Although the mouse spleen dendritic cell (DC) is perhaps the most intensively studied DC type, little has been published concerning its human equivalent. In this report, rare event flow cytometry and in situ immunofluorescence were used to study the surface phenotype and distribution of HLA-DR+CD3−14−16−19− human spleen DC. Spleens from organ donors with different clinical histories were used. Most (81% ± 9%; n = 14) spleen DCs expressed high levels of the integrin CD11c. CD11c+ DCs were distributed in 3 distinct regions—the peri-arteriolar T-cell zones, the B-cell zones, and the marginal zone, where they formed a ring of cells surrounding the white pulp, just inside a ring of CD14+ red pulp macrophages, apparently more regularly organized than the previously described marginating DC population in the mouse spleen. The T-cell zones contained CD86+ DCs, among which a subpopulation expressed CD83. These mature/activated CD86+DCs represented a minority (12% ± 8%) of total spleen DCs in most organ donors: most spleen DCs are immature. In 3 of 18 (17%) donors, however, most (54%-81%) of spleen DCs were CD86+, suggesting that in vivo DC activation had occurred. In one donor, a radical shift in DC distribution from the marginal zone to the T-cell zones was also observed. This activation of spleen DCs in vivo was reminiscent of the effects of experimental microbial product injection in mice, and it seemed to correlate with bacterial infection or multiple trauma.
Introduction
The initiation of a specific immune response implies the acquisition and presentation of antigen to naive T lymphocytes by dendritic cells (DCs).1-3 Like many other biologic functions, the timeliness and efficacy of this initiation are regulated by compartmentalization.4 The first level of compartmentalization is the existence of 2 distinct maturation stages in DCs: immature DCs are specialized in the uptake of antigens by macropinocytosis and endocytosis by Fc receptors5,6 and the macrophage mannose receptor,5,7,8 but they do not have a high capacity for T-cell costimulation. Conversely, mature or activated DCs express extremely high levels of major histocompatibility complex (MHC) and costimulatory molecules such as CD809and CD86,10,11 and they stimulate T cells far more effectively than any other cell type12,13 but internalize antigens poorly.14,15 The second level of compartmentalization is anatomic: naive T lymphocytes can probably only be activated in the T-cell areas of lymphoid organs and almost never in the periphery.3,4,16 Therefore, the initiation of a specific immune response requires a redistribution of antigen-loaded, maturing DC toward the T-cell areas of secondary lymphoid organs.17-20 Indeed, immature DCs are found as sentinels at nonlymphoid sites, in the interstitia of solid organs, and in the epithelia as Langerhans cells, whereas mature DCs are mostly found in the T-cell areas of lymphoid organs.21 However, much has to be done to understand precisely the micro-anatomy of the immune response in the lymphoid organs, especially in humans.
In the mouse, the integrin α chain CD11c is a marker for most DCs.22 Some DCs express the integrin α chain CD11b also expressed by monocyte/macrophages, some express the lectin molecule DEC-205,23,24 and others express the T-cell antigen CD8α.25 Hence, the mouse spleen contains CD11c+ CD11b+ CD8−DEC-205− marginal zone (marginating) DCs at the edge of the T-cell zones, and CD11c+ CD11b−CD8+ DEC-205+ central DCs that have a periarteriolar localization within the T-cell zones.21,26Marginating DCs appear to be the direct precursors of central DCs; activation of DCs in vivo by intravenous injection of lipopolysaccharide (LPS) or Toxoplasma gondii–derived antigen induces the migration of marginating DCs into the T-cell zones, accompanied by maturation and early, transient interleukin 12 (IL-12) secretion.18,19 Marginal zone DCs were shown to have a rapid turnover in vivo and to have phagocytic properties,27,28 whereas central DCs had a slow turnover.27
Human spleen DCs have been less extensively studied than either mouse spleen DCs or human DCs from other lymphoid organs. T-cell zone DCs were reported to express CD11c, MHC II,29 and CD83.30 In addition, germinal center DCs were observed in the B-cell zones.31 However, no human equivalent of the mouse marginating DC population has previously been described.
In the present work, we explore the phenotype of human spleen DCs by flow cytometry and in situ immunofluorescence to determine how many subpopulations of human spleen DCs can be defined on the basis of surface phenotype and distribution. In particular, we describe the human equivalent of the mouse marginating DC population. Additionally, evidence is presented indicating that spleen DCs in a subset of organ donors had been activated in vivo, with micro-anatomic distribution changes, perhaps in the process of antigen presentation and specific immune response induction.
Materials and methods
Human tissues
Spleens were obtained from organ transplant donors (Table1) at the Hôpital Pitié-Salpêtrière following national ethical guidelines regulating the use of human tissues. Whole blood from healthy donors was obtained from the Blood Transfusion Centre (Centre National de Transfusion Sanguine) at the Hôpital Pitié-Salpêtrière.
Proportion of total and activated spleen DCs and relevant clinical information on organ donors included in this study
| Donor no. . | CD86+ DC (%) . | DC (%) . | Age/sex . | Delay Trauma to death (d) . | Cause of death, clinical findings . |
|---|---|---|---|---|---|
| 176 | 81 | 0.3 | 29/M | 10 | Cardiogenic shock, pulmonary atelectasia, nosocomial infection (E coli,methicillin-resistant S aureus) |
| 93 | 75 | 1.8 | 53/F | 4 | Multiple trauma, hypovolemic shock |
| 106 | 54 | 0.5 | 23/M | 1 | Multiple trauma: cranial trauma, right arm fracture, and myocardial rupture |
| 173 | 30 | 0.3 | 24/M | 1 | Multiple trauma: cranial + compound elbow fracture |
| 174 | 28 | 0.4 | 39/M | NF | Multiple trauma |
| 60 | 16 | 0.4 | 47/M | 1 | Cranial trauma by firearm |
| 143 | 16 | 0.7 | 53/F | 1 | Cerebral aneurysmal rupture |
| 67 | 14 | 1.3 | 52/M | 2 | Cerebral hemorrhage |
| 180 | 13 | 0.9 | NF | NF | NF |
| 181 | 11 | 1.0 | 47/M | 1 | Cerebral hemorrhage |
| 58 | 9.3 | 0.5 | 51/F | 5 | Severe cardiac failure |
| 179 | 9.0 | 0.3 | 57/M | 4 | Cranial trauma |
| 108 | 7.3 | 1.7 | 38/M | 1 | Cerebral hemorrhage |
| 94 | 6.1 | 0.6 | 47/M | 1 | Cranial trauma |
| 76 | 4.9 | 0.9 | 34/M | 1 | Cranial sub dura hematoma |
| 71 | 4.7 | 0.6 | 29/M | 1 | Cranial trauma |
| 172 | 4.0 | 0.2 | 67/M | 1 | Cerebral aneurysmal rupture |
| 91 | < 5 | 0.7 | 39/F | 1 | Cranial trauma, ethyl intoxication |
| 92 | ND | < 0.1 | 47/M | NF | Cranial trauma |
| 147 | ND | ND | 32/M | NF | Cerebral hemorrhage |
| Median | 12 | 0.6 | 47 | 1 |
| Donor no. . | CD86+ DC (%) . | DC (%) . | Age/sex . | Delay Trauma to death (d) . | Cause of death, clinical findings . |
|---|---|---|---|---|---|
| 176 | 81 | 0.3 | 29/M | 10 | Cardiogenic shock, pulmonary atelectasia, nosocomial infection (E coli,methicillin-resistant S aureus) |
| 93 | 75 | 1.8 | 53/F | 4 | Multiple trauma, hypovolemic shock |
| 106 | 54 | 0.5 | 23/M | 1 | Multiple trauma: cranial trauma, right arm fracture, and myocardial rupture |
| 173 | 30 | 0.3 | 24/M | 1 | Multiple trauma: cranial + compound elbow fracture |
| 174 | 28 | 0.4 | 39/M | NF | Multiple trauma |
| 60 | 16 | 0.4 | 47/M | 1 | Cranial trauma by firearm |
| 143 | 16 | 0.7 | 53/F | 1 | Cerebral aneurysmal rupture |
| 67 | 14 | 1.3 | 52/M | 2 | Cerebral hemorrhage |
| 180 | 13 | 0.9 | NF | NF | NF |
| 181 | 11 | 1.0 | 47/M | 1 | Cerebral hemorrhage |
| 58 | 9.3 | 0.5 | 51/F | 5 | Severe cardiac failure |
| 179 | 9.0 | 0.3 | 57/M | 4 | Cranial trauma |
| 108 | 7.3 | 1.7 | 38/M | 1 | Cerebral hemorrhage |
| 94 | 6.1 | 0.6 | 47/M | 1 | Cranial trauma |
| 76 | 4.9 | 0.9 | 34/M | 1 | Cranial sub dura hematoma |
| 71 | 4.7 | 0.6 | 29/M | 1 | Cranial trauma |
| 172 | 4.0 | 0.2 | 67/M | 1 | Cerebral aneurysmal rupture |
| 91 | < 5 | 0.7 | 39/F | 1 | Cranial trauma, ethyl intoxication |
| 92 | ND | < 0.1 | 47/M | NF | Cranial trauma |
| 147 | ND | ND | 32/M | NF | Cerebral hemorrhage |
| Median | 12 | 0.6 | 47 | 1 |
Donors are listed in decreasing order of activated CD86+ DC percentage. ND, not determined; NF, not found.
Preparation of mononuclear cell suspensions
Blocks of spleen, measuring approximately 2 × 2 × 3 cm, were kept at 4°C in RPMI 1640 medium (ICN Flow, Irvine, Scotland) for the period between surgery and isolation of splenocytes, which varied from 4 to 12 hours. Blocks were cut into small pieces that were forced through the mesh of a sterile sieve using the plunger of a syringe. Cells were then dissociated enzymatically by digestion with type VII collagenase at 20 U/mL (Sigma, St Quentin Fallavier, France) and DNase I at 20 U/mL (Sigma) in RPMI supplemented with 2% fetal calf serum for 30 minutes at room temperature. Cell aggregates were further dissociated by the addition of EDTA to 10 mM and agitation for 5 minutes at room temperature as described.32 These conditions did not affect surface molecule expression compared to nonenzymatic dissociation procedures.33 Mononuclear cells were isolated from these splenocyte suspensions or from whole blood by centrifugation over Diatrizoate-Ficoll (density, 1.077 g/mL; Eurobio, Les Ulis, France).
Flow cytometry
Cells were incubated for 30 minutes at 4°C in RPMI containing 10% fetal calf serum and human immunoglobulins (Biotransfusion, Les Ulis, France) at 100 μg/mL and then were incubated with isotype controls or unconjugated antibodies listed in Table2, which were revealed with polyclonal goat antimouse immunoglobulin G (IgG) conjugated to fluorescein isothiocyanate (FITC) at 7 μg/mL (Caltag, San Francisco, CA). Residual antimouse antibodies were blocked by incubation with 100 μg/mL mouse IgG for 15 minutes at 4°C, and the cells were incubated with the following cocktail of 4 phycoerythrin (PE)-conjugated antibodies: anti-CD3 (S4.1 at 4 μg/mL; Caltag), anti-CD14 (My4 at 5 μg/mL; Coulter, Margency, France), anti-CD16 (3G8 at 2 μg/mL; Caltag), anti-CD19 (B4 at 5 μg/mL; Coulter), and anti–HLA-DR (HL38 at 4μg/mL; Caltag) conjugated to PE-Cy5. When directly FITC-coupled antibodies were used, they were added to cells simultaneously with the cocktail of PE- and PE–Cy5-conjugated antibodies immediately after the blocking step. Cells were washed once between incubation steps in 3 mL phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA), then centrifuged at 400g for 6 minutes at 4°C. All incubations were carried out in 100 μL PBS containing 1% BSA for 30 minutes on ice. After a final wash, cells were fixed in PBS containing 1% paraformaldehyde, then analyzed using a FACScan (Becton Dickinson, Le Pont de Claix, France).
Antibodies used for flow cytometry
| Antigen . | Monoclonal . | Conjugate . | Concentration for labeling . | Major specificities . | Source . |
|---|---|---|---|---|---|
| CD1a | BL6 | — | 5 μg/mL | LC, thymocytes | I |
| CD3 | S4.1 | PE | 4 μg/mL | T lymphocytes | Cal |
| CD4 | SK3 | FITC | 1/10 | T lymphocytes, monocytes, MΦ | BD |
| CD11a | 25.3 | FITC | 1/10 | Leukocytes | I |
| CD11b | Bear1 | FITC | 5 μg/mL | Monocytes, MΦ, granulocytes | Cal |
| CD11c | KB90 | FITC | 5 μg/mL | DCs, monocytes, MΦ, granulocytes | Cal |
| CD14 | My-4 | PE | 5 μg/mL | Monocytes, MΦ | C |
| CD16 | 3G8 | PE | 2 μg/mL | NK cells, granulocytes, MΦ | Cal |
| CD19 | SJ25-C1 | PE | 5 μg/mL | B lymphocytes | Cal |
| CD20 | HRC20-B9-E9 | — | 4 μg/mL | B lymphocytes | I |
| CD21 | BL13 | — | 4 μg/mL | B lymphocytes | I |
| CD32 | 2E1 | — | 5 μg/mL | Monocytes, MΦ, granulocytes, B cells | I |
| CD33 | My-9 | FITC | 1/10 | Monocytes, MΦ | C |
| CD35 | J3D3 | — | 5 μg/mL | Monocytes, MΦ, granulocytes, B cells | I |
| CD40 | MAB89 | — | 5 μg/mL | B lymphocytes, activated MΦ, DCs | I |
| CD54 | 84H10 | — | 5 μg/mL | Activated leukocytes and endothelial cells | I |
| CD64 | 22 | — | 5 μg/mL | Monocytes, MΦ | V |
| CD80 | MAB104 | — | 5 μg/mL | DCs, activated B lymphocytes | I |
| CD83 | HB15a | — | Ascites 1/1000 | DCs, activated B and T lymphocytes | T |
| CD86 | FUN-1 | — | 5 μg/mL | DC, activated B lymphocytes | P |
| HLA A, B, C | B9.12.1 | — | 5 μg/mL | All leukocytes | I |
| HLA-DR | HL38 | PE-Cy5 | 4 μg/mL | B lymphocytes, MΦ, DCs | Cal |
| HLA-DQ | SPVL3 | — | 4 μg/mL | B lymphocytes, MΦ, DCs | I |
| Antigen . | Monoclonal . | Conjugate . | Concentration for labeling . | Major specificities . | Source . |
|---|---|---|---|---|---|
| CD1a | BL6 | — | 5 μg/mL | LC, thymocytes | I |
| CD3 | S4.1 | PE | 4 μg/mL | T lymphocytes | Cal |
| CD4 | SK3 | FITC | 1/10 | T lymphocytes, monocytes, MΦ | BD |
| CD11a | 25.3 | FITC | 1/10 | Leukocytes | I |
| CD11b | Bear1 | FITC | 5 μg/mL | Monocytes, MΦ, granulocytes | Cal |
| CD11c | KB90 | FITC | 5 μg/mL | DCs, monocytes, MΦ, granulocytes | Cal |
| CD14 | My-4 | PE | 5 μg/mL | Monocytes, MΦ | C |
| CD16 | 3G8 | PE | 2 μg/mL | NK cells, granulocytes, MΦ | Cal |
| CD19 | SJ25-C1 | PE | 5 μg/mL | B lymphocytes | Cal |
| CD20 | HRC20-B9-E9 | — | 4 μg/mL | B lymphocytes | I |
| CD21 | BL13 | — | 4 μg/mL | B lymphocytes | I |
| CD32 | 2E1 | — | 5 μg/mL | Monocytes, MΦ, granulocytes, B cells | I |
| CD33 | My-9 | FITC | 1/10 | Monocytes, MΦ | C |
| CD35 | J3D3 | — | 5 μg/mL | Monocytes, MΦ, granulocytes, B cells | I |
| CD40 | MAB89 | — | 5 μg/mL | B lymphocytes, activated MΦ, DCs | I |
| CD54 | 84H10 | — | 5 μg/mL | Activated leukocytes and endothelial cells | I |
| CD64 | 22 | — | 5 μg/mL | Monocytes, MΦ | V |
| CD80 | MAB104 | — | 5 μg/mL | DCs, activated B lymphocytes | I |
| CD83 | HB15a | — | Ascites 1/1000 | DCs, activated B and T lymphocytes | T |
| CD86 | FUN-1 | — | 5 μg/mL | DC, activated B lymphocytes | P |
| HLA A, B, C | B9.12.1 | — | 5 μg/mL | All leukocytes | I |
| HLA-DR | HL38 | PE-Cy5 | 4 μg/mL | B lymphocytes, MΦ, DCs | Cal |
| HLA-DQ | SPVL3 | — | 4 μg/mL | B lymphocytes, MΦ, DCs | I |
LC, Langerhans cells; MΦ, macrophages; NK, natural killer. Antibodies were obtained from: BD, Becton-Dickinson, Pont-de-Claix, France; C, Coulter, Margency, France; Cal, Caltag, San Francisco, CA; I, Immunotech, Marseilles, France; P, Pharmingen, San Diego, CA; T, Dr T. F. Tedder, Duke University; V, Valbiotech, Paris, France.
For 3-color analysis of unseparated spleen mononuclear cells (SMCs), 10 000 ungated events were acquired in addition to 2000 to 5000 gated CD3−14−16−19−HLA-DR+ events, corresponding to DCs. Gated acquisition was improved when the forward scatter detection threshold was increased to exclude most lymphocytes. Number of spleen DCs as a percentage of total SMCs was calculated by determining the percentage of large DCs contained in the gated CD3−14−16−19−HLA-DR+ events (termed L). The percentage of the total SMC contained within the acquisition gate (termed A) was obtained either by noting the total number of cells that had run through the flow cytometer during acquisition or by determining the proportion of CD3−14−16−19−HLA-DR+ events in the whole SMC population using the same fluorescence gate as had been used for acquisition. The proportion of DCs (termed %DC) as a percentage of the whole SMC population was then calculated using the formula %DC = L × A/100. Analyzing 3 to 5 different data files for each sample proved that the results were reproducible and that both methods of obtaining the percentage A gave the same results.
In situ immunofluorescence
Small blocks of spleen tissue (approximately 5 mm × 5 mm × 5 mm) were snap-frozen in liquid nitrogen then stored at −80°C until 5-μm thin sections were prepared using a Leica CM1500 cryotome (Rueil Malmaison, France). Sections were dried overnight, fixed for 10 minutes in acetone, and used directly for labeling or stored at −20°C. After rehydration for 2 minutes in PBS, nonspecific sites were blocked by incubation in PBS containing 0.5% BSA and 0.5% gelatin for 30 minutes. Sections were then incubated for 60 minutes with primary antibodies listed in Table3, then washed 3 times in PBS containing 0.5% BSA. Sections were incubated for 30 minutes with biotinylated goat-antimouse IgG1 at 4 μg/mL (Caltag) and either FITC-conjugated goat-antimouse IgG2a or FITC-conjugated goat-antimouse IgG2b at 10 μg/mL (Caltag). After 3 further washes, biotinylated antibodies were revealed by a 15-minute incubation with 5 μg/mL avidin–Texas Red (Vector Laboratories, Burlingame, CA). Sections were washed again, and nuclei were counterstained by a 15-second incubation in 20 μg/mL Hoechst 33258 in PBS. Slides were mounted in 90% glycerol/10% PBS containing 25 mg/mL Dabco (Sigma).
Antibodies used for in situ immunofluorescence
| Antigen . | Monoclonal . | Isotype . | Conjugate . | Concentration for labeling . | Major specificities . | Source . |
|---|---|---|---|---|---|---|
| CD3 | UCHT1 | IgG1 | — | Ascites | T lymphocytes | PB |
| 1/200 | ||||||
| CD11b | OKM1 | IgG2b | — | 1/20 | Monocytes, MΦ, granulocytes | O |
| CD11c | KB90 | IgG1 | — | 1/10 | DCs, monocytes, MΦ, granulocytes | D |
| CD14 | My-4 | IgG2b | — | 25 μg/mL | Monocytes, MΦ | C |
| CD20 | B1 | IgG2a | — | 1/20 | B lymphocytes | C |
| CD83 | HB15a | IgG2b | — | Ascites | DCs, activated B and T lymphocytes | T |
| 1/500 | ||||||
| CD86 | FUN-1 | IgG1 | — | 10 μg/mL | DCs, activated B lymphocytes | P |
| HLA-DR | B8.12.2 | IgG2b | FITC | 1/10 | B lymphocytes, MΦ, DCs | BD |
| Antigen . | Monoclonal . | Isotype . | Conjugate . | Concentration for labeling . | Major specificities . | Source . |
|---|---|---|---|---|---|---|
| CD3 | UCHT1 | IgG1 | — | Ascites | T lymphocytes | PB |
| 1/200 | ||||||
| CD11b | OKM1 | IgG2b | — | 1/20 | Monocytes, MΦ, granulocytes | O |
| CD11c | KB90 | IgG1 | — | 1/10 | DCs, monocytes, MΦ, granulocytes | D |
| CD14 | My-4 | IgG2b | — | 25 μg/mL | Monocytes, MΦ | C |
| CD20 | B1 | IgG2a | — | 1/20 | B lymphocytes | C |
| CD83 | HB15a | IgG2b | — | Ascites | DCs, activated B and T lymphocytes | T |
| 1/500 | ||||||
| CD86 | FUN-1 | IgG1 | — | 10 μg/mL | DCs, activated B lymphocytes | P |
| HLA-DR | B8.12.2 | IgG2b | FITC | 1/10 | B lymphocytes, MΦ, DCs | BD |
LC, Langerhans cells; MΦ, macrophages; NK, natural killer; IgG, immunoglobulin G. Antibodies were obtained from: BD, Becton-Dickinson, Pont-de-Claix, France; C, Coulter, Margency, France; D, Dako, Denmark; P, Pharmingen, San Diego, CA; PB, Dr P. C. Beverley, University College London Medical School, United Kingdom; T, Dr T. F. Tedder, Duke University.
Cytokine measurement
Spleen mononuclear cells (1 × 106/mL) were cultured for 24 hours in RPMI 1640 complete medium containing 10% human AB serum in 24-well plates, with or without 100 ng/mL LPS34 (Escherichia coli serotype 026:B6; Sigma, St Louis, MO). IL-12 was measured in the supernatants using an enzyme-linked immunosorbent assay kit that detects both heterodimeric (p70) and homodimeric (p40-p40) forms (Biosource Europe, Fleurus, Belgium).
Results
Quantitation and phenotype of spleen dendritic cells by flow cytometry
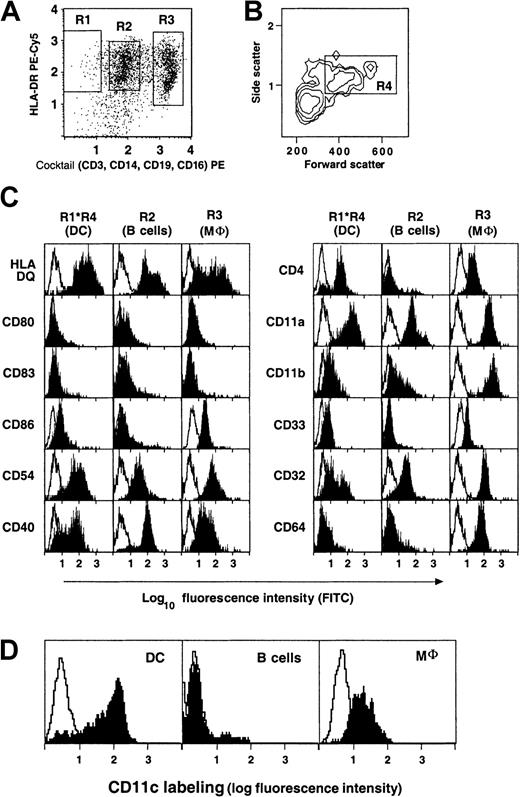
The surface phenotype of spleen DCs was examined by rare event flow cytometry, as previously described.35 Briefly, SMCs were stained for 3-color immunofluorescence with a cocktail of PE-conjugated mAbs (anti-CD3, -CD14, -CD56, and -CD19), PE-Cy5 conjugated anti–HLA-DR, and different mAbs directly or indirectly coupled to FITC. Data on DCs were collected by gated acquisition of cocktail− HLA-DR+ events (Figure1A), and large DCs within the acquired population were analyzed by scatter gating, as previously described (Figure 1B).35 Thus defined, DCs represented 0.7% ± 0.5% of SMCs (range, less than 0.1%-1.8%; n = 19; Table1), whereas macrophages and B lymphocytes made up 9.2% ± 6.9% (range, 1%-22%; n = 19) and 52% ± 16% (range, 16%-85%; n = 19) of SMCs, respectively.
Surface phenotype of spleen DCs, B lymphocytes, and macrophages by rare event flow cytometry.
Expression of surface molecules on gated DC (R1*R4), B lymphocytes (R2), and macrophages (R3). Gates were defined as follows: R1, Cocktail− HLA-DR+ events; R2, Cocktail+ HLA− DR+ events; R3, Cocktail++ events. The cocktail consisted of anti-CD3, CD14, CD16, and CD19 PE-conjugated antibodies as described in “Materials and methods.” (A-C) Data from donor 103. (D) Preferential expression of CD11c on Cocktail−HLA-DR+ DC. Data from donor 94.
Surface phenotype of spleen DCs, B lymphocytes, and macrophages by rare event flow cytometry.
Expression of surface molecules on gated DC (R1*R4), B lymphocytes (R2), and macrophages (R3). Gates were defined as follows: R1, Cocktail− HLA-DR+ events; R2, Cocktail+ HLA− DR+ events; R3, Cocktail++ events. The cocktail consisted of anti-CD3, CD14, CD16, and CD19 PE-conjugated antibodies as described in “Materials and methods.” (A-C) Data from donor 103. (D) Preferential expression of CD11c on Cocktail−HLA-DR+ DC. Data from donor 94.
Figure 1C shows the results of a typical experiment. Gated DCs were uniformly HLA-DQ+ CD11a+, CD11b−or weak, and most of them were CD33+ or weak and CD4+. Most of them were CD11c+ (81% ± 9%; n = 14; Figure 1D). Spleen DCs were clearly distinct from B lymphocytes, macrophages (Figure 1C-D), and Langerhans cells. They were distinct from macrophages in that they all expressed relatively high levels of HLA-DQ, they did not express high levels of CD11b, and they had a much lower expression of Fc-γ receptors: half (54% ± 3%; n = 3) of the gated DCs expressed high levels of FcγRII (CD32), whereas FcγRIII (CD16) was absent (not shown), and FcγRI (CD64) was either negative or only weakly positive on most DCs. One distinguishing feature was the higher expression of CD11c on DCs than on macrophages (relative fluorescence intensity was, on average, 2.8 times higher in 11 different donors, Figure 1D). In addition, spleen DCs were mostly negative for complement receptors 1 and 2 (CD35 and CD21, respectively) and CD1a (data not shown). However, they expressed similar levels of HLA A, B, and C antigens (data not shown) and CD4 as macrophages. CD54 (ICAM-1) and CD40 molecules were both expressed by DCs, the latter weakly. Most spleen DCs were negative or only weakly positive for CD86 and were negative for CD80 and CD83. In brief, in most donors, most spleen DCs had an immature phenotype, similar to that of the CD11c+ subset of blood “myeloid” DCs.36-40
Distribution of spleen dendritic cells
Because a difference in CD11c expression intensity between DC and macrophages was noted by flow cytometry (Figure 1), spleen sections were stained for CD11c to investigate the possibility that, as in the mouse, this molecule could be used as a relatively specific marker for spleen DCs in situ.41 Strongly CD11c+ cells were observed in 3 distinct regions—at the periphery of the white pulp, in the T-cell zones, and in the B-cell zones (Figure2A-F). Double labeling showed that these CD11c+ cells were CD14− (Figure 2B), CD3− (Figure 2D), CD20− (Figure 2F), CD11b−, and HLA-DR+ (not shown) and, therefore, that they had the same phenotype as the cocktail− HLA-DR+ DCs that had been analyzed by flow cytometry.
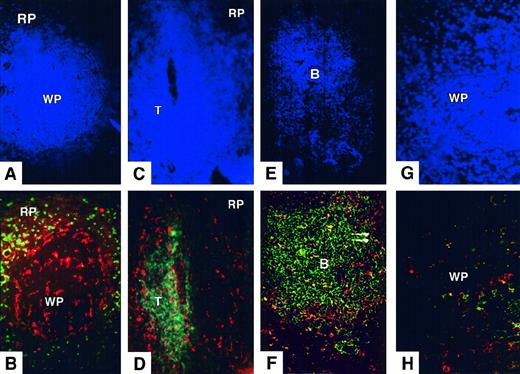
Distribution of CD11c+ spleen DC in normal donors.
(A,B) Triple staining for nuclei (Hoechst, A) CD11c (Texas Red, B), and CD14 (FITC, B). Donor 147; original magnification, ×200. Strongly labeled CD11c+ DCs are scattered throughout the white pulp (WP) and surround its periphery, just inside red pulp (RP) CD14+ macrophages. (C,D) Triple staining for nuclei (Hoechst, C), CD11c (Texas Red, D), and CD3 (FITC, D). Donor 108; original magnification, ×200. Strongly labeled CD11c+ DCs are found mostly in the T-cell zone (T) and the marginal zone. (E,F) Triple staining for nuclei (Hoechst, E), CD11c (Texas Red, F), and CD19+CD20 (FITC, F). Donor 173; original magnification, ×200. CD11c+ CD19−CD20− cells are found around the edge and in the interior of the B-cell zone (B). (G,H) Triple staining for nuclei (Hoechst, G), CD86 (Texas Red, H), and CD83 (FITC, H) in a section of white pulp. Donor 147; original magnification, ×400. Rare CD86+ or CD83+ cells are found in the white pulp. Distribution and phenotype of spleen DCs observed in these donors were representative of those found in 5 organ donors.
Distribution of CD11c+ spleen DC in normal donors.
(A,B) Triple staining for nuclei (Hoechst, A) CD11c (Texas Red, B), and CD14 (FITC, B). Donor 147; original magnification, ×200. Strongly labeled CD11c+ DCs are scattered throughout the white pulp (WP) and surround its periphery, just inside red pulp (RP) CD14+ macrophages. (C,D) Triple staining for nuclei (Hoechst, C), CD11c (Texas Red, D), and CD3 (FITC, D). Donor 108; original magnification, ×200. Strongly labeled CD11c+ DCs are found mostly in the T-cell zone (T) and the marginal zone. (E,F) Triple staining for nuclei (Hoechst, E), CD11c (Texas Red, F), and CD19+CD20 (FITC, F). Donor 173; original magnification, ×200. CD11c+ CD19−CD20− cells are found around the edge and in the interior of the B-cell zone (B). (G,H) Triple staining for nuclei (Hoechst, G), CD86 (Texas Red, H), and CD83 (FITC, H) in a section of white pulp. Donor 147; original magnification, ×400. Rare CD86+ or CD83+ cells are found in the white pulp. Distribution and phenotype of spleen DCs observed in these donors were representative of those found in 5 organ donors.
CD11c+ cells at the periphery of the white pulp surrounded both T-cell and B-cell zones, effectively separating them from the red pulp (Figure 2A-D). Macrophages expressing CD14 and CD11b were not present in this zone. Indeed white pulp CD11c+ cells and the first line of red pulp CD14+ macrophages seemed to form concentric rings around the white pulp (Figure 2A-B). These marginal zone CD11c+ cells had a distribution similar to that of marginating DCs in the mouse, though more consistently surrounding the white pulp.22,26 27
Large CD11c+ cells with clear dendritic morphology, corresponding to the previously described germinal center DCs,31 were scattered throughout the B-cell zones (Figure 2E-F). In some sections, they appeared to stain weakly positive for CD14 (not shown).
CD11c+ DCs were distributed evenly throughout the T-cell zones (Figure 2D). Although they were not rigorously counted, they seemed more densely distributed than B-cell zone DCs. Among them, a minority of mature or activated CD86+ DCs were found (Figure 2H), a subpopulation of which expressed CD83 (also see Figure5). No staining for CD83 or CD86 was observed in the B-cell zones (not shown).
Therefore, in 5 of 8 human spleens analyzed in situ, 3 populations of CD11c+ DCs were clearly identified: marginal zone DC, T-cell zone DC, and B-cell zone DC, with a minority of activated cells in the T-cell zone.
In vivo activation of dendritic cells in a subset of organ donors
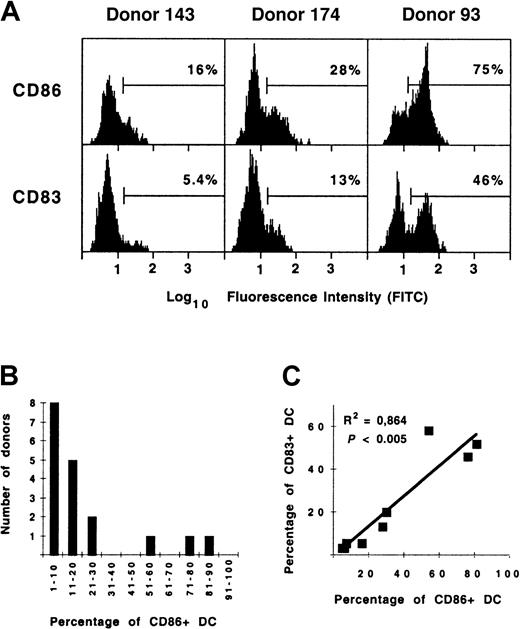
By flow cytometry, spleen DCs from most donors were found to be CD80−83−, and CD86 expression did not seem significant, implying that spleen DCs were not fully mature or activated, as previously observed.35 However, because both CD83+ and CD86+ DC were observed in situ, fluorescence histograms were analyzed in more detail to quantify these DC subpopulations (Figure 3A). The proportion of DCs expressing high levels of CD86 varied from 4.0% to 81% (median, 12%) but was greater than 50% in only 3 of 18 donors analyzed (donors 93, 106, and 176; Table 1). The frequency histogram of these percentages (Figure 3B) was suggestive of a bimodal distribution, though the low sample number does not allow this to be affirmed with certainty. CD83+ DCs made up 3.4% to 58% of spleen DCs (median, 5.8%; n = 9). As with CD86, high proportions of spleen DC from donors 93, 106, and 176 expressed CD83. Indeed, the expression of CD83 and CD86 by DCs was significantly correlated (Figure 3C;P < .005; n = 9), implying that these molecules are up-regulated in a coordinated fashion by DCs in vivo, as previously observed in vitro.35 42
Quantification of mature/activated CD83+ and CD86+ spleen DCs.
(A) Expression of CD83 and CD86 on gated cocktail−HLA-DR+ DC from 3 donors. (B) Frequency distribution of the proportion of CD86+ spleen DCs in organ donors (n = 19). (C) Correlation between the proportion of CD83+ and CD86+ spleen DCs in organ donors (n = 9).
Quantification of mature/activated CD83+ and CD86+ spleen DCs.
(A) Expression of CD83 and CD86 on gated cocktail−HLA-DR+ DC from 3 donors. (B) Frequency distribution of the proportion of CD86+ spleen DCs in organ donors (n = 19). (C) Correlation between the proportion of CD83+ and CD86+ spleen DCs in organ donors (n = 9).
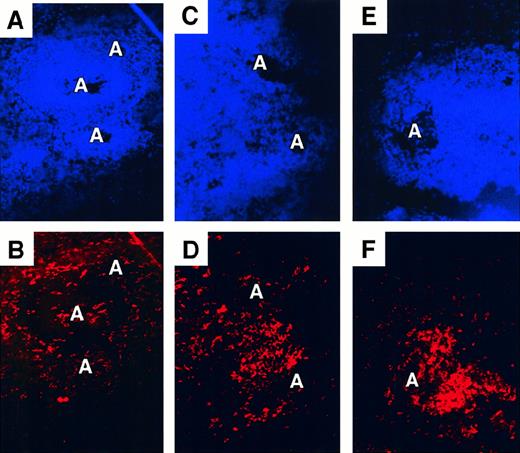
In donor 93, who had 75% CD86+ spleen DCs, the distribution and the surface phenotype of spleen DCs was strikingly different from those seen in most of the other donors. Extremely large numbers of CD11c+ DCs were concentrated in the T-cell zones, and the layer of CD11c+ DCs encircling the white pulp seemed to be absent, though CD11c+ DC were present in the B-cell zones (Figure 4E-F; Figure5). Activated CD83+ and CD86+ DCs were located exclusively in the DC-laden T-cell zones. As in other donors, CD83+ DC were a subpopulation of the CD86+ DCs (Figure 5C-D). In donors 106 and 176, the distribution of spleen DCs appeared more normal, with strongly CD11c+ cells clearly tracing the edge of the white pulp, but 1 to 2 T-cell zones per section did contain heavy concentrations of CD11c+ DCs (Figure 4C-D), many of them expressing CD83+ and CD86+.
Perturbed distribution of CD11c+ spleen DCs in some donors.
Double staining for nuclei (Hoechst, A,C,E) and CD11c (Texas Red, B,D,F). Original magnification, ×200. (A, B) Donor 147 showing a normal distribution of CD11c+ DCs around the edge of the white pulp and scattered CD11c+ DC around the arteriole (A). (C,D) Donor 106. (E,F) Donor 93. CD11c+ cells are concentrated in the white pulp in proximity to arterioles.
Perturbed distribution of CD11c+ spleen DCs in some donors.
Double staining for nuclei (Hoechst, A,C,E) and CD11c (Texas Red, B,D,F). Original magnification, ×200. (A, B) Donor 147 showing a normal distribution of CD11c+ DCs around the edge of the white pulp and scattered CD11c+ DC around the arteriole (A). (C,D) Donor 106. (E,F) Donor 93. CD11c+ cells are concentrated in the white pulp in proximity to arterioles.
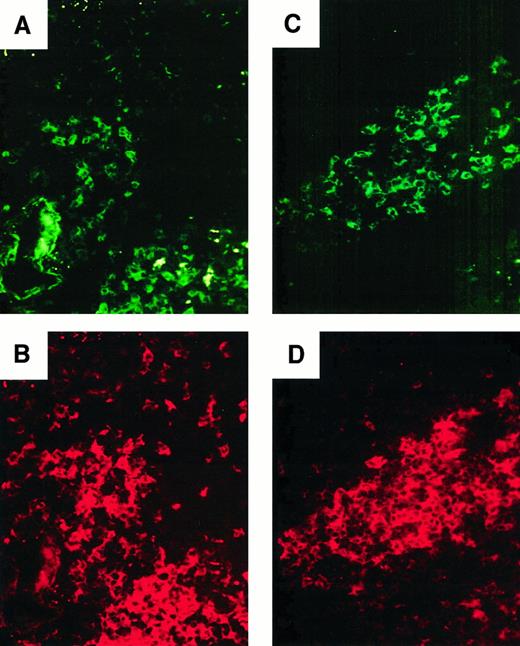
Distribution of activated DC in donor 93.
(A, B) Double staining for CD83 (FITC, A) and CD11c (Texas Red, B). Original magnification, ×400 of the section shown in panel F. A subset of CD11c+ DCs in the white pulp expresses CD83. (C,D) Double staining for CD83 (FITC, C) and CD86 (Texas Red, D). Original magnification, ×400. CD83+ cells are a subset of CD86+ cells.
Distribution of activated DC in donor 93.
(A, B) Double staining for CD83 (FITC, A) and CD11c (Texas Red, B). Original magnification, ×400 of the section shown in panel F. A subset of CD11c+ DCs in the white pulp expresses CD83. (C,D) Double staining for CD83 (FITC, C) and CD86 (Texas Red, D). Original magnification, ×400. CD83+ cells are a subset of CD86+ cells.
Because the phenotype and distribution of DCs in these 3 atypical donors suggested in vivo activation, the clinical records of the organ donors were examined to identify possible clinical correlates of spleen DC activation (Table 1). The duration between trauma and death might be a factor: 2 of 5 patients with 28% or more CD86high DCs were in intensive care for 10 and 4 days, respectively. However, 2 other patients with normal percentages of CD86high DC had hospital stays of 5 and 4 days, respectively. The median duration between trauma and death was 1 day (n = 16; Table 1). Particular attention was paid to bacterial infection given that LPS and endotoxins are known to result in DC activation in the mouse17,18,43and in the rat.44 One striking correlation was that donor 176 had a localized, nosocomial infection (lung abscess) with E coli and Staphylococcus aureus. There was no other recorded infection (all were organ donors and, therefore, extensively tested). It seems that the 2 other donors with more than 50% activated DCs had experienced more extensive trauma than the others: donor 106 had a myocardial trauma that might have resulted in the release of tumor necrosis factor (TNF)-α,45 and donor 93 had extensive multiple trauma with an occipital open lesion. Multiple trauma was the cause of the death in 5 donors, all of whom had 28% or more CD86high DCs compared to the median value of 12%. Hence, the activated DC phenotype in 3 donors might have been related to bacterial infection or to more extensive trauma.
IL-12 secretion
To determine whether these phenotypically activated DCs had a function related to their potential bacterial stimulation and to T-cell activation, the spleen cells from the donor with the most activated DCs (donor 176) were compared with those of a donor with a few activated DCs (donor 172) for the secretion of IL-12. The spontaneous secretion of IL-12 p40 after 24-hour culture was 1554 ± 967 pg/mL for donor 176 compared to 433 ± 0 for donor 172, indicating a functional activation of dendritic cells but perhaps also of other cell types.
Discussion
Human spleen dendritic cell labeling by flow cytometry and in situ
Although mouse spleen DCs have been intensively studied for the past 20 years, relatively little has been published concerning their human counterpart.29,33,35,46 In particular, the phenotype and distribution of human spleen DCs has not yet been described in great detail. In the present work, the surface phenotype of fresh human spleen DCs was studied by flow cytometry, and their distribution was studied by in situ immunofluorescence. Fresh human spleen DCs were defined as large, HLA-DR+ cells negative for markers of other cell lineages. No single DC-specific cell surface marker was found for these cells. For example, as in earlier immunocytochemical studies,29,46 CD1a+ DCs were not found in the spleen. In the mouse, CD11c expression is restricted to DCs, allowing the CD11c promoter to be used to direct specific expression of genes into DCs.41 In contrast, in human spleen and blood, DCs are not the only cells to express CD11c. Nevertheless, we found that DCs expressed CD11c at a higher level than any other cell type, including macrophages, which provided a discriminative tool for in situ immunofluorescence studies. In situ, a clear-cut exclusion between strong CD11c labeling and CD14 (My4) expression was found (except in some B-cell zone DCs), whereas immunocytochemical studies using other monoclonal antibodies had found DC-like cells to express CD14 at various levels.29
Overall, there was a close agreement between the flow cytometry and in situ results concerning the phenotype of CD11c+ DCs. By both methods, only a minority of CD86+ or CD83+DCs were detected in most donors, and if many CD83+ and CD86+ DCs were found in situ (see below), these DCs were also detected by flow cytometry. Hence, both methods analyzed the same cells, and in situ immunofluorescence did not reveal the existence of any DC subtypes that escaped analysis by flow cytometry, probably because all DCs had been efficiently released from tissue by DNAse/collagenase digestion of spleen.32 This combination of flow cytometric and in situ immunofluorescence data allowed us to divide spleen DC into 4 subpopulations: CD11c− DCs and 3 CD11c+ DC subpopulations, from the marginal zone, T-cell zone, and B-cell zone, respectively.
CD11c− DCs.
Most cells (81% ± 9%; n = 14) were CD11c+ spleen DCs, but some cocktail− HLA-DR+CD11c− DCs were also present. Although these cells were not investigated further in this study, we speculate that they may be related to the CD11c− DC precursors found in blood,36,39,47 which seem to be able to migrate directly to the T-cell zones of lymph nodes and tonsils by high endothelial venules.37,48 Their distribution within the spleen and their relation to other CD11c− DC populations could be investigated in additional studies using antibodies against IL-3Rα, which is a specific marker.37-39 In contrast, CD11c+ DCs could be divided into 3 subpopulations on the basis of their distribution as revealed by in situ immunofluorescence
Marginal zone DCs.
First, CD11c+ DCs were situated at the edge of the white pulp. These cells were negative for CD83 and CD86 and, hence, were not fully differentiated for optimal stimulation of T cells. They made up most of CD11c+ DC in most donors. In the human spleen, CD11c+ cells with this distribution have been previously termed marginal zone macrophages29; however, their lack of expression of CD11b and CD14 indicates that they are in fact the human equivalent of the mouse marginating DC population.21,26,27In the mouse, marginating DCs appear to play a sentinel role, as they retain the capacity to capture and process antigen efficiently.49 The phenotype and localization of their human equivalent, which we shall also refer to as marginal zone DCs, suggest that they fulfill a similar function, sampling blood-borne antigens as they pass through the marginal zone. By flow cytometry, human spleen DCs were found to be positive for the Fc receptors CD32 and CD64. These molecules could be involved in antigen capture by spleen DCs. However, the absence of CD11b, CD21, and CD35 implies that complement receptors are not used for antigen uptake by spleen DCs. In addition, the distribution of CD11c+cells in donor 93 (discussed below) implies a direct relation in vivo between the CD11c+ DCs at the periphery of the white pulp and those in the T-cell zones, analogous to the relation between mouse spleen marginating DCs and peri-arteriolar DCs.18 26
On the other hand, the distribution of CD11c+ marginal zone DCs in human spleen differed somewhat from that of mouse marginating DCs, which are clustered at the edge of the white pulp, usually adjacent to a T-cell zone.21,26,27 In human spleen, we did not observe such DC clusters. Rather, CD11c+ marginal zone DCs formed a ring surrounding both the T-cell zones and the B-cell zones, in a distribution reminiscent of mouse marginal zone metallophilic macrophages.50 Hence, human CD11c+ marginal zone DCs, while phenotypically equivalent to mouse marginating DCs, seem to have a slightly different anatomic localization.
T-cell zone DCs.
Second, numerous CD11c+ DCs were present in the T-cell zones. Some of these DCs were CD83+ and CD86+, and, hence, the T-cell zones appeared to be the site of spleen DC maturation. Labeling in situ showed that in all donors, CD83+ DCs were a subset of CD86+ DCs, and the strong positive correlation between the percentage of CD83+and CD86+ DCs showed that CD83+CD86+ mature DCs formed a relatively stable proportion (64% ± 20%; n = 9) of all CD86+DCs. Hence, spleen DCs in the T-cell zones seem to be progressing along a differentiation pathway in which immature or sentinel CD83−CD86− DCs would first become CD83−CD86+ before ultimately becoming fully mature or activated CD83+CD86+ DCs. This maturation program occurs with rapid kinetics in vitro, with CD83−CD86− DCs becoming CD83+CD86+ within 24 hours.35
B-cell zone DCs.
Finally, the B-cell zones also contained CD11c+ DCs, which by microscopy appeared larger than the CD11c+ cells in the T-cell zones and in some sections stained weakly positive for CD14. These 2 observations, together with the previously reported expression of CD11b by tonsil germinal center DCs,31 imply that B-cell zone DC differ in some respects from marginal zone and T-cell zone DCs and may be closer to macrophages. In addition, they were never observed to express CD83, CD80, or CD86 by immunofluorescence microscopy and were, therefore, not fully differentiated for T-cell stimulation, even in the 3 donors who had very high proportions of activated DCs. This indicates clearly that in vivo activation of these cells requires different signals from those that activate marginating and T-cell zone DCs.
DC activation in a subset of donors.
Very high proportions of CD83+ CD86+CD11c+ DCs, suggesting in vivo DC activation, were observed in a subset (3 of 18; 17%) of organ donors. Spleens of the same 3 donors (of 8 spleens examined by in situ immunofluorescence) also showed signs of DC activation in situ. Donor 93 in particular showed a striking alteration in the distribution of spleen DCs, with few marginal zone DCs and large numbers of CD83+CD86+ DCs concentrated in the T-cell zones. Similar changes in DC distribution were observed only in a few T-cell zones in donors 106 and 176. The high proportion of DCs (1.8% of SMC) in donor 93 may account for the fact that changes in DC distribution were more clearly visible in this donor. However, there was no relation between DC number and DC phenotype or distribution; donor 108, in whom DCs made up 1.7% of SMC, showed a normal distribution of DCs. Moreover, a high spontaneous secretion of IL-12 was found in the spleen cells from donor 176, who had the most activated DC phenotype and a localized bacterial infection. This interleukin may have been secreted by other cell types, but the data are compatible with a secretion by activated DC.3
The up-regulation of CD83 and CD86 on human DC in vitro occurs in response to TNF-α, IL-1, and microbial products such as LPS.5,51 In the mouse, the same stimuli provoke in vivo activation of DCs and their migration to the T-cell regions of lymphoid organs.43,52 In particular, relocalization of mouse spleen DCs from the marginal zone to the T-cell zones, with simultaneous up-regulation of CD86, is caused by the injection of LPS or ofT gondii–derived antigens.18,19 The similarity of the observed phenotype and distribution of DCs in donors 93, 106, and 176 to those in mice led us to search for a similar cause of DC activation. This was distinctly possible for donor 176, who was the only subject with an ongoing bacterial infection. The possible cause of DC activation in donors 93 and 106 was less clear and was perhaps related to more extensive trauma than in the other donors, which may have increased the serum levels of TNF-α, such as after myocardial infarction or cardiac surgery.45,53,54 However, we were unable to verify the TNF-α hypothesis because of the lack of serum samples. In addition, the kinetics of DC activation and redistribution after microbial challenge in mice is transient, with a maximum after 6 hours,17-19 55 and other organ donors may have displayed similar changes transiently.
DC activation in organ donors may have consequences for the fate of transplanted organs. Interstitial DCs in transplanted organs are good candidates for allostimulatory passenger leukocytes, postulated as initiators of graft rejection.4,56 Indeed, artificially increasing the numbers of phenotypically mature donor DCs in transplanted liver by donor pretreatment with Flt-3 ligand resulted in acute rejection after transplantation in mice.57 In conclusion, the data presented here indicate that the human spleen can be the site of activation and of microanatomic redistribution of DCs on bacterial stimulation or extensive trauma, pointing to events potentially occurring at the initiation of the immune response.
We thank Prof T. F. Tedder (Duke Medical University, Durham, NC) for generously providing the HB15a monoclonal; Dr C. Sylla for providing spleen samples; M. S. Laye for technical help; Drs G. Milon, J. G. Guillet, and E. Souil for kind advice; and Drs M. Moser and C. Müller for critical review of the manuscript.
Supported by grants from the Agence Nationale de Recherches sur le SIDA and the Fondation pour la Recherche Médicale (A.H. and D.M.), SIDAction (Ensemble Contre le SIDA) (A.S., C.T.), and the CAPES (F.G).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anne Hosmalin, Unité INSERM 445, 27 rue du Faubourg St Jacques, 75014 Paris, France; e-mail:hosmalin@cochin.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal