This study was undertaken to show that a high survival rate can be obtained in B-cell (Burkitt and large B-cell) lymphoma and L3 leukemia with multiagent chemotherapy adapted to the tumor burden (stage, resection status, percentage of blasts in bone marrow, and central nervous system [CNS] involvement) and early response to chemotherapy, to investigate actual prognostic factors, and to see if large B-cell lymphoma can be treated with the same regimen as Burkitt lymphoma. Patients were classified into 3 risk groups. Group A (resected stage I and abdominal stage II) received 2 courses of vincristine, cyclophosphamide, doxorubicin, and prednisone. Group B (patients not eligible for groups A or C) received 5 courses of chemotherapy with, in addition, high-dose methotrexate, 3 g/m2 over 3 hours; infusional cytarabine; and intrathecal (IT) methotrexate. Group C (patients with CNS involvement and acute lymphoblastic leukemia with at least 70% of blasts in bone marrow) received 8 courses with, in addition, high-dose methotrexate, 8 g/m2; high-dose cytarabine; etoposide; and triple IT. Except in group A, treatment started with a prephase (COP, low-dose vincristine and cyclophosphamide). It was intensified for patients who did not respond to COP in group B and any patient with residual viable cells after the consolidation phase. A total of 561 patients were enrolled in the SFOP LMB89 protocol (July 1989-June 1996). Five-year survival is 92.5% (95% confidence interval [CI], 90%-94%) and event-free survival (EFS) 91% (95% CI, 89%-93%). EFS is 98% (95% CI, 90%-100%), 92% (95% CI, 89%-95%), and 84% (95% CI, 77%-90%) for group A, B, and C, respectively. In group B, multivariate analysis of prognostic factors showed that a lactate dehydrogenase level more than 2-fold the normal value, no response after COP, and age of at least 15 years were associated with a lower EFS. CNS involvement was the only prognostic factor in group C.
Introduction
In the 1980s, the French Society of Pediatric Oncology (Société Française d'Oncologie Pédiatrique, or SFOP) conducted therapeutic trials for advanced stage Burkitt lymphomas and FAB (French-American-British) L3 acute lymphoblastic leukemia (L3ALL).
The first study, LMB81 (1981-1984), a 1-year intensive 9-drug chemotherapy regimen that included 114 patients, succeeded in increasing the survival rate of 93 patients with stages III and IV lymphoma and ALL, without central nervous system (CNS) involvement, to 75%.1 Only 1% of isolated CNS relapses occurred with CNS-directed treatment based on high-dose methotrexate (HDMTX, 3 g/m2 in a 3-hour infusion). However, toxicity-related mortality was high (10%). Two prognostic factors were identified as predictive of outcome: CNS involvement (21 patients, event-free survival [EFS] ± SD, 19% ± 16%) and the absence of complete remission (CR) after 3 multiagent chemotherapy courses.
The second study, LMB84 (1984-1987), demonstrated the efficacy of a short treatment for patients without CNS involvement (CNS−).2 It also showed that (1) patients who achieved a partial remission after 3 chemotherapy courses could be salvaged with high-dose chemotherapy (HDCT) and autologous bone marrow transplantation (ABMT)3; (2) patients whose tumors did not respond to the prephase, ie, the first week of treatment, ultimately failed (21 patients, EFS 22% ±20%); and (3) toxicity-related morbidity declined as investigators gained experience with the protocol even though the induction phase remained unchanged.
During the same period (1985-1989), the pilot LMB86 study was conducted for patients with CNS disease (CNS+) or L3ALL with more than 70% of blasts in BM (patients who appeared at a higher risk of CNS disease in our previous experience4,5)6. Treatment of CNS disease was intensified with a higher dose of MTX (8 g/m2 in a 4-hour infusion) in the induction phase, high-dose cytarabine (HDAra-C) and etoposide (VP-16) during the consolidation phase,7 and cranial irradiation. The EFS of these patients increased dramatically to 75% ± 9% (24 CNS+) and 82% ± 12% (11 ALL CNS−).
Based on these results, a new protocol was designed for all consecutive patients with B-cell lymphoma including the large B-cell type, whatever the stage, with low stages included. The aims of the study were to confirm the results of previous studies, especially LMB86 findings, to see whether a treatment strategy adapted to the tumor burden and to the initial tumor response to chemotherapy would maintain a good or improve a worse outcome, and to identify the remaining or any new prognostic factors.
We report on the results of this multicenter, prospective, nonrandomized study that began in July 1989 and accrued the last patient in June 1996. Follow-up has attained at least 20 months for all patients.
Patients and methods
Eligibility
Patients were eligible for the study if they were untreated; younger than 18 years of age; had no history of immunodeficiency or a previous malignant disease; were newly diagnosed as having a B-cell lymphoma, ie, the Burkitt (or small noncleaved cell) or the large cell, centroblastic (or diffuse large cell, now designated large B-cell in the REAL classification) types, or L3ALL; and in whom follow-up was possible for at least 18 months. A mature B-cell immunophenotype was defined as reactivity to B-cell antigens (CD10, CD19, CD20, CD22, CD24 in cell suspensions or frozen tissue, or L26, CD79a in fixed tissue) and monoclonality of surface immunoglobulins. When an immunophenotype study was not available or histology was not completely informative, lymphoma arising on the bowel and lymphoma displaying specific translocations t(8;14), t(8;22), and t(2;8) at cytogenetic analysis were included.
The St Jude system was used for staging.8 In the present study, “abdominal stage II ” disease is specifically defined as localized gut tumors having undergone complete resection without extensive surgery. Involvement of a node immediately adjacent to the tumor was accepted provided that the next nodes were demonstrated to be tumor free at histologic analysis; otherwise (involvement of several nodes or involvement of a distant mesenteric node or other signs of abdominal spread such as ascites and hemoperitoneum), the tumor was classifed as stage III disease.
CNS disease was defined as the presence of blasts in the cerebrospinal fluid (CSF), whatever the number; cranial nerve palsy not related to a facial tumor; clinical signs of spinal cord compression (otherwise a paraspinal tumor was classified as stage III disease); or an intracranial mass.
A prospective study of prognostic factors for EFS was planned for groups B and C. The following factors were investigated: age, performance and nutritional status, lactate dehydrogenase (LDH) level, classification of abdominal tumors into “a” and “b” according to the criteria defined by Philip et al9 (“a”: tumor restricted to the gut and mesentery, possibly with ascites; “b”: extension to other abdominal viscera and extraabdominal extension) and the percentage of regression after the prephase (> 80%, 50%-80%, 20%-49%).
Thirty-two SFOP and 4 affiliated French centers, one Belgian center, and one Dutch center participated in this study organized by the SFOP. Two other centers, one French and one Belgian, were excluded during the study for failure to comply with minimum requirements for participation.
The minimal investigations requested were a physical examination, chest and nasopharyngeal X-rays, abdominal ultrasound, a complete blood cell (CBC) count, 2 percutaneous iliac crest BM aspirations, and an analysis of CSF. A computed tomography scan was performed in patients with head and neck tumors to evaluate local tumor extension. A computed tomography scan was mandatory to confirm complete resection in patients with a resected abdominal tumor. A metabolic evaluation and an echocardiogram were also performed.
A national panel of 4 pathologists and 2 cytologists jointly reviewed pretreatment specimens regularly and classified them according to the updated Kiel classification10 and the Working Formulation (WF).11 Approval was obtained from the SFOP review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Chemotherapy regimen
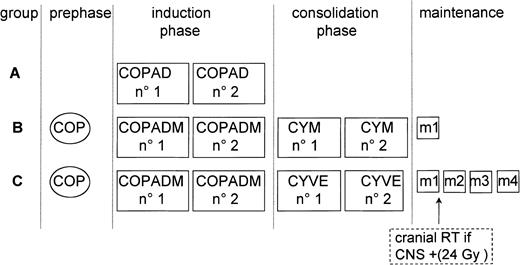
The chemotherapy regimen is shown in Figure1 and Table1. Patients were stratified into 3 groups, A, B, and C, with escalation of treatment intensity in ascending order. Treatment was also adapted to response to chemotherapy after one week and after the third or fourth courses.
LMB89 protocol schedule.
Patients were stratified into 3 risk groups: A, B, and C, depending on stage, resection status, percentage of blasts in BM, and CNS involvement. Treatment courses are listed in Table 1.
LMB89 protocol schedule.
Patients were stratified into 3 risk groups: A, B, and C, depending on stage, resection status, percentage of blasts in BM, and CNS involvement. Treatment courses are listed in Table 1.
LMB89 treatment courses
| Regimen . | Daily dose . | Administration . | Days . |
|---|---|---|---|
| Prephase | |||
| COP | |||
| CPM | 0.3 g/m2 | IV | 1 |
| VCR | 1 mg/m2 | IV | 1 |
| Prednisone | 60 mg/m2 | IV or orally (in 2 fractions) | 1-7 |
| MTX + HC (gr C: + Ara-C) | 15 mg (gr C: + 30 mg) | IT | 1 (gr C: + 3 + 5) |
| Induction phase | |||
| COPADM no. 1, started 1 wk after first day of prephase | |||
| VCR | 2 mg/m2 (max 2 mg) | IV | 1 |
| HDMTX | B: 3 g/m2 (gr C: 8 g/m2) | IV 3 h (gr C: 4 h) | 1 |
| CFR | 15 mg/m2 every 6 h | Orally | 2, 3, 4 |
| MTX + HC (gr C: + Ara-C) | 15 mg (gr C: + 30 mg) | IT | 2, 6 (gr C: + 4) |
| ADR | 60 mg/m2 | IV | 2 |
| Cyclophosphamide | 0.5 g/m2 | IV (in 2 fractions) | 2, 3, 4 |
| Prednisone | 60 mg/m2 | IV or orally | 1-6 |
| COPADM no. 2, similar to COPADM no. 1 except for: | |||
| Second VCR dose | 2 mg/m2(max 2 mg) | IV | 6 |
| Cyclophosphamide | 1 g/m2 | IV (in 2 fractions) | 2, 3, 4 |
| COPAD (group A), similar to COPADM no. 1, but without HDMTX and IT and with additional dose of VCR | 2 mg/m2 (max 2 mg) | IV | 6 |
| Consolidation phase | |||
| Group B: CYM no. 1 and 2 | |||
| HDMTX | 3 g/m2 | IV (3 h) | 1 |
| CFR | 15 mg/m2 every 6 h | Orally | 2, 3, 4 |
| MTX + HC | 15 mg | IT | 2 |
| Ara-C | 100 mg/m2 | CI (24 h) | 2-6 |
| Ara-C + HC | 30 mg + 15 mg | IT | 6 |
| Group C: CYVE no. 1 and 2 | |||
| Ara-C | 50 mg/m2 | CI (12 h) | 1-5(8 pm-8 am) |
| HDAra-C | 3 g/m2 | IV (3 h) | 2-5 (8-11 am) |
| VP-16 | 200 mg/m2 | IV | 2-5 (2-4pm) |
| Maintenance (monthly alternated courses) | |||
| m1 | |||
| VCR | 2 mg/m2 (max 2 mg) | IV | 1 |
| HDMTX | B: 3 g/m2 (gr C: 8 g/m2) | IV 3 h (gr C: 4 h) | 1 |
| CFR | 15 mg/m2 every 6 h | Orally | 2-4 |
| Prednisone | 60 mg/m2 | Orally | 1-5 |
| MTX + HC (gr C: + Ara-C) | 15 mg (gr C: + 30 mg) | IT | 2 |
| Cyclophosphamide | 0.5 g/m2 | IV | 1, 2 |
| ADR | 60 mg/m2 | IV | 2 |
| m3, similar to m1 but without HDMTX and IT | |||
| m2 or m4 | |||
| VP-16 | 150 mg/m2 | IV | 1-3 |
| Ara-C | 100 mg/m2 | SC (in 2 fractions) | 1-5 |
| Regimen . | Daily dose . | Administration . | Days . |
|---|---|---|---|
| Prephase | |||
| COP | |||
| CPM | 0.3 g/m2 | IV | 1 |
| VCR | 1 mg/m2 | IV | 1 |
| Prednisone | 60 mg/m2 | IV or orally (in 2 fractions) | 1-7 |
| MTX + HC (gr C: + Ara-C) | 15 mg (gr C: + 30 mg) | IT | 1 (gr C: + 3 + 5) |
| Induction phase | |||
| COPADM no. 1, started 1 wk after first day of prephase | |||
| VCR | 2 mg/m2 (max 2 mg) | IV | 1 |
| HDMTX | B: 3 g/m2 (gr C: 8 g/m2) | IV 3 h (gr C: 4 h) | 1 |
| CFR | 15 mg/m2 every 6 h | Orally | 2, 3, 4 |
| MTX + HC (gr C: + Ara-C) | 15 mg (gr C: + 30 mg) | IT | 2, 6 (gr C: + 4) |
| ADR | 60 mg/m2 | IV | 2 |
| Cyclophosphamide | 0.5 g/m2 | IV (in 2 fractions) | 2, 3, 4 |
| Prednisone | 60 mg/m2 | IV or orally | 1-6 |
| COPADM no. 2, similar to COPADM no. 1 except for: | |||
| Second VCR dose | 2 mg/m2(max 2 mg) | IV | 6 |
| Cyclophosphamide | 1 g/m2 | IV (in 2 fractions) | 2, 3, 4 |
| COPAD (group A), similar to COPADM no. 1, but without HDMTX and IT and with additional dose of VCR | 2 mg/m2 (max 2 mg) | IV | 6 |
| Consolidation phase | |||
| Group B: CYM no. 1 and 2 | |||
| HDMTX | 3 g/m2 | IV (3 h) | 1 |
| CFR | 15 mg/m2 every 6 h | Orally | 2, 3, 4 |
| MTX + HC | 15 mg | IT | 2 |
| Ara-C | 100 mg/m2 | CI (24 h) | 2-6 |
| Ara-C + HC | 30 mg + 15 mg | IT | 6 |
| Group C: CYVE no. 1 and 2 | |||
| Ara-C | 50 mg/m2 | CI (12 h) | 1-5(8 pm-8 am) |
| HDAra-C | 3 g/m2 | IV (3 h) | 2-5 (8-11 am) |
| VP-16 | 200 mg/m2 | IV | 2-5 (2-4pm) |
| Maintenance (monthly alternated courses) | |||
| m1 | |||
| VCR | 2 mg/m2 (max 2 mg) | IV | 1 |
| HDMTX | B: 3 g/m2 (gr C: 8 g/m2) | IV 3 h (gr C: 4 h) | 1 |
| CFR | 15 mg/m2 every 6 h | Orally | 2-4 |
| Prednisone | 60 mg/m2 | Orally | 1-5 |
| MTX + HC (gr C: + Ara-C) | 15 mg (gr C: + 30 mg) | IT | 2 |
| Cyclophosphamide | 0.5 g/m2 | IV | 1, 2 |
| ADR | 60 mg/m2 | IV | 2 |
| m3, similar to m1 but without HDMTX and IT | |||
| m2 or m4 | |||
| VP-16 | 150 mg/m2 | IV | 1-3 |
| Ara-C | 100 mg/m2 | SC (in 2 fractions) | 1-5 |
Italics indicate specific to group C.
COP indicates cyclophosphamide, Oncovin (vincristine), prednisone; CPM, cyclophosphamide; IV, intravenous; VCR, vincristine (maximum dose, 2 mg by injection); MTX, methotrexate; HC, hydrocortisone; Ara-C, cytarabine; IT, intrathecal; COPADM, cyclophosphamide, Oncovin (vincristine), prednisone, adriamycin (doxorubicin), methotrexate; HD, high dose; CFR, citrovorum factor rescue (leucovorin); ADR, adriamycin (doxorubicin); CYM, cytarabine (Ara-c), methotrexate; CI, continuous infusion; CYVE, cytarabine (Ara-c), VP-16 (etoposide); SC, subcutaneously.
Patients having undergone complete resection of stage I and abdominal stage II disease were assigned to group A. This selected group of patients, which we considered to have a very good prognosis, received only 2 cyclophosphamide, Oncovin (vincristine), prednisone, adriamycin (doxorubicin) (COPAD) courses and no intrathecal (IT) injections.
Patients with unresected stage I, nonabdominal stage II, and any stage III or IV disease and L3ALL CNS− (with < 70% of blasts in BM) were assigned to Group B. Treatment was similar to that of the short arm of the LMB84 protocol.2 If disease failed to respond after the prephase COP regimen, group B patients were switched to group C therapy (but were nonetheless analyzed with group B patients).
Patients in Group C, which we considered to have the worst prognosis, had CNS involvement or L3ALL with at least 70% of blasts in BM. Treatment was according to the LMB86 protocol with a higher dose of MTX (8 g/m2), triple IT injections, and more intensive consolidation with HDAra-C and VP-16. Cranial irradiation (24 Gy) was delivered only to patients with blasts in the CSF or cranial palsies.
If CR was not obtained after the third or fourth induction-consolidation courses in group B and group C, respectively, treatment was intensified with HDCT and ABMT. The preparative regimen was the previously described BEAM regimen (BCNU, VP-16, Ara-C, and melphalan).3
Details of treatment are given in Table 1. HDMTX was administered with alkaline hyperhydration over 48 hours in group B and 72 hours in group C with folinic acid rescue (15 mg/m2 every 6 hours × 12) started 24 hours later. The IT injection on day 2 was to precede the initiation of rescue therapy with folinic acid. Intervals between induction and consolidation courses were to be as short as possible in all groups, and chemotherapy was to be started as soon as the absolute neutrophil count attained more than 1.5 × 109/L (≥ 1500/μL) and platelets more than 100 × 109/L (≥ 100 000/μL). The course following a COPADM course was generally started at a median of 18 days later.
Supportive care
At diagnosis, vigorous alkaline diuresis was to be obtained, with furosemide if necessary, and a uricolytic (allopurinol, or urate-oxydase) was to be instituted before the initiation of treatment. All blood products were to be irradiated. Indications for transfusion were to be according to the standard policy in each center. However, erythrocyte transfusions were given when the hemoglobin level was below 80 or 70 g/L (8 or 7 g/dL) and platelet transfusions when the platelet count was below 20 × 109/L or 10 × 109/L (20 000 or 10 000/μL). Prophylaxis against Pneumocystis carinii pneumonia was mandatory in group C.
From January 1994 to June 1996, patients were proposed participation in a prospective, randomized trial of prophylactic granulocyte colony-stimulating factor given or not after COPAD(M) and CYVE courses. Part of the results of this prospective study have been reported elsewhere.12
Response criteria
CR was defined as the complete disappearance of all tumor masses confirmed at clinical examination, on X-rays, and ultrasound studies; a normal BM examination; and no evidence of CNS disease.
CR had to be assessed after the first CYM in group B and the second CYVE in group C. At this point, any residual mass had to be removed surgically or widely biopsied if resection was impossible without mutilation. CR was confirmed in the absence of residual tumor cells; on the other hand, documented proof of viable malignant cells was required for an incomplete remission.
Statistical methods
The study cutoff limit was December 1999.
Survival was calculated from the first day of chemotherapy to death due to any cause or to the date of the last follow-up contact for patients who were alive.
EFS was calculated from the first day of chemotherapy to an event (death due to any cause, progression after a PR, relapse or a second malignancy) or to the date of the last follow-up contact for patients who did not experience any event.
Overall survival and EFS were estimated with the Kaplan-Meier method.13 The 95% confidence intervals (95% CI) of the actuarial rates were calculated with the Rothman method.14The prognostic analysis was based on EFS. The log-rank test15 was used for the univariate analysis and the Cox model for multivariate analysis.
Results
Patients
A total of 579 patients were enrolled between July 1989 and June 1996. Three patients did not receive any treatment after complete resection due to a personal decision (2 relapses). Fifteen patients were excluded after review by the panel of pathologists for the following reasons: malignant disease other than non-Hodgkin lymphoma, 3; other types of lymphoma, 8 (1 T-lymphoblastic, 3 B-lymphoblastic, 1 T-pleiomorphic, 1 T-cell–rich B-cell, 1 anaplastic large cell, and 1 subcutaneous unclassified lymphoma; 5 patients alive); other types of leukemia, 3 (L2ALL, surface immunoglobulin–positive, with no specific translocation; 2 patients alive); and a staging error and modified adapted treatment after the first chemotherapy course, 1 (alive). A total of 561 patients were eligible for the analysis. Ages ranged from 2 months to 18 years with a median of 8 years. There were 436 boys and 125 girls, for a sex ratio of 3.5:1.
Primary tumor sites and stages are shown in Table2. Most patients had abdominal stage III disease. Six cases had initial removal of the tumor but were classified as having stage III disease because of enlarged mesenteric nodes without histologic evidence to confirm or exclude tumor involvement. However, after review of data concerning these patients, one was reclassified as stage II and is reported as such in this paper (but is included in the analysis of group B where he was treated). All abdominal stage II tumors presented as abdominal emergencies (intussusception or appendicitis). The tumor size ranged from 2 to 12 cm (20 of 38 were < 5 cm), and a node was positive in 5 of 26 patients for whom complete surgical and histologic documentation was available for review.
Primary sites and lactate dehydrogenase level according to stage
| . | Stage I . | Stage II . | Stage III . | Stage IV . | L3ALL . | Total . |
|---|---|---|---|---|---|---|
| Primary site | ||||||
| Abdomen | — | 38 | 230 | 35 (12) | 27 (4) | 330 (16) |
| Head and neck | 10 | 40 | 10 | 17 (13) | 18 (10) | 95 (23) |
| Peripheral node | 17 | 9 | 6 | 2 (1) | 1 (1) | 35 (2) |
| Thorax | — | — | 18 | 1 | 1 | 20 |
| Other sites* | 4 | 1 | 14 | 6 (4) | 1 | 26 (4) |
| Without | 0 | 0 | 0 | 1 (1) | 54 (21) | 55 (22) |
| LDH | ||||||
| ≤ 2N†(%) | 28 (100%) | 80 (95%) | 127 (49%) | 24 (41%) | 3 (3%) | 262 (50%) |
| > 2N (%) | 0 (0%) | 3 (5%) | 132 (51%) | 34 (59%) | 90 (97%) | 258 (50%) |
| Unknown | 3 | 5 | 19 | 4 | 10 | 41 |
| Total | 31 | 88 | 278 | 62 (31) | 102 (36) | 561 (67) |
| . | Stage I . | Stage II . | Stage III . | Stage IV . | L3ALL . | Total . |
|---|---|---|---|---|---|---|
| Primary site | ||||||
| Abdomen | — | 38 | 230 | 35 (12) | 27 (4) | 330 (16) |
| Head and neck | 10 | 40 | 10 | 17 (13) | 18 (10) | 95 (23) |
| Peripheral node | 17 | 9 | 6 | 2 (1) | 1 (1) | 35 (2) |
| Thorax | — | — | 18 | 1 | 1 | 20 |
| Other sites* | 4 | 1 | 14 | 6 (4) | 1 | 26 (4) |
| Without | 0 | 0 | 0 | 1 (1) | 54 (21) | 55 (22) |
| LDH | ||||||
| ≤ 2N†(%) | 28 (100%) | 80 (95%) | 127 (49%) | 24 (41%) | 3 (3%) | 262 (50%) |
| > 2N (%) | 0 (0%) | 3 (5%) | 132 (51%) | 34 (59%) | 90 (97%) | 258 (50%) |
| Unknown | 3 | 5 | 19 | 4 | 10 | 41 |
| Total | 31 | 88 | 278 | 62 (31) | 102 (36) | 561 (67) |
Numbers in parentheses represent patients with CNS involvement.
ALL indicates acute lymphoblastic leukemia; LDH, lactate dehydrogenase.
Other sites include bone, 15; kidney, 5; breast, 1; brain, 2; testis, 1; intraspinal, 1; and subcutaneous, 1.
2N indicates 2-fold the upper limit of the institutional normal.
The LDH level was known in 520 patients (Table 2). A high LDH level, defined as more than 2-fold the institutional upper normal value, was observed in half of the patients. This high value was observed in 0%, 45%, and 85% of patients in groups A, B, and C and in 0%, 4%, 49%, 59%, and 97% of patients with stage I, II, III, IV disease and ALL, respectively.
A total of 141 patients had blasts in their BM, 102 with more than 25% and 65 with circulating blasts. Sixty-seven patients had CNS involvement with various associated conditions, including blasts in CSF (37 cases), cranial nerve palsies (38 cases), intracranial mass (13 cases), and cord compression (12 cases). Among these 67 patients, 18 had only blasts in CSF, 16 only cranial nerve palsies, 3 only an intracranial mass (primary cerebral lymphoma in 2), and 5 only symptoms of cord compression (dumbbell tumors in 2 cases).
The diagnosis was based on histology in 247 cases, cytology in 158, and both in 156. A total of 542 slides (97%) were reviewed by the national panel: 420 were subclassified as Burkitt lymphoma (according to the WF, 247 were small noncleaved Burkitt and 173 small noncleaved non-Burkitt), 63 as large B-cell lymphomas, and 59 were not subclassified because samples were too small or there were technical fixation problems. These unclassified cases were nonetheless considered eligible for the study because they were diagnosed as malignant high-grade B-cell lymphoma.
The B-cell phenotype was confirmed in all but 40 patients in whom a phenotype analysis was either not performed or proved unsuccessful. The 40 patients were classified locally as Burkitt lymphoma and after the national panel review (4 were not reviewed but were abdominal primaries), 34 were classified as Burkitt, 10 of whom exhibited a t(8;14), and 2 were not classified but were stage IV abdominal tumors with BM and CNS involvement. Surface immunologlobulins were positive in 283 of the 293 patients studied. A cytogenetic analysis was performed in 175 patients and showed no abnormality in 28 patients; various translocations in 129, including 116 t(8;14), 10 t(8;22), 1 t(2;8), and 2 t(3;22); and other abnormalities in 18. Whereas variant translocations t(8;22) and t(2 ;8) were seen only in Burkitt and t(3;22) only in large cell lymphomas, the t(8;14) was found in 101 Burkitt, 2 large cell, 10 unclassified lymphomas, and in 3 that were not reviewed.
Therapy results
Fifty-two patients were treated in group A, 386 in group B, and 123 in group C (Table 3). In 2 patients the treatment group was wrongly assigned (1 already mentioned, with abdominal stage II disease in group B, and 1 with multifocal stage III bone disease in group C).
Distribution of patients in treatment groups
| Group . | No. of patients . | . | Subcategories . |
|---|---|---|---|
| Group A | 52 patients | 15 | Resected stage I |
| 37 | Abdominal stage II | ||
| Group B | 386 patients | 16 | Unresected stage I |
| 1 | Abdominal stage II | ||
| 50 | Nonabdominal stage II | ||
| 277 | Stage III | ||
| 31 | Stage IV CNS− (BM 1%-24%) | ||
| 11 | ALL with BM < 70%, CNS− | ||
| Group C | 123 patients | 1 | Stage III |
| 31 | Stage IV CNS+ (23, BM−; 8, BM 2%-23%) | ||
| 55 | ALL with BM ≥ 70%, CNS− | ||
| 36 | ALL CNS+ (BM 52%-99%) |
| Group . | No. of patients . | . | Subcategories . |
|---|---|---|---|
| Group A | 52 patients | 15 | Resected stage I |
| 37 | Abdominal stage II | ||
| Group B | 386 patients | 16 | Unresected stage I |
| 1 | Abdominal stage II | ||
| 50 | Nonabdominal stage II | ||
| 277 | Stage III | ||
| 31 | Stage IV CNS− (BM 1%-24%) | ||
| 11 | ALL with BM < 70%, CNS− | ||
| Group C | 123 patients | 1 | Stage III |
| 31 | Stage IV CNS+ (23, BM−; 8, BM 2%-23%) | ||
| 55 | ALL with BM ≥ 70%, CNS− | ||
| 36 | ALL CNS+ (BM 52%-99%) |
CNS indicates central nervous system; BM, bone marrow; ALL, acute lymphoblastic leukemia.
Response to prephase treatment
Response was not evaluated or not evaluable in 89 patients. Among the 420 other patients treated in group B and C, 36 achieved a CR, 99 had a response of at least 80%, 176 between 50% and 79%, 88 between 20% and 49%, and 21 had no response (stability defined as response below 20% or tumor progression).
Eighteen of these nonresponders were group B patients, and 14 of them were switched to treatment of group C, as stipulated by the protocol.
Remission
A total of 545 patients (97%) achieved a CR: 532 (95%) with the planned protocol at the planned time, 2 after a modified protocol (1 toxicity-related problem, 1 was switched to group C after the first COPADM because the tumor seemed to be growing), 1 after cervical radiotherapy (extension of cranial irradiation to the cervical epidural site), and 10 after HDCT because a partial remission was observed at the time of CR assessment (9 with documented viable cells in the residual mass and 1 without histologic documentation). One CNS+ patient also had HDCT with ABMT, although he was in CR at the time of assessment because transient reemergence of blasts in CSF was detected following an optional spinal tap during the induction phase.
Sixteen patients did not achieve a CR and died—5 because of early toxicity before the evaluation of response and 11 because of failure to respond to initial treatment even though 4 had been switched from group B to group C treatment and 3 had received HDCT with ABMT because of viable cells in the residual mass.
Of note, 126 patients were diagnosed as having residual masses or suspicious images: 13 had no exploration, 57 underwent biopsies, and 56 had complete removal of the residual mass. Only 12 of these patients had residual viable tumor cells.
Relapses
Twenty-seven patients relapsed. Table4 shows the distribution of relapses according to stages and treatment groups.
Events and survivors according to treatment group and stage
| . | All patients . | Group . | Stage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A . | B (→C) . | C . | I . | II . | III . | IV + ALL CNS− . | IV + ALL CNS+ . | ||
| No. of patients | 561 | 52 | 386 (14) | 123 | 31 | 88 (3) | 278 (11) | 97 | 67 |
| Events | |||||||||
| Initial treatment failures | 11 | 0 | 6 (4) | 5 | 0 | 0 | 6 (4) | 0 | 5 |
| Toxicity-related death | 8 | 0 | 3 | 5 | 0 | 0 | 3 | 1 | 4 |
| Relapse | 27 | 1 | 18 (1) | 8 | 2 | 1 | 14 (1) | 5 | 5 |
| Late event | 6 | 0 | 4 | 2 | 0 | 0 | 4 | 1 | 1 |
| Outcome | |||||||||
| Death | 41 | 0 | 23 (5) | 18 | 0 | 1 | 20 (5) | 6 | 14 |
| Alive in CR1 | 509 | 52 | 355 (9) | 103 | 29 | 87 (3) | 251 (6) | 90 | 52 |
| Alive after event | 11 | 0 | 8 | 2 | 2 | 0 | 7 | 1 | 1 |
| . | All patients . | Group . | Stage . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A . | B (→C) . | C . | I . | II . | III . | IV + ALL CNS− . | IV + ALL CNS+ . | ||
| No. of patients | 561 | 52 | 386 (14) | 123 | 31 | 88 (3) | 278 (11) | 97 | 67 |
| Events | |||||||||
| Initial treatment failures | 11 | 0 | 6 (4) | 5 | 0 | 0 | 6 (4) | 0 | 5 |
| Toxicity-related death | 8 | 0 | 3 | 5 | 0 | 0 | 3 | 1 | 4 |
| Relapse | 27 | 1 | 18 (1) | 8 | 2 | 1 | 14 (1) | 5 | 5 |
| Late event | 6 | 0 | 4 | 2 | 0 | 0 | 4 | 1 | 1 |
| Outcome | |||||||||
| Death | 41 | 0 | 23 (5) | 18 | 0 | 1 | 20 (5) | 6 | 14 |
| Alive in CR1 | 509 | 52 | 355 (9) | 103 | 29 | 87 (3) | 251 (6) | 90 | 52 |
| Alive after event | 11 | 0 | 8 | 2 | 2 | 0 | 7 | 1 | 1 |
Numbers in parentheses indicate nonresponders in group B after the prephase who were switched to group C regimen. They are analyzed with group B patients.
Relapses occurred at a single site in 15 patients (7 locoregional, 1 BM, 6 CSF, 1 elsewhere) and at multiple sites in 12. They occurred 3 to 32 months after the diagnosis at a median of 5 months. Only 3 relapses occurred after 14 months (at 21, 31, and 32 months) and concerned only large B-cell lymphomas. Seven patients who relapsed at a single site (5 locoregional, 2 CSF) are alive in second CR with a long-term follow-up after second-line HDCT with ABMT.
Toxicity-related deaths
Toxicity-related deaths are shown in Table 4. Eight patients (3 in group B, 5 in group C) died, 7 due to treatment-related complications. One death occurred after the COP course (due to sepsis), 3 after the first induction course (1 hemorrhage, 1 postsurgical pelvic abscess, and 1 complications due to enterocolitis), 1 after the second induction course (due to sepsis), and 2 after the first consolidation course (1 pneumocystis pneumonia and 1 febrile encephalitis of an undetermined origin). The eighth death was due to a congenital atrioventricular block in a patient at home in CR who was waiting to be fitted with a pacemaker.
Treatment-related toxicity and morbidity
Treatment-related toxicity and morbidity is shown in Table5. Myelosuppression was the main treatment complication, especially during the COPADM and CYVE courses. More than 75% of the patients experienced febrile neutropenia requiring intravenous antibiotics, and more than 50% required transfusions. Morbidity was higher in group C and was correlated with the higher dose of MTX and HDAra-C but also with initial BM involvement for most of the patients in this group. Mucositis, the second main complication in the COPADM courses, was due to the combination of HDMTX and doxorubicin, because it did not occur in COPAD or CYM courses where only one of these drugs was administered. A parallel increased incidence of mucositis was observed when the doxorubicin infusion was prolonged (data not shown).
Main toxicities of induction and consolidation courses
| . | COPAD . | COPADM (MTX = 3) . | CYM . | COPADM (MTX = 8) . | CYVE . |
|---|---|---|---|---|---|
| No. of courses | 103 | 750 | 716 | 261 | 271 |
| Red cell transfusion | 13% | 58% | 31% | 71% | 84% |
| Platelet transfusion | 3% | 17% | 15% | 53% | 82% |
| Mucositis grade 3 | 5% | 23% | 1% | 33% | 6% |
| grade 4 | 0 | 15% | 0 | 21% | 0 |
| Febrile neutropenia | 44% | 82% | 25% | 85% | 73% |
| Severe infection5-150 | 3% | 12% | 5% | 20% | 21% |
| Life-threatening | 0 | 2% | 0.8% | 5% | 0.7 |
| Toxicity-related deaths | 0 | 2 | 1 | 2 | 1 |
| . | COPAD . | COPADM (MTX = 3) . | CYM . | COPADM (MTX = 8) . | CYVE . |
|---|---|---|---|---|---|
| No. of courses | 103 | 750 | 716 | 261 | 271 |
| Red cell transfusion | 13% | 58% | 31% | 71% | 84% |
| Platelet transfusion | 3% | 17% | 15% | 53% | 82% |
| Mucositis grade 3 | 5% | 23% | 1% | 33% | 6% |
| grade 4 | 0 | 15% | 0 | 21% | 0 |
| Febrile neutropenia | 44% | 82% | 25% | 85% | 73% |
| Severe infection5-150 | 3% | 12% | 5% | 20% | 21% |
| Life-threatening | 0 | 2% | 0.8% | 5% | 0.7 |
| Toxicity-related deaths | 0 | 2 | 1 | 2 | 1 |
For abbreviations, see Table 1.
Severe infections include sepsis, urine infection, extensive skin infection, pneumonitis, and “shock” without bacteriologic documentation.
Late events
Table 6 shows late events. Six patients experienced events after 3 years. One patient developed a myeloproliferative disorder, interpreted as related to the treatment of the L3ALL 45 months earlier, and died despite allogeneic BMT. The cytogenetic analysis revealed an isochromosome, 17q, in BM cells. Five patients developed second malignancies linked to a particular familial history in 2 of them. Two of these malignancies were non-Hodgkin lymphoma, but it was not possible to perform a successful molecular study on clonal differences between the first and the second malignant growths. Four patients were alive in CR at the time of the last follow-up contact, and one had died.
Late events
| Patient sex, age . | Lymphoma/leukemia characteristics and treatment group . | Familial history . | Second event . | ||
|---|---|---|---|---|---|
| Disease . | Time after lymphoma . | Outcome after second event . | |||
| Male, 3 y | Stage III, liver | Mother, thymoma brother, Burkitt lymphoma Hepatitis B | Stage IV, liver | 5 y | Alive more than 4 y |
| Burkitt lymphoma | Burkitt lymphoma | ||||
| Group B | |||||
| Male, 14 y | L3ALL CNS+ | Unknown | Hemisphere PNET | 5 y | Alive more than 2 y |
| Group C | Acoustic neurinoma | 6½ y | |||
| Male, 10 y | Stage III, abdomen | Familial lamellar ichthyosis | Anaplastic astrocytoma | 2 y | Alive more than 2¾ y |
| Large B-cell lymphoma | Grade IV | ||||
| Group B | |||||
| Male, 10 y | Stage II, cervical nodes | No | Abdomen | 6 y | Alive more than 2 y |
| Burkitt lymphoma | Large B cell lymphoma | ||||
| Group B | |||||
| Male, 3 y | L3ALL CNS≥roup C | No | Myeloproliferative disorder | 3¾ y | Died 9 m later (despite allograft) |
| Male, 4 y | Stage III, abdomen | Unknown | Ewing sarcoma | 4½ y | Died 11 m later |
| Burkitt lymphoma | |||||
| Group B | |||||
| Patient sex, age . | Lymphoma/leukemia characteristics and treatment group . | Familial history . | Second event . | ||
|---|---|---|---|---|---|
| Disease . | Time after lymphoma . | Outcome after second event . | |||
| Male, 3 y | Stage III, liver | Mother, thymoma brother, Burkitt lymphoma Hepatitis B | Stage IV, liver | 5 y | Alive more than 4 y |
| Burkitt lymphoma | Burkitt lymphoma | ||||
| Group B | |||||
| Male, 14 y | L3ALL CNS+ | Unknown | Hemisphere PNET | 5 y | Alive more than 2 y |
| Group C | Acoustic neurinoma | 6½ y | |||
| Male, 10 y | Stage III, abdomen | Familial lamellar ichthyosis | Anaplastic astrocytoma | 2 y | Alive more than 2¾ y |
| Large B-cell lymphoma | Grade IV | ||||
| Group B | |||||
| Male, 10 y | Stage II, cervical nodes | No | Abdomen | 6 y | Alive more than 2 y |
| Burkitt lymphoma | Large B cell lymphoma | ||||
| Group B | |||||
| Male, 3 y | L3ALL CNS≥roup C | No | Myeloproliferative disorder | 3¾ y | Died 9 m later (despite allograft) |
| Male, 4 y | Stage III, abdomen | Unknown | Ewing sarcoma | 4½ y | Died 11 m later |
| Burkitt lymphoma | |||||
| Group B | |||||
PNET indicates primitive neuro-ectodermal tumor; CNS, central nervous system.
Survival
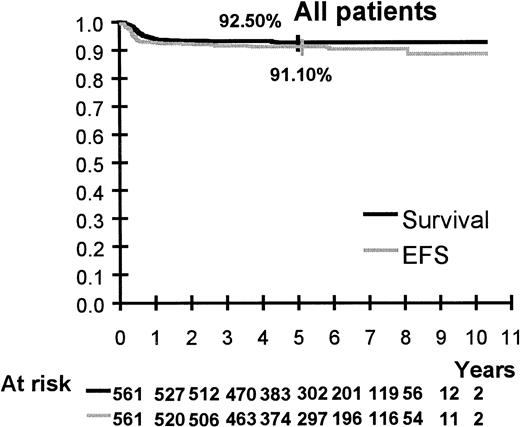
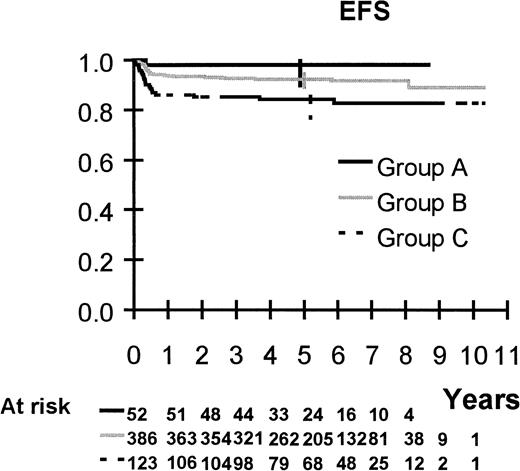
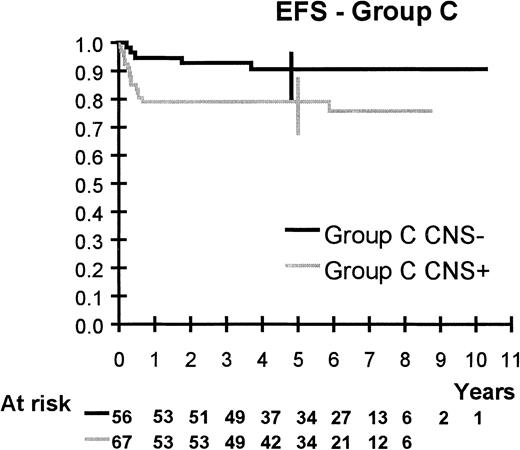
Survival is shown in Figures 2 to 6. Median follow up is 64 months (range 20 to 123 months). Five-year overall survival and EFS are 92.5% (95% CI, 90%-94%) and 91%, (95% CI, 89%-93%), respectively, for the 561 patients. Five-year survival and EFS are, respectively, 100% and 98% (95% CI, 90%-100%) for group A, 94% (95% CI, 91%-96%) and 92% (95% CI, 89%-95%) for group B, and 85% (95% CI, 78%-90%) and 84% (95% CI, 77%-90%) for group C.
EFS of group C patients according to CNS involvement.
CNS was the only prognostic factor found in group C.
EFS of group C patients according to CNS involvement.
CNS was the only prognostic factor found in group C.
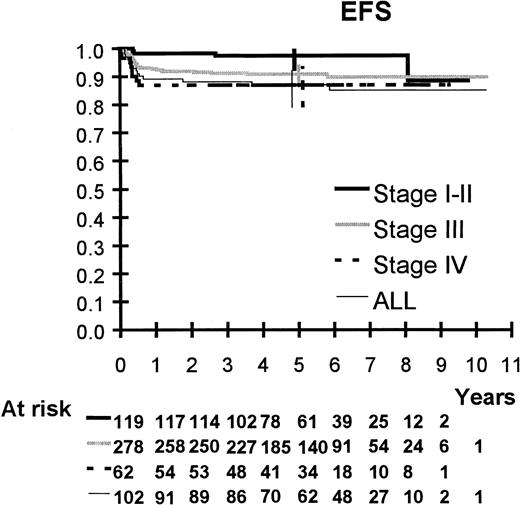
Five-year survival and EFS are, respectively, 100% and 93% (95% CI, 78%-98%) for stage I, 99% and 99% (95% CI, 94%-100%) for stage II, 93% (95% CI, 89%-95%) and 91% (95% CI, 87%-94%) for stage III, 87% and 87% (95% CI, 77%-93%) for stage IV, and 88% (95% CI, 80%-93%) and 87% (95% CI, 79%-92%) for ALL.
Five-year EFS is 79% (95% CI, 68%-87%) for CNS+ patients and 77% (95% CI, 66%-86%) after exclusion of the 5 patients with isolated cord compression.
If HDCT recipients had been considered as failures, “failure-free survival” would be 1% to 3% lower than EFS for some groups of patients, ie, 90% for group B patients and 97%, 88%, 86%, and 78% for patients with stage II and stage III disease, ALL, and CNS involvement, respectively.
Concerning the LDH level, 5-year EFS was significantly better (P < .001) among patients with a low LDH level: 95% (95% CI, 92%-97%) versus 87% (95% CI, 83%-91%) in patients with a high LDH level. No further decline was observed in the EFS when the LDH level increased beyond 2-fold the upper limit of the normal value. For stage III patients, EFS attained 94% (95% CI, 89%-97%) versus 89% (95% CI, 82%-93%) when the LDH level was less or more than 2-fold the normal value respectively (P = .06).
EFS of patients with Burkitt (92%; 95% CI, 89%-94%) and large B-cell (89%; 95% CI, 79%-95%) lymphoma was not significantly different (P = .59), and there was no difference within the Burkitt group when it was subdivided according to the WF (small noncleaved Burkitt, 92%; 95% CI, 87%-95%; and small noncleaved non-Burkitt, 91%; 95% CI, 86%-95%) (P = .55).
Prognostic study
In the univariate analysis of EFS performed in group B patients, the performance status, stage (including substages IIIa and IIIb), and histologic subtype were not prognostic factors. The nutritional status was of borderline prognostic significance but there were only 6 patients in the group with the lowest score. A high LDH level, no response after COP, and age of at least 15 years were poor prognostic factors. In the multivariate analysis, these 3 factors appeared independent (Table 7). In group C, the only prognostic factor was CNS involvement (P = .04). The performance status, LDH level, response to COP, age, histologic type, percentage of blasts in BM, and the presence of blasts in blood were not predictive of outcome.
Multivariate analysis of event-free survival in group B
| . | No. of patients . | Relative risk . | P value . |
|---|---|---|---|
| Serum LDH level | .04 | ||
| ≤ 2N7-150 | 198 | 1 | |
| > 2N7-150 | 162 | 2.3 (1.0-5.2) | |
| Age7-151 | .01 | ||
| < 8 years | 174 | 1 | |
| 8-14 y | 167 | 1.4 (0.6-3.4) | |
| ≥ 15 y | 19 | 6.7 (2.2-20.4) | |
| Response to COP7-151 | .006 | ||
| > 80% | 128 | 1 | |
| 20%-79% | 188 | 2.3 (0.76-7.0) | |
| < 20% | 16 | 12.3 (3.2-47.1) | |
| Not evaluated | 28 | 3.2 (0.71-14.8) |
| . | No. of patients . | Relative risk . | P value . |
|---|---|---|---|
| Serum LDH level | .04 | ||
| ≤ 2N7-150 | 198 | 1 | |
| > 2N7-150 | 162 | 2.3 (1.0-5.2) | |
| Age7-151 | .01 | ||
| < 8 years | 174 | 1 | |
| 8-14 y | 167 | 1.4 (0.6-3.4) | |
| ≥ 15 y | 19 | 6.7 (2.2-20.4) | |
| Response to COP7-151 | .006 | ||
| > 80% | 128 | 1 | |
| 20%-79% | 188 | 2.3 (0.76-7.0) | |
| < 20% | 16 | 12.3 (3.2-47.1) | |
| Not evaluated | 28 | 3.2 (0.71-14.8) |
Two-fold the upper limit of the institutional normal value.
Figures are based on 360 patients because the LDH value was missing in 26 patients.
Discussion
This report presents the results of a multicenter, prospective study in a large series of 561 patients with “mature” B-cell lymphoma (Burkitt and large B-cell) or L3ALL treated with the French LMB89 protocol. It shows that survival can attain 90% with a strategy adapted to the tumor burden (stage and resection status) and to previously identified prognostic factors (CNS involvement, no tumor response after the first week of treatment, and no CR after the third or fouth course), with escalation of treatment intensity when required during treatment.
It is established that early stage lymphomas do not require as intensive treatment as advanced stages. In the LMB89 protocol, we sought to identify a group of patients with a very good prognosis who can be cured with only 2 courses of chemotherapy without CNS-directed treatment. This group comprises patients with resected stage I and abdominal stage II tumors. At the end of the 1970s, CNS-directed treatment was demonstrated to be unnecessary for localized stage abdominal tumors.16-18 Our experience in the COPAD study (1974-1980) corroborated this finding, but the treatment we administered lasted one year.19 20 We shortened the duration of therapy to 6 months during the 1980s (C. P., unpublished data, 1989) and to 2 courses in this study.
The results obtained in group A are excellent, with a 5-year EFS rate of 98% (95% CI, 90%-99.7%). Only one patient relapsed. The site of recurrence was the CNS with lymphomatous infiltration of the L5 root without blasts in the CSF. During treatment, this patient complained of pain in the foot. Thought to be related to vincristine, this pain was in fact due to CNS disease, which became obvious several weeks later. This case illustrates the concern of some clinicians about the noninclusion of CNS-directed therapy, especially for Burkitt head and neck tumors. It may lead to overinterpretation of certain complete tumor resections as doubtful and to the assignment of such cases to more aggressive treatment. This raises the question of whether an additional course of HDMTX should be added to each COPAD course for resected Burkitt head and neck tumors, as is the case in other protocols that administer additional IT or an intermediate dose of MTX.17,21 22
For patients with any stage I and II lymphoma, except for the lymphoblastic type, the Pediatric Oncology Group (POG) recently confirmed that 3 courses of CHOP was as efficient as the same treatment with local radiotherapy or maintenance therapy, yielding an EFS of 89% ± 8%.21,23 In LMB89, the EFS rate for such stages is 97% (95% CI, 92%-99%). Half of these patients were treated in group B, and it could be contended that some of them were overtreated. Less intense therapy is being investigated for group B patients in our ongoing protocol. However, it should be borne in mind that stage II Waldeyer ring tumors, especially nasopharyngeal lesions, fared worse than stage II disease of other sites in the COPAD study, mainly because of CNS progression despite CNS-directed therapy with IT MTX and cranial irradiation.19 24
As shown in the LMB84 trial, the result of 3 1/2 months of treatment is very good in stage III patients, with a 5-year EFS of 91% (95% CI, 87%-94%). This EFS compares favorably with the 76% obtained in 44 patients in the United Kingdom Children's Cancer Study Group (UKCCSG) study NHL 86,25 the 79% in 58 patients treated with “total B ” therapy in the POG 8616 study,26 the 87.5% in 48 patients (including 2 patients with high-risk stage I and II disease) in the National Cancer Institute (NCI) 89-C-41 protocol,27 and the 88% ± 6% in 171 patients in the Berlin-Frankfurt-Munster (BFM) 90 study.22 However, patients with stage III disease form a heterogeneous group with a wide range of LDH levels, reflecting differences in tumor burden, which may account for differences in results. An element that can be compared with other series is the 89% (95% CI, 82%-93%) EFS of patients whose LDH level was more than 2-fold the upper limit of the normal value.
This study confirms the results of the pilot LMB86 study on a larger series of patients, which showed an improvement in the outcome of patients with initial CNS involvement and ALL with massive infiltration of BM (≥ 70% blasts). This intensified treatment associates an intensive consolidation phase (the CYVE courses, demonstrated to be efficient for systemic and CNS disease in phase II studies7) and intensified CNS-directed therapy with a higher dose of MTX (8 mg/m2), HDAra-C, and triple ITs.
In contrast with our previous studies, we included patients with large B-cell lymphomas in this study. Like the BFM group28 and the NCI,29 we observed a high cure rate in this group of patients that was similar to that of Burkitt lymphomas treated with the same regimen. POG also observed a high cure rate but with a different protocol from that used to treat Burkitt lymphomas.30 The results of all these series appear to be better than those observed in adults. However, for a valid comparison, pediatric patients should be stratified like adult patients according to the international prognostic index.
Our first treatment stratification took into account the stage, which was refined by the completeness of resection for early stages, and the percentage of blasts in BM and the presence of CNS disease in advanced stages and ALL. This allowed us to identify 3 groups of patients with a different prognosis. Although the LDH level is a widely recognized “marker” of the tumor burden and progression and a prognostic factor in many series, it was not taken into account for stratification in ours because it was not investigated in our previous studies. Outcome remained significantly different between the 3 groups. Group A has already been discussed. Group B comprised a very heterogeneous group of patients with a wide spectrum of stages, ranging from stage I to ALL. Notably, stages and “substages” in this group did not appear to be predictive of outcome, as found in the UKCCSG 9002 study with the same protocol given to the same category of stage III and IV patients.31 On the other hand, the LDH level was a prognostic factor in our series. However, the difference in EFS between patients with a low and a high LDH level was only 5.5% (95% vs 89.5%). This small difference could be attributed to the use of HDMTX. The systemic effect of HDMTX was clearly demonstrated by the BFM 86 and BFM 90 studies,28 where EFS rose from 50% ± 10% to 78% ± 6% in ALL and from 43% ± 10% to 81% ± 4% in abdominal stage III disease with LDH at least 500 U/L when the MTX dose was increased from 500 mg/m2 to 5 g/m2.
An unexpected adverse prognostic factor was age of at least 15 years. This factor was not identified in other series where adults and children with B-cell neoplasia were treated with the same regimen,29,32 although the POG 8617 study of stage IV lymphoma and ALL did single out age below 10 years as a favorable factor.33 Our finding must be considered with caution because the number of patients in this age group was very small, because age was a prognostic factor for EFS in group B but not for survival, and because it was not prognostic in group C.
CNS involvement was the only prognostic factor found in group C. CNS+ patients continue to fare worse even though EFS was as high as 79% (95% CI, 68%-87%). Is this because CNS disease is more difficult to cure because of the blood-brain barrier or, as others claim, because it is associated with more advanced disease?34 In our study, it is remarkable that among the 15 failures in group C, 5 were toxicity-related deaths (one unrelated to treatment while the patient was in CR), 7 systemic failures associated or not associated to CNS failure, and only 3 isolated CNS failures. The role of cranial irradiation is of dubious value because it is deferred until the fourth or fifth month of treatment: All our failures occurred before. In the following study, cranial irradiation was eliminated. Would intraventricular instead of IT injections improve outcome, as suggested by the BFM group?22 How can treatment intensity be escalated and sustained when toxicity-related death is a cause of failure in these patients?
Our second stratification for treatment was response to chemotherapy, with the recommendation that “poor responders” switch to the higher-intensity treatment during the first phases of therapy. Response after only one week from the initiation of therapy and thus after very small doses of chemotherapy (COP course) still allowed us to identify a small group of more “resistant” patients with a 12.3 relative risk of events in group B. Although early intensification by switching nonresponders in group B to group C clearly improved their outcome, increasing EFS from 22% in LMB84 to 72% in this study, treatment still needs to be improved. The second phase of treatment intensification was after the consolidation phase in case of partial remission: this concerned 14 patients, of whom 10 are alive.
Toxicity is a major cause for concern. Toxicity-related mortality was 1.2% for the whole series and was linked to the intensity of treatment: 0 in group A, 0.8% in group B, and 3.2% in group C. However, this rate is acceptable when compared with that of other groups: 14 of 413 (3.4%) in the BFM 90 study22 and 15 of 133 (11.3%) in the POG 8617 study for stage IV and ALL.33Our results are partly due to the long experience of SFOP investigators with LMB protocols. No death occurred in relation to tumor lysis syndrome. The prephase provides an opportunity to solve metabolic problems without dealing with other toxicities such as aplasia and mucositis encountered in the induction phase. In addition, the use of Uricozyme, which prevents uratic nephropathy, could explain the absence of mortality related to this tumor lysis syndrome.35-37The only patient who died after COP (sepsis after dialysis) was from The Netherlands, where Uricozyme is not available. In the UKCCSG 9003 study, using the same protocol but without Uricozyme, early toxic death and relapse, occurring in most patients for whom treatment had to be delayed or modified because of metabolic early complications, were important causes of treatment failure.38
Morbidity is nonetheless high, and adequate supportive care is needed for metabolic disorders, febrile neutropenia, mucositis, and potential blood product transfusions. Such morbidity may compromise the use of such intensive protocols in less privileged countries with a high incidence of Burkitt lymphoma. Treatment needs to be adapted without compromising cure rates, because it is clear that improved cure rates were always obtained with early intensification of chemotherapy and especially with the introduction of HDMTX, which led to increased morbidity. The current international FAB LMB96 protocol is investigating the possibility of decreasing therapy in a randomized study.39
Six patients developed another malignancy. The myeloproliferative disorder can be considered treatment-related. Two patients developed another lymphoma 5 and 6 years after the first one. Such a long interval between the 2 malignancies points to a second lymphoma rather than a relapse, especially in the child who had Burkitt lymphoma and a particular familial history. The 3 other second malignancies are not usually described as related to anticancer therapy.
In conclusion, a high survival rate of 90% was obtained in B-cell lymphoma and L3ALL with multiagent chemotherapy adapted to the tumor burden that was defined according to the stage, the initial resection status and the percentage of blast cells in BM, and the initial tumor response to treatment. Outcome was also improved substantially in patients with a high LDH level, CNS involvement, or no tumor response after the first week of therapy, but these factors, especially the second and the third, continue to confer the worse prognosis and are therefore still a therapeutic challenge. In the other patients, the possibility of decreasing the intensity and duration of therapy is being addressed in the ongoing international FAB LMB96 study.
The authors are grateful to Lorna Saint-Ange for editing.
Participating institutions and investigators: Institut Gustave Roussy, Villejuif, Dr Patte, Dr Brugieres, Dr Kalifa, Dr Oberlin, Dr Valteau, Dr Hartmann, Dr Pein; Institut Curie, Paris, Dr Michon, Prof Zucker, Dr Pacquement, Dr Doz, Dr Quintana; Emma Kinder Ziekenhuis, AMC Amsterdam, Amsterdam, Dr Behrendt; Hopital Trousseau, Paris, Prof Leverger, Dr Landmann-Parker; Leon Berard, Lyon, Dr Frappaz, Prof Philip; Hospices Civils, Strasbourg, Prof Lutz, Dr Babin Boilletot; Hopital De La Timone, Marseille, Dr Coze, Dr Gentet; CHU Bordeaux, Dr Perel; Hopital Huriez, Lille, Dr Nelken; Lyon Debrousse, Dr Bertrand, Prof Philippe; CHU Nantes, Dr Mechinaud-Lacroix; CHU Purpan, Toulouse, Dr Robert, Dr Rubie; CHRU Nancy, Prof Sommelet, Dr Schmitt; CHRU Hopital Sud, Rennes, Dr Bergeron, Prof Legall; Hopital St Louis, Paris, Prof Baruchel, Dr Leblanc; Hopital C. Nicolle, Rouen, Prof Vannier; CHU, Hopital Lenval, Nice, Prof Thyss, Dr Deville, Dr Monpoux; Hotel Dieu, Clermont Ferrand, Prof Demeocq; Hopital Americain, Reims, Dr Behar, Dr Munzer; Hopital J. Bernard, Poitiers, Dr Millot; CHU Grenoble, Dr Plantaz; Hopital D'enfants, Dijon, Dr Couillault; Hopital Fabiola, Bruxelles, Dr Devalck, Dr Sariban; Hopital Robert Debre, Paris, Prof Vilmer, Dr Rohrlich; Hopital Clocheville, Tours, Prof Lamagnere, Dr Lejars; CHU Amiens, Dr Pautard; Hopital St Jacques, Chu Besancon, Dr Plouvier; CHU Angers, Dr Rialland; CHU, Caen, Dr Boutard; CHU Dupuytren, Limoges, Prof De Lumley; Hopital Nord De St Etienne, Dr Stephan; Centre Oscar Lambret, Lille, Dr Demaille, Dr Baranzelli; CHR Le Mans, Dr Damay; Lille St Antoine, Dr Dusol. National panel of pathologists: Dr Terrier-Lacombe (chairman), Dr Berger (Lyon), Dr Dumont (I. Curie, Paris), Prof Raphaël. Cytologists: Dr Bayle (IGR, Villejuif), Dr Gentilhomme, and Dr Felman (Lyon).
A complete list of participants in the Société Française d'Oncologie Pédiatrique is given in an at the end of this article.
Supported in part by the Association pour la Recherche contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Catherine Patte, Pediatrics Dept, Institut Gustave Roussy, Rue Camille Desmoulins, 94805 Villejuif Cedex, France; e-mail:patte@igr.fr.