Cryoglobulin activity associated with murine immunoglobulin G3 (IgG3) has been shown to play a significant role in the development of murine lupuslike glomerulonephritis. A fraction, but not all, IgG3 monoclonal antibodies are capable of inducing a severe acute lupuslike glomerulonephritis as a result of direct localization of IgG3 cryoglobulins, suggesting the importance of qualitative features of cryoglobulins in their nephritogenic activities. Here a remarkable difference is shown in the renal pathogenicity of 2 murine IgG3 monoclonal cryoglobulins, identical in the amino acid sequences of their heavy and light chains but different in galactosylation patterns of oligosaccharide side chains because of their synthesis in different myeloma cells. The antibody lacking the capacity to induce severe glomerulonephritis displayed an increased proportion of galactosylated heavy chains. Changes in conformation, as revealed by gel filtration analysis, reduced cryoglobulin activity, and accelerated clearance could account for the lack of the renal pathogenicity of the more galactosylated variant. This observation provides a direct demonstration for the role of IgG galactosylation in the pathogenic potential of cryoglobulins.
Introduction
Pathologic cryoglobulinemia occurs in lymphoproliferative disorders, auto-immune diseases such as systemic lupus erythematosus and rheumatoid arthritis, and various chronic infectious diseases.1 2 Conventionally, cryoglobulins are classified as type 1 or type 2; type 1 cryoglobulins are usually monoclonal immunoglobulin M (IgM) or IgG; type 2 cryoglobulins are rheumatoid factors (RF) that form cryoprecipitating complexes with polyclonal IgG. The presence of these cryoglobulins could result in a wide range of vascular, renal, and neurologic complications. However, the precise cellular and molecular mechanisms involved in the induction of the cryoglobulin-associated disease have not been well defined.
Murine IgG3 is able to self-associate as a result of nonspecific IgG3 Fc-Fc interaction, and most IgG3 monoclonal antibodies (mAbs) generate cryoglobulins independently of their specificities.3,4Variable and constant regions are likely to contribute to this property, as shown by the influence of charged residues in the variable region of IgG3 antibodies.5,6 With regard to auto-immune cryoglobulin-associated disease, a fraction of IgG3 monoclonal auto-antibodies, such as anti-IgG2a RF and anti-DNA—derived from lupus-prone mice—are highly nephritogenic, generating wire loop–like glomerular lesions characteristically described in human lupus nephritis.3,7-10 However, the development of identical glomerular lesions by IgG3 RF monoclonal cryoglobulins in Ig-deficient mice lacking the corresponding auto-antigens11,12 and the absent development of these lesions by IgM and IgG1 switch variants of the same mAb13,14 indicate that the direct localization of IgG3 monoclonal cryoglobulins without the involvement of immune complex formation is responsible for the development of such lesions. The fact that certain IgG3 monoclonal cryoglobulins fail to induce significant glomerular lesions further suggests that qualitative features of cryoglobulins are critical in the nephritogenic activity of IgG3 cryoglobulins. It can be speculated that a unique sequence present in the immunoglobulin variable regions, as a result of either a particular combination of heavy and light chains or of somatic mutations, may confer the nephritogenic activity of IgG3 cryoglobulins.15
Alternatively, possible differences in the structure of carbohydrate side chains present in the CH2 domain of IgG may have a determining role for their nephritogenic activities. Asparagine-linked bi-antennary complex-type oligosaccharide chains attached there are thought to stabilize the IgG molecule and to contribute to various effector functions associated with the Fc regions, such as complement activation and Fc receptor interaction.16,17 Each of these oligosaccharide chains, most of which are nonsialylated and neutral, ends by 2 terminal galactose residues, one terminal galactose and one terminal N-acetylglucosamine or 2 terminalN-acetylglucosamines.18 It has been shown that in patients with rheumatoid arthritis, the proportion of IgG lacking galactose is significantly increased, in correlation with the progression of the disease.18,19 The same pattern is observed in lupus-prone MRL-lpr/lpr mice, which spontaneously develop glomerulonephritis,20,21 in which IgG3 cryoglobulin formation correlates with the development of the disease.22-24 In a murine model of collagen-induced arthritis, in vitro treatment of polyclonal IgG molecules (containing anticollagen antibodies) with β-galactosidase enhanced their arthritogenic activity,25 suggesting that the level of galactosylation of IgG autoantibodies may be related to their pathogenicity.
To explore more directly the possible relation between the pathogenic potential of murine IgG3 cryoglobulins and their patterns of glycosylation, we have generated by 2 different means (cell fusion and transfection) 2 cell lines secreting hybrid IgG3 mAb made of the same γ3 heavy chains (derived from 6-19 anti-IgG2a RF) with potentially different glycosylation pattern (because of their synthesis in different myeloma cells) and of the λ1 light chains from J558 anti–α1-3 dextran antibodies. The assessment of renal pathogenicity of these 2 mAbs, identical in the amino acid sequence of their heavy and light chains, revealed that the lack of the pathogenic activity by one of these IgG3 hybrid mAbs was associated with an increased level of galactosylation, which apparently provoked conformational changes of IgG3 molecules.
Materials and methods
DNA constructions
The LVDJH6-19–Cγ3 plasmid containing the complete 6-19 IgG3 heavy-chain gene was constructed using the following DNA fragments: the rearranged LVDJ (leader-variable-diversity-joining) region isolated from complementary cDNA (cDNA) encoding the heavy chain of the 6-19 mAb,11 the promoter region isolated from pSV-Vμ1,26 the heavy-chain enhancer region isolated from pSVE2-neo (a kind gift of Dr K. Rajewsky, Cologne, Germany), and the Cγ3 region derived from the genomic clone pJW7.27
Monoclonal antibodies
The L8D mAb made of γ3 heavy chains from 6-19 anti-IgG2a RF mAb and λ1 light chains from J558 anti–α1-3 dextran antibodies was obtained after the fusion of 6-19 hybridoma cells (derived from MRL-lpr/lpr mice)3 with J558L heavy-chain loss mutant myeloma cells (derived from BALB/c mice),28 as described previously.11 The 6-19J mAb made of the same heavy and light chains was generated as follows: J558L cells were transfected by electroporation with the LVDJH6–19-Cγ3 plasmid, together with a plasmid containing the hygromycin-resistant gene. After selection for resistance to hygromycin and secretion of IgG3 antibodies, transfected cells secreting IgG3 were cloned by limiting dilutions.
Reverse transcriptase–polymerase chain reaction and cDNA sequencing
RNA was prepared from cultured cell lines by Rneasy Mini kit (Qiagen AG, Basel, Switzerland). The first strand of cDNA was synthesized with an oligo(dT) primer and total RNA. For amplification with Taq DNA polymerase (Boehringer Mannheim, Mannheim, Germany), the following primers were used: Vκ6-19 leader primer (5′-GTTGCCTCCTCAAATG-3′) and Cκ primer (5′-TGGATGGTGGGAAGATG-3′) for the 6-19 κ light chain; Vλ1 J558 leader primer (5′-TCTCCTGGCTCTCAGCTCAG-3′) and Cλ1 primer (5′-CTTCAGAGGAAGGTGGAAACAGGGTG-3′) for the J558 λ1 light chain; VH1 back primer (5′-AGGTSMARCTGCAGSAGTCWGG-3′)29 and Cγ3-CH1 primer (5′-CGATAGACAGATGG-3′) for the 6-19 γ3 heavy chain. Polymerase chain reaction products were visualized after electrophoresis through 2% agarose gels by staining with ethidium bromide.
For the nucleotide sequencing of the entire variable and constant regions for the heavy and light chains of the L8D and 6-19J mAb, cDNA was amplified with Pfu DNA polymerase (Stratagene Cloning Systems, La Jolla, CA), by using the following pairs of oligonucleotides: 3′-UT VH primers (5′-CACTGACTTTCACCATG-3′ or 5′-CAGTTCTCTCTACAGTTA-3′) and 3′-UT Cγ3 primer (5′-GACCCGAGGAATGGC-3′) for the 6-19 γ3 heavy chain; Vλ1 J558 leader primer and 3′-UT λ1 primer (5′-TTCTGATCTCAGCCTCTGTG-3′) for the J558 λ1 light chain. The nucleotide sequence corresponding to the variable and constant regions of the 6-19 γ3 heavy chain and of the J558 λ1 light chain was determined by the dideoxynucleotide chain termination method.30
In vivo studies
To study the pathogenicity of IgG3 mAb, pristane-treated (MRL × BALB/c)F1 mice were injected intraperitoneally with 107 L8D or 6-19J cells and killed when moribund. Kidneys were obtained at autopsy, processed for histologic examination, and stained with periodic acid Schiff (PAS) or with fluorescein isothiocyanate-conjugated rabbit antimouse IgG. Serum cryoglobulin activities were determined as described.8 To determine the clearance of L8D or 6-19J mAb, 0.2 mL (0.5 mg) each mAb was injected intravenously into immunoglobulin-deficient μMT C57BL/6 mice31 to facilitate the quantification of the injected mAb. Mice were bled at serial time points after the injection. Serum concentrations of L8D and 6-19J mAb were determined by enzyme-linked immunosorbent assay (ELISA). The amount of each mAb remaining in blood was calculated relative to the value 1 minute after the injection.
ELISA
IgG3 concentrations in culture supernatants, sera, or cryoglobulins were quantitated by ELISA as described.8 For the detection of IgG3 bearing λ1-chains, κ-chains, or 6-19 idiotype (Id), various concentrations of purified mAb (1-10 μg/mL) were added to microtiter wells coated with a rat antimouse γ3-chain mAb, H139.61.1.32 Assays were developed with antimouse λ1-chain, LS-136,33 antimouse κ-chain, H139.52.1,32 or anti–6-19 Id34 mAb conjugated with alkaline phosphatase, as described previously.11 Anti-IgG2a RF activities were determined by ELISA as described.8
Analysis of oligosaccharide structures
IgG3 mAb or polyclonal IgG was purified from cultured supernatants or sera from C3H mice by protein A column chromatography, followed by fast-performance liquid chromatography (FPLC) using a Superdex 200 pg column (2.6 × 60 cm; Pharmacia, Uppsala, Sweden) equilibrated with 50 mM sodium phosphate buffer (pH 7.2) containing 0.15 M NaCl. The purity of IgG samples thus obtained was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Ouchterlony double-immunodiffusion assay. Purified IgG samples were then subjected to gas-phase hydrazinolysis for 3 hours at 90°C using Hydraclub S204 (Honen, Tokyo, Japan) followed byN-acetylation to quantitatively liberate N-linked oligosaccharides.35-37 The oligosaccharides liberated from the IgG samples were purified by high-performance liquid chromatography (HPLC) using a Shodex RSpak DC-613 column (0.6 × 15 cm; Showa Denko, Tokyo, Japan) and then labeled with P-aminobenzoic acid ethyl ester (ABEE; Nacalai Tesque, Kyoto, Japan) by reductive amination according to the method previously described.38 The ABEE-labeled oligosaccharides were subjected to HPLC using a Cosmogel diethylaminoethyl (DEAE) column (0.75 × 7.5 cm; Nacalai Tesque). The column was equilibrated with 0.5 mM sodium acetate, and the elution was performed by a linear gradient to a final concentration of 150 mM sodium acetate over 30 minutes at a flow rate of 1 mL/min at room temperature. ABEE-oligosaccharides from the IgG samples were separated into neutral, monosialo, and disialo oligosaccharide fractions.
Neutral oligosaccharide mixtures obtained by sialidase treatment of ABEE-oligosaccharide fraction were subjected to HPLC using a Wakosil 5C18-200 octadecylsilane (ODS) column (0.46 × 25 cm; Wako Pure Chemical Industries, Kyoto, Japan). A mixture of solvent A (5% acetonitrile containing 50 mM acetic acid) and solvent B (15% acetonitrile containing 50 mM acetic acid) was used for the elution. The column was equilibrated with a mixture of solvents A:B, 70:30 (vol/vol), and, after injection of the sample, the elution was performed using a linear gradient to solvent A:B, 40:60 (vol/vol) over 60 minutes at a flow rate of 0.8 mL/min at 60°C. Desialylated ABEE-oligosaccharides were separated into 8 oligosaccharide fractions eluted at the same position as a series of authentic bi-antennary complex-type oligosaccharides.
Mannose-binding lectin-binding assay
Human mannose-binding lectin (MBL) was purified from human sera, as described previously.39 To determine the MBL-binding by IgG in vitro, 5 μg IgG3 mAb in 0.1 mL binding buffer (50 mM Tris-HCl buffer, pH 7.4, containing 0.15 M NaCl and 50 mM CaCl2) were dot-blotted on a polyvinylidene difluoride membrane (Bio-Rad Laboratories, CA). Then the membrane was heated at 100°C for 10 minutes, blocked with binding buffer containing 3% bovine serum albumin for 2 hours at room temperature, and incubated with sodium iodide (I125)–labeled MBL (3 × 105 cpm/mL binding buffer containing 0.1% bovine serum albumin) for 2 hours at 4°C. After the membrane was washed, binding of MBL to IgG sample dots was assessed by autoradiography as described previously.40 41
Results
Differences in pathogenic activities of the L8D and 6-19J IgG3 mAbs identical in the amino acid sequences of their heavy and light chains
To obtain cell lines secreting mAbs made of the same 6-19 γ3 heavy chains with potentially different glycosylation pattern, 2 approaches were used: (1) fusion of 6-19 RF hybridoma cells (derived from MRL-lpr/lpr mice) with mutant myeloma cells (J558L) secreting only λ1 light chains (derived from BALB/c mice), resulting in the L8D cell line11; (2) transfection of the J558L myeloma cell line with a plasmid containing the LVDJH6–19-Cγ3 DNA sequence, resulting in the 6-19J cell line. Both L8D and 6-19J cloned cell lines were selected for the secretion of comparable amounts (approximately 10 μg/mL) of hybrid antibodies made only of 6-19 γ3 heavy chains and J558 λ1 light chains. As a consequence of light-chain replacement, both hybrid mAbs lost 6-19 RF activity and were no longer recognized by an anti–6-19 anti-Id mAb (Figure1A). Notably, κ-chain–specific messenger RNA (mRNA) was undetectable in L8D and 6-19J cells, as assessed by amplifying cDNA with Vκ6-19– and Cκ-specific primers (Figure 1B). Furthermore, cDNA nucleotide sequence analysis of the entire variable and constant regions for the heavy and light chains of the L8D and 6-19J mAbs revealed a complete identity (data not shown) corresponding to the published nucleotide or amino acid sequences of the 6-19 γ3 heavy chain and the J558 λ1 light chain.11,27,42 43 This indicates that these 2 antibodies are identical in the amino acid sequences of their heavy and light chains
Expression of λ1 and κ light chains, 6-19 Id, and anti-IgG2a RF activities in L8D, 6-19J, and 6-19 IgG3 mAbs and expression of 6-19 κ, J558 λ1, and 6-19 γ3 mRNA in 6-19, L8D, and 6-19J cells.
(A) For the detection of IgG3 bearing λ1, κ, or 6-19 Id, purified L8D (■), 6-19J (▨), and 6-19 (▪) mAb (10 μg/mL) were added to microtiter wells coated with rat antimouse γ3 mAb. Assays were developed with anti-λ1, anti-κ, or anti–6-19 Id mAb conjugated with alkaline phosphatase, as described previously.11 Anti-IgG2a RF activities were determined by ELISA as described.8 Results are expressed as OD at 405 nm. (B) The presence of κ-, λ1-, and γ3-specific mRNA was examined by amplifying cDNA made from the cultured cell lines with 6-19 κ, J558 λ1, and 6-19 γ3-specific primers. Polymerase chain reaction (PCR) products were visualized after electrophoresis through 2% agarose gels by staining with ethidium bromide. Lane 1, 6-19; lane 2, L8D; lane 3, 6-19J; lane 4, PCR mix without cDNA. M, molecular weight marker (DNA molecular weight marker VI; Boehringer Mannheim).
Expression of λ1 and κ light chains, 6-19 Id, and anti-IgG2a RF activities in L8D, 6-19J, and 6-19 IgG3 mAbs and expression of 6-19 κ, J558 λ1, and 6-19 γ3 mRNA in 6-19, L8D, and 6-19J cells.
(A) For the detection of IgG3 bearing λ1, κ, or 6-19 Id, purified L8D (■), 6-19J (▨), and 6-19 (▪) mAb (10 μg/mL) were added to microtiter wells coated with rat antimouse γ3 mAb. Assays were developed with anti-λ1, anti-κ, or anti–6-19 Id mAb conjugated with alkaline phosphatase, as described previously.11 Anti-IgG2a RF activities were determined by ELISA as described.8 Results are expressed as OD at 405 nm. (B) The presence of κ-, λ1-, and γ3-specific mRNA was examined by amplifying cDNA made from the cultured cell lines with 6-19 κ, J558 λ1, and 6-19 γ3-specific primers. Polymerase chain reaction (PCR) products were visualized after electrophoresis through 2% agarose gels by staining with ethidium bromide. Lane 1, 6-19; lane 2, L8D; lane 3, 6-19J; lane 4, PCR mix without cDNA. M, molecular weight marker (DNA molecular weight marker VI; Boehringer Mannheim).
Intraperitoneal implantation of L8D cells into (MRL × BALB/c)F1 mice induced a rapid increase in serum levels of IgG3 within 4 days (1.3 ± 0.8 mg/mL), reaching 3 to 5 mg/mL at approximately 7 days. By day 10, all 8 mice developed severe acute glomerulonephritis similar to that induced by the original 6-19 RF hybridoma cells (Table1, Figure2A). In contrast, in mice inoculated with 6-19J cells, increases in serum IgG3 were much slower and only detectable on day 7 (0.9 ± 0.7 mg/mL). Nevertheless, within 2 to 3 weeks, serum levels of IgG3 became nearly comparable (2-4 mg/mL) to those seen in mice 7 days after the injection of L8D cells (Table 1). Strikingly, none of the 6-19J–injected mice developed appreciable glomerular lesions when assessed approximately 3 weeks after the implantation of the cells (Table 1, Figure 2B). Significantly, the amount of serum cryoglobulins, as determined at the time of euthanasia (8-10 days and approximately 3 weeks after the injection of L8D and 6-19J cells, respectively), was lower in mice injected with 6-19J cells than in L8D-injected mice (Table 1). The lower cryoglobulin activity of the 6-19J mAb was confirmed by the analysis on purified mAb. After overnight incubation of 1 mL purified mAb (1 mg/mL) at 4°C, only one third of the cryoprecipitates were recovered after centrifugation of the 6-19J mAb (12 μg), compared with the L8D mAb (43 μg). An essential difference in the pathogenic property of the L8D and the 6-19J mAb was further confirmed by the observation of IgG deposits within glomeruli of mice injected 24 hours earlier with 10 mg L8D mAb, whereas only minimal glomerular deposits were seen in mice injected with the 6-19J mAb (Figure 2C-D), though this dose of the L8D mAb was not sufficient to provoke glomerular inflammation (data not shown).
Differences in renal pathogenic activity between L8D and 6-19J monoclonal antibodies
| Antibody* . | IgG3* . | Cryoglobulin* . | Kidney lesions† . |
|---|---|---|---|
| L8D | 3.8 ± 1.6 | 14.6 ± 6.8 | 8/8 |
| 6-19J | 3.4 ± 2.1 | 4.1 ± 2.7 | 0/17 |
| Antibody* . | IgG3* . | Cryoglobulin* . | Kidney lesions† . |
|---|---|---|---|
| L8D | 3.8 ± 1.6 | 14.6 ± 6.8 | 8/8 |
| 6-19J | 3.4 ± 2.1 | 4.1 ± 2.7 | 0/17 |
IgG indicates immunoglobulin G.
107 cells secreting L8D or 6-19J IgG3 mAb were inoculated intraperitoneally into (MRL × BALB/c)F1 mice. L8D- and 6-19J–injected mice were killed 8 to 10 days and approximately 3 weeks later, respectively, for serologic and histologic analysis. Serum levels of IgG3 (mg/mL) and cryoglobulins (μg/mL), measured at euthanasia, are shown (means ± 1 SD). Serum levels of IgG3 and cryoglobulins before the injection of hybridoma cells were 0.4 ± 0.2 mg/mL and < 0.1 μg/mL, respectively.
Incidence of glomerulonephritis. When the severity of glomerular lesions was semiquantitatively evaluated on a 0 to 4+ scale, mice injected with L8D cells developed very severe lesions, the mean was 3.1 ± 0.5. In contrast, none of the mice injected with 6-19J cells developed significant glomerular lesions.
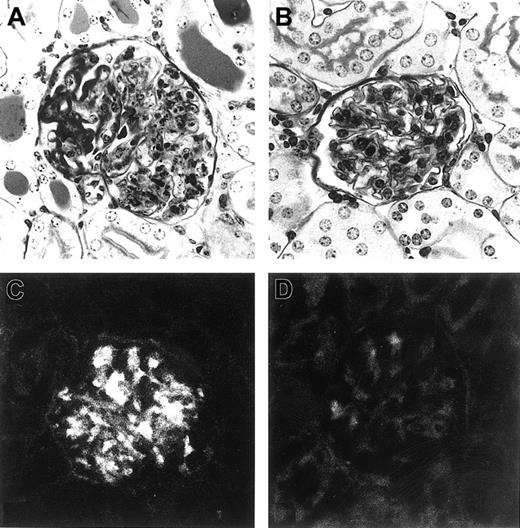
Representative histologic appearance of glomeruli in mice injected with L8D or 6-19J cells and glomerular IgG deposits in mice injected with purified L8D or 6-19J IgG3 mAb.
Mice injected with L8D cells (A) developed severe glomerular lesions characterized by the massive deposition of PAS-positive materials along the glomerular capillary walls, corresponding to the “wire-loop” lesions, with some proliferative and exudative changes, whereas no significant glomerular changes were seen in 6-19J–injected mice (B) (PAS; magnification, 200×). Note substantial glomerular deposits of IgG 24 hours after intraperitoneal injection of 10 mg L8D mAb (C), which markedly contrast with the lack of deposits in mice injected with the same dose of 6-19J mAb (D) (magnification, 200 ×).
Representative histologic appearance of glomeruli in mice injected with L8D or 6-19J cells and glomerular IgG deposits in mice injected with purified L8D or 6-19J IgG3 mAb.
Mice injected with L8D cells (A) developed severe glomerular lesions characterized by the massive deposition of PAS-positive materials along the glomerular capillary walls, corresponding to the “wire-loop” lesions, with some proliferative and exudative changes, whereas no significant glomerular changes were seen in 6-19J–injected mice (B) (PAS; magnification, 200×). Note substantial glomerular deposits of IgG 24 hours after intraperitoneal injection of 10 mg L8D mAb (C), which markedly contrast with the lack of deposits in mice injected with the same dose of 6-19J mAb (D) (magnification, 200 ×).
Different levels of galactosylation in the L8D and 6-19J IgG3 mAbs
To determine whether the observed different pathogenic activities of the L8D and 6-19J mAbs can be attributed to a possible structural difference in the carbohydrate side chains present in the CH2 domain, the oligosaccharide structures liberated from the 2 mAbs were compared. Analysis by DEAE-HPLC showed comparable molar ratios of neutral asialo (N), acidic monosialo (A1), and acidic disialo (A2) oligosaccharides, and a very large majority of neutral nonsialylated oligosaccharides (Table 2). Neutral oligosaccharides obtained after sialidase treatment of total oligosaccharide fractions were then analyzed by ODS-HPLC. No significant differences in the level of fucosylation of the 3 mAbs were found; fucosylated oligosaccharides amounted to more than 90% of total oligosaccharides (Figure3). In contrast, differences in galactosylation were observed. The content in nongalactosylated oligosaccharides (G0; no galactose, terminating inN-acetylglucosamine) was higher with the pathogenic L8D mAb than with the nonpathogenic 6-19J mAb, whereas the content of digalactosylated oligosaccharides (G2; 2 terminal galactose residues) was higher in 6-19J mAb than in L8D mAb (Figure 3, Table 2). Consequently, the ratio of the galactosylated (G1 + G2) versus nongalactosylated (G0) oligosaccharides was 2 times higher in the nonpathogenic 6-19J mAb than in the pathogenic L8D mAb. This ratio of 6-19J mAb was also greater than that found in polyclonal IgG isolated from nonautoimmune C3H mice (Table 2).
Structural analysis of N-linked oligosaccharide chains purified from 6-19J and L8D immunoglobulin G3 monoclonal antibodies and from serum polyclonal immunoglobulin G of C3H mice
| IgG . | Sialylated glycoforms* . | Galactosylated glycoforms† . | |||||
|---|---|---|---|---|---|---|---|
| N (%) . | A1 (%) . | A2 (%) . | G0 (%) . | G1 (%) . | G2 (%) . | G1 + G2 G0 . | |
| 6-19J | 92.4 | 7.4 | 0.2 | 21.9 | 55.4 | 22.7 | 3.57 |
| L8D | 92.3 | 6.5 | 1.2 | 36.4 | 49.3 | 14.3 | 1.75 |
| C3H | 90.3 | 6.8 | 2.9 | 33.3 | 46.6 | 20.1 | 2.00 |
| IgG . | Sialylated glycoforms* . | Galactosylated glycoforms† . | |||||
|---|---|---|---|---|---|---|---|
| N (%) . | A1 (%) . | A2 (%) . | G0 (%) . | G1 (%) . | G2 (%) . | G1 + G2 G0 . | |
| 6-19J | 92.4 | 7.4 | 0.2 | 21.9 | 55.4 | 22.7 | 3.57 |
| L8D | 92.3 | 6.5 | 1.2 | 36.4 | 49.3 | 14.3 | 1.75 |
| C3H | 90.3 | 6.8 | 2.9 | 33.3 | 46.6 | 20.1 | 2.00 |
Molar ratios of neutral asialo (N), acidic monosialo (A1), and acidic disialo (A2) oligosaccharides of IgG3 mAb and polyclonal IgG purified from 4-month-old C3H mice.
Molar ratios of nongalactosylated (G0), monogalactosylated (G1), and digalactosylated (G2) glycoforms in desialylated oligosaccharides of monoclonal and polyclonal IgG.
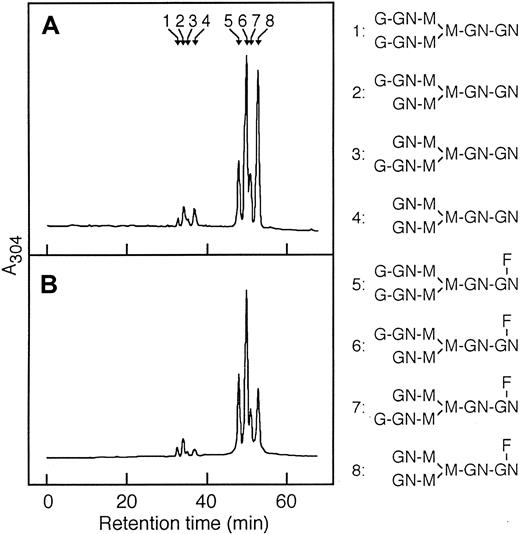
Separation of desialylated oligosaccharides of L8D and 6-19J IgG3 mAb by ODS-HPLC.
Desialylated ABEE-oligosaccharides of L8D (A) and 6-19J (B) IgG3 were separated into 8 oligosaccharide fractions that were eluted as a series of authentic bi-antennary complex-type oligosaccharide structures (positions 1 to 8) of ±Galβ1-4GlcNAcβ1-2Manα1-6(± Galβ1-4GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-4(± Fucα1-6)GlcNAc shown in the right column. Peaks 1 and 5 correspond to digalactosylated oligosaccharide chains with or without a fucose residue (G2). Peaks 2, 3, 6, and 7 correspond to monogalactosylated oligosaccharide chains with or without a fucose residue (G1). Peaks 4 and 8 correspond to nongalactosylated oligosaccharide chains with or without a fucose residue (G0). G and Gal, galactose; GN and GlcNAc,N-acetylglucosamine; M and Man, mannose; F and Fuc, fucose.
Separation of desialylated oligosaccharides of L8D and 6-19J IgG3 mAb by ODS-HPLC.
Desialylated ABEE-oligosaccharides of L8D (A) and 6-19J (B) IgG3 were separated into 8 oligosaccharide fractions that were eluted as a series of authentic bi-antennary complex-type oligosaccharide structures (positions 1 to 8) of ±Galβ1-4GlcNAcβ1-2Manα1-6(± Galβ1-4GlcNAcβ1-2Manα1-3)Manβ1-4GlcNAcβ1-4(± Fucα1-6)GlcNAc shown in the right column. Peaks 1 and 5 correspond to digalactosylated oligosaccharide chains with or without a fucose residue (G2). Peaks 2, 3, 6, and 7 correspond to monogalactosylated oligosaccharide chains with or without a fucose residue (G1). Peaks 4 and 8 correspond to nongalactosylated oligosaccharide chains with or without a fucose residue (G0). G and Gal, galactose; GN and GlcNAc,N-acetylglucosamine; M and Man, mannose; F and Fuc, fucose.
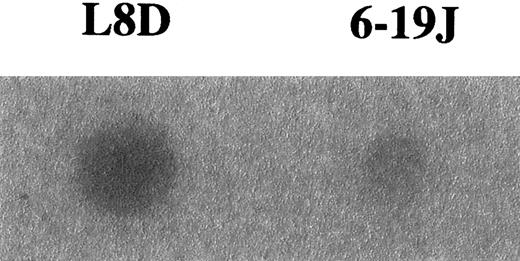
To confirm differences in the level of galactosylation between the L8D and 6-19J mAbs, we assessed the capacity of these mAbs to bind MBL, which is able to preferentially bind nongalactosylated IgG by interacting with the terminal N-acetylglucosamine residues exposed by the lack of galactosylation.44-46 In good correlation with the results obtained by the biochemical analysis on oligosaccharides liberated from these 2 mAbs, the pathogenic L8D mAbs exhibited marked binding to MBL, whereas little binding was observed with the nonpathogenic 6-19J mAb (Figure4). It should be stressed, however, that significant binding to MBL was detectable only after the heat denaturation of the L8D mAb.
Binding of MBL to L8D and 6-19J IgG3 mAb.
Five micrograms purified mAb were adsorbed on a polyvinylidene difluoride membrane heated at 100°C for 10 minutes, incubated with125I-labeled MBL, and subjected to autoradiography for 24 hours for the detection of MBL binding.
Binding of MBL to L8D and 6-19J IgG3 mAb.
Five micrograms purified mAb were adsorbed on a polyvinylidene difluoride membrane heated at 100°C for 10 minutes, incubated with125I-labeled MBL, and subjected to autoradiography for 24 hours for the detection of MBL binding.
Changes in conformation and accelerated clearance of the more galactosylated 6-19J IgG3 mAb
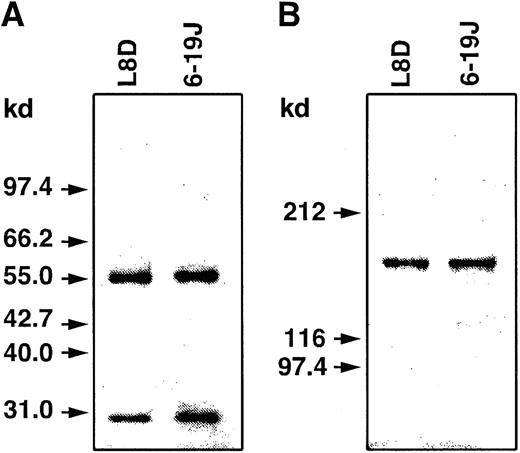
The observed increase in relative content of galactose residues in the oligosaccharide side chains present on the 6-19J heavy chains could result in conformational changes of the 6-19J molecules, thereby abolishing their nephritogenic activity. To explore this possibility, the 6-19J and L8D mAbs were subjected to Superdex (Pharmacia) 200 HR gel filtration chromatography. Both IgG3 mAbs eluted as single peaks. However, the 6-19J mAb eluted at an unusual lower molecular mass of 89.3 kd, in marked contrast to the L8D mAb exhibiting a size consistent with monomeric IgG (151.8 kd) (Figure5A). This difference was confirmed when both mAbs were simultaneously applied on gel filtration chromatography (Figure 5B). When analyzed by conventional nonreducing and reducing SDS-PAGE, the 6-19J mAb migrated as typical IgG, heavy and light chains of expected sizes, excluding the generation of an abnormal truncated form of heavy chains by the 6-19J cells and an aberrant assembly of 6-19J heavy and light chains (Figure 6). These results indicated that the 6-19J mAb apparently has a unique conformation with a smaller hydrodynamic volume than the L8D mAb and is thereby eluted as the aberrantly lower molecular mass material by gel filtration chromatography.
Elution profiles of 6-19J and L8D IgG3 mAbs on FPLC with a gel filtration column.
Twenty micrograms purified mAb was subjected to a Superdex 200 HR column (1 × 30 cm) equilibrated with 50 mM phosphate buffer (pH 7.4) containing 0.15 M NaCl, and elution was performed using the same buffer at a flow rate of 0.4 mL/min at 4°C with continuously monitored absorbance at 280 nm. (A) Solid line, L8D mAb; broken line, 6-19J mAb. (B) Mixture of L8D and 6-19J mAb. Arrows indicate elution positions of molecular weight markers: 1, human IgM (900 kd); 2, human IgG (150 kd); 3, human serum albumin (66 kd); 4, ovalbumin (45 kd).
Elution profiles of 6-19J and L8D IgG3 mAbs on FPLC with a gel filtration column.
Twenty micrograms purified mAb was subjected to a Superdex 200 HR column (1 × 30 cm) equilibrated with 50 mM phosphate buffer (pH 7.4) containing 0.15 M NaCl, and elution was performed using the same buffer at a flow rate of 0.4 mL/min at 4°C with continuously monitored absorbance at 280 nm. (A) Solid line, L8D mAb; broken line, 6-19J mAb. (B) Mixture of L8D and 6-19J mAb. Arrows indicate elution positions of molecular weight markers: 1, human IgM (900 kd); 2, human IgG (150 kd); 3, human serum albumin (66 kd); 4, ovalbumin (45 kd).
SDS-PAGE analysis of 6-19J and L8D IgG3 mAbs.
Purified mAbs were subjected to SDS-PAGE under reducing (A; 10% gel) or nonreducing (B; 6% gel) conditions. Proteins were stained with Coomassie Brilliant Blue R-250. Molecular size markers are indicated on the left.
SDS-PAGE analysis of 6-19J and L8D IgG3 mAbs.
Purified mAbs were subjected to SDS-PAGE under reducing (A; 10% gel) or nonreducing (B; 6% gel) conditions. Proteins were stained with Coomassie Brilliant Blue R-250. Molecular size markers are indicated on the left.
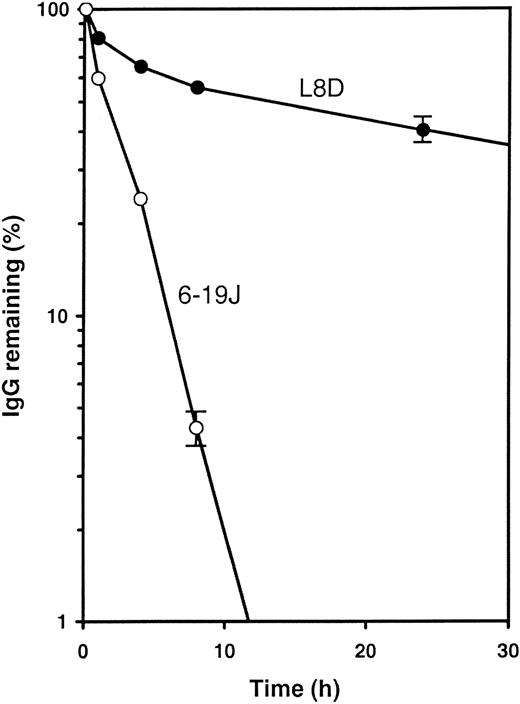
To analyze the stability of the aberrant form of the 6-19J mAb in vivo, we tested the clearance of the 6-19J and L8D mAbs in immunoglobulin-deficient mice after intravenous injection. As shown in Figure 7, serum levels of the L8D mAb were gradually diminished with time, whereas this process was markedly accelerated for the 6-19J mAb. Twenty-four hours after injection, the 6-19J mAb was hardly detectable (less than 1%), but approximately 40% of the L8D mAb was still present in the circulating blood. These results indicated that the 6-19J mAb was more rapidly eliminated in vivo than the L8D mAb.
In vivo clearance of 6-19J and L8D IgG3 mAbs.
Half a milligram each mAb (○, 6-19J; ●, L8D) was injected intravenously into immunoglobulin-deficient μMT mice (3 mice per group). Blood samples were collected at the time points indicated. The amount of each mAb remaining in blood was calculated relative to the value 1 minute after the injection. Error bars (± 1 SD) are shown only on last points; fractional errors of other points are similar or lower.
In vivo clearance of 6-19J and L8D IgG3 mAbs.
Half a milligram each mAb (○, 6-19J; ●, L8D) was injected intravenously into immunoglobulin-deficient μMT mice (3 mice per group). Blood samples were collected at the time points indicated. The amount of each mAb remaining in blood was calculated relative to the value 1 minute after the injection. Error bars (± 1 SD) are shown only on last points; fractional errors of other points are similar or lower.
Discussion
The present study was designed to define a possible role of IgG glycosylation in the pathogenic potential of murine IgG3 mAb with cryoglobulin activity. Our results have demonstrated a remarkable difference in pathogenic activities of 2 IgG3 mAbs, L8D and 6-19J, identical in the amino acid sequence of their heavy and light chains but different in galactosylation pattern because of their synthesis in different myeloma cells. The structural analysis of oligosaccharide side chains, the assessment of MBL-binding capacity, and the gel filtration analysis of these 2 mAbs indicate that the lack of the renal pathogenicity of the 6-19J mAb is associated with an increase in the relative content of galactose residues in the oligosaccharide side chains on their heavy chains, which apparently leads to conformational changes of the 6-19J molecules.
Analysis of the oligosaccharide structures of the pathogenic L8D and the nonpathogenic 6-19J IgG3 mAb has shown that the only difference is the level of galactosylation in neutral nonsialylated oligosaccharides. Glycosylation of the IgG3 mAb studied should only occur in the constant region of their heavy chains because of the lack of potential glycosylation sites in their VH and VLregions.11,42 Notably, in murine IgG3, there is an additional potential glycosylation site in its CH3 domain.27 The presence of the CH3 oligosaccharides has been suggested by a recent demonstration that the self-associating ability was significantly reduced in a murine IgG3 RF mutant mAb lacking the glycosylation site in the CH3 domain.47However, the interpretation of this result is still tentative because the observed difference could be due to the modification of amino acid sequence as a result of the mutation in the CH3 domain. Thus, it has not yet been formally proved whether murine IgG3 is indeed glycosylated in this domain. We did not find any significant differences between the total contents of oligosaccharides recovered from the 6-19J and L8D mAbs and those of IgG1 mAb lacking the CH3 potential glycosylation site (I.M. et al, unpublished data, May 2000), suggesting that the amounts of the CH3 oligosaccharide chains, if present, should be a minor component.
It should be mentioned that the galactosylation and gel filtration patterns of the 6-19J mAb are somehow unique because the relative content of galactose residues in their oligosaccharide chains is still higher than that seen in polyclonal IgG from nonautoimmune C3H mice and because the presence of IgG molecules with the smaller hydrodynamic volume has not been readily observed in sera. The presence of immunoglobulin molecules with the aberrant conformation resulting from increased galactosylation may occur more frequently. However, such IgG molecules may be hard to detect, probably because of their rapid elimination from the circulating blood. In fact, we observed a markedly accelerated clearance of the aberrant 6-19J mAb compared with the L8D mAb, which is consistent with a much slower increase in serum IgG3 levels in mice injected with 6-19J cells than in those with L8D cells. Thus, the increased galactosylation may be a mechanism that promotes the clearance and degradation of potentially more pathogenic antibodies, thereby avoiding deleterious immunopathologic consequences.
Essentially all the 6-19J mAbs, despite the presence of 6-19J molecules bearing differently galactosylated oligosaccharide chains, were uniformly cleared more quickly than the L8D mAb. This could be related to the fact that murine IgG3 self-associates and forms IgG3-IgG3 complexes.3,4 48 Consequently, fewer galactosylated 6-19J molecules could be cleared rapidly together with more galactosylated 6-19J molecules. Thus, relative contents of galactosylated oligosaccharide chains of IgG3 antibodies likely play a determining role in the in vivo clearance, and hence the nephritogenic potential, of IgG3 cryoglobulins.
What could be the mechanism(s) of the different pathogenicity for glomeruli between the L8D and the 6-19J IgG3 mAbs? The demonstration of an unusual lower molecular mass of the 6-19J mAb (a size of 89.3 kd) by gel filtration chromatography strongly suggests that an increased galactose content in the oligosaccharide chains of the 6-19J heavy chains leads to conformational changes, which are likely to be responsible for accelerated clearance in vivo. Consequently, such an aberrant form of the IgG3 antibodies may be less efficient at inducing their glomerular localization and the subsequent development of glomerular inflammation. In addition, this could, in part, be related to a reduced cryoglobulin activity of the 6-19J mAb, a property closely associated with the nephritogenic potential of IgG3 mAb.6,14,49 On the other hand, it has recently been suggested that the interaction of nongalactosylated IgG with MBL may promote the pathogenicity of IgG antibodies through complement activation.46 However, the lack of effect of C3 depletion on the development of glomerulonephritis by the 6-19 IgG3 mAb,50 which was undergalactosylated—like the L8D mAb (I.M. et al, unpublished observation, October 1999)—argues against such a possibility in this model of glomerulonephritis. In addition, the binding to MBL was only observed with heat-denatured L8D and 6-19 mAb but not with the native form. We cannot, however, exclude the possibility that a concentration-dependent self-association of IgG3 mAb, especially when displaying cryoglobulin activity, might promote this interaction. If so, it can be speculated that the IgG3-MBL complexes may be more efficiently phagocytosed by glomerular mesangial cells, the initial site of localization of pathogenic IgG3 mAb,7 and subsequent activation of mesangial cells might then lead to the generation of inflammatory cytokines, thereby provoking glomerular inflammation.
The observation that more galactosylated IgG has a lesser pathogenic potential raises an important question: what controls IgG galactosylation? It has been reported that B-lymphocytes from patients with rheumatoid arthritis and from MRL-lpr/lpr mice have a reduced galactosyltransferase activity,51-53 though this was not confirmed in another study.54 Our recent study has demonstrated a marked, but not complete, reduction in the proportion of galactosylated IgG in mice deficient in β-1,4–galactosyltransferase, indicating the involvement of additional galactosyltransferase(s) for IgG galactosylation (manuscript in preparation). In addition, we have observed an increased percentage of nongalactosylated IgG in MRL-lpr/lpr and MRL-gld/gld mice, but not in MRL-+/+ mice nor in C57BL/6 and C3H mice bearing thelpr or gld mutation (manuscript in preparation). It thus appears that a decrease in the level of galactosylated IgG, putatively resulting from a decreased level of galactosyltransferase in B cells, involves the lpr (Fas) and the gld (Fas ligand) mutations in combination with the MRL genetic background. These mutations probably result in increased lifespans of activated B cells because of functional defects in Fas-mediated apoptosis. In an auto-immune-prone genetic background, such as exists in MRL mice, the expansion of some autoreactive B-cell clones may be accompanied by a progressive decrease in galactosyltransferase activity of these aging cells, thus favoring the appearance of undergalactosylated auto-antibodies with immunopathologic consequences. If so, the dysregulated expression of galactosyltransferase involved in IgG galactosylation could be a significant genetic factor predisposing to the development of autoantibody-mediated tissue lesions. Clearly, further identification of the molecular basis of IgG galactosylation and elucidation of its pathogenic role would help in the understanding and development of new therapeutic approaches for auto-immune rheumatic diseases.
We thank Dr P. Vassalli for critically reading the manuscript and Ms G. Leyvraz and Mr G. Brighouse for their excellent technical help.
Supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture and from the Ministry of Health and Welfare of Japan, Proposal-Based New Industry Creative Type Technology R & D Promotion Program from the New Energy and Industrial Technology Development Organization of Japan, the Swiss National Foundation for Scientific Research, and the Association pour la Recherche sur le Cancer.
T.M., Y.P., and K.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shozo Izui, Department of Pathology, CMU, 1211 Geneva 4, Switzerland; e-mail: shozo.izui@medecine.unige.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal