A population of metachromatic cells with mast cell (MC) and basophil features was identified recently in the peripheral blood of patients with several allergic disorders. This study now shows that these metachromatic cells express on their surface the high-affinity IgE receptor (FcεRI), CD4, and the chemokine receptors CCR3, CCR5, and CXCR4, but not the T-cell surface protein CD3 and the monocyte/macrophage surface protein CD68. This population of MCs/basophils can be maintained ex vivo for at least 2 weeks, and a comparable population of cells can be generated in vitro from nongranulated hematopoietic CD3−/CD4+/CD117− progenitors. Both populations of MCs/basophils are susceptible to an M-tropic strain of human immunodeficiency virus 1 (HIV-1). Finally, many patients with acquired immunodeficiency syndrome have HIV-1–infected MCs/basophils in their peripheral blood. Although it is well known that HIV-1 can infect CD4+ T cells and monocytes, this finding is the first example of a human MC or basophil shown to be susceptible to the retrovirus.
Introduction
Mast cells (MCs) and basophils are histamine-containing, metachromatic granulocytes that express the high-affinity IgE receptor, FcεRI, on their surfaces.1,2When activated via this receptor, both populations of cells immediately exocytose their granule constituents and then more slowly generate and release cytokines, chemokines, and arachidonic acid metabolites. Thus, these granulocytes play extremely important roles in IgE-mediated inflammatory responses. Although human MCs and basophils are similar in certain aspects, their substantial biochemical and morphologic differences in normal individuals have led to the concept that the 2 populations of cells probably are developmentally unrelated. MCs, for example, reside in tissues and generally possess a centrally positioned, oval nucleus. In contrast, basophils generally reside in the blood and contain a segmented nucleus. The MCs in the lung and skin of most normal individuals contain chymase, carboxypeptidase A, elastase, cathepsin G, and multiple tryptases in their granules.3-13 Basophils in the peripheral blood of most normal individuals contain cathepsin G and elastase4 but lack carboxypeptidase A and chymase.14,15 Tryptase has been detected in normal human basophils. However, in the initial reports, Schwartz and coworkers concluded that normal human MCs contain on average about 250-fold more tryptase protein16 and about 50 000-fold more tryptase messenger RNA (mRNA)15than normal human basophils.
Although it was originally thought that the phenotype of a MC was fixed and irreversibly predetermined before its progenitor exited the bone marrow, it is now apparent that human and mouse MCs exhibit substantial plasticity in terms of what genes they can express. Because certain populations of MCs are long-lived,17 these effector cells have developed the capacity to reversibly alter their phenotypes to respond to rapid changes in the immune status of their microenvironments. It was first demonstrated in the mouse that the phenotype of a MC at any stage in its life span is a consequence of the factors the cell encounters in its previous and current tissue microenvironments,18-25 and it is now understood that certain populations of human MCs also can reversibly alter their granule phenotype in vivo.26
The developmental relationship between basophils and MCs remains unclear, in part, because no defined biochemical marker has been identified that can distinguish these 2 populations of cells in an animal that can be experimentally manipulated. A population of FcεRI+/metachromatic cells that possesses segmented nuclei is present in the peripheral blood of helminth-infected mice. Because of their location and nuclear profiles, it was originally thought that these mouse cells were basophils; however, recent studies have shown that they are actually senescent MCs trafficking from the jejunum to the spleen.27 We have described a population of cells with both MC and basophil features in the blood of patients with asthma, allergy, and allergic drug reactions.28 These metachromatic cells resemble normal mature basophils in terms of their peripheral blood location, segmented nuclei, and surface expression of the Bsp-1 epitope. However, they more closely resemble normal mature MCs in terms of their surface expression of CD117 (c-kit) and their granule expression of multiple proteases.
In the current study, we present additional biochemical evidence that the unusual population of metachromatic cells we identified in the blood of patients with various allergic disorders belongs in the MC/basophil lineage. While characterizing their surface receptors to understand their chemokine-dependent migration potential, we discovered that these cells are susceptible to an M-tropic strain of human immunodeficiency virus 1 (HIV-1) because they express CD4 and the chemokine receptors CXCR4, CCR3, and CCR5. Similar findings were obtained with human MCs differentiated in vitro. Although these findings were unexpected because of the reports that MCs/basophils differentiated in vivo do not express CD4,29 30 analysis of the metachromatic cells in the blood of 11 patients with acquired immunodeficiency syndrome (AIDS) revealed that HIV-1–infected MCs/basophils are present in many of these patients.
Materials and methods
Antibodies
Mouse antihuman tryptase α/β IgG (free and conjugated to alkaline phosphatase [AP]) and mouse antihuman chymase IgG (free and biotinylated) were obtained from Chemicon International (Temecula, CA). Rabbit antibodies that recognize residues 221 to 236 in human transmembrane tryptase (TMT) were obtained with an antipeptide approach.13 Normal rabbit serum, rabbit antimouse IgG, AP anti-AP, rabbit antimouse IgG conjugated to horseradish peroxidase (HRP), and mouse antihuman CD68 antibody were obtained from Dako (Glostrup, Denmark). Sheep antirabbit IgG-HRP, goat antirabbit IgG-AP, and mouse antihuman FcεRI IgG31 were from Silenus (Melbourne, Australia), Sigma (St Louis, MO), and Dr J.-P. Kinet (Boston, MA), respectively. Mouse antihuman CD117 IgG conjugated to phycoerythrin (PE), antihuman IgG-PE, antihuman CD3 IgG conjugated to PE/cyanin (PC5), and antihuman IgG-PC5 antibody were obtained from Beckman Coulter (Fullerton, CA). Mouse antihuman CD4 IgG and antihuman CD3 IgG (free and conjugated to fluorescein isothiocyanate [FITC]) and antihuman IgG-FITC were obtained from Becton Dickinson (San Jose, CA). Mouse antihuman CCR5, mouse antihuman CXCR4, and rat antihuman CCR3 IgG were obtained from R&D Systems (Minneapolis, MN). Mouse anti–HIV-1 p24(142-207) antibody was obtained from ICN Biomedicals (Costa Mesa, CA).
Cell isolation and culture
Peripheral blood from 3 patients with asthma, a patient with an allergic drug reaction, and 4 normal laboratory workers was collected in heparinized tubes (Greiner Labortechnik, Frickenhausen, Germany). The tubes were centrifuged at 800g for 10 minutes, and the leukocyte-enriched buffy coats were removed and resuspended for 10 minutes in erythrocyte lysis buffer (Sigma). The nonlysed cells remaining were washed with Dulbecco phosphate-buffered saline (PBS), and samples were analyzed immediately as described below. In some instances, the cells were resuspended at a density of 3 × 106 cells/mL in enriched medium supplemented with 50 ng/mL recombinant stem cell factor/c-kit ligand (KL) (Amgen, Thousand Oaks, CA) and 50% (vol/vol) human bone marrow–derived mastocytosis (HBM)–M cell-conditioned medium.32The cells were cultured at 37°C in a 5% CO2 humidified atmosphere with the medium changed every 3 to 4 days as previously described.28
Fluorescence-activated cell sorter analysis and cell sorting
Two- and 3-color flow cytometry and cell sorting were performed on a MoFlo MLS Flow Cytometer (Cytomation, Fort Collins, CO) equipped with an argon-ion laser tuned at 488 nm. Data acquisition was performed with Cyclops Summitt software (Cytomation). Fluorescence emission was collected at 510 to 550 nm, 555 to 590 nm, and 650 to 690 nm for FITC, PE, and PC5, respectively. Compensation parameters were determined using single stained cell populations. For 2-color fluorescence-activated cell sorter (FACS) analyses, about 106 human cells were suspended in 100 μL buffer containing anti–CD4-FITC (0.6 μg/mL) and anti–CD117-PE (2.5 μg/mL) IgG. Anti–CD3-PC5 IgG (5 μg/mL) was also added to the buffer when 3-color FACS analysis was carried out. The resulting cell suspensions were incubated for 45 minutes in the dark, washed twice with ice-cold PBS containing 5% fetal calf serum, and resuspended in 0.5 mL PBS. Isotype-matched, irrelevant mouse antihuman IgG-PE, antihuman IgG-FITC, and antihuman IgG-PC5–conjugated monoclonal antibodies were used to assess the degree of nonspecific binding to each cell preparation. The net percentage of positive cells was calculated by subtracting the percentage of positive cells obtained with an isotype-matched, negative control monoclonal antibody from the percentage of positive cells in each preparation. Cell sorting was performed by first selecting live, single cells by forward and side scatter and pulse width, and then gating on the regions in the compensated bivariate histograms that contained those cells that expressed both CD4 and CD117. Gates were set at 99%. The resulting cells were sorted into minimal essential media supplemented with 10% fetal calf serum.
Immunocytochemistry
Approximately 2 × 104 sorted cells were centrifuged for 5 minutes at 200g onto glass slides, air-dried, and then processed. For CD117 and FcεRI analyses, slides containing unsorted or sorted human cells were fixed in acetone for 10 minutes at room temperature and then incubated with mouse antihuman CD117 (0.5 μg/mL) or antihuman FcεRI (1.4 μg/mL) IgG overnight at 4°C. The stained cells were washed with PBS/1% bovine serum albumin and incubated with rabbit antimouse IgG-AP for 1 hour at room temperature. The resulting slides were developed with AP/anti-AP complex and AP substrate.
Double immunocytochemistry was used to assess the coexpression of tryptase or chymase with CD4, CCR3, CCR5, or CXCR4. For these analyses, slides were sequentially placed in Carnoy fixative, 0.3% H2O2 in methanol, and normal rabbit serum (1:10 vol/vol) for 15, 10, and 10 minutes, respectively. The fixed and blocked slides were then incubated overnight at 4°C with mouse antihuman CXCR4 (2 μg/mL), antihuman CCR5 (2 μg/mL), or antihuman CD4 (0.5 μg/mL) IgG. The resulting slides were incubated with rabbit antimouse IgG-HRP (6 μg/mL) for 1 hour at room temperature, and then with a freshly prepared 3,3′-diaminobenzidine (DAB) substrate solution. CCR3 staining was performed with rat antihuman CCR3 IgG (5 μg/mL) followed by HRP/rabbit antirat IgG (13 μg/mL). Tryptase expression was evaluated as described28 with the AP method. In each instance, nonspecific staining was assessed with slides not exposed to the primary antibody.
The coexpression of TMT with chymase, tryptase α, tryptase βI, tryptase βII, or tryptase βIII was assessed. Fixed cells were sequentially incubated for 2 hours at 37°C with rabbit antihuman TMT IgG (5 μg/mL, 1:200) for 1 hour at room temperature with sheep antirabbit IgG-HRP (1.3 μg/mL) and then with DAB substrate. The treated slides were incubated at room temperature for 2 hours with mouse antihuman tryptase α/β IgG-AP (2 μg/mL) before development with AP substrate. Alternately, the treated cells were incubated at room temperature for 1 hour with biotinylated mouse antihuman chymase IgG (0.3 μg/mL), and then at room temperature for 1 hour with AP-conjugated avidin before development with AP substrate. To identify those cells that coexpress TMT and FcεRI, the cells were incubated for 2 hours at 37°C with rabbit antihuman TMT IgG and then at room temperature for 1 hour with goat antirabbit IgG-AP (1:200) before development with AP substrate. The resulting cells were sequentially incubated with mouse antihuman FcεRI IgG (1.4 μg/mL), rabbit antimouse IgG-HRP (6 μg/mL), and then DAB substrate. Immunohistochemistry was used on the sorted CD4+/CD117+ cells for expression of CD3 or CD68. After the slides were fixed and blocked as described above, they were incubated with either mouse antihuman CD3 (0.6 μg/mL) or mouse antihuman CD68 (3 μg/mL) IgG, followed by HRP/rabbit antimouse IgG (6 μg/mL), and then with DAB substrate. Cell preparations were scored blind both for immunohistochemistry and electron microscopy (see below).
HIV infection of human MCs/basophils differentiated in vivo and in vitro
The CD3−/CD4+/CD117+MCs/basophils were isolated by FACS from the peripheral blood of a patient with asthma and a patient with an allergic drug reaction. Phenotypically similar cells were generated in vitro from the peripheral blood of 2 normal individuals. Approximately equal numbers of the resulting 4 populations of sorted cells were separately exposed for 4 hours to the same virus inoculate (0.025 infectious virus particles/cell) of either the M-tropic BAL strain or the T-tropic NL4-3 strain of HIV-1 (AIDS Reagent Program, National Institutes of Health, Bethesda, MD). On days 0, 3, 6, and/or 12 after infection, the levels of the p24 viral antigen in the conditioned medium was measured with an enzyme-linked immunosorbent assay (ELISA) kit (Coulter, Miami, FL). The levels of HIV-1 DNA in the treated cells were determined with a semiquantitative polymerase chain reaction assay, as described.33 In this assay, DNA from the HIV-1–infected T-cell line 8E5 was used to construct the standard curve.
In other studies, the MCs/basophils in the peripheral blood of 11 patients with AIDS were evaluated for the presence of HIV-1. Three of these AIDS patients also had asthma; one also had an allergic rhinitis. In the double-staining approach described above, the freshly isolated cells from these patients were fixed and then stained with anti–HIV-1 p24 antibody followed by antihuman tryptase antibody. Standard electron microscopic methodologies also were carried out to evaluate the ultrastructure of the MCs/basophils in the blood of 2 patients with AIDS and asthma.
Results and discussion
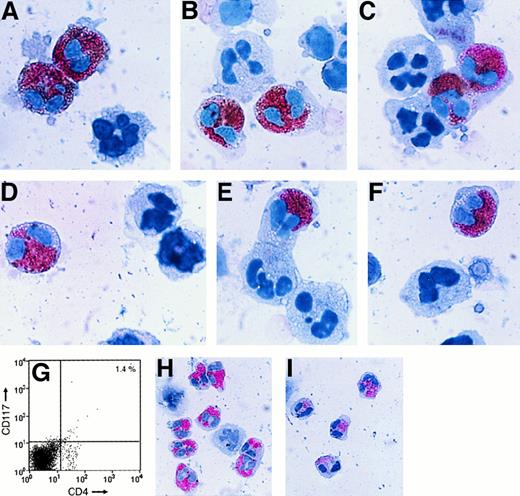
We recently identified a population of metachromatic cells in the peripheral blood of 3 groups of patients with allergic disorders that have some features of normal MCs and some features of normal basophils.28 These metachromatic cells resemble normal mature basophils in terms of their peripheral blood location, segmented nuclei, and surface expression of the Bsp-1 epitope. However, they more closely resemble normal mature MCs in terms of their prominent surface expression of CD117 and their granule expression of the neutral proteases carboxypeptidase A, chymase, and tryptases α, βI, βII, and/or βIII. In this study, we found that these cells also express the high-affinity IgE receptor FcεRI and the MC granule protease TMT (Figure 1). These new findings support the conclusion that the circulating metachromatic cells belong in the MC/basophil lineage. Although it was initially reported that human basophils contain virtually no tryptase,15,16 Foster and coworkers recently reported that the FcεRI+ basophils in the blood of some humans actually contain more total tryptase protein than that found in any tissue MC.34 The accumulated data now indicate that the varied tryptases are not MC-specific proteases as previously thought. Alternately, human MCs and basophils are more closely related than previously appreciated.
TMT and FcεRI expression.
Nonsorted, freshly isolated cells from asthma patient 1 (A,B) and asthma patient 2 (C-E) were stained with antihuman TMT IgG alone (A,C). Cells from patient 1 were also sequentially stained with anti-TMT IgG (brown) and then with an antibody that recognizes human tryptases α, βI, βII, and βIII (pink) (B). Cells from patient 2 were sequentially stained with anti-TMT IgG (brown [D] or pink [E]) and then with either antichymase IgG (pink) (D) or anti-FcεRI IgG (brown) (E). Note that the metachromatic cells in the blood of both patients express a number of the granule proteases that normal, mature MCs express, but nearly all of these cells possess segmented nuclei like normal blood basophils. Magnification ∼×400.
TMT and FcεRI expression.
Nonsorted, freshly isolated cells from asthma patient 1 (A,B) and asthma patient 2 (C-E) were stained with antihuman TMT IgG alone (A,C). Cells from patient 1 were also sequentially stained with anti-TMT IgG (brown) and then with an antibody that recognizes human tryptases α, βI, βII, and βIII (pink) (B). Cells from patient 2 were sequentially stained with anti-TMT IgG (brown [D] or pink [E]) and then with either antichymase IgG (pink) (D) or anti-FcεRI IgG (brown) (E). Note that the metachromatic cells in the blood of both patients express a number of the granule proteases that normal, mature MCs express, but nearly all of these cells possess segmented nuclei like normal blood basophils. Magnification ∼×400.
It is now known that MCs and basophils can express varied chemokine receptors. For example, it was reported recently that the MCs that reside in normal human skin express CCR3,35 whereas the MCs developed in vitro from cord blood progenitors express CXCR4.36 Using negative selection approaches, Jinquan30 and Uguccioni29 and their coworkers reported that the basophils in the blood of normal humans express the chemokine receptors CCR1, CCR2, CCR3, and CXCR4, but not the chemokine receptor CCR5. Nevertheless, Ochi and coworkers37 were able to generate an immature population of metachromatic cells in vitro that transiently expressed CCR5 before these cells fully granulated by culturing cord blood–derived progenitors in medium supplemented with KL, interleukin 6 (IL-6), and IL-10. To evaluate their potential for chemokine-dependent migration into tissues, the more mature MCs/basophils, differentiated in vivo, identified in the blood of our patients were examined by FACS and double-immunostaining approaches for their expression of CCR3, CCR5, and CXCR4. The helper/inducer subset of T cells38 is the major cell type in the blood that expresses CD4. Although it has been reported that normal basophils do not express CD4,29,30 hematopoietic progenitors that initially express CD4 and CD3439 can be induced to differentiate into relatively mature human MCs in vitro.40Thus, we also investigated whether or not the MCs/basophils in the blood of our allergic patients expressed CD4 on their surface.
In double-immunostaining approaches, nearly all of the tryptase+/chymase+ cells in the blood of our 3 patients with asthma and one with an allergic drug reaction expressed CD4, CCR5, CXCR4 (Figure 2), and CCR3 (data not shown). Most of those CD4+cells that failed to express tryptase or chymase generally were small in size and possessed a monolobed nucleus typical of a T cell. Anti-CD4 IgG is routinely used to remove contaminating T cells during the purification of human basophils.29 30 Because many of the MCs/basophils in the blood of our asthma and drug-reactive patients expressed CD4, the widespread use of this negative selection approach now appears to be one of the reasons that comparable cells had not been identified previously.
CD4, CCR5, and CXCR4 expression.
Nonsorted (A-F) and CD4+/CD117+ sorted (G-I) freshly isolated cells from patients with asthma were stained pink with antichymase (A-C,H) or antitryptase (D-F,I) IgG. In some instances, the resulting cells were then stained brown with anti-CD4 (A,D), anti-CCR5 (B,E), or anti-CXCR4 (C,F) IgG. As assessed by this double-immunohistochemical procedure, most of the tryptase+/chymase+ cells in the blood of these patients expressed CD4, CCR5, and CXCR4. The sorted cells depicted in panels H and I are shown at a lower magnification than the cells in panels A through F to better evaluate the efficiency of the purification procedure. As noted in the nonfixed cells in panel G, CD4 is expressed on the surface of the MCs/basophils. The apparent intracellular location of CD4 in panels A and D could be a consequence of the fixation step used in this immunohistochemistry procedure.
CD4, CCR5, and CXCR4 expression.
Nonsorted (A-F) and CD4+/CD117+ sorted (G-I) freshly isolated cells from patients with asthma were stained pink with antichymase (A-C,H) or antitryptase (D-F,I) IgG. In some instances, the resulting cells were then stained brown with anti-CD4 (A,D), anti-CCR5 (B,E), or anti-CXCR4 (C,F) IgG. As assessed by this double-immunohistochemical procedure, most of the tryptase+/chymase+ cells in the blood of these patients expressed CD4, CCR5, and CXCR4. The sorted cells depicted in panels H and I are shown at a lower magnification than the cells in panels A through F to better evaluate the efficiency of the purification procedure. As noted in the nonfixed cells in panel G, CD4 is expressed on the surface of the MCs/basophils. The apparent intracellular location of CD4 in panels A and D could be a consequence of the fixation step used in this immunohistochemistry procedure.
CD117 is the receptor for KL, and CD117 is expressed on the surface of nearly all immature and mature MCs.41 A minor population of natural killer cells expresses CD117,42 but no mature T cell has been found in peripheral blood that expresses this cytokine receptor. The observation that the metachromatic cells in the blood of our patients expressed CD117, coupled with the observation that less than 0.6% of these cells expressed CD3, indicated that they were not T cells. The cells also failed to express the monocytes/macrophage marker CD68. To obtain further evidence that the CD3−/CD4+/CD68−/CD117+/CCR3+/CCR5+/CXCR4+/FcεRI+/chymase+/tryptase+cells are not related to T cells or monocytes/macrophages, the buffy coat preparation of cells from patients with asthma were phenotyped after being subjected to a FACS enrichment step (Figure 2G). Of the CD4+/CD117+ cells in the sorted preparation, 94% ± 2.7% expressed chymase and 90% ± 3.6% (mean ± SD, n = 3) expressed at least one tryptase (Figure2H,I). When these cells were evaluated for their chemokine receptor expression, 92% ± 3.2% of the CXCR4+ cells and 67% ± 3.7% of the CCR5+ cells in the CD4+/CD117+ sorted population also expressed at least one tryptase.
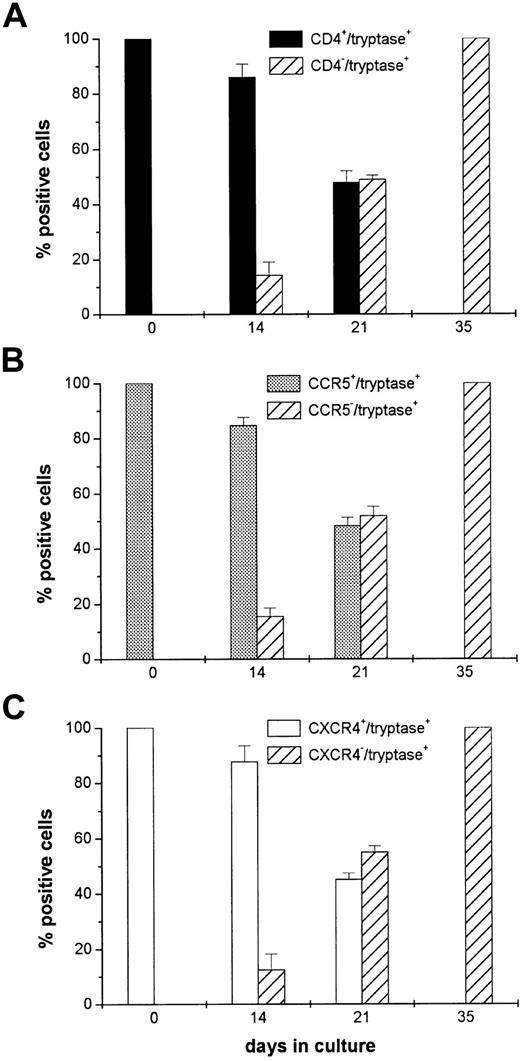
Sorted CD3−/CD4+/CD117+ cells from the buffy coat preparation were cultured in the presence of KL and HBM–M cell-conditioned medium. At various time intervals, the cells were examined by double immunostaining for their granule expression of tryptase and for their surface expression of CD4, CCR5, or CXCR4 to determine whether or not in vivo–differentiated human MCs/basophils can alter their surface phenotype ex vivo. In the 3 experiments depicted in Figure 3, 5 × 105, 7 × 105, and 8 × 105 sorted CD3−/CD4+/CD117+ cells were placed in culture; the viability of these cells ranged from 93% to 96%. At day 21, there were 1 × 105, 2 × 105, and 2 × 105 cells in the corresponding cultures and the cell viabilities ranged from 51% to 57%. Although these data indicate that substantial numbers of the in vivo– differentiated MCs/basophils died during the culture, virtually all of the starting cells and more than 80% of tryptase+ cells in the 2-week cultures expressed CD4, CXCR4, and CCR5. Thus, tryptase+/CD4−/CXCR4−/CCR5−cells should have been detected in the starting population of cells if there were 2 distinct populations of MCs/basophils in the initial cultures having fixed surface phenotypes. We cannot rule out the possibility that a tryptase+/CD4−/CXCR4−/CCR5−population of cells proliferated and slowly took over the cultures. Nevertheless, this scenario is highly unlikely because the starting MCs/basophils possessed segmented nuclei (Figures 1 and 2) and such cells do not divide. Moreover, the number of MCs/basophils in the cultures decreased. It is well known that human and mouse MCs can reversibly alter their expression of granule and lipid mediators. The findings depicted in Figure 3 now suggest that human MCs or basophils also can reversibly alter their expression of surface cytokine and chemokine receptors. Ochi and coworkers37 recently obtained similar findings with in vitro differentiated, immature human MCs developed by culturing cord blood progenitors in the presence of IL-6, IL-10, and KL.
Reversible expression of CD4, CCR5, and CXCR4.
Sorted CD3−/CD4+/CD117+ in vivo differentiated MCs/basophils from 3 patients with asthma were placed in culture for up to 35 days to assess whether or not these cells can alter their expression of CD4, CCR5, and CXCR4 ex vivo. At each time point, cells were stained with antitryptase IgG and then with either anti-CD4 (A), anti-CCR5 (B), or anti-CXCR4 (C) IgG. The hatched bars indicate the percentage of tryptase+ cells (mean ± SD) that fail to express CD4 or one of the chemokine receptors. The solid bars indicate the percentage of tryptase+ cells that continue to express CD4, CCR5, and CXCR4. Note that none of the tryptase+ cells in the 35-day cultures expressed CD4, CCR5, or CXCR4. Similar findings were obtained when the double-stained cells were first incubated with either antichymase or anti-TMT IgG (data not shown).
Reversible expression of CD4, CCR5, and CXCR4.
Sorted CD3−/CD4+/CD117+ in vivo differentiated MCs/basophils from 3 patients with asthma were placed in culture for up to 35 days to assess whether or not these cells can alter their expression of CD4, CCR5, and CXCR4 ex vivo. At each time point, cells were stained with antitryptase IgG and then with either anti-CD4 (A), anti-CCR5 (B), or anti-CXCR4 (C) IgG. The hatched bars indicate the percentage of tryptase+ cells (mean ± SD) that fail to express CD4 or one of the chemokine receptors. The solid bars indicate the percentage of tryptase+ cells that continue to express CD4, CCR5, and CXCR4. Note that none of the tryptase+ cells in the 35-day cultures expressed CD4, CCR5, or CXCR4. Similar findings were obtained when the double-stained cells were first incubated with either antichymase or anti-TMT IgG (data not shown).
To obtain larger numbers of a phenotypically similar population of MCs/basophils for investigation, we searched for a poorly granulated progenitor in the blood of normal individuals and individuals with allergic disorders that could give rise in vitro to cells that phenotypically resemble the in vivo–differentiated MCs/basophils circulating in the blood of our allergic patients. The cells in the buffy coat preparations of allergic individuals were subdivided by FACS into distinct pools of cells that differed in their surface expression of CD3, CD4, and CD117. The sorted cells were then cultured for up to 3 weeks in the presence of KL and HBM–M cell-conditioned medium. At the start of the culture, there were essentially no tryptase+or chymase+ cells in the sorted population that possessed a CD3−/CD4+/CD117− phenotype. However, after this nongranulated population of progenitors was cultured for 14 days, 20% ± 4.6%, 20% ± 5.4%, and 7.8% ± 1.0% (mean ± SD, n = 3) of the resulting cells expressed tryptase, chymase, and CD117, respectively. As assessed by double immunohistochemical approaches, these MCs/basophils also expressed CCR5 and CXCR4. Although fewer in number, phenotypically similar MCs/basophils also could be generated when CD3−/CD4+/CD117− sorted cells from normal individuals (n = 3) were cultured as described above.
During HIV-1 infection, the envelope glycoprotein gp120 initially binds to CD4 on the surface of the target cell.43 The chemokine receptors CXCR4 and CCR5 are the major coreceptors that mediate entry of the retrovirus into T cells and macrophages.44 CXCR4 is a receptor for stromal-derived factor 1 and is the major coreceptor used by those strains of HIV-1 that infect circulating T cells and T-cell lines.45,46 CCR5 is a receptor for macrophage inflammatory protein-1 and RANTES,47 and is the major coreceptor used by those strains of HIV-1 that infect macrophages.48-52 The tryptase+/chymase+ cells in the blood of our allergic patients express those receptors needed for efficient HIV-1 infection. MCs can be easily immortalized by retroviruses,53 and these cells are believed to be somewhat developmentally related to monocytes.54 All of these observations raised the possibility that the human MCs/basophils we isolated from the blood of our allergic patients could be infected with M-tropic strains of HIV-1.
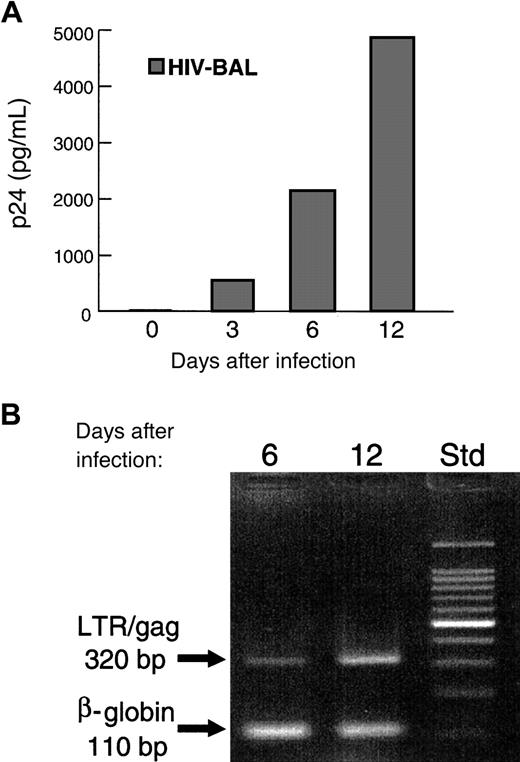
The freshly isolated, in vivo differentiated MCs/basophils from the individual with asthma proved highly susceptible to the M-tropic BAL strain of HIV-1 and died quickly after exposure. In contrast, the freshly isolated in vivo differentiated sorted CD3−/CD4+/CD117+ MCs/basophils from the patient with an allergic drug reaction not only survived the initial HIV-1 infection but produced low levels of the retrovirus over a 2-week period. Viral DNA was readily detected in these cells 12 days after treatment with the BAL strain of HIV-1, and the level of viral antigen p24 in the conditioned medium was about 400 pg/mL. The in vitro– differentiated MCs/basophils also survived the initial HIV-1 infection. However, these cells produced substantially higher levels of the retrovirus, and viral antigen p24 could be detected in the conditioned medium 3 days after the cells were exposed to the BAL strain of HIV-1 (Figure 4A). The levels of viral antigen p24 increased progressively during the next 12 days of culture to about 5000 pg/mL. A corresponding increase in HIV-1 genomic DNA was also observed (Figure 4B). Because macrophages adhere to plastic, the fact that the in vivo– and in vitro–differentiated cells were subjected to plastic adherence steps before exposure to the M-tropic strain is additional evidence that the human MCs/basophils in the culture are the cells in the tested preparations that are HIV-1 infected.
HIV-1 infection.
In the depicted experiment, the peripheral blood cells in the buffy coat of a normal individual were placed in culture for 3 weeks. Immediately after sorting, the CD3−/CD4+/CD117+ population of in vitro–generated MCs/basophils was examined for its susceptibility to HIV-1. Three, 6, and 12 days after infection, samples of the cultures were evaluated for the presence of the viral protein p24 (A) and the viral “LTR/gag” genomic fragment (B). For a positive control in the polymerase chain reaction assay, replicate samples of the isolated genomic DNA were evaluated for the presence of the humanβ-globin gene. Molecular weight standards (Std) are indicated on the right. MCs/basophils freshly isolated from a patient with an allergic drug reaction and a patient with asthma also were found to be susceptible to the BAL strain of HIV-1 (data not shown). In those latter 2 experiments, the sorted CD3−/CD4+/CD117+ in vivo–differentiated MCs/basophils were exposed to the retrovirus immediately after their purification from the peripheral blood. As assessed immunohistochemically, less than 1% of the cells in the varied sorted population used in these HIV-1 susceptibility studies expressed CD3 and only about 1.5% expressed CD68.
HIV-1 infection.
In the depicted experiment, the peripheral blood cells in the buffy coat of a normal individual were placed in culture for 3 weeks. Immediately after sorting, the CD3−/CD4+/CD117+ population of in vitro–generated MCs/basophils was examined for its susceptibility to HIV-1. Three, 6, and 12 days after infection, samples of the cultures were evaluated for the presence of the viral protein p24 (A) and the viral “LTR/gag” genomic fragment (B). For a positive control in the polymerase chain reaction assay, replicate samples of the isolated genomic DNA were evaluated for the presence of the humanβ-globin gene. Molecular weight standards (Std) are indicated on the right. MCs/basophils freshly isolated from a patient with an allergic drug reaction and a patient with asthma also were found to be susceptible to the BAL strain of HIV-1 (data not shown). In those latter 2 experiments, the sorted CD3−/CD4+/CD117+ in vivo–differentiated MCs/basophils were exposed to the retrovirus immediately after their purification from the peripheral blood. As assessed immunohistochemically, less than 1% of the cells in the varied sorted population used in these HIV-1 susceptibility studies expressed CD3 and only about 1.5% expressed CD68.
Although the MCs/basophils circulating in the blood of our patients with asthma and allergic drug reactions expressed CD4 and CXCR4, the viral strain NL4-3 was unable to infect these cells. It is possible that other CXCR4-utilizing strains of HIV-1 can infect human MCs/basophils. Nevertheless, the fact that no cell in the 4 analyzed cell preparations was susceptible to the NL4-3 strain of HIV-1 supports the conclusion that the viral DNA and protein in the BAL-treated cultures did not originate from a T-cell contaminant. Although it is unknown why the MCs/basophils are not susceptible to the NL4-3 strain, similar findings have been reported for a range of myeloid cells, including macrophages.33 55 It is possible that CD4 preferentially associates with CCR5 rather than CXCR4 on the surface of these cells, that the surface density of CXCR4 receptors is insufficient for efficient viral infection, or that CXCR4 is not functional as a viral coreceptor.
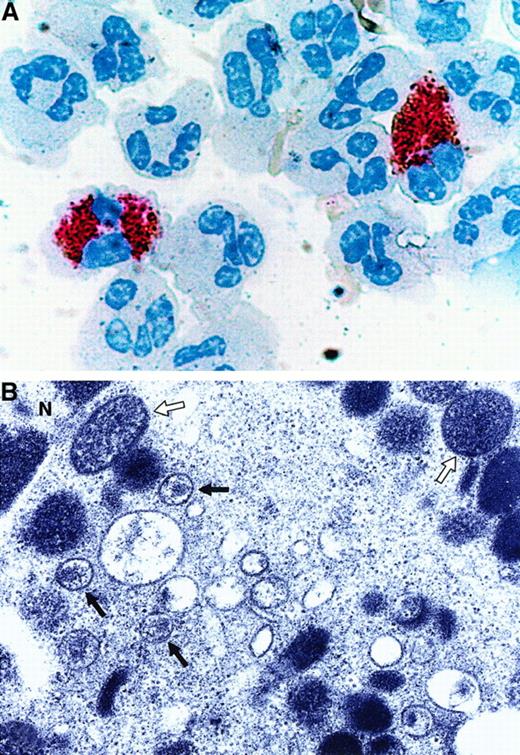
The finding that the in vivo–differentiated MCs/basophils in the blood of patients with asthma and allergic drug reactions are susceptible to an M-tropic strain of HIV-1 ex vivo raised the possibility that some AIDS patients might have HIV-1–infected MCs/basophils in their peripheral blood. The metachromatic cells in the blood of 11 patients were therefore examined to evaluate that possibility. As assessed immunohistochemically using a double-staining procedure, 7 of the 11 patients had p24+ cells in their blood. The failure to identify HIV-1–infected T cells and monocytes in the other 4 patients probably is a consequence of the low sensitivity of the immunohistochemical procedure, the variable stage of the disease in each patient, or the effectiveness of the drug treatments used to decrease the patient's viral load. Of the 7 p24+ patients, 6 had many p24+/tryptase+ MCs/basophils in their blood (Figure 5A). Tryptase+/p24+ MCs were found in the AIDS patient with allergic rhinitis and 2 of the 3 patients with AIDS and asthma. Ultrastructural studies revealed the presence of intact retrovirus in the MCs/basophils in the blood of the latter 2 patients (Figure 5B). Because no p24+ lymphocytes or monocytes were detected in 4 patients, the number of AIDS patients containing HIV-1–infected MCs/basophils probably is underestimated.
Retroviral analysis of the in vivo–differentiated MCs/basophils in the blood of patients with AIDS.
In the depicted experiment, the cells in the peripheral blood of a patient with both AIDS and asthma were stained with anti-HIV-1 p24 IgG (brown) followed by anti-tryptase IgG (pink) (A). Similar tryptase+/p24+ MCs/basophils were found in the blood of 5 other patients with AIDS. In the negative control experiments, immunoreactive p24 was not detected in the tryptase+ MCs/basophils present in 3 asthma patients that were not infected with HIV-1 (data not shown). Because the immunohistochemical data indicated the presence of viral protein, electron microscopy was carried out to evaluate whether or not the MCs/basophils in 2 of these patients contain intact retrovirus (B). The open and closed arrows in the depicted electron micrograph indicate secretory granules and intact retrovirus, respectively, in these MCs/basophils. N refers to the nucleus. Although certain populations of human eosinophils are susceptible to HIV-1 ex vivo,59 the depicted HIV-1–infected cell clearly is not an eosinophil, T cell, or macrophage based on its ultrastructure.
Retroviral analysis of the in vivo–differentiated MCs/basophils in the blood of patients with AIDS.
In the depicted experiment, the cells in the peripheral blood of a patient with both AIDS and asthma were stained with anti-HIV-1 p24 IgG (brown) followed by anti-tryptase IgG (pink) (A). Similar tryptase+/p24+ MCs/basophils were found in the blood of 5 other patients with AIDS. In the negative control experiments, immunoreactive p24 was not detected in the tryptase+ MCs/basophils present in 3 asthma patients that were not infected with HIV-1 (data not shown). Because the immunohistochemical data indicated the presence of viral protein, electron microscopy was carried out to evaluate whether or not the MCs/basophils in 2 of these patients contain intact retrovirus (B). The open and closed arrows in the depicted electron micrograph indicate secretory granules and intact retrovirus, respectively, in these MCs/basophils. N refers to the nucleus. Although certain populations of human eosinophils are susceptible to HIV-1 ex vivo,59 the depicted HIV-1–infected cell clearly is not an eosinophil, T cell, or macrophage based on its ultrastructure.
The biologic consequences of HIV-1–infected MCs/basophils in patients with AIDS remain to be determined. It has been difficult to eradicate HIV-1 in humans, in part because of its ability to infect long-lived cells such as resting CD4+ T cells.56 Certain populations of MCs and their progenitors are extremely long-lived,17 and the ability of HIV-1 to infect a MC/basophil that resides in the peripheral blood where the virus is present raises the possibility that these cells or their progenitors in some patients are another long-lived target for certain strains of the retrovirus. Phenotypically similar metachromatic cells have been found in the blood of patients who have experienced an allergic drug reaction.28 In this regard, it is of interest to note that individuals infected with HIV-1 experience a much higher frequency of allergic drug reactions than noninfected individuals.28 57
The presence of FcεRI on the surface of CD4+/CCR3+/CCR5+/CXCR4+human MCs/basophils raises the possibility that these cells will release tumor necrosis factor-α and other cytokines, lipid mediators, granule mediators, and infectious virus during an IgE-mediated allergic event that, in turn, exacerbates the infection. Equally, HIV-1–induced dysfunction of MCs or basophils could contribute to the frequency and severity of allergic drug reactions in the mucosa of AIDS patients before the infected cells are destroyed. Finally, the fact that MCs are so important in acquired and innate immunity raises the possibility that an HIV-1–induced dysfunction of CD4+/CCR3+/CCR5+/CXCR4+MCs/basophils contributes to the inability of patients with advanced AIDS to combat opportunistic infections.
Compared to normal humans, very few MCs are present in the intestinal mucosa of advanced patients with AIDS.58 The in vivo– differentiated MCs/basophils isolated from one patient died quickly after they became infected with HIV-1. Although it has been proposed that the reduced number of MCs in patients with advanced AIDS is a secondary effect caused by a depletion of CD4+ T cells that are producing MC-regulatory factors,58 our findings now suggest that the MC deficiency is primarily a direct result of HIV-1 infection of either the mature MCs in the jejunum or their progenitors.
Anti-FcεRI Ig was kindly provided by Dr J.-P. Kinet (Department of Pathology, Beth Israel Hospital, Boston, MA). We also thank Dr Daniel Friend (Brigham and Women's Hospital, Boston, MA) for his comments.
Supported by grants from the National Health and Medical Research Council (Australia), the Clive and Vera Ramaciotti Foundation (Australia), and the Australian National Centre for HIV Research; and by grants AI-23483, HL-36110, and HL-63284 from the National of Institutes of Health (United States).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Steven A. Krilis, Department of Immunology, Allergy, and Infectious Diseases, St George Hospital, 2 South St Centre, Kogarah, NSW 2217, Australia; e-mail:s.krilis@unsw.edu.au; or Richard L. Stevens, Department of Medicine, Brigham and Women's Hospital, Smith Bldg, Rm 616B, 1 Jimmy Fund Way, Boston, MA 02115; e-mail:rstevens@rics.bwh.harvard.edu.

![Fig. 1. TMT and FcεRI expression. / Nonsorted, freshly isolated cells from asthma patient 1 (A,B) and asthma patient 2 (C-E) were stained with antihuman TMT IgG alone (A,C). Cells from patient 1 were also sequentially stained with anti-TMT IgG (brown) and then with an antibody that recognizes human tryptases α, βI, βII, and βIII (pink) (B). Cells from patient 2 were sequentially stained with anti-TMT IgG (brown [D] or pink [E]) and then with either antichymase IgG (pink) (D) or anti-FcεRI IgG (brown) (E). Note that the metachromatic cells in the blood of both patients express a number of the granule proteases that normal, mature MCs express, but nearly all of these cells possess segmented nuclei like normal blood basophils. Magnification ∼×400.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/11/10.1182_blood.v97.11.3484/6/m_h81111135001.jpeg?Expires=1769103195&Signature=rpwIZKYgLtZUeJaZWFTM2VdBnWqPUjViq09tOyppzp~OhRqEnij2FlkkP69NbcBOOIB5zeWbavP-Q8O7wRWjjO6smGfH4-nqrxYMdRiV8MXcBjMhloYv1khh4mCfhERg~JTgMI6eTgXXvuaCyC8JNmdyqh0hr3AXMv8eciTu9u8g3XwRDDceRcpIRxSrP9WqmEmnMx5H6ddPEfj8Ezhi-ZPoeZ6Xs~Yh69ZaiajmX6stt4XzkcHLDvr6HJJY5el5VStcAU3No6MjqlUPJH~akxja2pz5IRN3OV2omrv8A5~aelpA5bhXJ-hjryWRUxO4oG0FmV2kWKJy0yIecXDN2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal