Abstract
Dendritic cells (DCs) are important for the initiation of immune responses to foreign antigens. Their antigen uptake and presentation capacities enable them to prime and activate T cells. Immature DCs capture antigens; however, they must be activated to mature before serving as efficient antigen-presenting cells. The antigen-presenting capacity of DCs can be diminished during viral infection and as a consequence of tumor formation. Chronic infection with hepatitis C virus (HCV) has been shown to affect the allostimulatory function of DCs. In this study, it is demonstrated that monocyte-derived DCs from patients with chronic HCV infection do not respond to maturation stimuli. Instead, they maintain their immature phenotype, reflected by the pattern of cell surface markers and by their continued capacity to uptake antigen. Moreover, their allostimulatory abilities are impaired compared with those of mature DCs derived from healthy donors. To investigate a possible correlation between viral clearance and this DC maturation defect, patients with resolved HCV infection after a course of antiviral therapy were studied. Results demonstrate that DCs from patients who cleared HCV behaved like DCs from healthy donors: in response to maturation stimuli, they decrease antigen uptake, up-regulate expression of appropriate surface markers, and are potent stimulators of allogeneic T cells.
Introduction
Dendritic cells (DCs) are important for the initiation of immune responses to foreign antigens because of their “professional” competence to capture and present antigen to T cells. The 2 functions, antigen uptake and presentation, are temporally and spatially separated. Thus, after antigen internalization, the DCs themselves undergo a process of maturation, migration, and relocation (for review, see Bell et al1). During maturation, DCs up-regulate major histocompatibility complex (MHC), adhesion, and costimulatory molecules, including CD80 (B7.1), CD86 (B7.2), CD54 (ICAM-1), CD58 (LFA-3), CD11a, CD11c, and CD40, whereas they down-regulate the expression of Fc and mannose receptors.2Mature DCs also secrete high levels of interleukin-12 (IL-12), a Th-1–polarizing cytokine that promotes the maturation of cytotoxic T cells (CTLs).3
Several viruses, such as herpes simplex virus,4 measles virus,5 and Epstein-Barr virus, have been shown to diminish DC function. Recently, hepatitis C virus (HCV) infection has also been shown to affect the function of DCs. Compared to monocyte-derived DCs from healthy donors, DCs from patients with chronic HCV infection showed impaired ability to stimulate allogeneic T cells and to produce interferon γ (IFN-γ).6 An independent study also demonstrated impaired stimulatory capacity of DCs derived from HCV-infected patients with hepatocellular carcinoma. However, this dysfunction was not unique to HCV because DCs from hepatitis B virus–infected patients with hepatocellular carcinoma also showed reduced stimulatory activity, suggesting that the liver damage itself might affect DC function.7 These studies examined only the DCs of patients with chronic HCV infection. To the best of our knowledge, studies investigating DC derived from patients with cleared HCV infection have not yet been reported.
Hepatitis C virus causes an often inapparent acute infection that is cleared only in a minority of patients.8 Patients who are able to clear the virus mount a vigorous T-cell response. Spontaneous viral clearance is correlated with both HCV-specific, IFN-γ–producing CD8+ T cells and a strong proliferative CD4+ T-cell response during the first 6 months after infection.9 Similarly, patients who cleared the virus mounted a CTL response to a larger number of viral epitopes than those who became chronic carriers.10 In addition, persistent CTL activity could be detected in patients whose HCV was resolved but not in patients with chronic HCV infection.11 The clearance of an HCV infection has also been correlated with a strong HCV-specific T-helper cell response.12-14
Because DCs are essential for T-cell activation, we questioned whether viral clearance was affected by their abilities to process and present antigen. We therefore compared DCs from chronically infected patients to those from patients who cleared the virus after a course of antiviral therapy and to those of healthy donors. DCs were generated in a 2-step protocol.15 First, monocytes were isolated from peripheral blood mononuclear cells (PBMCs) and cultured in the presence of IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) for 5 days. Subsequently, immature DCs were stimulated to mature with tumor necrosis factor-α (TNF-α) for 2 additional days.
We demonstrate that monocytes and immature DCs from patients with chronic HCV infection do not differ in phenotype or function from those derived from healthy donors. Yet DCs from chronically infected patients cannot be stimulated to mature. They resemble immature DCs from healthy donors. However, DCs from patients whose HCV infection resolves after antiviral therapy behave like DCs from healthy donors; they can be stimulated to mature and to function as potent stimulators of allogeneic mixed leukocyte reaction (mLR).
Patients, materials, and methods
Donors
Buffy coats from 13 healthy donors were obtained from the Stanford Blood Center. All donors tested serologically negative for HCV, human immunodeficiency virus (HIV), and hepatitis B virus. Informed consent was obtained from all patients. HCV-infected patients fell into 2 groups. Group 1 included 15 patients who had chronic HCV infection. All 15 patients tested positive for the presence of antibodies to HCV and HCV RNA by reverse transcription–polymerase chain reaction (RT-PCR). They all tested negative for HIV and had normal results on complete and differential blood counts at the time their blood samples were obtained. Some of these patients had previously been treated for HCV but did not achieve an antiviral response. Their antiviral therapy was discontinued at least 6 months before sample collection. Group 2 consisted of 8 patients who had prior HCV infection but who cleared the virus after antiviral therapy with either IFN-α or IFN-α plus Ribavirin (Schering, Kenilworth, NJ), as documented by negative findings on a minimum of 2 subsequent RT-PCR assays for HCV. They also tested negative for HIV and had normal complete and differential blood counts when samples were obtained. Table 1 shows additional information on all HCV-infected patients (including age, gender, liver enzymes, histology, duration of disease, genotype, and prior treatment for HCV).
Characteristics of patients with hepatitis C virus infection
| Patient . | Age (y) . | Gender . | ALT/AST . | Viral load . | RT-PCR . | Geno type . | Liver histology . | Duration of disease (y) . | Prior Treatment . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | 108/99 | 1.2 × 104 | Quant. | 3a | Cirrhosis | Unknown | IFN-α |
| 2 | 48 | M | 183/137 | 3.9 × 106 | Quant. | 3a | Cirrhosis | 28 | None |
| 3 | 48 | F | 78/57 | 3.5 × 105 | Quant. | 3a | Cirrhosis | 25 | None |
| 4 | 51 | M | 107/92 | 10 × 106 | Quant. | 3a | Cirrhosis | 20 | None |
| 5 | 49 | M | 93/89 | 3.2 × 106 | Quant. | 1a | Fibrosis | 30 | None |
| 6 | 51 | M | 84/61 | 2.2 × 106 | Quant. | 1b | Fibrosis | 30 | None |
| 7 | 62 | F | 73/76 | 1.5 × 104 | Quant. | 4c | Fibrosis | 20 | IFN-α+Ribavirin |
| 8 | 40 | F | 70/34 | 1.8 × 104 | Quant. | 1d | Cirrhosis | 25 | None |
| 9 | 54 | F | 70/58 | 1 × 106 | Quant. | 2a | Cirrhosis | 30 | None |
| 10 | 52 | F | 47/33 | 3 × 105 | Quant. | 1b | Fibrosis | 30 | None |
| 11 | 35 | M | 164/130 | 5 × 106 | Quant. | 1a/1b | Fibrosis | 30 | None |
| 12 | 48 | M | 238/99 | 3 × 106 | Quant. | 1a | Fibrosis | 6 | IFN-α |
| 13 | 45 | M | Unknown | 7.5 × 105 | Quant. | 1a | Fibrosis | 25 | IFN-α+Ribavirin |
| 14 | 41 | M | 36/24 | Pos | Qual. | Unknown | Cirrhosis | 20 | IFN-α |
| 15 | 52 | M | 115/68 | 2.2 × 104 | Quant. | 1a | Fibrosis | 30 | IFN-α+Ribavirin |
| 16 | 50 | F | 36/34 | Neg. | Qual. | 3a | Unknown | 22 | IFN-α+Ribavirin |
| 17 | 49 | F | 10/16 | Neg. | Qual. | 1b | Unknown | 3 | IFN-α+Ribavirin |
| 18 | 45 | F | 15/26 | Neg. | Qual. | 1a | Unknown | 20 | IFN-α+Ribavirin |
| 19 | 46 | M | 29/21 | Neg. | Qual. | 2 | Normal | 32 | IFN-α |
| 20 | 48 | M | 15/16 | Neg. | Quant. | 2b | Fibrosis | 10 | IFN-α+Ribavirin |
| 21 | 53 | F | 15/22 | Neg. | Quant. | Unknown | Unknown | 4 | IFN-α+Ribavirin |
| 22 | 45 | F | Norm. | Neg. | Quant. | 1a, 1b | Fibrosis | 7 | IFN-α+Ribavirin |
| 23 | 50 | M | Norm. | Neg. | Quant. | 2b | Unknown | 32 | IFN-α+Ribavirin |
| Patient . | Age (y) . | Gender . | ALT/AST . | Viral load . | RT-PCR . | Geno type . | Liver histology . | Duration of disease (y) . | Prior Treatment . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | M | 108/99 | 1.2 × 104 | Quant. | 3a | Cirrhosis | Unknown | IFN-α |
| 2 | 48 | M | 183/137 | 3.9 × 106 | Quant. | 3a | Cirrhosis | 28 | None |
| 3 | 48 | F | 78/57 | 3.5 × 105 | Quant. | 3a | Cirrhosis | 25 | None |
| 4 | 51 | M | 107/92 | 10 × 106 | Quant. | 3a | Cirrhosis | 20 | None |
| 5 | 49 | M | 93/89 | 3.2 × 106 | Quant. | 1a | Fibrosis | 30 | None |
| 6 | 51 | M | 84/61 | 2.2 × 106 | Quant. | 1b | Fibrosis | 30 | None |
| 7 | 62 | F | 73/76 | 1.5 × 104 | Quant. | 4c | Fibrosis | 20 | IFN-α+Ribavirin |
| 8 | 40 | F | 70/34 | 1.8 × 104 | Quant. | 1d | Cirrhosis | 25 | None |
| 9 | 54 | F | 70/58 | 1 × 106 | Quant. | 2a | Cirrhosis | 30 | None |
| 10 | 52 | F | 47/33 | 3 × 105 | Quant. | 1b | Fibrosis | 30 | None |
| 11 | 35 | M | 164/130 | 5 × 106 | Quant. | 1a/1b | Fibrosis | 30 | None |
| 12 | 48 | M | 238/99 | 3 × 106 | Quant. | 1a | Fibrosis | 6 | IFN-α |
| 13 | 45 | M | Unknown | 7.5 × 105 | Quant. | 1a | Fibrosis | 25 | IFN-α+Ribavirin |
| 14 | 41 | M | 36/24 | Pos | Qual. | Unknown | Cirrhosis | 20 | IFN-α |
| 15 | 52 | M | 115/68 | 2.2 × 104 | Quant. | 1a | Fibrosis | 30 | IFN-α+Ribavirin |
| 16 | 50 | F | 36/34 | Neg. | Qual. | 3a | Unknown | 22 | IFN-α+Ribavirin |
| 17 | 49 | F | 10/16 | Neg. | Qual. | 1b | Unknown | 3 | IFN-α+Ribavirin |
| 18 | 45 | F | 15/26 | Neg. | Qual. | 1a | Unknown | 20 | IFN-α+Ribavirin |
| 19 | 46 | M | 29/21 | Neg. | Qual. | 2 | Normal | 32 | IFN-α |
| 20 | 48 | M | 15/16 | Neg. | Quant. | 2b | Fibrosis | 10 | IFN-α+Ribavirin |
| 21 | 53 | F | 15/22 | Neg. | Quant. | Unknown | Unknown | 4 | IFN-α+Ribavirin |
| 22 | 45 | F | Norm. | Neg. | Quant. | 1a, 1b | Fibrosis | 7 | IFN-α+Ribavirin |
| 23 | 50 | M | Norm. | Neg. | Quant. | 2b | Unknown | 32 | IFN-α+Ribavirin |
Chronic HCV infection (patients 1-15), resolved HCV infection (patients 16-23).
ALT/AST indicates alanine amino transferase/aspartate amino transferase; RT-PCR, reverse transcription–polymerase chain reaction; IFN-α, interferon α; HCV, hepatitis C virus.
Cell culture
Buffy coats from healthy donors or 60 mL peripheral blood from HCV-infected patients were used to generate DCs, as previously described.15 Briefly, PBMCs were isolated by Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation. Isolated PBMCs were plated at 5 × 106 cells/mL in X-Vivo 15 media (BioWhittaker, Walkersville, MD) under serum-free conditions. Cells were allowed to adhere for 2 hours at 37°C, and nonadherent cells were removed by pipetting medium gently over the adherent cells. Fresh X-Vivo 15 medium containing 1000 IU/mL IL-4 (R&D Systems, Minneapolis, MN) and 100 ng/mL GM-CSF (Immunex, Seattle, WA) was added, and the cells were cultured for 7 days. Fresh medium and cytokines were substituted every other day. During the last 2 days of culture, 10 μg/mL TNF-α (R&D Systems) was added to IL-4 and GM-CSF as a maturation stimulus. Cell differentiation was monitored by light microscopy and by flow cytometry. Monocytes were harvested after culturing adherent cells without cytokines for 1 day. Immature dendritic cells were collected on day 5 before exposure to TNF-α, and mature dendritic cells were harvested on day 7 after incubation with TNF-α.
Immunophenotyping
Antibodies used for cell surface staining included CD3, CD11c, CD14, CD19, CD40, CD54, HLA-DR (Becton Dickinson, San Jose, CA), CD1a, CD40, CD86 (Pharmingen, San Diego, CA), and CD83 (Immunotech, Marseilles, France). All antibodies were mouse monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE). An irrelevant mouse isotype control (Becton Dickinson) was included in the analysis. Data acquisition and analysis were performed on a FACS Scan flow cytometer (Becton Dickinson, Mountain View, CA) using Cellquest software.
Antigen uptake
Antigen uptake by DCs was performed as previously described.2 Briefly, 50 μg/mL FITC-labeled bovine serum albumin (BSA-FITC; Sigma, St Louis, MO) was added to 1 × 106 cells at 37°C. Uptake of the same concentration of BSA-FITC at 4°C served as a negative control. Both groups were incubated for 30 minutes in the dark. After incubation, cells were washed twice in cold PBS plus 1% BSA and analyzed by FACS. Data represent the difference in mean fluorescence intensity (MFI) of DCs after BSA-FITC uptake at 37°C and at 4°C.
Allogeneic mixed leukocyte reaction
The stimulatory ability of DCs was assessed in an allogeneic mLR. For all assays, PBMCs from the same healthy donor served as a source of responder cells. Donor PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation, as previously described, and resuspended in fetal calf serum (FCS) containing 10% dimethyl sulfoxide (Sigma), and aliquots were frozen in liquid nitrogen. Responder PBMCs were thawed immediately before use, washed, and resuspended in RPMI + 10% FCS and plated at 1 × 105 responder cells per well. Dendritic cells (day 7) were treated with 25 μg/mL mitomycin C (Sigma) at 37°C for 30 minutes and subsequently washed 3 times in media (RPMI + 10% FCS) before plating. Decreasing numbers of DCs were mixed with responder PBMCs, as indicated in the legend to Figure 3. DCs cultured in the absence of responder cells served as control for background proliferation. Cells in triplicate wells of a 96-well flat-bottom plate (Corning, Corning, NY) were cultured for 5 days and subsequently pulsed with 1 μCi [3H] thymidine during the last 16 hours of culture. [3H] Thymidine incorporation was measured using a liquid scintillation counter (Wallac-Perkin-Elmer, Wallac, Finland).
Results
Changes in surface marker expression in response to stimulation with TNF-α
DCs were generated from adherent PBMCs in a 2-step maturation protocol.15 The levels of CD14 and MHC class II expression on monocyte cultures from healthy donors, patients with chronic HCV infection, and patients who cleared the virus were similar (data not shown). CD14 was expressed on 51% ± 24%, 67% ± 22%, and 53% ± 33% of their cells, respectively. MHC class II was expressed on 97% ± 3%, 98% ± 2%, and 95% ± 3% of their cells, respectively. Monocytes from all 3 groups responded similarly to IL-4 and GM-CSF and differentiated into immature DCs, monitored by immunophenotyping and light microscopy (data not shown).
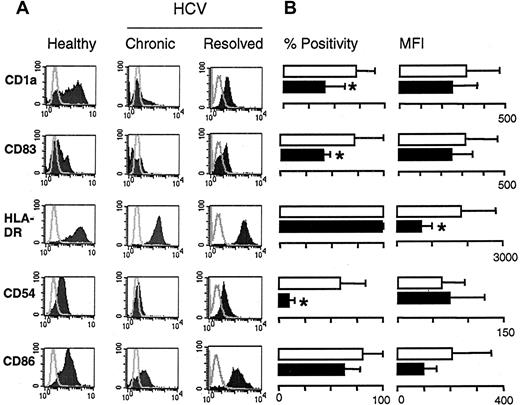
We then analyzed the maturation of immature DCs from the 3 groups. DCs from healthy donors displayed characteristic features of mature myeloid DCs, including veiled morphology by light microscopy, nonadherence to plastic, and positivity for a distinct set of cell surface markers. However, these characteristics were not observed in DC cultures generated from patients with chronic HCV infection. As shown in a representative example, (Figure 1A, left panels), day 7 DCs from a healthy donor expressed the mature DC markers CD1a and CD83. These DCs also expressed high levels of MHC class II, the adhesion molecule CD54, and the costimulatory molecule CD86. In contrast, DCs derived from a patient with chronic infection expressed lower levels of these markers, though they were cultured under identical conditions (Figure 1A, middle panels). Cumulative data regarding the maturation status of DCs derived from 15 patients with chronic HCV infection and 13 healthy donors are shown in Figure 1B. These results demonstrate that the percentage of cells expressing mature DC markers is reduced in patients with chronic HCV infection in comparison with healthy donors. Noticeably, the differences in the percentages of CD1a+ cells (70% vs 40%;P ≤ .01), CD54 cells (58% vs 9%;P ≤ .01), and CD83 cells (38% vs 8%;P ≤ .01) were statistically significant. MHC class II was expressed on 100% of the cells; however, its expression level was reduced considerably in patients with chronic HCV infection (1810 vs 679 MFI; P = .01). In addition, the expression levels of CD1a, CD83, and CD86 were consistently lower in DCs derived from HCV-infected patients, reflecting a less mature phenotype. Taken together, these data suggest that immature DCs of patients with chronic HCV infection do not respond appropriately to stimulation with TNF-α, as shown by their inability to mature and to display cell surface markers typical of mature DCs. In contrast, DCs generated from the 8 patients whose HCV infection resolved could be stimulated to mature, as observed for healthy donors. These mature DCs expressed the dendritic cell markers CD1a (59% ± 25% positive cells), CD83 (31% ± 11% positive cells), CD54 (88% ± 13% positive cells), and CD86 (97% ± 3% positive cells). Their MHC class II molecules were also up-regulated to the range of that seen for healthy DC (MFI 1499) (Figure 1A, right panels).
Immunophenotyping of DCs stimulated to mature with TNF-α.
(A) Representative example of DCs (day 7) of a healthy donor (left panels), a patient with chronic HCV infection (middle panels), and a patient whose HCV infection resolved (right panels). Surface expression of the indicated markers (shaded areas) are overlaid with isotype control staining (gray line). (B) Cumulative flow cytometry data on 15 patients with chronic HCV infection (▪) and 13 healthy donors (■). Data are expressed as mean percentage of positive cells ± SD (left panels) and mean fluorescence intensity ± SD (right panels). *P ≤ .01 between the 2 groups.
Immunophenotyping of DCs stimulated to mature with TNF-α.
(A) Representative example of DCs (day 7) of a healthy donor (left panels), a patient with chronic HCV infection (middle panels), and a patient whose HCV infection resolved (right panels). Surface expression of the indicated markers (shaded areas) are overlaid with isotype control staining (gray line). (B) Cumulative flow cytometry data on 15 patients with chronic HCV infection (▪) and 13 healthy donors (■). Data are expressed as mean percentage of positive cells ± SD (left panels) and mean fluorescence intensity ± SD (right panels). *P ≤ .01 between the 2 groups.
Antigen uptake by dendritic cells
Immature DCs capture antigen efficiently and yet lose this capacity on maturation.16 Therefore, the decrease in antigen uptake has been used to monitor DC maturation.17Because DCs of patients with chronic HCV infection showed an immature phenotype, we used this functional assay and tested the uptake of a labeled antigen, (BSA-FITC) by the DCs of the 3 groups. As expected, DC on day 5, before stimulation with TNF-α, captured BSA-FITC at 37°C but not at 4°C (Figure 2A, day 5). Immature DCs from a healthy donor (Figure 2A), from a patient with chronic HCV infection (Figure 2B), and from a patient whose HCV infection resolved (Figure 2C) internalized comparable amounts of the antigen. However, after stimulation with TNF-α, only DCs from patients with chronic HCV infection continued to capture BSA-FITC (Figure 2B, day 7). Cumulative results of antigen uptake on day 5 by DCs obtained from healthy donors or from patients with chronic or resolved HCV infection are similar for all 3 groups (Figure 2D, day 5) (P = .32 for HCV-infected patients vs healthy donors;P = .11 for healthy donors vs patients with resolved HCV infection). In contrast, after TNF-α stimulation (day 7), DCs from HCV-infected patients continued to capture antigen at levels comparable to those on day 5 (P = .19) and significantly higher than DCs on day 7 from healthy donors (P = .02) and patients with resolved HCV infection (P = .03). After the maturation stimulus, DCs from healthy donors and patients with resolved HCV infection decreased their antigen uptake to the same low level (P = .85). Thus, DCs from healthy donors and from patients who cleared HCV infection down-regulated antigen capture on stimulation with TNF-α. In contrast, though stimulated to mature for 2 days with TNF-α, DCs from patients with chronic HCV infection continued to uptake antigen.
Antigen uptake by DCs.
BSA-FITC uptake at 37°C (shaded area) overlaid with the control uptake at 4°C (gray line) on day 5 (left panels) before TNF-α stimulation and on day 7 after TNF-α stimulation (right panels). Representative analysis of antigen uptake by DCs from a healthy donor (A), from a patient with chronic HCV infection (B), and from a patient whose HCV infection resolved (C). The difference in uptake (ΔMFI) on day 5 and day 7 for all persons is shown in panel D. Single symbols represent individuals: ■, healthy donor; ▪, patient with chronic HCV infection; ○, patient with resolved HCV infection. Horizontal bars represent group means. *P ≤ .03 for antigen uptake on day 7 between healthy donors and patients whose HCV infection resolved versus carriers of chronic HCV.
Antigen uptake by DCs.
BSA-FITC uptake at 37°C (shaded area) overlaid with the control uptake at 4°C (gray line) on day 5 (left panels) before TNF-α stimulation and on day 7 after TNF-α stimulation (right panels). Representative analysis of antigen uptake by DCs from a healthy donor (A), from a patient with chronic HCV infection (B), and from a patient whose HCV infection resolved (C). The difference in uptake (ΔMFI) on day 5 and day 7 for all persons is shown in panel D. Single symbols represent individuals: ■, healthy donor; ▪, patient with chronic HCV infection; ○, patient with resolved HCV infection. Horizontal bars represent group means. *P ≤ .03 for antigen uptake on day 7 between healthy donors and patients whose HCV infection resolved versus carriers of chronic HCV.
Immunostimulatory properties of dendritic cells in allogeneic mLR
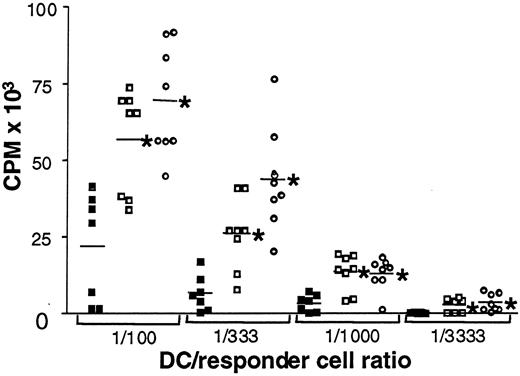
DCs increase their antigen presentation capabilities on maturation because of the up-regulation of MHC class I and II and the costimulatory molecules on their cell surfaces. Thus, their state of maturation reflects their ability to stimulate allogeneic mLR. To test the allostimulatory potential of mature (day 7) DCs in this study, we used PBMCs from a single healthy donor as responders in all assays. Cells were incubated for 5 days, and cell proliferation was assessed by [3H] thymidine incorporation. Figure3 summarizes the results of these assays. Individuals are represented by single symbols, and group means are depicted as horizontal bars at the indicated stimulator-to-responder ratio. Stimulatory capacity of DCs from HCV-infected patients was significantly decreased in comparison with DCs from healthy donors and with those from patients who had cleared the virus at all 4 stimulator-to-responder ratios (P ≤ .02). No difference was observed between healthy donors and patients who had cleared HCV infection. In summary, significantly decreased allostimulatory capacity was observed for DCs from patients with chronic HCV infection compared with DC derived from healthy controls. However, DCs from patients whose HCV infection resolved showed allostimulatory capacity similar to that of healthy controls.
Impaired allogeneic mLR stimulation by DCs from patients with chronic HCV infection.
Proliferative responses of PBMCs to allogeneic DCs from patients with chronic HCV infection, ▪; healthy donors, ■; and resolved HCV infection, ○. Single symbols represent individuals. Horizontal bars represent group means. Counts per minute (CPM) reflect cell proliferation, shown at different DC-to-responder cell ratios (1:100; 1:333; 1:1000; 1:3333). *P ≤ .02 for healthy donors and patients whose HCV infection resolved versus carriers of chronic HCV.
Impaired allogeneic mLR stimulation by DCs from patients with chronic HCV infection.
Proliferative responses of PBMCs to allogeneic DCs from patients with chronic HCV infection, ▪; healthy donors, ■; and resolved HCV infection, ○. Single symbols represent individuals. Horizontal bars represent group means. Counts per minute (CPM) reflect cell proliferation, shown at different DC-to-responder cell ratios (1:100; 1:333; 1:1000; 1:3333). *P ≤ .02 for healthy donors and patients whose HCV infection resolved versus carriers of chronic HCV.
Discussion
Chronic HCV infection is a major public health problem, with more than 100 million people worldwide estimated to be infected with the virus.8,18 The clinical course of HCV is highly variable. In most patients, the initial infection remains clinically asymptomatic, and neither the time nor the route of infection is known. HCV infection becomes chronic in 85% of patients and resolves only in 15% of patients.19 20 The virus achieves persistence in most patients by circumventing both humoral and cellular immune responses. However, the mechanism applied remains to be detected. Thus, better understanding is needed of how antiviral immune responses are modulated in acute and chronic HCV infection.
Viral clearance has been correlated with a vigorous T-cell response encompassing a large number of viral epitopes. This was shown in infected humans21 and chimpanzees experimentally infected by the virus.22 In contrast, in patients and chimpanzees with chronic HCV, the virus elicits only a weak T-cell response.21 22 Therefore, a defective antiviral immune response might be an important reason for HCV persistence.
Induction of an effective immune response requires the active participation of host APCs, the most potent of which are DCs.23 Strategies using DCs have been effective in overcoming self-tolerance when they are used as antitumor vaccines.24-26 To date, few studies have analyzed the role of DCs in HCV infection, and these studies tested only DCs from patients with chronic HCV infection. The stimulatory capacity of such DCs was shown to be significantly decreased in an allogeneic mLR when compared to those of healthy donors. Our results confirm these earlier findings by demonstrating a diminished ability of DCs from chronic HCV carriers to stimulate allo-mLR.
In addition, we investigated the underlying cause for this functional impairment and have shown that DCs from patients with chronic HCV infection have a maturation defect. Unlike DCs from healthy donors who respond to TNF-α by up-regulating cell surface proteins involved in antigen presentation (Figure 1) and down-regulating antigen uptake (Figure 2), DCs from chronically infected patients behave like immature DCs (Figures 1, 2). This inability to mature is probably the cause of their poor allostimulation in the mLR (Figure 3).
To determine whether this maturation impairment was associated with viral persistence, we investigated DCs from 8 patients whose HCV infection resolved after treatment (Table 1). Our data clearly demonstrate that DCs generated from these patients behaved phenotypically and functionally like those obtained from healthy controls. This is the first study to analyze DCs from patients who cleared viral infection. It demonstrates that their responses to maturation stimuli and their functional abilities are identical to those of DCs from healthy donors. This might suggest that the defect seen in the chronically infected patients is limited to the period of viral infection but can be reversed after viral clearance. Alternatively, a possibility exists that these patients' DCs matured normally—hence their ability to eliminate the virus. Unfortunately, earlier blood samples from these patients are not available to test this possibility.
Viral clearance of patients whose HCV infection resolved has frequently been associated with vigorous T-cell responses. Moreover, CD4+ and CD8+ T cells that were active in clearing the acute phase of the infection were maintained after viral clearance.9-13 Could the potency of the T-cell response depend on efficient antigen presentation? Increasing T-cell responses have been correlated with the maturation stage of DCs and the generation of tumor immunity. DCs expressing high levels of costimulatory and adhesion molecules were able to induce protective tumor immunity against a poorly immunogenic tumor. Mature DCs induced the most potent response.27 It remains to be determined whether the correlation between viral clearance and effective antigen presentation by DCs, seen in this study, is also correlated with more potent antiviral T-cell responses.
DC maturation and function are modulated by their microenvironment. For example, DCs require T cells for functional maturation in vivo and in vitro,28-30 yet anergic T cells can inhibit the allostimulatory capacity of DCs and down-regulate MHC class II, CD80, and CD86 on their surfaces.31 The fact that anergic T cells can negatively influence DC function is important in the context of HCV. HCV-specific CD8+ cells have been detected in the circulation of patients with chronic HCV infection. However, it has recently been shown that 90% of these CD8+ cells were negative for the activation markers CD38 and CD69. This anergic phenotype was maintained despite stimulation by their specific antigen.32 Thus, it is possible that the impaired maturation of DCs, seen in this study, is a consequence of their interaction with anergic T cells.
In summary, the immune response in patients with chronic HCV infection was previously shown to be impaired at the effector cell level. Here we demonstrate that impaired maturation of DCs correlated with persistent HCV infection. In contrast, patients who cleared the virus have normal DCs. In the future, it will be important to determine whether the virus affects DCs directly or whether the impaired phenotype and function are secondary to the chronicity of infection.
We thank Lucinda Porter and Susanna Lam for their help with blood sample collection and Debra K. Czerwinski for her help with immunophenotyping. We thank Ron Levy, John Timmermann, Elizabeth R. Quinn, and Mike Flint for reviewing the manuscript.
Supported by National Institutes of Health grant CA34233 (S.L.). E.K. is funded by the Stanford Hutchinson Program. S.A.-G. is supported by a grant from Dr Mildred Scheel Stiftung fuer Krebsforschung, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shoshana Levy, Division of Oncology, Department of Medicine, Stanford University School of Medicine, Stanford, CA 94305-5151; e-mail: levy@cmgm.stanford.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal