Abstract
Intractable autoimmune diseases in chimeric resistant MRL/lpr mice were treated by a new bone marrow transplantation (BMT) method consisting of fractionated irradiation, 5.5 Gy × 2, followed by intra–bone marrow (IBM) injection of whole bone marrow cells (BMCs) from allogeneic normal C57BL/6 (B6) mice (5.5 Gy × 2 + IBM). In MRL/lpr mice treated with this method, the number of donor-derived cells in the bone marrow, spleen, and liver rapidly increased (almost 100% donor-derived cells by 14 days after the treatment), and the number of donor-derived hemopoietic progenitor cells concomitantly increased. Furthermore, donor-derived stromal cells were clearly detected in the cultured bone pieces from MRL/lpr mice treated with 5.5 Gy × 2 + IBM. All the recipients thus treated survived more than 1 year (> 60 weeks after birth) and remained free from autoimmune diseases. Autoantibodies decreased to almost normal levels, and abnormal T cells (Thy1.2+/B220+/CD4−/CD8−) disappeared. Hematolymphoid cells were reconstituted with donor-derived cells, and newly developed T cells were tolerant to both donor (B6)-type and host (MRL/lpr)-type major histocompatibility complex determinants. Successful cooperation was achieved among T cells, B cells, and antigen-presenting cells when evaluated by in vitro antisheep red blood cell responses. These findings clearly indicate that this new strategy (IBM-BMT) creates the appropriate hemopoietic environment for the early recovery of hemopoiesis and donor cell engraftment, resulting in the complete amelioration of intractable autoimmune diseases in chimeric resistant MRL/lpr mice without recourse to immunosuppressants. This strategy would therefore be suitable for human therapy.
Introduction
Using various autoimmune-prone mice, we have previously shown that conventional allogeneic bone marrow transplantation (allo BMT) can be used to prevent and treat a range of autoimmune diseases.1-6 These findings have recently been confirmed even in humans.7-15 However, in humans, the success rate of BMT across major histocompatibility complex (MHC) barriers is lowered by graft-versus-host disease (GVHD), graft rejection, and incomplete T-cell recovery. Therefore, autologous BMT (auto BMT) or peripheral blood stem cell transplantation (auto PBSCT) is the preferred treatment for autoimmune diseases.16-18 There have, however, been reports on the rapid recurrence or persistence of autoimmune diseases after auto BMT or auto PBSCT.19Therefore, it is important to establish a safe new method for allo BMT.
We have recently found that the MRL/lpr mouse is a suitable model for establishing a safe new strategy for allo BMT because the MRL/lpr mouse itself is radiosensitive (< 8.5 Gy), whereas the abnormal hemopoietic stem cells (HSCs) of the MRL/lpr mouse are radioresistant (> 8.5 Gy); conventional BMT (8.5 Gy irradiation plus allo BMT) has a transient effect on autoimmune diseases, which recur 3 months after the BMT.20 However, we have found that BMT plus bone grafts (to recruit donor stromal cells) completely prevents the recurrence of autoimmune diseases in MRL/lpr mice21; donor-derived stromal cells seem to play a crucial role in successful allo BMT,21,22 because there is an MHC restriction between HSCs and stromal cells.23 We have, however, found that the combination of BMT plus bone grafts has no effect on the treatment of autoimmune diseases in MRL/lpr mice,24 because MRL/lpr mice become more radiosensitive after the onset of lupus nephritis due to uremic enterocolitis. To reduce the cytotoxic effect of radiation on the intestine, we carried out fractionated irradiation and attempted to devise a new strategy.
Recently, we have found that most donor HSCs are trapped and retained in the liver when they are injected either via the portal vein (PV) or intravenously (IV),25 and that the HSCs induce anergy to host CD8+ T cells.26 In addition, we have found a strategy (the PV plus the supplemental IV injections of donor whole bone marrow cells [BMCs]) that induces persistent tolerance in the skin allograft system.27 On the basis of these findings, we have very recently established a new strategy for allo BMT: fractionated irradiation (5.5 Gy × 2) and the PV plus IV injections of whole BMCs.28 However, this method has 2 drawbacks for patients: laparoscope-guided injection of BMCs via the PV is necessary, and an additional IV injection is essential for obtaining a 100% success rate.28 We have analyzed the mechanism underlying the tolerance induced by the PV injection of BMCs and noted the importance of donor-derived stromal cells trapped in the liver, which facilitate the proliferation and differentiation of donor HSCs. Based on these findings, we attempted to inject whole BMCs (including stromal cells) directly into the bone marrow (intra–bone marrow [IBM] injection). In the present study, we show that IBM-BMT is a powerful strategy for the treatment of intractable autoimmune diseases in chimeric resistant MRL/lpr mice; IBM-BMT reduces the radiation dose from 5.5 Gy × 2 to 5 Gy × 2, and obviates the need for the supplemental IV injection of BMCs.
Materials and methods
Mice
Female MRL/Mp-lpr/lpr (MRL/lpr, H-2k), C57BL/6 (B6, H-2b), BALB/c (H-2d) mice were purchased from SLC (Shizuoka, Japan) and maintained until use in our animal facilities under specific pathogen-free conditions.
Preparation of donor BMCs and IBM injection of BMCs
The BMCs were collected from the femurs and tibias of B6 mice. The IBM injection was carried out as follows. The region from the inguen to the knee joint was shaved of hair with a razor and a 5-mm incision was made on the thigh. The knee was flexed to 90° and the proximal side of the tibia was drawn to the anterior. A 26-gauge needle was inserted into the joint surface of the tibia through the patellar tendon and then inserted into the bone marrow cavity. Using a microsyringe (50 μL; Hamilton, Reno, NV) containing the donor BMCs (3 × 107/30 μL), the donor BMCs were injected from the bone hole into the bone marrow cavity.
PV injection of BMCs
The BMCs were injected via the PV, as described previously.25 In brief, a midline incision was made on the abdomen to expose the viscera. The donor BMCs (3 × 107/300 μL) were injected via the superior mesenteric vein using a 27-gauge needle.
Experimentalprotocols
The onset of autoimmune diseases in MRL/lpr mice was monitored by proteinuria (> 2.5+) and lymphadenopathy. The mice (4-5 months of age) with autoimmune diseases were irradiated in fractionated doses (5.5 Gy × 2 = 11 Gy; 4-hour interval). One day after the irradiation, the mice were transplanted with the whole BMCs (3 × 107) by IBM injections (abbreviated as 5.5 Gy × 2 + IBM). Two groups were prepared as controls. The first group consisted of mice that were irradiated (5.5 Gy × 2) and transplanted with the whole BMCs (3 × 107) via the PV (abbreviated as 5.5 Gy × 2 + PV). The second group of mice was irradiated (5.5 Gy × 2) and transplanted with the whole BMCs (3 × 107) via the tail vein (abbreviated as 5.5 Gy × 2 + IV). Furthermore, recipients that had received reduced irradiation doses (5 Gy × 2, or 4.5 Gy × 2) plus IBM-BMT, PV-BMT, and IV-BMT were also prepared.
Preparationof hepatic mononuclear cells (HMNCs)
The HMNCs were prepared as described previously.28In brief, the liver was perfused with collagenase (Type IV, 400 U/mL; Sigma Chemical, St Louis, MO) via the PV, and then incubated at 37°C for 40 minutes. A single-cell suspension was placed on Lympholyte-Mammal density solution (1.0860 g/mL; Cedarlane Laboratories, Hornby, ONT, Canada), and centrifuged for 30 minutes at 2800 rpm. The HMNCs were collected from the defined layer at the interface.
Analyses for surface marker antigens
The spleen cells, HMNCs, and BMCs were prepared from the recipient mice, and the cells were then stained with fluorescein isothiocyanate (FITC)-conjugated anti-H-2Db and phycoerythrin (PE)-conjugated anti-H-2Kk mAbs (Pharmingen, San Diego, CA) to identify the donor-derived cells. The cell surface phenotypes were also analyzed by FITC- or PE-conjugated mAbs against Thy1.2, B220, CD4, CD8, CD11b, and Gr-1 (Pharmingen). Furthermore, the cells were stained with biotinylated mAbs against lineage (Lin) markers (anti-CD4, anti-CD8 [Caltag, Burlingame, CA], anti-B220, anti-Gr-1, and anti-CD11b mAbs [Pharmingen]), followed by streptavidin-RED670 (Gibco BRL, Rockville, MD), then further stained with PE-anti-c-kit mAb and FITC-anti-H-2Db or anti-H-2Kk (Pharmingen). The cells with the immunophenotype of Lin−/c-kit+/H-2b+ were categorized as donor-derived hemopoietic progenitors. The stained cells were analyzed by a FACScan (Becton Dickinson, Mountain View, CA).
Analyses for donor-derived stromal cells
To prepare the bone marrow-derived stromal cells, the humeri from which the BMCs had been extensively washed out were cut into pieces, and the bone pieces were then cultured in a flask containing RPMI with 10% fetal bovine serum (FBS) at 37°C in 5% CO2 in air. The medium in the culture flask was replaced with the same volume of fresh medium weekly. Three weeks later, nonadherent cells, if any, were extensively removed, and the adherent cells were then collected from the surface of the flask using Cell Dissociation Solution (Sigma). To detect donor-derived stromal cells, the cultured cells were stained with anti-PA6 mAbs, which were reactive to the stromal cells,29 followed by PE-anti-rat IgM (Pharmingen). After blocking with normal rat IgM (Pharmingen), the cells were further stained with FITC-anti-H-2Db or anti-H-2Kk mAb, and analyzed by a FACScan. The cultured cells stained with the isotype-matched immunoglobulins served as a negative control.
Measurement of autoantibodies
Rheumatoid factors (RFs; IgG and IgM) and antisingle-stranded DNA (ssDNA) Abs (IgG and IgM) in the sera of the recipient mice were determined by a standard enzyme-linked immunosorbent assay (ELISA) as described previously.28Autoantibodies were measured by absorbance at OD 405 nm, which is the maximum absorbance using the phosphatase substrate tablet (Sigma 104; Sigma).
Analyses for immunologic functions
Antibody production against sheep red blood cells (SRBCs) and mixed leukocyte reaction (MLR) were performed as immunologic functions of the treated mice. Anti-SRBC antibody response was evaluated, as described previously.28 In brief, the spleen cells (4 × 106) were cultured with the same number of SRBCs for 5 days, and anti-SRBC antibody production was measured by the modified Jerne plaque-forming cell (PFC) assay. MLR was performed as follows. The splenic T cells (2 × 105) were cultured with 2 × 105 responder T cells and 2 × 105 irradiated (12 Gy) stimulator spleen cells for 72 hours and pulsed with 0.5 μCi of [3H]-thymidine for the last 16 hours of the culturing period.
Pathologic findings
The kidneys of the recipient mice were removed and fixed in 10% formalin, and the sections were stained with hematoxylin and eosin (H-E). For the immunofluorescence study, the specimens were frozen in dry ice/acetone, as previously described.6 Briefly, 3-μm sections were stained with FITC-conjugated rabbit antimouse IgG or FITC-conjugated antimouse C3 (Medical and Biological Laboratories, Nagoya, Japan).
At 14 days after the treatment with 5.5 Gy × 2 + IBM, the tibia (directly injected with the BMCs) and the femur (not injected with BMCs) were removed and fixed in 10% formalin, and the sections were then stained with H-E. As control groups, the tibias and femurs of the mice treated with 5.5 Gy × 2 + IV were stained with H-E. For the immunofluorescence study, the bone marrow specimens were frozen, and sectioned samples were stained with anti-PA6 mAb followed by PE-antirat IgM, then blocked by normal rat IgM. These sections were further stained with FITC-anti-H-2Db or anti-H-2Kk mAbs. The stained samples were examined on a confocal laser scanning microscope (LSM-GB200, Olympus, Tokyo, Japan) equipped with a × 20 objective lens.
Statisticalanalyses
Statistical analyses of the survival rate of the recipient mice were performed using a log-rank test.
Proteinuria
Proteinuria was measured using testing papers (Albustix, Miles-Sankyo, Tokyo, Japan).
Results
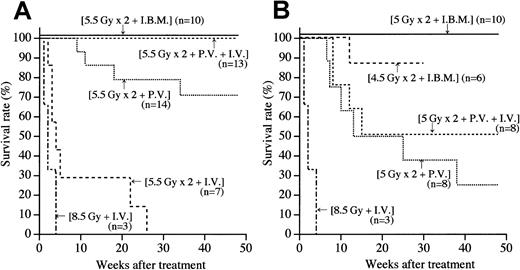
Survival rates after BMT
The MRL/lpr mice with lymphadenopathy and a high level of proteinuria (after the onset of autoimmune diseases) were treated with BMT via the IBM, PV, or IV. As shown in Figure1A, all the mice treated with 8.5 Gy + IV died within 4 weeks due to the side effects of radiation, as we previously reported.24 The ineffectiveness of BMT via the IV was again observed when the recipients received fractionated irradiation to reduce the side effects of radiation (5.5 Gy × 2 + IV): all the mice died within 30 weeks (Figure 1A). In contrast, more than 70% of MRL/lpr mice treated with fractionated irradiation and BMT via the PV (5.5 Gy × 2 + PV) survived more than 1 year after the treatment. These findings suggest that the PV injection of BMCs is more effective in prolonging survival than the IV injection. The supplemental injection via the IV (5.5 Gy × 2 + PV + IV) completely cured the autoimmune diseases in the MRL/lpr mice; 100% of the mice survived 1 year after the treatment, indicating that the supplemental IV injection is helpful for successful engraftment, as we previously reported.28
Survival rates in MRL/lpr mice treated by various methods.
(A) MRL/lpr mice irradiated with 5.5 Gy × 2 were injected with BMCs via the peripheral vein (intravenously, IV), via the portal vein (PV), or into the bone marrow cavity (intra–bone marrow, IBM). As a negative control, the mice irradiated with 8.5 Gy received BMCs via the IV. Numbers in parentheses represent the numbers of mice used in each group. Statistical analyses were carried out by a log-rank test:P < .001, 5.5 Gy × 2 + IBM versus 5.5 Gy × 2 + PV. (B) MRL/lpr mice irradiated with 5 Gy × 2 were injected with BMCs via the IV, via the PV, or via the IBM. Furthermore, the mice that had been irradiated with 4.5 Gy × 2 were injected with BMCs via the IBM.
Survival rates in MRL/lpr mice treated by various methods.
(A) MRL/lpr mice irradiated with 5.5 Gy × 2 were injected with BMCs via the peripheral vein (intravenously, IV), via the portal vein (PV), or into the bone marrow cavity (intra–bone marrow, IBM). As a negative control, the mice irradiated with 8.5 Gy received BMCs via the IV. Numbers in parentheses represent the numbers of mice used in each group. Statistical analyses were carried out by a log-rank test:P < .001, 5.5 Gy × 2 + IBM versus 5.5 Gy × 2 + PV. (B) MRL/lpr mice irradiated with 5 Gy × 2 were injected with BMCs via the IV, via the PV, or via the IBM. Furthermore, the mice that had been irradiated with 4.5 Gy × 2 were injected with BMCs via the IBM.
We next examined whether the radiation dose could be reduced. MRL/lpr mice treated with either 5 Gy × 2 + PV or 5 Gy × 2 + PV + IV showed survival rates of 30% and 50%, respectively (Figure 1B). These findings indicate that the 5 Gy × 2 irradiation alone (without using cyclophosphamide) is insufficient to prevent graft rejection.24 Therefore, we attempted to establish a new method for BMT that prevents the graft rejection even under the reduced radiation dose. BMCs were injected directly into the bone marrow (IBM). Surprisingly, all the recipients that received IBM-BMT survived 48 weeks after the treatment without showing any signs of graft rejection or recurrence of autoimmune diseases when treated with 5 Gy × 2 + IBM (Figure 1B) as well as 5.5 Gy × 2 + IBM (Figure 1A). Furthermore, more than 85% of the MRL/lpr mice survived 30 weeks after the treatment even when treated with 4.5 Gy × 2 + IBM (Figure 1B).
Analyses of donor-derived hemopoietic cells
The percentages of the donor-derived hemopoietic cells in the bone marrow, spleen, and liver were kinetically examined on days 3, 7, 10, and 14 after the treatment with 5.5 Gy × 2 + IBM, and compared with those from the recipients that received 5.5 Gy × 2 + IV or 5.5 Gy × 2 + PV. As shown in Figure2B, when treated with 5.5 Gy × 2 + IV, the percentages of donor-derived cells in the hemopoietic organs had increased by 10 days after the treatment, but then gradually decreased. When treated with 5.5 Gy × 2 + PV, the percentages of donor-derived cells had rapidly increased by 10 days and reached almost 100% on day 14. When treated with 5.5 Gy × 2 + IBM, the percentages of donor-derived cells remarkably increased to approximately 60% both in the bone marrow from the tibia (directly injected with BMCs) and the femur (not injected with BMCs) and to approximately 40% in the spleen on day 3 after the treatment, reaching almost 100% in both the bone marrow (the tibia and femur) and spleen on day 10 (Figure 2B). The representative FACS profiles (on days 3 and 10) confirming the kinetic data are shown in Figure 2A.
Percentages of donor-derived cells in MRL/lpr mice treated with 5.5 Gy × 2 + IBM, 5.5 Gy × 2 + PV, or 5.5 Gy × 2 + IV.
(A) Representative dot-plot profiles of BMCs, spleen cells, and HMNCs obtained from MRL/lpr mice 3 or 10 days after the treatment with 5.5 Gy × 2 + IBM, 5.5 Gy × 2 + PV, or 5.5 Gy × 2 + IV are shown. The cells were collected from the recipients and stained with donor (FITC-anti-H-2b)- and recipient (PE-anti-H-2k)-specific mAbs. (B) Kinetic analysis of donor-derived cells. Donor-derived cells in the bone marrow, spleen, and liver (HMNCs) were analyzed at the days indicated on the X-axis after staining with FITC-anti-H-2b and PE-anti-H-2k mAbs. The results are expressed as the mean ± SD of 6 mice. Symbols representing the sites of the injection of BMCs are as follows: ● represent donor-derived BMCs collected from the tibia where the donor BMCs were directly injected, and ♦ show donor-derived BMCs obtained from the femur where the donor BMCs were not injected; ▴ indicates PV, and ▪, IV.
Percentages of donor-derived cells in MRL/lpr mice treated with 5.5 Gy × 2 + IBM, 5.5 Gy × 2 + PV, or 5.5 Gy × 2 + IV.
(A) Representative dot-plot profiles of BMCs, spleen cells, and HMNCs obtained from MRL/lpr mice 3 or 10 days after the treatment with 5.5 Gy × 2 + IBM, 5.5 Gy × 2 + PV, or 5.5 Gy × 2 + IV are shown. The cells were collected from the recipients and stained with donor (FITC-anti-H-2b)- and recipient (PE-anti-H-2k)-specific mAbs. (B) Kinetic analysis of donor-derived cells. Donor-derived cells in the bone marrow, spleen, and liver (HMNCs) were analyzed at the days indicated on the X-axis after staining with FITC-anti-H-2b and PE-anti-H-2k mAbs. The results are expressed as the mean ± SD of 6 mice. Symbols representing the sites of the injection of BMCs are as follows: ● represent donor-derived BMCs collected from the tibia where the donor BMCs were directly injected, and ♦ show donor-derived BMCs obtained from the femur where the donor BMCs were not injected; ▴ indicates PV, and ▪, IV.
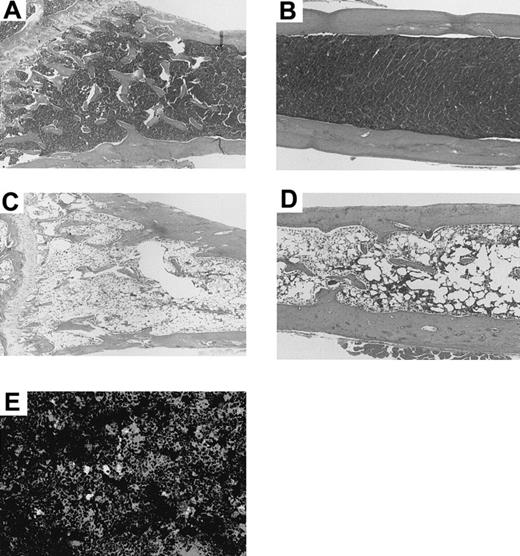
These findings were confirmed histologically; both the tibia (Figure3A; directly injected with the BMCs) and the femur (Figure 3B; not injected with BMCs) showed a remarkable proliferation of hemopoietic cells in comparison with the tibia (Figure3C) and femur (Figure 3D) of the mice treated with 5.5 Gy × 2 + IV when examined 14 days after the treatment. The tibia and femur of the mice treated with 5.5 Gy × 2 + IV showed hypoplastic bone marrow. Furthermore, the tibia from the recipients that had been treated with 5.5 Gy × 2 + IV and injected with 30 μL medium alone (instead of BMCs), also showed hypoplastic bone marrow, and the most mice died within 2 months (data not shown). This indicates that the IBM injection actually facilitates the donor cell engraftment and does not merely modulate the homing pattern of BMCs.
Histologic findings in tibia and femur of a MRL/lpr mouse 14 days after treatment with 5.5 Gy × 2 + IBM or 5.5 Gy × 2 + IV.
The tibia (directly injected with BMCs, A) and femur (not injected, B) of the MRL/lpr mouse treated with 5.5 Gy × 2 + IBM show hyperplastic bone marrow 14 days after the treatment. In contrast, the tibia (C) and femur (D) of the mouse treated with 5.5 Gy × 2 + IV show hypoplastic bone marrow 14 days after the treatment. (E) Immunohistochemical analysis of bone marrow treated with 5.5 Gy × 2 + IBM. The frozen specimen of the bone marrow from the tibia was stained with anti-PA6 mAb followed by PE-antirat IgM and further stained with FITC-anti-H-2Db mAb. The stained samples were examined on a confocal laser scanning microscope. Note that there is a scattering of cells stained both with anti-PA6 mAb and anti-H-2Db mAb (yellow-colored). Donor-derived BMCs are stained green.
Histologic findings in tibia and femur of a MRL/lpr mouse 14 days after treatment with 5.5 Gy × 2 + IBM or 5.5 Gy × 2 + IV.
The tibia (directly injected with BMCs, A) and femur (not injected, B) of the MRL/lpr mouse treated with 5.5 Gy × 2 + IBM show hyperplastic bone marrow 14 days after the treatment. In contrast, the tibia (C) and femur (D) of the mouse treated with 5.5 Gy × 2 + IV show hypoplastic bone marrow 14 days after the treatment. (E) Immunohistochemical analysis of bone marrow treated with 5.5 Gy × 2 + IBM. The frozen specimen of the bone marrow from the tibia was stained with anti-PA6 mAb followed by PE-antirat IgM and further stained with FITC-anti-H-2Db mAb. The stained samples were examined on a confocal laser scanning microscope. Note that there is a scattering of cells stained both with anti-PA6 mAb and anti-H-2Db mAb (yellow-colored). Donor-derived BMCs are stained green.
Donor-derived cells with mature lineage markers (B220+, CD4+, CD8+, CD11b+, and Gr-1+) had also been generated in the bone marrow, spleen, and liver by 14 days after the treatment with 5.5 Gy × 2 + IBM (Table 1), and remained at normal levels 1 year after the treatment (data not shown). In contrast, 14 days after the treatment with 5.5 Gy × 2 + IV, hardly any donor-derived cells bearing lineage markers could be detected in the hemopoietic organs. These findings indicate that IBM-BMT significantly facilitates the early engraftment and continuous proliferation and differentiation of donor cells.
Analyses of surface antigens on donor-derived cells in MRL/lpr mice treated with 5.5 Gy × 2 + IBM
| Mice‡ . | Cell surface antigen (%)† . | ||||
|---|---|---|---|---|---|
| B220 . | CD4 . | CD8 . | CD11b . | Gr-1 . | |
| MRL/lpr mice treated with | |||||
| 5.5 Gy × 2 + IBM | |||||
| Spleen cells | 18.5 ± 8.9 | 3.7 ± 2.0 | 6.9 ± 2.4 | 40.2 ± 4.2 | 42.2 ± 3.5 |
| BMCs | 16.5 ± 4.6 | 1.9 ± 1.5 | 2.9 ± 1.2 | 55.3 ± 8.8 | 65.6 ± 3.9 |
| HMNCs | 18.0 ± 2.3 | 1.2 ± 0.2 | 19.1 ± 2.5 | 40.4 ± 8.6 | 46.5 ± 9.6 |
| 5.5 Gy × 2 + PV | |||||
| Spleen cells | 10.8 ± 6.3 | 1.0 ± 0.5 | 4.7 ± 1.1 | 36.3 ± 5.9 | 38.5 ± 2.0 |
| BMCs | 9.9 ± 3.9 | 0.5 ± 0.1 | 2.0 ± 0.7 | 48.9 ± 0.5 | 57.2 ± 5.2 |
| HMNCs | 10.3 ± 4.3 | 1.5 ± 0.3 | 5.5 ± 1.91-160 | 42.2 ± 2.8 | 48.5 ± 5.8 |
| 5.5 Gy × 2 + IV | |||||
| Spleen cells | 0.4 ± 0.5* | 0.2 ± 0.2 | 0.5 ± 0.5* | 3.8 ± 2.31-160 | 4.3 ± 4.01-160 |
| BMCs | 2.7 ± 1.41-160 | 0.4 ± 0.2 | 1.1 ± 0.8 | 27.9 ± 8.6* | 26.0 ± 3.91-160 |
| HMNCs | 0.5 ± 0.41-160 | 0.4 ± 0.3* | 0.4 ± 0.41-160 | 9.6 ± 4.6* | 13.0 ± 11.0* |
| Normal B6 mice | |||||
| Spleen cells | 51.8 ± 6.7 | 18.3 ± 3.3 | 15.3 ± 3.3 | 6.6 ± 1.2 | 7.4 ± 1.0 |
| BMCs | 13.8 ± 0.3 | 1.5 ± 0.4 | 1.1 ± 0.1 | 48.0 ± 1.4 | 54.0 ± 13.2 |
| HMNCs | 36.9 ± 2.3 | 24.3 ± 0.3 | 7.5 ± 0.2 | 21.8 ± 1.8 | 20.6 ± 2.5 |
| Mice‡ . | Cell surface antigen (%)† . | ||||
|---|---|---|---|---|---|
| B220 . | CD4 . | CD8 . | CD11b . | Gr-1 . | |
| MRL/lpr mice treated with | |||||
| 5.5 Gy × 2 + IBM | |||||
| Spleen cells | 18.5 ± 8.9 | 3.7 ± 2.0 | 6.9 ± 2.4 | 40.2 ± 4.2 | 42.2 ± 3.5 |
| BMCs | 16.5 ± 4.6 | 1.9 ± 1.5 | 2.9 ± 1.2 | 55.3 ± 8.8 | 65.6 ± 3.9 |
| HMNCs | 18.0 ± 2.3 | 1.2 ± 0.2 | 19.1 ± 2.5 | 40.4 ± 8.6 | 46.5 ± 9.6 |
| 5.5 Gy × 2 + PV | |||||
| Spleen cells | 10.8 ± 6.3 | 1.0 ± 0.5 | 4.7 ± 1.1 | 36.3 ± 5.9 | 38.5 ± 2.0 |
| BMCs | 9.9 ± 3.9 | 0.5 ± 0.1 | 2.0 ± 0.7 | 48.9 ± 0.5 | 57.2 ± 5.2 |
| HMNCs | 10.3 ± 4.3 | 1.5 ± 0.3 | 5.5 ± 1.91-160 | 42.2 ± 2.8 | 48.5 ± 5.8 |
| 5.5 Gy × 2 + IV | |||||
| Spleen cells | 0.4 ± 0.5* | 0.2 ± 0.2 | 0.5 ± 0.5* | 3.8 ± 2.31-160 | 4.3 ± 4.01-160 |
| BMCs | 2.7 ± 1.41-160 | 0.4 ± 0.2 | 1.1 ± 0.8 | 27.9 ± 8.6* | 26.0 ± 3.91-160 |
| HMNCs | 0.5 ± 0.41-160 | 0.4 ± 0.3* | 0.4 ± 0.41-160 | 9.6 ± 4.6* | 13.0 ± 11.0* |
| Normal B6 mice | |||||
| Spleen cells | 51.8 ± 6.7 | 18.3 ± 3.3 | 15.3 ± 3.3 | 6.6 ± 1.2 | 7.4 ± 1.0 |
| BMCs | 13.8 ± 0.3 | 1.5 ± 0.4 | 1.1 ± 0.1 | 48.0 ± 1.4 | 54.0 ± 13.2 |
| HMNCs | 36.9 ± 2.3 | 24.3 ± 0.3 | 7.5 ± 0.2 | 21.8 ± 1.8 | 20.6 ± 2.5 |
Spleen cells, BMCs, and HMNCs in MRL/lpr mice 14 days after the treatment with 5.5 Gy × 2 + IBM, 5.5 Gy × 2 + PV, or 5.5 Gy × 2 + IV were stained with FITC-anti-H-2Db or PE-conjugated mAbs specific for mature lineage markers (listed in the table), and analyzed using a FACScan.
The results are expressed as the mean ± SD of 6 mice. Asterisks represent P values of 5.5 Gy × 2 + IBM versus 5.5 Gy × 2 + PV or 5.5 Gy × 2 + IV;
P < .01,
P < .001.
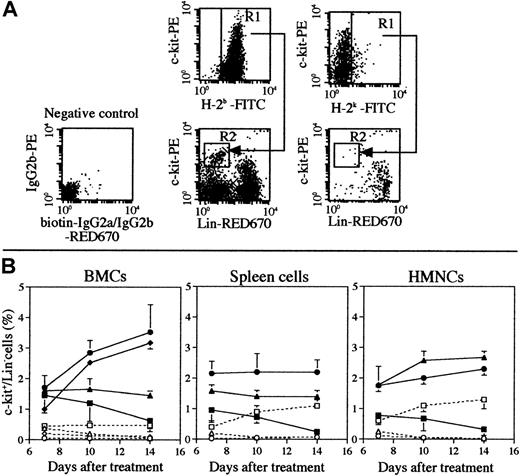
Analyses of donor-derived progenitor cells
The engraftments of hemopoietic stem cells and progenitor cells are crucially important to maintain hemopoiesis. Because many mice (> 10 mice in each group) are necessary to analyze the pluripotent hemopoietic stem cells (P-HSCs) (c-kit<low/Lin−),30 we examined the frequency of donor-derived progenitor cells (c-kit+/Lin− cells) in the hemopoietic organs. As shown in Figure 4B, the percentages of donor-derived progenitor cells (H-2b+/c-kit+/Lin− cells) in the bone marrow (either in the tibia or femur where the BMCs were directly injected or not injected, respectively) significantly increased when treated with 5.5 Gy × 2 + IBM in comparison with 5.5 Gy × 2 + PV or 5.5 Gy × 2 + IV 14 days after the treatment, and almost no progenitor cells of host origin (H-2k+/c-kit+/Lin− cells) could be detected (Figure 4B, dashed line; it should be noted that the residual host cells gradually decreased to 0% when treated with 5.5 Gy × 2 + IBM, as shown in Figure 2B). As shown in Figure 4A, 14 days after the treatment with 5.5 Gy × 2 + IBM, almost all the progenitor cells were of donor origin. It should be noted that the percentages of donor-derived progenitor cells had increased even in the spleen of recipients treated with 5.5 Gy × 2 + IBM, although the percentages in the spleens in the other groups had decreased. The percentages of donor-derived progenitor cells in the bone marrow remained more than 3% up to 1 year after the treatment (data not shown). These findings indicate that IBM-BMT accelerates and maintains the proliferation of donor-derived progenitor cells, possibly P-HSCs.
Percentages of donor-derived c-kit+/Lin− cells in MRL/lpr mice treated with 5.5 Gy × 2 + IBM, 5.5 Gy × 2 + PV, or 5.5 Gy × 2 + IV.
(A) A representative dot-plot profile of BMCs (the femur) obtained from MRL/lpr mice 14 days after the treatment with 5.5 Gy × 2 + IBM is shown. The cells were collected from the recipients and stained with FITC-anti-H-2b mAb or FITC-anti-H-2k mAb to detect the donor- or host-derived cells (gated as R1). The cells were then stained with PE-anti-c-kit and biotinated mAbs (anti-CD4, anti-CD8, anti-B220, anti-CD11b, and anti-Gr-1 mAbs) plus streptavidin-RED670. The donor- or host-derived hemopoietic progenitor cells (c-kit+/Lin− cells) were observed (gated as R2), and the percentages of these cells to total cells were calculated, respectively. The FACS profile of the negative control, in which the cells were stained with isotype-matched biotinated-IgG2a/IgG2b plus streptavidin RED670 and PE-IgG2a (instead of PE-anti-c-kit mAb), is also shown. (B) Kinetic analysis of donor- or host-derived progenitor cells. The percentages of donor (closed symbols and straight lines) or host (open symbols and dashed lines) of c-kit+/Lin− progenitor cells in the bone marrow, spleen, and liver (HMNCs) in MRL/lpr mice treated with 5.5 Gy × 2 + IBM (●, ○, tibia; ♦, ⋄, femur), 5.5 Gy × 2 + PV (▴, ▵), or 5.5 Gy × 2 + IV (▪, ■) are shown. The results are expressed as the mean ± SD of 6 mice. Symbols representing the sites of the injection of BMCs are shown next to the right-hand figure. (●) Represent donor-derived BMCs collected from the tibia, where the donor BMCs were directly injected, and the (♦) show donor-derived BMCs obtained from the femur, where no donor BMCs were injected.
Percentages of donor-derived c-kit+/Lin− cells in MRL/lpr mice treated with 5.5 Gy × 2 + IBM, 5.5 Gy × 2 + PV, or 5.5 Gy × 2 + IV.
(A) A representative dot-plot profile of BMCs (the femur) obtained from MRL/lpr mice 14 days after the treatment with 5.5 Gy × 2 + IBM is shown. The cells were collected from the recipients and stained with FITC-anti-H-2b mAb or FITC-anti-H-2k mAb to detect the donor- or host-derived cells (gated as R1). The cells were then stained with PE-anti-c-kit and biotinated mAbs (anti-CD4, anti-CD8, anti-B220, anti-CD11b, and anti-Gr-1 mAbs) plus streptavidin-RED670. The donor- or host-derived hemopoietic progenitor cells (c-kit+/Lin− cells) were observed (gated as R2), and the percentages of these cells to total cells were calculated, respectively. The FACS profile of the negative control, in which the cells were stained with isotype-matched biotinated-IgG2a/IgG2b plus streptavidin RED670 and PE-IgG2a (instead of PE-anti-c-kit mAb), is also shown. (B) Kinetic analysis of donor- or host-derived progenitor cells. The percentages of donor (closed symbols and straight lines) or host (open symbols and dashed lines) of c-kit+/Lin− progenitor cells in the bone marrow, spleen, and liver (HMNCs) in MRL/lpr mice treated with 5.5 Gy × 2 + IBM (●, ○, tibia; ♦, ⋄, femur), 5.5 Gy × 2 + PV (▴, ▵), or 5.5 Gy × 2 + IV (▪, ■) are shown. The results are expressed as the mean ± SD of 6 mice. Symbols representing the sites of the injection of BMCs are shown next to the right-hand figure. (●) Represent donor-derived BMCs collected from the tibia, where the donor BMCs were directly injected, and the (♦) show donor-derived BMCs obtained from the femur, where no donor BMCs were injected.
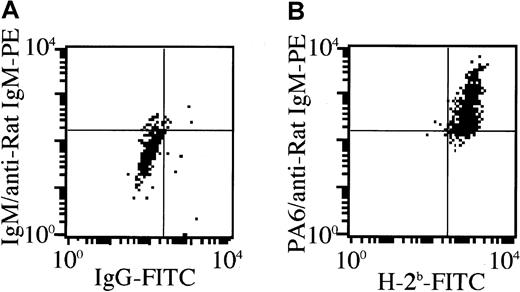
Analyses of donor-derived stromal cells
We have recently found that donor-derived stromal cells are necessary for successful allogeneic BMT, because there is an MHC restriction between P-HSCs and stromal cells.23 To examine whether donor-derived stromal cells are actually present in the recipient bone marrow after the treatment, bone pieces without BMCs from the treated MRL/lpr mice were cultured for 3 weeks, and the cultured stromal cells were then collected. The number of adherent cells from the recipient bone treated with 5.5 Gy × 2 + IV was 1.5 ± 1.5 × 104/culture (humerus), and that of 5.5 Gy × 2 + PV was 4.2 ± 1.2 × 105, whereas that of 5.5 Gy × 2 + IBM was 5.9 ± 0.7 × 105 (the mean ± SD of 5 mice). Furthermore, as shown in Figure 5, these cultured stromal cells from the recipients treated with 5.5 Gy × 2 + IBM were positive for H-2Db and stained by stromal cell-specific anti-PA6 mAb,29 indicating the replacement of stromal cells by donor-derived stromal cells. This was also confirmed by the immunohistochemical analyses where the stromal cells that were stained by anti-PA6 mAb were also detected by anti-H-2Db mAb (Figure 3E, cells colored yellow), but not by anti-H-2Kk mAb (data not shown), again indicating that the stromal cells were of donor origin. These findings indicate that the donor-derived stromal cells can efficiently proliferate in the recipient bone marrow when IBM-BMT is carried out. In addition, abnormal T cells (Thy1.2+/B220+) of MRL/lpr mice disappeared, and all the cells in the spleen and lymph nodes had been replaced by donor-derived cells by 40 weeks after the treatment of 5.5 Gy × 2 + IBM (at the age of 60 weeks), indicating that the hematolymphoid cells are completely reconstituted with donor-derived cells (data not shown).
Generation of donor-derived stromal cell.
The bone pieces without BMCs from MRL/lpr mice 10 days after the treatment with 5.5 Gy × 2 + IBM were cultured for 3 weeks, and the cultured stromal cells were then collected. The cells were stained with anti-PA6 mAb followed by PE-anti-rat IgM, then blocked with normal rat IgM. They were further stained with FITC-anti-H-2Db mAb. Quadrants in the figures were set by the staining profile of the cells treated with isotype-matched Ig controls.
Generation of donor-derived stromal cell.
The bone pieces without BMCs from MRL/lpr mice 10 days after the treatment with 5.5 Gy × 2 + IBM were cultured for 3 weeks, and the cultured stromal cells were then collected. The cells were stained with anti-PA6 mAb followed by PE-anti-rat IgM, then blocked with normal rat IgM. They were further stained with FITC-anti-H-2Db mAb. Quadrants in the figures were set by the staining profile of the cells treated with isotype-matched Ig controls.
Immunologic and immunopathologic findings in MRL/lpr mice treated with 5.5 Gy × 2 + IBM
Nontreated MRL/lpr mice at the age of 20 weeks showed increased anti-ssDNA Abs (IgG and IgM) and RFs (IgG and IgM), whereas MRL/lpr mice treated with 5.5 Gy × 2 + IBM showed almost normal values in all these parameters 40 weeks after the treatment (Figure 6). In addition, the immunologic functions were completely restored after the treatment (40 weeks) when assessed by in vitro anti-SRBC antibody response (number of PFC/culture: 198.3 ± 16.1 in the recipients treated with 5.5 Gy × 2 + IBM and 257.7 ± 13.2 in normal B6 mice). Furthermore, newly developed T cells showed tolerance to both host (MRL/lpr)-type and donor (B6)-type MHC determinants, whereas they showed normal responsiveness to third party (BALB/c) cells when examined in MLR (data not shown). These findings indicate that successful cooperation can be achieved among newly developed T cells, B cells, and antigen-presenting cells in MRL/lpr mice treated with 5.5 Gy × 2 + IBM.
Serum autoantibody levels in MRL/lpr mice treated with 5.5 Gy × 2 + IBM.
The levels of anti-ssDNA Abs and RFs were measured 40 weeks after the treatment (striped bars). Sera from B6 (black bars) and nontreated MRL/lpr mice (white bars) at 20 weeks of age served as positive and negative controls, respectively. Results are expressed as the mean ± SD of 6 mice. Asterisks represent P values of treated versus nontreated MRL/lpr mice; *P < .01 and **P < .001.
Serum autoantibody levels in MRL/lpr mice treated with 5.5 Gy × 2 + IBM.
The levels of anti-ssDNA Abs and RFs were measured 40 weeks after the treatment (striped bars). Sera from B6 (black bars) and nontreated MRL/lpr mice (white bars) at 20 weeks of age served as positive and negative controls, respectively. Results are expressed as the mean ± SD of 6 mice. Asterisks represent P values of treated versus nontreated MRL/lpr mice; *P < .01 and **P < .001.
The glomeruli in the nontreated MRL/lpr mice showed the proliferation of mesangial cells and marked IgG deposits (Figure7A,B), whereas the glomeruli in the MRL/lpr mice treated with 5.5 Gy × 2 + IBM showed normal appearance without IgG deposits (Figure 7C,D).
Pathologic findings in glomeruli of MRL/lpr mouse kidneys.
A nontreated MRL/lpr mouse (20 weeks old) shows the proliferation of mesangial cells in the glomerulus (A) and marked IgG deposits (B). A MRL/lpr mouse (60 weeks old) treated with 5.5 Gy × 2 + IBM shows normal appearance (C) without C3 deposits (D) 40 weeks after the treatment.
Pathologic findings in glomeruli of MRL/lpr mouse kidneys.
A nontreated MRL/lpr mouse (20 weeks old) shows the proliferation of mesangial cells in the glomerulus (A) and marked IgG deposits (B). A MRL/lpr mouse (60 weeks old) treated with 5.5 Gy × 2 + IBM shows normal appearance (C) without C3 deposits (D) 40 weeks after the treatment.
Discussion
Allogeneic BMT has become an established technique and is generally performed as the treatment of preference for leukemia and aplastic anemia. However, problems such as graft failure and graft-versus-host reaction have been reported; only 60% of patients with leukemia or 55% of patients with aplastic anemia survive disease free.31-33 We have previously found that allo BMT via the IV or the PV with or without bone grafts (to recruit donor stromal cells) has curative effects on autoimmune diseases in various autoimmune-prone mice except MRL/lpr mice.34,35 In humans, allo BMT has been performed on patients with autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, psoriasis, and Crohn disease, and has been reported to be effective in treating these autoimmune diseases.7-13Moreover, these diseases do not recur after allo BMT during long-term observation (range 7-20 years).14 15 In general, patients with autoimmune diseases will have undergone long-term administration of anti-inflammatory drugs, immunosuppresants, and cytotoxic reagents, all of which produce various side effects. Therefore, a safe new strategy for allo BMT has been long awaited.
We have previously found that MHC-matched stromal cells are required for the proliferation and differentiation of P-HSCs, and that the donor-derived stromal cells play a crucial role in successful allo BMT.21-27 In addition, we have recently found that allo BMT via the PV plus supplemental IV injections completely cures autoimmune diseases in chimeric-resistant MRL/lpr mice even after the onset of autoimmune diseases, and that all the recipients thus treated survived more than 1 year without any recurrence of the diseases.28 These findings suggest that the PV injection of donor whole BMCs (including donor stromal cells) creates a suitable microenvironment in the liver, and that this facilitates the proliferation of donor HSCs in collaboration with MHC-matched donor stromal cells. Indeed, we have very recently found that donor-derived cells trapped in the liver play a crucial role in the success of BMT36; when adherent cell-depleted BMCs were injected, 75% of recipients died within 90 days, whereas all the recipients injected with adherent cell-depleted BMCs with cultured stromal cells survived more than 80 days.37 Although this method is effective, there are risks associated with the abdominal section, and supplemental IV injection is required. In addition, the radiation dose could not be reduced to 5 Gy × 2.
It has been reported that the transplantation of a large number of HSCs overcomes MHC barriers.38,39 We have very recently found that a large number of HSCs can proliferate in collaboration with MHC-mismatched stromal cells,40 although there is an MHC restriction between HSCs and stromal cells.23 However, in humans, this strategy suffers from the difficulty of obtaining a large number of HSCs from one donor. In the present study, we attempted to inject the allogeneic whole BMCs (including a small number [< 3%] of T cells, HSCs, and stromal cells) directly into the recipient bone marrow cavity (IBM-BMT) so that donor-derived hemopoietic cells including stromal cells can effectively accumulate in the bone marrow. To our knowledge, this is the first report on the effect of IBM-BMT on autoimmune diseases in MRL/lpr mice, although there are a few reports in which bone marrow cells aspirated from the bone marrow cavity were used for serial BMT41 and gene therapy.42 All the MRL/lpr mice survived more than 1 year (> 60 weeks after birth) without the recurrence of autoimmune diseases, and immunologic functions were completely restored even when the radiation dose was reduced to 5 Gy × 2 (5 Gy × 2 + IBM). It should be noted that more than 85% of MRL/lpr mice treated only with 4.5 Gy × 2 + IBM also survived more than 30 weeks after the treatment. These findings suggest that IBM-BMT can be used to treat intractable autoimmune diseases under reduced radiation doses without using any immunosuppressants. This seems to be attributable to the enhanced engraftment of donor-derived cells in the early stage after this treatment; the percentage of donor-derived cells reached almost 100%, and donor-derived cells bearing mature lineage markers were generated in the bone marrow, spleen, and liver by 14 days after the treatment with IBM-BMT (Figure 2 and Table 1). Furthermore, the percentages of donor-derived progenitor cells in the bone marrow of both the tibia (BMCs were directly injected) and the femur (BMCs were not injected) significantly increased by 14 days after the treatment (Figure 4). Therefore, the IBM injection rapidly accelerates the proliferation of donor-derived progenitor cells and simultaneously maintains hemopoietic progenitor cells, resulting in the recovery of hemopoiesis.
In the present study, we have shown that IBM-BMT is, so far, the best strategy for allo BMT: (1) no GVHD develops even if T cells are not depleted from the bone marrow; (2) no graft failure occurs even if the dose of radiation as the conditioning for BMT is reduced to 5 Gy × 2; (3) hemopoietic recovery is rapid; and (4) the restoration of T-cell functions is complete even in donor-recipient combinations across the MHC barriers. We believe that this IBM-BMT is applicable to humans, because intraosseous (IO) infusion (IBM injection) is now an established method for administering fluids, drugs, and blood to critically ill patients, particularly infants.43-46Indeed, Hagglund and coworkers have recently compared the effectiveness of IO infusion with that of IV infusion in human allo BMT47; they have concluded that allo BMT can be safely performed by IO infusion, but the incidences of acute and chronic GVHD, transplantation-related mortality, and survival rates are similar. However, they aspirated the donor BMCs from the iliac bones and infused these BMCs into the iliac bones of the recipients. Using cynomolgus monkeys, we have just established a new method (perfusion method) for collecting BMCs from the long bones (femur, humerus, and so on) without peripheral blood contamination.48 This method has various merits: (1) no GVHD develops even in cynomolgus monkeys, because the percentage of T cells in the BMCs thus collected is less than 3%, and (2) a large number of BMCs can be collected quickly and safely. We therefore believe that this method (IBM-BMT) will become a powerful new strategy for not only allo BMT but also organ transplantation in conjunction with BMT.
The authors thank Ms Y. Tokuyama, Ms M. Shinkawa, Ms S. Miura (First Department of Pathology) and Mr K. Kobayashi (Central Research Center) for their expert technical assistance, and Mr Hilary Eastwick-Field and Ms K. Ando for their help in the preparation of the manuscript.
Supported by a grant from Haiteku Research Center of the Ministry of Education, a grant-in-aid for scientific research (B) 11470062, grants-in-aid for scientific research on priority areas (A)10181225 and (A)11162221, a grant from Millennium of Science and Technology Agency, and also a grant from Japan Immunoresearch Laboratories Co, Ltd (JIMRO).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Susumu Ikehara, First Department of Pathology, Kansai Medical University, 10-15 Fumizono-cho, Moriguchi City, Osaka 570-8506, Japan; e-mail: ikehara@takii.kmu.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal