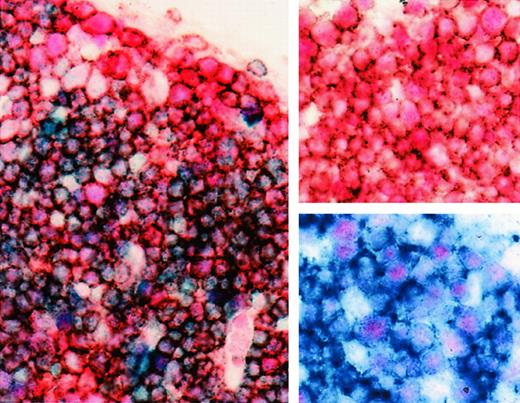
The BCL-6 protein was expressed by 60% to 90% of cortical thymocytes and by 10% to 20% of the medullary thymocytes in the 6 normal fetal thymuses analyzed (Figure2A). BCL-6 was expressed as early as 14 weeks of gestational age. The pattern of expression was similar in the 6 postnatal thymuses. Most cortical thymocytes expressed both BCL-6 and CD1a, CD2, CD3, CD4, CD5, CD7, or CD8 (shown for CD3 in Figure 2B). However, a small fraction of BCL-6–expressing cortical thymocytes were negative for these antigens. The rare BCL-6+ cells in the medulla also expressed CD2, CD3, CD4, CD5, CD7, or CD8 (not shown). Three-color staining for BCL-6, CD4, and CD8 showed that BCL-6 was expressed by most double-positive (CD4+, CD8+) cortical thymocytes (Figure 3).
BCL-6 expression in normal thymus.
(A) BCL-6 was expressed by 60% to 90% of thymocytes in the cortex. Less than 20% of the cells in the medulla were BCL-6+. (B) Double staining for BCL-6 and CD3 in thymus shows that most CD3+ cortical thymocytes (cytoplasmic, blue) express BCL-6 (nuclear, brown). BCL-6+ thymocytes also express CD1a, CD2, CD4, CD5, CD7, and CD8 (21 weeks gestational age). Original magnification × 200 (A), × 1000 (B).
BCL-6 expression in normal thymus.
(A) BCL-6 was expressed by 60% to 90% of thymocytes in the cortex. Less than 20% of the cells in the medulla were BCL-6+. (B) Double staining for BCL-6 and CD3 in thymus shows that most CD3+ cortical thymocytes (cytoplasmic, blue) express BCL-6 (nuclear, brown). BCL-6+ thymocytes also express CD1a, CD2, CD4, CD5, CD7, and CD8 (21 weeks gestational age). Original magnification × 200 (A), × 1000 (B).
BCL-6 expression in double-positive (CD4+, CD8+) cortical fetal thymocytes.
Three-color staining for BCL-6 (nuclear, pink-red), CD4 (cytoplasmic, brown), and CD8 (cytoplasmic, blue) of cortical thymocytes. Most cortical thymocytes expressing BCL-6 (nuclear, pink-red) are CD4+, CD8+ double-positive thymocytes (cytoplasm, muddy brown-blue). Insert, upper right corner, shows double staining for BCL-6 (nuclear, pink-red) and CD4 (cytoplasmic, brown) of cortical thymocytes; insert, lower right corner, shows double staining for BCL-6 (nuclear, pink-red) and CD8 (cytoplasmic, blue) (21 weeks gestational age). Original magnification × 1000.
BCL-6 expression in double-positive (CD4+, CD8+) cortical fetal thymocytes.
Three-color staining for BCL-6 (nuclear, pink-red), CD4 (cytoplasmic, brown), and CD8 (cytoplasmic, blue) of cortical thymocytes. Most cortical thymocytes expressing BCL-6 (nuclear, pink-red) are CD4+, CD8+ double-positive thymocytes (cytoplasm, muddy brown-blue). Insert, upper right corner, shows double staining for BCL-6 (nuclear, pink-red) and CD4 (cytoplasmic, brown) of cortical thymocytes; insert, lower right corner, shows double staining for BCL-6 (nuclear, pink-red) and CD8 (cytoplasmic, blue) (21 weeks gestational age). Original magnification × 1000.
Expression of BCL-2 and BCL-XL in normal thymus and T-LBL and coexpression with BCL-6
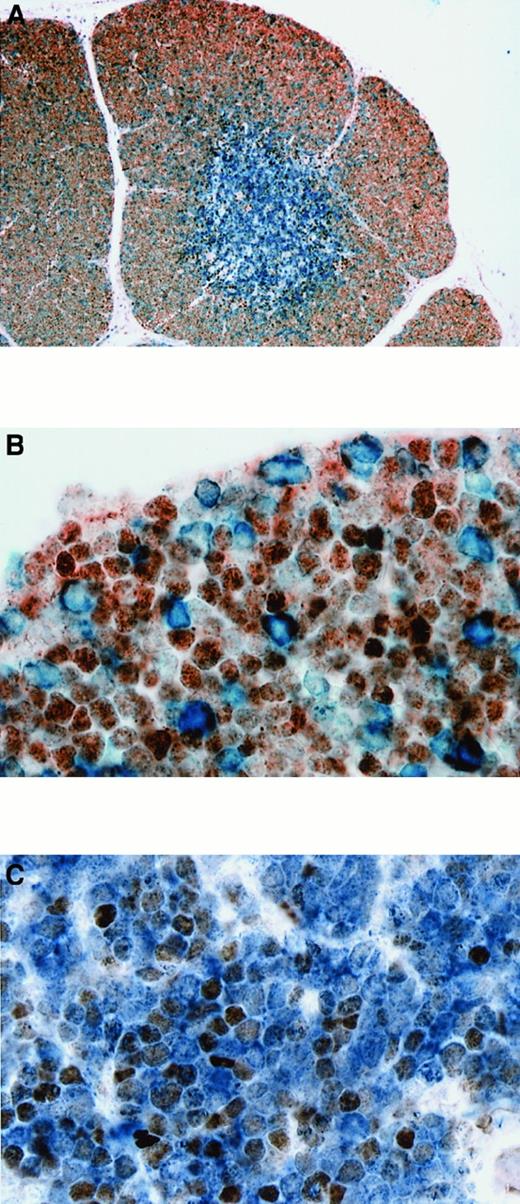
In the normal fetal and postnatal thymuses, BCL-2 was uniformly expressed by the mature medullary thymocytes; however, only scattered cortical thymocytes were BCL-2+ (Figure4A). The BCL-2+ cortical thymocytes did not coexpress BCL-6 (Figure 4B), except for the very rare cell. Conversely, BCL-XL was expressed by most cortical thymocytes but only by scattered medullary thymocytes. BCL-XL+ cortical thymocytes also coexpressed BCL-6 (not shown). All 4 BCL-6+ T-LBLs, as well as all 4 BCL-6− T-LBLs, coexpressed BCL-2 (Figure 4C) and BCL-XL (not shown). The BCL-2 expression was usually strong, while BCL-XL expression was variable.
BCL-6 and BCL-2 expression in normal fetal thymus and T-LBL.
(A) Double staining for BCL-6 and BCL-2 in thymus. BCL-6+cells (nuclear, brown) were primarily present in the cortex, while BCL-2+ cells (cytoplasmic, blue) were primarily seen in the medulla. (B) Higher magnification showing that most cortical thymocytes expressing BCL-6 are BCL-2−. Only scattered cortical thymocytes are BCL-2+ and do not express BCL-6 (21 weeks gestational age). (C) Double staining for BCL-6 and BCL-2 in T-LBL (case No. 2) showing that neoplastic T lymphoblasts coexpress BCL-6 (nuclear, brown) and BCL-2 (cytoplasmic, blue). Original magnification × 100 (A), × 1000 (B), × 1000 (C).
BCL-6 and BCL-2 expression in normal fetal thymus and T-LBL.
(A) Double staining for BCL-6 and BCL-2 in thymus. BCL-6+cells (nuclear, brown) were primarily present in the cortex, while BCL-2+ cells (cytoplasmic, blue) were primarily seen in the medulla. (B) Higher magnification showing that most cortical thymocytes expressing BCL-6 are BCL-2−. Only scattered cortical thymocytes are BCL-2+ and do not express BCL-6 (21 weeks gestational age). (C) Double staining for BCL-6 and BCL-2 in T-LBL (case No. 2) showing that neoplastic T lymphoblasts coexpress BCL-6 (nuclear, brown) and BCL-2 (cytoplasmic, blue). Original magnification × 100 (A), × 1000 (B), × 1000 (C).
Comparison between the pattern of BCL-6, BCL-2, and BCL-XL expression in normal thymus and BCL-6+T-LBL to that in follicle centers of reactive lymph nodes and follicular lymphoma
The pattern of BCL-6 and BCL-2 expression in thymic cortex and BCL-6+ T-LBL paralleled that seen in follicle centers of reactive lymph nodes and follicular lymphoma, respectively. Follicle centers of reactive lymph nodes expressed BCL-6, while BCL-2+ cells were seen primarily outside follicles (Figure5A). BCL-XL was expressed by most follicle center cells (not shown). Only scattered cells in follicle centers were BCL-2+ and did not express BCL-6 (Figure 5B), except for the very infrequent cell. Follicular lymphoma, similarly to BCL-6+ T-LBL, showed abnormal coexpression of BCL-6 and BCL-2 (Figure 5C) and also coexpressed BCL-XL(not shown).
BCL-6 and BCL-2 expression in reactive lymph node and follicular lymphoma.
(A) Double staining for BCL-6 and BCL-2 in reactive lymph node showing analogy between BCL-6 and BCL-2 expression in thymic cortex (Figure4A,B) and germinal centers of lymphoid follicles. BCL-6+cells (nuclear, brown) are present in germinal centers, while BCL-2+ cells (cytoplasmic, blue) are seen primarily outside germinal centers. (B) Higher magnification shows that most germinal center cells express BCL-6 and are BCL-2−. Only scattered cells in germinal centers are BCL-2+ and do not express BCL-6. (C) Double staining for BCL-6 and BCL-2 in a case of follicular lymphoma showing analogy between the pattern of BCL-6 (nuclear, brown) and BCL-2 (cytoplasmic, blue) coexpression between follicular lymphoma and T-LBL (Figure 4C). Original magnification × 100 (A), × 1000 (B), × 1000 (C).
BCL-6 and BCL-2 expression in reactive lymph node and follicular lymphoma.
(A) Double staining for BCL-6 and BCL-2 in reactive lymph node showing analogy between BCL-6 and BCL-2 expression in thymic cortex (Figure4A,B) and germinal centers of lymphoid follicles. BCL-6+cells (nuclear, brown) are present in germinal centers, while BCL-2+ cells (cytoplasmic, blue) are seen primarily outside germinal centers. (B) Higher magnification shows that most germinal center cells express BCL-6 and are BCL-2−. Only scattered cells in germinal centers are BCL-2+ and do not express BCL-6. (C) Double staining for BCL-6 and BCL-2 in a case of follicular lymphoma showing analogy between the pattern of BCL-6 (nuclear, brown) and BCL-2 (cytoplasmic, blue) coexpression between follicular lymphoma and T-LBL (Figure 4C). Original magnification × 100 (A), × 1000 (B), × 1000 (C).
Analysis of BCL-6 gene rearrangements and mutations
Southern blot analysis was performed to assess the presence of rearrangement in the 5′ region of the BCL-6 gene. All 6 T-LBLs examined showed a distinct band in the germline configuration, and no rearrangements were identified using this method (results not shown). None of the 6 T-LBLs examined showed evidence of mutations or deletions in the 5′ noncoding region of the BCL-6 gene, as evaluated by single-strand conformation polymorphism analysis.
Discussion
In this study we show that BCL-6 is expressed by a significant proportion of precursor T-cell lymphoblastic lymphomas. Four of 8 cases of T-LBL that we investigated expressed high levels of BCL-6. None of these cases had rearrangements or mutations in the regulatory region of the BCL-6 gene. This result indicates that, while structural alterations of the BCL-6 gene are uncommon in T-LBL, BCL-6 protein expression can be identified in a significant proportion of cases.
Of the 4 T-LBLs in our series expressing the BCL-6 protein, 3 were double-positive (CD4+, CD8+), displaying a common thymocyte phenotype. The fourth T-LBL had a double-negative phenotype (CD4−, CD8−), corresponding to a prethymocyte stage of differentiation. In this case the proportion of BCL-6+ cells was lower. All BCL-6+ cases of T-LBL were confirmed to have TCRαβ or γ chain gene rearrangements by Southern blot, polymerase chain reaction analysis, or TCRβ expression, except for the single BCL-6+, double-negative (CD4−, CD8−) T-LBL, which showed germline TCRβ and γ chain genes. Three BCL-6− T-LBLs displayed the phenotype of immature double-negative (CD4−, CD8−) prethymocytes, and one case had characteristics of immature single-positive (CD4+, CD8−) prethymocytes. These data suggest that BCL-6 expression in T-LBLs is highly clustered to the double-positive lymphomas, phenotypically corresponding to common cortical thymocytes. However, because the number of T-LBLs tested was small, it remains to be determined whether phenotypic profiles other than the observed ones can actually occur in BCL-6+ T-LBLs and, if this is the case, how often.
Staining for BCL-6 on normal prenatal and postnatal thymus showed that BCL-6 is expressed by most cortical thymocytes and is down-regulated in the medulla, where it is expressed only by a small proportion of thymocytes. Two-color immunohistochemistry of normal thymus showed that BCL-6 is expressed by double-negative (CD4−, CD8−) and double-positive (CD4+, CD8+) cortical thymocytes and by a small number of single CD4+ and CD8+ thymocytes in the medulla. This distinct pattern suggests that BCL-6 expression is tightly regulated during progression through thymocyte maturation in the cortex. The potential role of BCL-6 in thymocyte ontogeny and T-cell maturation is suggested moreover by (1) studies in prenatal and postnatal mice, which have shown that during ontogeny BCL-6 is expressed at discrete stages of development and anatomic distribution in a number of tissues including thymus;27and (2) studies on BCL-6–deficient mice, which provided evidence of the important role of BCL-6 in the formation of germinal centers and in the development of T-cell–controlled antibody immune responses and Th2 immunity.28 29
Recent in vitro studies have suggested that BCL-6 may function as an anti-apoptotic molecule. These studies have shown that cross-linking of B-cell antigen receptor (BCR) with anti-IgM on a BCL-6+, Epstein-Barr virus− Burkitt's lymphoma cell line, mimicking antigen stimulation, leads to apoptosis of these cells. The apoptotic death was preceded by down-regulation of BCL-6 expression. This down-regulation was shown to be due to mitogen-activated protein kinase (MAPK)-mediated phosphorylation of BCL-6 followed by degradation by the ubiquitin-proteasome pathway.30 Blocking of BCL-6 degradation via transfection with a BCL-6 mutant resistant to MAPK phosphorylation or ubiquitin pathway degradation conferred resistance to BCR-induced apoptosis, which correlated with the level of BCL-6 expression.2 These results suggest that dysregulation of BCL-6 expression may contribute to increased apoptosis resistance in B-cell lymphoma. Recent additional evidence has indicated that BCL-6 inhibits differentiation-induced apoptotic death in mouse myogenic cells.3
In contrast to our understanding of the surface receptors that regulate progression through thymocyte development, downstream signal transduction pathways are not completely understood.31Nevertheless, considerable progress has been made in unraveling the nature of signaling pathways involved in T-cell ontogeny, largely resulting from studies on gene-manipulated animals in which the activity of some of those molecules was either knocked out or enhanced, as well as by the availability of more specific inhibitors of signal transduction pathways.32 For αβ T cells a number of control points regulating further development have been identified. In particular, it has been shown that expression of pre-TCR is required for progression from the double-negative (CD4−, CD8−) to the double-positive stage (CD4+, CD8+), while positive selection mediated by TCRαβ is needed for the progression of double-positive thymocytes into single-positive T cells.33 Because there are strong analogies between the B- and T-cell antigen receptor signaling pathways,32 and because experimental data provide evidence that T-cell development is tightly controlled by TCR genes, it may be suggested that TCR signaling through MAPK in thymocytes leads to BCL-6 phosphorylation and ubiquitin-mediated degradation, which is necessary for progression from double- to single-positive thymocytes. Further supporting the suggestion that BCL-6 might be involved in T-cell development, several recent studies provide evidence that the MAPK pathway is critically involved in the positive selection of double-positive thymocytes.34 35 Therefore, BCL-6 down-regulation in thymocytes may be essential for differentiation, which would be analogous to this process in germinal center B cells. This notion is supported by the high level of BCL-6 expression in double-positive cortical thymocytes and expression by only scattered single-positive medullary thymocytes.
To further address a possible role of apoptosis in T-LBL lymphomagenesis, we analyzed the expression of 2 other anti-apoptotic genes, BCL-2 and BCL-XL, in T-LBLs and selected thymuses and determined their coexpression with BCL-6. Within the thymus, BCL-2 and BCL-XL showed a characteristic reciprocal pattern of expression, as previously reported.36-39 Flow cytometry has shown that BCL-2 is expressed in nearly all CD4+ and CD8+ single-positive cells but in only a few CD4+, CD8+ (double-positive) immature thymocytes. Conversely, BCL-XL is highly expressed in immature double-positive cells but absent from mature single-positive thymocytes.36-38 Moreover, there is evidence to suggest that expression of BCL-2 and BCL-XL may be coupled to TCR-mediated signals. BCL-2 expression is up-regulated during positive selection and persists in mature T cells in the periphery,40,41 while BCL-XL appears to play an essential role in the survival of double-positive thymocytes prior to selection signals. Interestingly, all 4 of our BCL-6+T-LBL cases expressed BCL-2 and showed variable levels of BCL-XL. The abnormal coexpression of BCL-6, BCL-2, and BCL-XL may be useful in the differential diagnosis of small biopsies from anterior mediastinal masses in distinguishing T-LBL from residual normal thymus. Whether coexpression of BCL-6, BCL-2, and BCL-XL represents a marker of malignancy or may occur also as a result of microenvironmental alterations in thymocyte maturation and development, such as observed in thymomas,42 has yet to be determined.
The abnormal coexpression of BCL-2, BCL-XL, and BCL-6 in T-LBL is also highly reminiscent of coexpression in follicular lymphomas 43 and suggests that, in conjunction, expression of these genes may contribute to T-LBL evolution. In addition, the pattern of BCL-2, BCL-6, and BCL-XL expression in thymus and normal germinal centers shows striking similarities, because most BCL-6+ cortical thymocytes lack BCL-2 expression and express BCL-XL, analogous to normal germinal center B cells.20,23,37,39 44
Our results show that, in our series, BCL-6 protein is expressed by cortical thymocytes and a subset of T-LBLs, in particular all of those with a double-positive phenotype. These results suggest that T-LBLs expressing BCL-6 may arise from normal cortical thymocytes, perhaps through inability to down-regulate BCL-6 during positive selection. The dysregulated BCL-6 expression does not appear to be caused by structural alterations in the regulatory region of this gene, and the mechanism causing this dysregulation remains to be elucidated. Nevertheless, the pattern of BCL-6, BCL-2, and BCL-XLexpression in normal thymuses is remarkably similar to that identified in germinal centers, and the abnormal coexpression of BCL-2, BCL-XL, and BCL-6 is seen in both T-LBL and follicular lymphoma.17,43 44 Expression of BCL-6 may be regulated by signals important for T-cell development, differentiation, and survival, and dysregulation of those signals may contribute to thymic lymphomagenesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Daniel M. Knowles, Department of Pathology, Weill Medical College of Cornell University, 1300 York Ave, New York, NY 10021; e-mail: dknowles@med.cornell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal