Chronic granulomatous disease (CGD) is an inherited immunodeficiency in which the absence of the phagocyte superoxide-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase results in recurrent bacterial and fungal infections. A murine model of X-linked CGD (X-CGD) was used to explore variables influencing reconstitution of host defense following bone marrow transplantation and retroviral-mediated gene transfer. The outcomes of experimental infection with Aspergillus fumigatus, Staphylococcus aureus, orBurkholderia cepacia were compared in wild-type, X-CGD mice, and transplanted X-CGD mice that were chimeric for either wild-type neutrophils or neutrophils with partial correction of NADPH oxidase activity after retroviral-mediated gene transfer. Host defense to these pathogens was improved in X-CGD mice even with correction of a limited number of neutrophils. However, intact protection against bacterial pathogens required relatively greater numbers of oxidant-generating phagocytes compared to protection against A fumigatus. The host response also appeared to be influenced by the relative level of cellular NADPH oxidase activity, particularly forA fumigatus. These results may have implications for developing effective approaches for gene therapy of CGD.
Introduction
Chronic granulomatous disease (CGD) is a group of inherited disorders characterized by recurrent, often life-threatening suppurative infections as well as chronic inflammation with granuloma formation.1-4 The disease, which has an estimated incidence of 1 in 250 000 individuals, results from mutations in any one of 4 subunits of a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase found in neutrophils and other phagocytic leukocytes.1 5 This plasma and phagosomal membrane-associated enzyme generates large quantities of superoxide in the respiratory burst, the precursor to potent microbicidal oxidants that are an essential component of innate immunity. Approximately two-thirds of cases of CGD are due to mutations in an X-linked gene encoding gp91phox, the 91-kD subunit of flavocytochrome b558, a membrane heterodimer that is the redox center of the oxidase. Defects in the gene encoding the 22-kD subunit of flavocytochrome b558, p22phox, are found in a rare autosomal recessive form of CGD. Additional autosomal recessive cases of CGD involve mutations in either p47phox or p67phox, 2 soluble proteins that interact with flavocytochrome b558 to form the enzymatically active NADPH oxidase complex.
Patients with CGD have defective killing of many bacterial and fungal pathogens and typically present in infancy or childhood with recurrent, often difficult to treat, infections.1,4,6-8 Frequently involved sites include skin and its draining lymph nodes, lungs, bone, liver, and gastrointestinal tract. CGD patients are particularly susceptible to organisms that contain catalase, which eliminates microbe-generated hydrogen peroxide, which could otherwise be scavenged within the phagolysosome to promote microbial killing. Commonly isolated species include Staphylococcus aureus,Aspergillus species, and a variety of gram-negative bacilli, including Salmonella species, Serratia marcescens, and Burkholderia cepacia. Current management has improved the overall prognosis in CGD and includes the use of prophylactic antibiotics and interferon-γ, along with aggressive treatment of acute infections. However, morbidity secondary to infection or granulomatous complications remains significant for many patients, particularly in the X-linked form,6,9 and Mardiney et al10 have estimated that current overall mortality is approximately 2% per year.
CGD is a promising candidate disease for somatic gene therapy targeted at hematopoietic stem cells.11-13 All CGD cases studied to date result from single gene defects in one of the 4 genetic subgroups, and insertion of a functional copy of the affected gene into hematopoietic stem cells (HSCs) could reconstitute NADPH oxidase activity in circulating and tissue phagocytes to provide a life-long cure of the disease. Allogeneic bone marrow transplantation (BMT), although rarely used for CGD because of the potential complications of BMT, has been curative, even in a case in which mixed chimerism resulted in only 15% to 20% of circulating neutrophils being of donor origin.14-17 Additional insights into the relative level of enzymatic correction required to rescue the defect in host defense in CGD comes from the study of patients and their families. Observations of female carriers of X-linked CGD (X-CGD), who have a dimorphic population of both normal and oxidase-negative neutrophils, suggest that full restoration or respiratory burst activity to approximately 10% of circulating neutrophils could ameliorate the clinical manifestations of CGD.10,18-20 “Variant” patients with X-CGD with small amounts of residual NADPH oxidase activity (2% to 10% of normal) often have a milder clinical course,21-24 although some still develop significant infectious or granulomatous complications.21 25-28 Hence, only partial correction of superoxide generation may be insufficient to restore full microbicidal function to phagocytes. It is unknown how incomplete correction of superoxide-generating activity, as might occur if vector-driven expression of the missing phoxsubunit is less than normal, might influence the clinical benefit of HSC-targeted gene therapy when only a minority of phagocytes is corrected.
Gene targeting has been used to develop mouse models for both X-CGD and an autosomal recessive (p47phox−/−) form.29,30 Murine CGD appears to be a good model for the human disease, with defects in both host defense and inflammation that are similar to their human counterpart.10,29-31 For example, CGD mice exhibit an increased susceptibility to infection withS aureus, B cepacia, and A fumigatus. Retroviral-mediated gene transfer into murine CGD bone marrow (BM) cells has been successful in restoring NADPH oxidase activity to phagocytes in vivo and improving resistance to infection. In mice with X-CGD, correction of approximately 20% of cellular NADPH oxidase activity in approximately 50% of neutrophils was sufficient to prevent the development of A fumigatus pneumonia.32 In the p47phox-deficient mouse, transient correction of NADPH oxidase activity in approximately 3% of circulating neutrophils prolonged survival in a model of B cepacia sepsis.10 These were some of the first studies to demonstrate that gene therapy could improve the clinical symptoms of an inherited disorder.
The goal of the current study was to further examine determinants for correcting the defect in host defense in CGD using the murine model of X-CGD. The outcomes of experimental infection with A fumigatus, S aureus, or B cepacia were compared in wild-type, X-CGD mice, and transplanted X-CGD mice that were chimeric for either wild-type neutrophils or neutrophils with partial correction of NADPH oxidase activity after retroviral-mediated gene transfer. The results indicate that host defense to these pathogens was improved in X-CGD mice even with correction of a limited number of neutrophils. A substantially higher percentage of corrected neutrophils was required to improve host defense against both S aureus and B cepacia compared with A fumigatus. The relative cellular level of NADPH oxidase activity was an important determinant in the response to A fumigatus when a minority of neutrophils were corrected. These results may have important implications for developing effective approaches for gene therapy of CGD.
Materials and methods
Mice
X-CGD mice with a null allele for gp91phox had been generated by targeted disruption of the gp91phox locus in 129-SV murine embryonic stem cells.29 Both male and female X-CGD mice were used in this study and obtained from litters generated after 10 generations or more of backcrossing female carriers with wild-type C57Bl/6J males. Genotyping of mice was performed using polymerase chain reaction of tail blood and confirmed by nitroblue tetrazolium (NBT) testing of peripheral blood neutrophils.29 Mice were maintained under specific pathogen-free conditions and fed autoclaved food and acidified water. For controls, untransplanted X-CGD mice from our colony and wild-type C57Bl/6J mice from either our own colony or purchased from Jackson Laboratories (Bar Harbor, ME) were used.
Retroviral infection and BMT
Isolation, transduction with the MSCV-m91Neo retrovirus, and transplantation of murine X-CGD or wild-type BM cells were essentially as previously described.32 Lethally irradiated 8- to 10-week-old X-CGD recipients (Cesium 137, 11 Gy given as a split dose approximately 3 hours apart) were transplanted with either mixtures of mock-transduced and MSCV-m91Neo-transduced marrow (MSCV-m91Neo/X-CGD chimeras) or mixtures of wild-type and X-CGD marrow (wild-type/X-CGD chimeras). A total of 2 to 3 × 106 cells per recipient mouse were given by tail vein injection. NBT testing was performed on tail blood samples32 at regular intervals post-transplant to assess chimerism of oxidase-positive versus oxidase-negative neutrophils. A stable percentage of NBT-positive neutrophils was typically seen by 6 weeks post-transplant for wild-type/X-CGD chimeras and by 3 to 4 months post-transplant for MSCV-m91Neo/X-CGD chimeras. Mice were typically 4 to 7 months of age when challenged with bacteria or fungus in the experimental protocols outlined below. NBT tests on peripheral blood were obtained on at least 2 occasions within the 2 months prior to challenge, including one test within 2 weeks prior to challenge; using the protocols above, the percentage of NBT-positive neutrophils is stable after 2 months post-transplant.32,33The mean value was used as a measure of the percentage of NBT-positive neutrophils at the time of challenge. In some cases, peripheral blood counts and differentials were also obtained on tail blood samples within 2 weeks prior to challenge with the infectious agent.32 Where indicated, wild-type or X-CGD mice were used as controls, which in most cases were age matched for the transplanted mice under study.
A fumigatus infection
Mice were infected by intratracheal instillation of various numbers of A fumigatus conidia (spores) obtained from a clinical isolate (ATCC No. 90240; American Tissue Culture Center, Rockville, MD), as previously described.29,32 The number of conidia in the inoculum was confirmed by plate culture. As prophylaxis against secondary bacterial infection, mice were given intramuscular injection of ceftriaxone (Rocephin; Hoffman-La Roch, Nutley, NJ) 1.25 mg per animal immediately before infection and again 24 hours later, followed by oral tetracycline (Polyotic; American Cyanamid, Wayne, NJ) 5 mg/mL in the drinking water for the remainder of the experiment. Mice were examined daily and killed when either moribund or 17 to 21 days after fungal challenge. Lungs were fixed for histologic examination of paraffin-embedded sections stained with hematoxylin and eosin for assessment of pathologic changes and with Grocott methamine silver for assessment of hyphae. Findings used to score for A fumigatus lung disease, based on previous studies in murine X-CGD,31 32 included areas of purulent bronchopneumonia, granulomas with mixed inflammatory cell infiltrate, and presence of hyphae or abscesses.
S aureus infection
Mice were injected intraperitoneally with 0.2 mL suspension of 1 × 108/mL S aureus strain 502A (ATCC No. 27217; ATCC), as previously described.29 The number of bacteria in the inoculum was confirmed by plate culture of serial dilutions. Mice were examined daily and killed 7 days after peritoneal challenge. The presence of Staphylococcal intraperitoneal abscesses was assessed by visual inspection, and the organism was confirmed by culture and Gram stain.
B cepacia infection
Mice were injected intraperitoneally with a 0.5 cc saline suspension containing various numbers of B cepacia bacilli (clinical isolate from bronchial washings; ATCC No. 25609; ATCC). The numbers of bacteria in the inoculum were confirmed by plate culture of serial dilutions. Animals were monitored daily and killed if moribund. Animals were otherwise followed for 7 to 8 days after challenge with 106 colony-forming unit (CFU) or greater per mouse and for 15 to 17 days after challenge with 105 CFU or less per mouse. In some experiments, blood cultures were obtained from tail vein blood samples at 2, 4, 7, or 14 days after challenge, and bacteremia was quantitated by plate culture.
Statistical analysis
Statistical analysis using the Fischer exact test or the Mann-Whitney nonparametric test with 2-tailed P values was performed by using Instat 2.0 software. Log rank-tests for equality of survival was performed using GB-Stat version 6.5 software (Dynamic Microsystems, Silver Spring, MD).
Results
To investigate variables important for correction of host defense in murine CGD, X-CGD mice chimeric for varying percentages of NADPH oxidase-positive neutrophils were generated by BMT and challenged with either bacterial or fungal pathogens. Groups of mice were transplanted with either mixtures of wild-type and X-CGD BM (also referred to as wild-type chimeric mice) or with mixtures of mock-transduced and MSCV-m91Neo-transduced BM (also referred to as gene therapy chimeric mice). Although the MSCV-m91Neo retrovirus has been shown to reconstitute NADPH oxidase activity in neutrophils and macrophages for more than 18 months post-transplantation, expression of recombinant gp91phox is low (approximately 5% to 10% of wild-type protein levels), and superoxide production per cell is estimated to be corrected to only about 20% to 25% of wild type.32 33 Chimerism for NADPH oxidase-positive peripheral blood neutrophils in both wild-type and MSCV-m91Neo chimeric mice was monitored post-transplant by the NBT test, which detects superoxide generation in individual cells.
A fumigatus
X-CGD mice have a marked impairment in host defense to the opportunistic fungus, A fumigatus. Although wild-type mice are resistant to respiratory challenge with millions of A fumigatus conidia, as few as 50 conidia always resulted in chronic and sometimes fatal bronchopneumonia in X-CGD mice.29,31Using X-CGD mice transplanted with mixtures of wild-type and X-CGD BM, we had previously found that X-CGD mice with 4% to 8% wild-type neutrophils were protected against respiratory challenge with 150 to 500 conidia, although all mice with only 2% to 3% wild-type neutrophils developed A fumigatus pneumonia at this challenge dose.32 We had also shown that X-CGD mice with approximately 50% NADPH oxidase-positive neutrophils after MSCV-m91Neo-mediated gene transfer were protected when challenged with doses in this same range.32
In the current study, we examined whether the reduced level of NADPH oxidase activity in MSCV-m91Neo-corrected neutrophils was sufficient to protect X-CGD mice against challenge with A fumigatus when less than 50% of neutrophils were corrected. All X-CGD mice that were not transplanted had histologic evidence of A fumigatuspneumonia after challenge with 150 to 200 conidia (Table1). In contrast, all X-CGD mice with 11% to 21% MSCV-m91Neo-corrected neutrophils were protected. However, 4 of 6 mice with 5% to 10% gene therapy-corrected neutrophils developedA fumigatus lung infection at this dose range, including 3 that died of fungal pneumonia. This finding is significantly different from results obtained for X-CGD mice chimeric for 4% to 8% wild-type neutrophils, in which none of 7 mice studied had evidence of lung disease after this inoculum (Table 1). We also examined the resistance of chimeric mice to higher numbers of A fumigatus conidia. X-CGD mice with 19% to 30% NADPH oxidase-positive neutrophils after transplantation with MSCV-m91Neo-transduced BM were fully resistant to challenge with 4000 conidia, as were wild-type chimeric mice with 15% to 33% oxidase-positive neutrophils challenged with 5000 conidia (Table 1). Finally, X-CGD chimeric mice chimeric for 21% to 36% wild-type neutrophils were protected against challenge with even 50 000 A fumigatus conidia (Table 1), a dose that would otherwise be expected to result in death of X-CGD mice within approximately 7 days.31
Aspergillus fumigatus pneumonia in X-CGD transplantation chimera mice
| Group . | Conidia . | N . | NBT (%) . | A fumigatus lung disease . |
|---|---|---|---|---|
| X-CGD | 150-200 | 10 | 0 | 10/10* |
| MSCV-m91Neo/X-CGD | 150-200 | 5 | 1-3 | 5/5 |
| 6 | 5-10 | 4/6†,‡ | ||
| 3 | 11-15 | 0/3*,‡ | ||
| 3 | 18-21 | 0/3*,‡ | ||
| 4 000 | 7 | 19-30 | 0/7 | |
| WT/X-CGD | 150-200 | 7 | 2-3 | 7/71-153 |
| 7 | 4-8 | 0/7†,1-154 | ||
| 5 000 | 6 | 15-33 | 0/6 | |
| 50 000 | 5 | 21-36 | 0/5 |
| Group . | Conidia . | N . | NBT (%) . | A fumigatus lung disease . |
|---|---|---|---|---|
| X-CGD | 150-200 | 10 | 0 | 10/10* |
| MSCV-m91Neo/X-CGD | 150-200 | 5 | 1-3 | 5/5 |
| 6 | 5-10 | 4/6†,‡ | ||
| 3 | 11-15 | 0/3*,‡ | ||
| 3 | 18-21 | 0/3*,‡ | ||
| 4 000 | 7 | 19-30 | 0/7 | |
| WT/X-CGD | 150-200 | 7 | 2-3 | 7/71-153 |
| 7 | 4-8 | 0/7†,1-154 | ||
| 5 000 | 6 | 15-33 | 0/6 | |
| 50 000 | 5 | 21-36 | 0/5 |
X-linked chronic granulomatous disease (X-CGD) mice were transplanted with either mixtures of wild-type (WT) and X-CGD bone marrow (BM) or X-CGD BM transduced with MSCV-m91Neo and mock-transduced X-CGD BM. As described in the “Materials and methods” section, transplanted mice were challenged with the indicated dose of A fumigatus conidia.
P < .001 (X-CGD vs MSCV-m91Neo/X-CGD, 11% to 21% nitroblue tetrazolium [NBT]-positive neutrophils), Fisher exact test.
P = .02 (MSCV-m91Neo/X-CGD, 5% to 10% NBT-positive neutrophils vs WT/X-CGD, 4% to 8% NBT-positive neutrophils), Fisher exact test.
P = .06 (MSCV-m91Neo/X-CGD, 5% to 10% NBT-positive neutrophils vs 11% to 21% NBT-positive neutrophils), Fisher exact test.
Includes data on 4 mice studied previously31and 3 mice in current study.
Includes 6 mice studied previously31 and one in current study.
S aureus
We next investigated the requirements for correction of the host defense defect in X-CGD mice against S aureus. S aureus is a common cause of soft tissue or visceral abscesses in CGD patients.1 We had previously found that clearance ofS aureus from the peritoneal cavity was impaired in X-CGD mice compared with wild-type mice after intraperitoneal injection of a sublethal dose of S aureus.29 In addition, 7 of 11 X-CGD mice developed abscesses within the abdominal cavity 7 days after inoculation, in contrast to only one of 9 wild-type mice.
We examined the incidence of intra-abdominal abscess formation inS aureus peritonitis for X-CGD mice chimeric with varying percentages of wild-type neutrophils. In chimeras with 5% to 20% wild-type neutrophils, approximately one-half of the mice developed abscesses at 7 days after inoculation, which was similar to the incidence observed in X-CGD mice and significantly higher than that seen in wild-type mice, in which only approximately 10% developed abscesses (Table 2). There was a trend toward a reduced incidence of abscess formation in transplantation chimeras with more than 20% wild-type neutrophils, but this improvement did not achieve statistical significance. The failure to reduce intra-abdominal abscess formation following S aureuschallenge in transplanted X-CGD mice with as many as 20% wild-type neutrophils was unexpected. To rule out an effect of the transplant procedure itself on the resistance to S aureus in this model, 10 wild-type mice were transplanted with wild-type BM. No abscesses were observed in this group (Table 2). Peripheral blood neutrophil counts (approximately 19 000 per cubic milliliter) and differentials (approximately 10% neutrophils) were similar in both X-CGD chimera and wild-type transplant groups, suggesting that a difference in the total number of circulating neutrophils did not account for the different incidence of abscess formation. A small number of MSCV-m91Neo/X-CGD chimeras with 5% to 20% NADPH oxidase-corrected neutrophils were also studied. The incidence of abscess formation was similar to untreated X-CGD mice and wild-type/X-CGD chimeras (Table 2). Additional mice chimeric for higher percentages of oxidase-positive neutrophils treated with gene therapy were not evaluated because of the large numbers of mice (estimated to be at least 30) that would be required to show a statistically significant reduction in S aureus abscess formation.
Intraperitoneal abscess formation following inoculation with Staphylococcus aureus
| Group . | N . | NBT (%) . | Intra-abdominal abscess . |
|---|---|---|---|
| WT | 33 | 100 | 3/33*,† |
| X-CGD | 27 | 0 | 12/27* |
| Transplantation chimeras | |||
| WT/X-CGD | 8 | 5-10 | 5/8† |
| 10 | 11-20 | 4/10† | |
| 5 | 21-35 | 0/5 | |
| 5 | 35-50 | 2/5 | |
| MSCV-m91Neo/X-CGD | 4 | 5-10 | 3/4† |
| 4 | 11-20 | 2/4 | |
| WT Transplants | 10 | 100 | 0/10 |
| Group . | N . | NBT (%) . | Intra-abdominal abscess . |
|---|---|---|---|
| WT | 33 | 100 | 3/33*,† |
| X-CGD | 27 | 0 | 12/27* |
| Transplantation chimeras | |||
| WT/X-CGD | 8 | 5-10 | 5/8† |
| 10 | 11-20 | 4/10† | |
| 5 | 21-35 | 0/5 | |
| 5 | 35-50 | 2/5 | |
| MSCV-m91Neo/X-CGD | 4 | 5-10 | 3/4† |
| 4 | 11-20 | 2/4 | |
| WT Transplants | 10 | 100 | 0/10 |
X-linked chronic granulomatous disease (X-CGD) mice were transplanted with either mixtures of wild-type (WT) and X-CGD bone marrow (BM) or X-CGD BM transduced with MSCV-m91Neo and mock-transduced X-CGD BM. A separate group of WT mice were also transplanted with WT BM. As described in the “Materials and methods” section, WT, X-CGD, and transplanted mice were inoculated by intraperitoneal injection with 2 × 107Staphylococcus aureus, and abscess formation was scored at 1 week.
P < .005 Fisher exact test (WT vs X-CGD).
P < .05 Fisher exact test (WT vs WT/X-CGD or MSCV-m91Neo/X-CGD).
B cepacia
A third group of studies examined the relative numbers of NADPH oxidase-positive neutrophils required for effective host defense in experimental infection with B cepacia. B cepaciais an opportunistic gram-negative pathogen that can produce serious infections in patients with CGD, including pneumonia and associated sepsis. In these experiments, varying numbers of B cepaciabacilli were injected into the peritoneal cavity of mice to establish peritonitis, which was also often associated with bacteremia. Mice were followed for up to 15 to 17 days after the challenge. As summarized in Table 3, all wild-type mice survived following inoculation with 108 organisms or less, whereas even as few as 30 B cepacia bacilli produced a fatal infection in X-CGD mice. When challenged with doses of more than 106 organisms, X-CGD mice succumbed within 2 days, whereas time to symptoms or death increased at reduced doses, with survival up to approximately 1 week at the lowest dose tested (data not shown). At these lower doses, asymptomatic bacteremia was present in the majority of X-CGD mice in the first days after challenge (data not shown). X-CGD mice then typically became moribund within an approximate 12-hour period, which was associated with a marked increase in the number of bacteria in the bloodstream.
Survival of wild-type, X-CGD, and X-CGD transplantation chimera mice in Burkholderia cepacia infection
| % NBT . | WT 100% . | X-CGD 0% . | Female X-CGD carrier 40%-60%3-150 . | WT/X-CGD . | MSCV-m91Neo/X-CGD . | |||
|---|---|---|---|---|---|---|---|---|
| 9%-16% . | 20%-26% . | 32%-41% . | 16%-17% . | 30%-42% . | ||||
| 109 | 0/5 | |||||||
| 108 | 8/8 | 0/6 | ||||||
| 107 | 9/9 | 0/6 | ||||||
| 106 | 0/3 | |||||||
| 105 | 0/10 | 10/10 | 0/6 | |||||
| 104 | 0/4 | |||||||
| 103 | 3/3 | 0/15 | 2/9 | 3/53-151 | 8/93-151 | 0/2 | 7/73-151 | |
| 102 | 0/4 | |||||||
| 30 | 0/4 | |||||||
| % NBT . | WT 100% . | X-CGD 0% . | Female X-CGD carrier 40%-60%3-150 . | WT/X-CGD . | MSCV-m91Neo/X-CGD . | |||
|---|---|---|---|---|---|---|---|---|
| 9%-16% . | 20%-26% . | 32%-41% . | 16%-17% . | 30%-42% . | ||||
| 109 | 0/5 | |||||||
| 108 | 8/8 | 0/6 | ||||||
| 107 | 9/9 | 0/6 | ||||||
| 106 | 0/3 | |||||||
| 105 | 0/10 | 10/10 | 0/6 | |||||
| 104 | 0/4 | |||||||
| 103 | 3/3 | 0/15 | 2/9 | 3/53-151 | 8/93-151 | 0/2 | 7/73-151 | |
| 102 | 0/4 | |||||||
| 30 | 0/4 | |||||||
X-linked chronic granulomatous disease (X-CGD) mice were transplanted with either mixtures of wild-type (WT) and X-CGD bone marrow (BM) or X-CGD BM transduced with MSCV-m91Neo and mock-transduced X-CGD BM. WT, X-CGD mice, and X-CGD carrier females were also studied, as indicated. Data show number of mice surviving of total challenged by intraperitoneal injection with the indicated number of B cepacia conidia (as quantitated by colony assay).
Mean percentage of nitroblue tetrazolium (NBT) for carrier females studied was 44 ± 6 (n = 10).
P < .006 for X-CGD vs transplantation chimera X-CGD mice (Fisher exact test).
Additional studies were performed to determine the relative numbers of NADPH oxidase-positive neutrophils required to protect mice at challenge doses of 105 or 103B cepacia. Female X-CGD carrier mice (gp91phox+/− heterozygotes, that had 44% ± 6% oxidase-positive neutrophils) survived challenge with 105 organisms (Table 3), although 3 of 10 mice were bacteremic at day 5 postinoculation (200 to 1000 CFU/mL blood). In contrast, all 6 X-CGD mice with 9% to 16% NBT-positive neutrophils following transplantation with a mixture of wild-type and X-CGD neutrophils developed either symptoms consistent with sepsis or were found dead within 6 days of challenge with 105B cepacia.
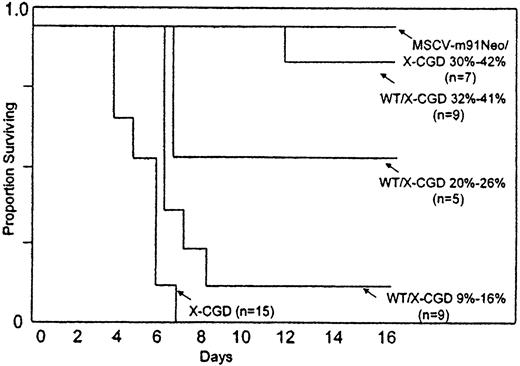
Survival and bacteremia following challenge with an even lower dose ofB cepacia was then compared between X-CGD mice and X-CGD chimeras with varying percentages of oxidase-positive neutrophils. All but 1 of 16 X-CGD mice with 30% to 42% NADPH-oxidase positive neutrophils following transplantation with either wild-type BM or with MSCV-m91-Neo-transduced X-CGD BM were resistant to intraperitoneal challenge with 103B cepacia (Table 3; Figure1). However, only 2 of 9 X-CGD chimeras with 9% to 16% wild-type neutrophils survived, although cumulative survival was significantly improved compared with untransplanted X-CGD mice (P = .008, log-rank test for equality of survival). Survival of mice with 20% to 26% wild-type neutrophils fell in between that of the 9% to 16% group and the 30% to 42% group, although this intermediate value was not statistically significantly different from either, likely because of the smaller numbers of mice in the 20% to 26% group. The peripheral white blood cell count and percentage of neutrophils were similar in all groups of X-CGD mice (Table 4). For reasons that are not clear, mice with 32% to 41% NBT-positive neutrophils had a higher mean total white blood cell count and therefore total neutrophil count; this difference, however, was not statistically significant from the other groups. Taken together, these results indicate that survival of mice challenged with B cepacia was directly related to the relative number of NADPH oxidase-positive neutrophils. Correction of at least 30% of NADPH oxidase activity in peripheral blood neutrophils of X-CGD mice by either transplantation with wild-type BM or MSCV-m91Neo-mediated gene transfer was required to achieve high rates of survival.
Survival of X-CGD mice after intraperitoneal challenge with B cepacia.
Untransplanted X-CGD control mice or X-CGD mice previously transplanted with X-CGD BM mixed with varying amounts of either wild-type or MSCV-m91Neo-transduced X-CGD BM were challenged with 103CFU B cepacia by intraperitoneal injection. The fraction surviving in each group is shown as a function of the day after inoculation. Groups are as indicated.
Survival of X-CGD mice after intraperitoneal challenge with B cepacia.
Untransplanted X-CGD control mice or X-CGD mice previously transplanted with X-CGD BM mixed with varying amounts of either wild-type or MSCV-m91Neo-transduced X-CGD BM were challenged with 103CFU B cepacia by intraperitoneal injection. The fraction surviving in each group is shown as a function of the day after inoculation. Groups are as indicated.
Peripheral blood leukocyte counts in mice challenged withBurkholderia cepacia
| Group4-150 . | N . | White blood cells . | Neutrophils (%) . | Neutrophil count . | NBT-positive neutrophils . |
|---|---|---|---|---|---|
| X-CGD | 6 | 18 500 ± 5 300 | 10 ± 6 | 1 920 ± 1 576 | 0 |
| WT/X-CGD chimera | |||||
| 9%-16% | 9 | 17 200 ± 5 700 | 8 ± 5 | 1 298 ± 582 | 68 ± 86 |
| 20%-26% | 5 | 19 100 ± 5 600 | 8 ± 4 | 1 562 ± 1 030 | 359 ± 238 |
| 32%-41% | 9 | 25 400 ± 10 500 | 11 ± 6 | 2 686 ± 2 068 | 1 024 ± 866 |
| MSCVm91Neo/X-CGD chimera | |||||
| 30%-42% | 7 | 15 200 ± 4 500 | 13 ± 5 | 1 988 ± 1 057 | 740 ± 461 |
| Group4-150 . | N . | White blood cells . | Neutrophils (%) . | Neutrophil count . | NBT-positive neutrophils . |
|---|---|---|---|---|---|
| X-CGD | 6 | 18 500 ± 5 300 | 10 ± 6 | 1 920 ± 1 576 | 0 |
| WT/X-CGD chimera | |||||
| 9%-16% | 9 | 17 200 ± 5 700 | 8 ± 5 | 1 298 ± 582 | 68 ± 86 |
| 20%-26% | 5 | 19 100 ± 5 600 | 8 ± 4 | 1 562 ± 1 030 | 359 ± 238 |
| 32%-41% | 9 | 25 400 ± 10 500 | 11 ± 6 | 2 686 ± 2 068 | 1 024 ± 866 |
| MSCVm91Neo/X-CGD chimera | |||||
| 30%-42% | 7 | 15 200 ± 4 500 | 13 ± 5 | 1 988 ± 1 057 | 740 ± 461 |
The percentage of nitroblue tetrazolium (NBT)-positive peripheral blood neutrophils is as indicated for the transplantation chimera groups. Total white blood cells, neutrophils, and NBT-positive neutrophils are indicated per cubic millimeter and were obtained prior to challenge with B cepacia.
X-CGD indicates X-linked chronic granulomatous disease; WT, wild-type.
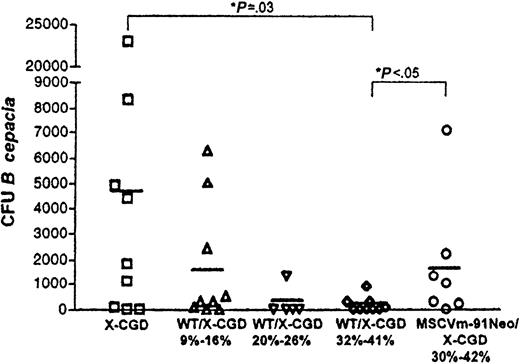
Bacteremia at 4 days after intraperitoneal challenge with 103B cepacia was also compared between the untransplanted X-CGD mice and different transplantation groups as another approach for assessing host response in the setting of variable numbers of NADPH oxidase-positive neutrophils (Figure2). Untransplanted X-CGD mice had a mean of 4844 ± 7470 CFU B cepacia/mL blood, a value that does not include data from 4 mice in which the CFU per milliliter blood could not be quantitated but had at least 10 000 CFU/mL 4 days after the challenge. In all groups of wild-type or gene therapy chimera X-CGD mice, bacteremia was reduced relative to untransplanted X-CGD mice, although this reduction was statistically significant only in the wild-type chimeric X-CGD mice with 30% to 40% NBT-positive neutrophils (P = .03, Mann-Whitney test). In wild-type chimera X-CGD mice, bacteremia appeared to correlate with the level of chimerism. In mice with 9% to 16% wild-type neutrophils, 1656 ± 2404 CFU/mL were detected compared with 179 ± 299 CFU/mL in mice with 32% to 41% wild-type neutrophils, although this difference did not quite reach statistical significance (P = .09, Mann-Whitney test). It is also noteworthy that there was a significant difference in the level of bacteremia 4 days after the challenge between the wild-type (179 ± 299) and gene therapy (1720 ± 2488) chimera X-CGD mice with NBT-positive neutrophils in the 30% to 40% range (P = .03, Mann-Whitney test). The mean CFU per milliliter for the latter group was 833 ± 836 if data from the one “outlier” mouse is excluded, which is not quite statistically significant from the mean obtained for the wild-type chimeras in this range (P = .066, Mann-Whitney test).
Bacteremia after intraperitoneal challenge with B cepacia.
Four days after intraperitoneal challenge with 103 CFUB cepacia, tail blood samples were cultured, and B cepacia CFU per milliliter of peripheral blood was determined. Data from individual mice were plotted, and the horizontal bar drawn for each group of data represents the mean CFU per milliliter for each group. Significant differences between groups for mean CFU per milliliter are as indicated (Mann-Whitney test with 2-tailedP value).
Bacteremia after intraperitoneal challenge with B cepacia.
Four days after intraperitoneal challenge with 103 CFUB cepacia, tail blood samples were cultured, and B cepacia CFU per milliliter of peripheral blood was determined. Data from individual mice were plotted, and the horizontal bar drawn for each group of data represents the mean CFU per milliliter for each group. Significant differences between groups for mean CFU per milliliter are as indicated (Mann-Whitney test with 2-tailedP value).
Discussion
Developing successful strategies for gene therapy of inherited disorders can benefit greatly from testing approaches in an animal model of the disease. Although observations made in such a model might not precisely parallel the clinical setting, critical parameters, such as the relative level of expression of the transferred genetic sequences required to prevent or reverse disease manifestations, can be evaluated. In this study, we used a murine model of X-CGD to examine how reconstitution of host defense to A fumigatus, S aureus, or B cepacia was influenced by correction of variable numbers of phagocytes by either transplantation of wild-type BM or retrovirus-transduced X-CGD BM. A striking finding in this study was that substantially higher numbers of NBT-positive neutrophils were required to fully restore host defense in experimental infection with either S aureus or B cepacia compared withA fumigatus. The results also suggest that the host response may be influenced by the relative level of cellular NADPH oxidase activity when a minority of neutrophils were corrected, particularly for A fumigatus infection.
A fumigatus is an opportunistic fungus that remains an important cause of life-threatening pneumonia and other serious infections in CGD patients.1,34,35 Respiratory burst-derived oxidants are of particular importance in neutrophil-mediated killing of hyphae that germinate from conidia-escaping macrophages, which otherwise efficiently kill conidia by largely nonoxidative mechanisms.36 37 We had previously found that as few as 5% respiratory burst-positive neutrophils protected X-CGD mice against respiratory challenge with approximately 200 A fumigatus conidia, using chimeric X-CGD mice generated by transplantation with mixtures of wild-type and X-CGD BM. In the current study, X-CGD mice with only partial correction of neutrophil NADPH oxidase activity after retroviral-mediated gene transfer least 10% of NBT-positive neutrophils were required to confer protection against this challenge dose of A fumigatus. Taken together, these data indicate that the relative level of NADPH oxidase activity per cell can be an important factor in determining the outcome ofA fumigatus challenge when small numbers of neutrophils are corrected. With higher numbers of oxidase-positive neutrophils, even partial reconstitution of NADPH oxidase activity protected as well as wild-type neutrophils when mice were challenged with low to moderate numbers of A fumigatus.
Compared with the challenge with A fumigatus, relatively greater numbers of oxidant-generating phagocytes were required for full protection against B cepacia or S aureus, 2 important bacterial pathogens in CGD. S aureus is overall the most common microbe isolated from sites of infection in CGD patients and is typically associated with abscesses in skin, lymph node, liver, or bone.1,35B cepacia is an important cause of bacteremia or sepsis, often in the setting of pneumonia, and was second only to Aspergillus as a cause of death in a recent report from a registry of CGD patients in the United States.35 In experimental infection with S aureus, in which intra-abdominal abscess formation is assessed 7 days after intraperitoneal injection, we found that the incidence of abscesses in X-CGD mice with as many as 20% wild-type neutrophils was no different than that for untransplanted X-CGD mice. In a peritonitis and sepsis model of B cepacia infection, X-CGD mice chimeric for 9% to 16% wild-type neutrophils all died at a challenge dose of 1 × 105 CFU, approximately 3 logs below the median lethal dose for wild-type mice. At a dose approximately 5 logs below the wild-type median lethal dose, survival in mice with 9% to 16% wild-type neutrophils was slightly improved, and full protection was seen only in mice with at least 30% oxidase-positive neutrophils. Although X-CGD gene therapy chimeric mice with partial correction of NADPH oxidase activity in at least 30% of neutrophils all survived challenge at this dose, transient bacteremia was higher compared with X-CGD mice chimeric for similar numbers of wild-type neutrophils. This finding suggests that the relative level of cellular NADPH oxidase activity may also be a determinant in host defense against B cepacia, which could influence the outcome with larger numbers of inocula or with correction of smaller numbers of neutrophils.
The markedly enhanced virulence of B cepacia in X-CGD mice relative to wild-type mice observed in this study is noteworthy and points to the critical importance of respiratory burst-derived oxidants in the host response to this organism. The unusual susceptibility of CGD patients to B cepacia is a distinctive feature of this disease.38,39 This organism is a rare cause of infection in other immunocompromised patients, although it is often found colonizing the lungs of patients with cystic fibrosis. Mardiney et al10 have reported that p47phox−/− CGD mice also exhibit a marked susceptibility to B cepacia in a study aimed at evaluating the effect of retroviral-mediated gene transfer of p47phox on host defense. There were some differences in the relative susceptibility of the CGD mice to B cepacia between that study10 and the current study. Intraperitoneal challenge with 2.5 × 105 CFU B cepaciawas fatal in untreated p47phox−/− mice, although 50% survived for 2 weeks, in contrast to our study in which untreated X-CGD mice died within 7 days even when challenged with as few as 30 CFU. In addition, 2 of 10 p47phox−/−CGD mice appeared to clear their infection after challenge with B cepacia when having 3.4% ± 1.04% oxidase-positive neutrophils following gene transfer,10 whereas X-CGD mice with 9% to 16% wild-type neutrophils all died when challenged with a similar dose in the current study. These differences may be due to the different isolates used in the 2 studies or may reflect an increased severity of the disease phenotype in X-CGD mice compared with p47phox deficiency, as has been seen in the clinical experience.9 35
Our observations on reconstitution of host defense in murine X-CGD, suggesting that fewer numbers of NADPH oxidase-positive neutrophils are required for protection against A fumigatus infection compared with bacterial pathogens, correlate with clinical observations of individuals who have a small fraction of oxidase-positive neutrophils. Most of these reported cases are female carriers of X-CGD who, by chance, have skewed X-linked inactivation and only a small percentage of oxidase-positive cells. In addition, 2 rare X-CGD kindreds have been reported in which affected males have 5% to 10% oxidase-positive neutrophils, because of a mutation in a regulatory region of the gp91phox gene that permits gp91phox expression in a subset of neutrophil progenitors.27,40 Some reported individuals with 3% to 10% oxidase-positive neutrophils are healthy.18,41However, others with oxidase-positive neutrophils in this same range have experienced recurrent, deep-seated bacterial infections typical of full-blown CGD, including those caused by S aureus, B cepacia, and Serratia marcescens.18-20,27,42,43 Interestingly, only one case of Aspergillus has been reported in this group, in a woman who consistently had less than 5% oxidase-positive neutrophils.18 One plausible explanation for the beneficial effect of small numbers of respiratory burst oxidase-positive cells in A fumigatus infection is that killing of A fumigatus hyphae, which are often too large for neutrophils to ingest, involves synergy between oxidants and granule contents of digestive and cytotoxic proteins that are released from the surrounding neutrophils. Consistent with these observations, the addition of a small proportion of normal neutrophils to CGD neutrophils reconstituted normal killing of A fumigatus hyphae in vitro.44 In contrast, bacteria are typically ingested and killed within the phagolysosome of individual neutrophils, and any contribution of oxidants from adjacent neutrophils may be less effective.
Similarly, observations suggesting that the relative cellular level of NADPH oxidase activity can be a factor in host defense is also consistent with clinical findings in variant X-CGD patients with low levels of residual phagocyte NADPH oxidase activity because of a partially functional flavocytochromeb558.21-26,28,45-47 Although many of the reported patients have a mild clinical course even with as little as 3% to 5% neutrophil superoxide-generating activity, serious bacterial infections or other complications typical of CGD do occur, including severe pneumonia, pulmonary fibrosis, and hepatic or perirectal abscesses. One patient reported by Jendrossek et al47 with approximately 70% weakly NBT-positive neutrophils and 10% residual NADPH oxidase activity had numerous episodes of bacterial pneumoniae and 3 liver abscesses, as well as pulmonary infection with A fumigatus.
As with any animal study, these experimental infections in murine CGD do not reflect all aspects pertinent to the defect in host defense in human CGD, and the clinical benefit of gene replacement therapy will ultimately need to be addressed in clinical trials. However, the results obtained in this study generally correlate with clinical observations of individuals with a skewed distribution of oxidase-positive and -negative cells and of variant X-CGD patients with partial NADPH oxidase activity and, thus, are useful as a general guideline for variables that can influence correction of the manifestations of human CGD. These observations are also relevant for treatment of CGD by allogeneic BMT if mixed chimeric states are attained following donor engraftment. Although significant protection against A fumigatus infection may be achieved by correction of only about 5% of phagocytes, maximal protection against bacterial pathogens will likely require higher levels of correction. In addition, complete restoration of superoxide generation is likely to be important for reconstituting host defense against a wide range of bacterial and fungal pathogens, particularly if NADPH oxidase activity is reconstituted in only a minority of phagocytes.
We thank Roopa Sehsadri for advice on statistical analysis; W. Scott Goebel and Frank Smith for helpful comments; and Teri Mallory for assistance with manuscript preparation.
Supported by grants P01 HL53586 and RO1 HL52565 from the National Heart, Lung, and Blood Institute and by a Clinical Research Award from the March of Dimes (1FY97). The Wells Center for Pediatric Research is a Center for Excellence in Molecular Hematology funded by the National Institute of Diabetes and Digestive and Kidney Diseases (P50 DK49218).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary C. Dinauer, Cancer Research Institute R4, Indiana University School of Medicine, 1044 W Walnut St, Rm 402, Indianapolis, IN 46202; e-mail: mdinauer@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal