In patients suspected of having drug-induced immune thrombocytopenia, antibodies reactive with normal platelets in the presence of the suspect drug can sometimes be identified, but negative results are often obtained. One reason for this is that drug metabolites, formed in vivo, can be the sensitizing agents, but very little is known about the specific metabolites that can cause this complication. Five patients were studied who developed thrombocytopenia after taking the nonsteroidal anti-inflammatory drug naproxen (3 cases) or acetaminophen (2 cases) but in whom drug-dependent antibodies could not be detected by means of the unmodified drugs. In each case, antibodies that reacted with normal target platelets in the presence of a known drug metabolite (naproxen glucuronide or acetaminophen sulfate) were identified. Four of the antibodies were specific for the glycoprotein (GP) IIb/IIIa complex, but one acetaminophen sulfate–dependent antibody reacted preferentially with GPIb/IX/V. In patients with a clinical picture suggestive of drug-induced immune thrombocytopenia, tests for metabolite-dependent antibodies can be helpful in identifying the responsible agent.
Introduction
Antibody-mediated thrombocytopenia is a recognized side effect of many medications. In patients exposed only to a single drug, recovery after its discontinuation provides circumstantial evidence, but not proof, that the thrombocytopenia was caused by drug sensitivity.1 In patients taking multiple medications, doubt often remains as to which drug, if any, was responsible. In such cases, it is sometimes possible to identify the sensitizing drug by demonstrating antibodies that bind to normal platelets in the presence of drug.2-4 However, negative results are often obtained. Factors responsible for the failure to demonstrate drug-dependent antibodies include the fact that some sensitizing drugs are poorly soluble in water and thus difficult to work with in vitro; a possible requirement that autologous cells be used for testing5; and insufficient sensitivity of the available assays. It is also possible, of course, that drug exposure was coincidental.
Another explanation for failure to obtain laboratory confirmation in patients with apparent drug-induced immune cytopenia is that the sensitizing agent can be a structurally modified form of the sensitizing drug resulting from in vivo metabolism,6-8 but the frequency of this complication and the range of metabolites that can cause it are poorly defined. We recently encountered 5 patients who experienced thrombocytopenia while taking the nonsteroidal anti-inflammatory drug (NSAID) naproxen or the antipyretic acetaminophen. Assays for platelet-binding, drug-dependent antibodies were negative with patient serum and unmodified drug, but positive reactions were obtained when testing was done with known drug metabolites. Our findings provide further evidence that sensitivity to drug metabolites should be considered in patients with a history suggestive of drug-induced thrombocytopenia in whom antibodies cannot be detected by means of the primary drug.
Patients, materials, and methods
Case reports
Case 1.
A 57-year-old man was started on 250 mg naproxen 3 times a day (TID) for treatment of plantar fasciitis. Ten days later, he experienced the acute onset of widespread petechial hemorrhages on the lower extremities. A complete blood count showed a platelet level of 3 × 109/L but was otherwise normal. Naproxen was discontinued, and the patient was given 60 mg prednisone per day. His platelet count rose gradually to normal during a 5-day period. Prednisone was discontinued after 2 weeks, and his platelet count has remained normal since that time.
Case 2.
A 56-year-old man was started on 1.0 g naproxen daily because of knee pain related to a cartilage injury. After 25 days, he experienced the acute onset of extensive petechial hemorrhages on the arms, legs, and abdomen and gastrointestinal bleeding. A complete blood count showed a platelet level of 8 × 109/L but was otherwise normal. Treatment with 60 mg prednisone per day for 1 week was followed by resolution of bleeding symptoms and a progressive rise in platelet levels to normal over a 7-day period. His platelet level remained normal thereafter.
Case 3.
A 71-year-old man was treated with 250 mg naproxen TID for joint pain related to rheumatoid arthritis. After 3 weeks, he noted widespread petechial hemorrhages on his legs and arms. A complete blood count demonstrated a platelet level of 6 × 109/L but was otherwise normal. On treatment with prednisone, 50 mg/d, his platelet level returned to normal in 5 days. Prednisone was discontinued, and he has maintained a normal platelet count since that time.
Case 4.
A 30-year-old man took 1.0 g acetaminophen intermittently for headaches over a period of 5 years. A complete blood count done at the time of a routine checkup showed a platelet level of 50 × 109/L but was otherwise normal. He was advised to discontinue acetaminophen, and 1 week later, his platelet level was 325 × 109/L. He was subsequently lost to follow-up.
Case 5.
A 66-year-old woman with alcoholic cirrhosis but satisfactory liver function and only minimal splenomegaly had been taking acetaminophen 1.0 g pro re nata for headache and other nonspecific indications. A routine blood count showed a platelet level of 45 × 109/L but was otherwise normal. Acetaminophen was discontinued and platelets rose to 165 × 109/L during the next 10 days. She was subsequently lost to follow-up.
Reagents
Naproxen, uridine diphosphoglucuronic acid (UDPGA), bovine serum albumin (BSA), 4-acetamidophenol, p-acetamidophenyl-β-D-glucuronide, and dimethyl sulfoxide-d6 (DMSO d6) were purchased from Sigma Chemical (St Louis, MO). Fluorescein isothiocyanate (FITC)–conjugated, affinity-purified F(ab′)2 goat antihuman immunoglobulin G (IgG) heavy and light chains; FITC-conjugated, affinity-purified F(ab′)2 goat antihuman IgG Fc; and FITC-conjugated F(ab′)2 donkey antihuman IgM Fc were from Jackson Immunoresearch Laboratory (West Grove, PA). Alkaline phosphatase–conjugated and FITC-conjugated mouse antihuman IgA were from Zymed Laboratories (South San Francisco, CA). High-pressure liquid chromatography (HPLC)–grade acetonitrile and triflouroacetic acid (TFA) were from Aldrich Chemical (Milwaukee, WI). We synthesized 6-0-desmethyl naproxen from naproxen by the method of Khorana and Pishawikar.9 Acetaminophen sulfate was synthesized by the method of Barco et al.10 The following monoclonal antibodies (mAbs) were used: AP1 (anti–glycoprotein Ib [GPIb]) from Dr Robert Montgomery, The Blood Center of Southeastern Wisconsin (Milwaukee); AP2 (anti-GPIIb/IIIa complex) from Dr Thomas Kunicki, Scripps Medical Research Institute (La Jolla, CA); MBC 142.11 (anti-GPIb) and MBC 132.4 (anti-GPIV) from the Monoclonal Antibody Laboratory of The Blood Center of Southeastern Wisconsin.
Flow cytometric detection of antibodies
The assay has been described in detail previously.2,3 We incubated 25 μL patient serum with 5 × 106 platelets suspended in 25 μL of a solution containing drug and 1% BSA in Ringer's citrate dextrose (RCD-BSA), pH 6.5. After incubation for 1 hour at room temperature, the platelets were washed twice with RCD-BSA containing drug at the same concentration as in the primary mixture. The washed platelets were suspended in 25 μL RCD-BSA, containing drug, and 25 μL FITC-labeled anti-IgG antibody (heavy- and light-chain specific), diluted 1:40, was added. For antibody isotype determination, labeled secondary antibodies specific for IgG, IgM, or IgA were used. An aliquot of each reaction mixture was diluted in 0.5 mL RCD immediately before being analyzed by flow cytometry (FACScan, Becton Dickinson, Mountain View, CA). A positive reaction was defined as a mean platelet fluorescence intensity at least twice that of platelets processed identically except for the absence of drug. This value always exceeded the mean obtained with control serum by at least 3.0 SDs. In some studies, platelets from a patient with the Bernard-Soulier syndrome (BSS) and from a patient with type I Glanzmann thrombasthenia (GT) were used as targets. Methods and nature of the molecular defect in these patients have been described previously.11
Modified antigen capture enzyme-linked immunosorbent assay
The modified antigen capture enzyme-linked immunosorbent assay (MACE) was performed as previously described.3 12 In brief, 1.6 × 108 platelets were incubated with antibody and drug, washed in buffer containing drug, and solubilized with nonionic detergent, also in the presence of drug. Aliquots of the lysate containing 1 × 107 platelet equivalents were added to the wells of microtiter plates containing 0.5 μg immobilized mAb specific for GPIIb/IIIa (AP-2), GPIb (AP-1 or MBC 142.1), or GPIV (MBC 132.4) that were incubated overnight to permit capture of the GP for which each mAb was specific. After washing, human antibodies associated with the captured GP were identified by enzyme-linked immunosorbent assay by means of alkaline phosphatase-conjugated anti-immunoglobulin.
Preparation of rat liver microsomes
Fractions containing microsomes were isolated from the livers of Sprague Dawley rats as described by Radominska-Pyrek and coworkers.13 All procedures were carried out between 0°C and 4°C. Frozen rat livers were thawed at 4°C in homogenizing buffer (0.25 M sucrose, 1 mmol ethylenediaminetetraacetic acid (EDTA), 5 mmol Tris, pH 7.6). Each liver was blotted dry, weighed, suspended in cold homogenizing buffer adjusted to 4 times the weight of the livers, minced, and homogenized separately. The pooled homogenates were centrifuged at 5000g for 15 minutes. The supernatants were collected and centrifuged at 17 000g for 30 minutes. The supernatants were again collected, and their content of microsomes was pelleted at 100 000g for 1 hour. The pelleted microsomes were washed once in cold homogenizing buffer and were resuspended in 1 mL storage solution (0.25 M sucrose, 5 mmol Tris, pH 7.5) for each liver. Aliquots were then frozen at −80°C until used. On average, the microsomal preparations contained 40 mg protein per milliliter determined by the bicinchoninic acid method.14
Microsomal glucuronidation of naproxen
A detailed description has been published.15 Rat liver microsomes (0.1 mL containing 4 mg protein) were added to 0.25 mL Tris buffer (200 mmol Tris, 20 mmol MgCl2, pH 7.4) and 0.1 mL of a solution containing naproxen (1 mg/mL naproxen in 5% BSA with 25 mmol D-saccharic 1,4 lactone). After a 15-minute preincubation at 37°C, 50 μL of a 30-mmol solution of UDPGA in water was added. Aliquots (50 μL) were removed immediately and at various times thereafter, and were added to 100 μL acetonitrile. After centrifugation to remove protein, the supernatants were stored at −20°C pending HPLC analysis.
HPLC analysis
A Beckman system gold model 126 pump and 167 variable wavelength dual channel detector set at 254 and 275 nm (Beckman Coulter, Fullerton, CA) was used. A C18 guard column from Vydac (Hesperia, CA) was used as the precolumn. The column was a C18 column (4.6 mm × 250 mm), also from Vydac. Solvent A consisted of 0.1% TFA in de-ionized distilled water. Solvent B consisted of 0.08% TFA in HPLC-grade acetonitrile. The flow rate was 1 mL/min. The program for an 18-minute procedure was as follows: 2 minutes at isocratic conditions using 80% solvent A and 20% solvent B, a linear gradient over the next 10 minutes to a mixture of 15% solvent A and 85% solvent B, and an isocratic flush at 5% solvent A and 95% solvent B for 3 minutes. The program ended with a 5-minute re-equilibration at 80% solvent A and 20% solvent B. Peaks of interest were collected, frozen at −80°C, and lyophilized.
Isolation of drug metabolites from urine
Urinary metabolites were isolated by passing 15 mL of a 24-hour urine collection from a normal subject taking naproxen (250 mg TID) through a 600-mg Maxi-clean solid phase (C18) extraction cartridge (Alltech, Deerfield, IL) and eluting with acetonitrile as previously described.15 The effluent was frozen at −80°C and lyophilized. The residue was reconstituted in RCD-BSA for serologic testing and HPLC analysis.
Results
Sera from patients 1 through 3 contained antibodies that recognized normal platelets in the presence of urinary metabolites of naproxen
Sera from patients 1 through 3 were initially tested for drug-dependent antibodies reactive with naproxen with negative results. Negative reactions were also obtained when urine from a normal subject taking naproxen, 250 mg TID, was used as the source of “drug” in the assay. However, as shown in Figure 1for patient 1, antibody binding was detected with metabolites extracted from the same urine. Essentially the same results were obtained with sera from patients 2 and 3 (not shown). With immunoglobulin class–specific secondary probes, it was found that each of the antibodies was of the IgG class only. Antibodies reactive with platelets in the presence of naproxen metabolites were not detected in sera from 8 normal subjects or 2 persons taking naproxen who had normal platelet counts.
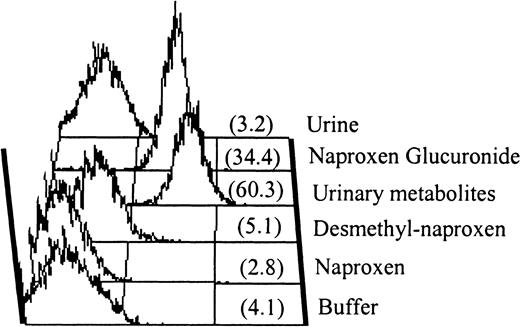
IgG antibody in serum from patient 1.
Serum from patient 1 contained an IgG antibody that recognized normal platelets in the presence of naproxen glucuronide. Antibody binding was not promoted by naproxen or by urine from a normal individual taking naproxen. However, positive reactions were obtained with drug metabolites isolated from the same urine (“urinary metabolites”) and with purified naproxen glucuronide. No reaction was obtained with 6-0-desmethyl naproxen, another metabolite of naproxen. Mean fluorescence intensity values are shown in parentheses.
IgG antibody in serum from patient 1.
Serum from patient 1 contained an IgG antibody that recognized normal platelets in the presence of naproxen glucuronide. Antibody binding was not promoted by naproxen or by urine from a normal individual taking naproxen. However, positive reactions were obtained with drug metabolites isolated from the same urine (“urinary metabolites”) and with purified naproxen glucuronide. No reaction was obtained with 6-0-desmethyl naproxen, another metabolite of naproxen. Mean fluorescence intensity values are shown in parentheses.
The active naproxen metabolite was identified as naproxen glucuronide
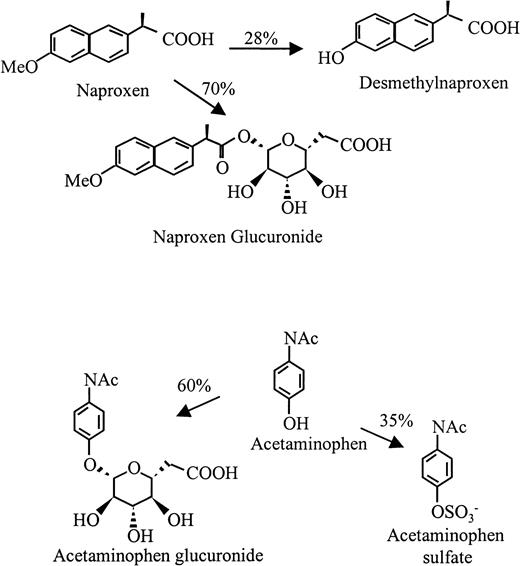
The major urinary metabolites of naproxen are shown in Figure2 (top). During metabolism, naproxen undergoes demethylation at the 6 position and/or esterification with glucuronide at its carboxyl residue.16By HPLC analysis, we found that the major metabolite in urine from a subject taking naproxen had a retention time identical to that of naproxen glucuronide synthesized by rat liver microsomes (Figure3). Both the major peak from urine and synthesized naproxen glucuronide promoted binding of the antibodies from patients 1 through 3 to normal target platelets (shown for patient 1 in Figure 1). However, negative reactions were obtained with 6-0-desmethyl naproxen.
Structures of naproxen, acetaminophen, and their major urinary metabolites.
Percentages represent the approximate reaction of each drug that is metabolized by each pathway.
Structures of naproxen, acetaminophen, and their major urinary metabolites.
Percentages represent the approximate reaction of each drug that is metabolized by each pathway.
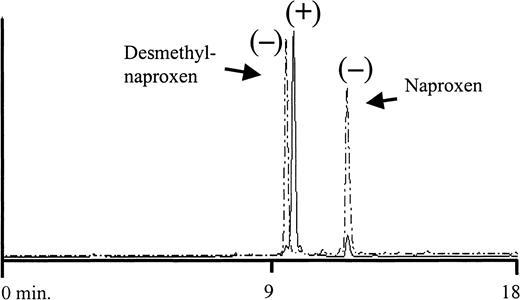
HPLC analysis of metabolites isolated from urine of a normal subject taking naproxen.
The 6-0-desmethyl naproxen and naproxen standards had retention times of 9.9 and 12.1 minutes, respectively (dashed peaks). Retention time of the major urinary peak (solid line) was 10.3 minutes, which was identical to the retention time of a naproxen glucuronide standard (not shown). The ability of each peak to promote binding of antibodies from patients 1 through 3 to platelets is shown (+/−). Positive results were obtained only with the peak corresponding to naproxen glucuronide.
HPLC analysis of metabolites isolated from urine of a normal subject taking naproxen.
The 6-0-desmethyl naproxen and naproxen standards had retention times of 9.9 and 12.1 minutes, respectively (dashed peaks). Retention time of the major urinary peak (solid line) was 10.3 minutes, which was identical to the retention time of a naproxen glucuronide standard (not shown). The ability of each peak to promote binding of antibodies from patients 1 through 3 to platelets is shown (+/−). Positive results were obtained only with the peak corresponding to naproxen glucuronide.
Sera from patients 4 and 5 contained antibodies that recognized normal platelets in the presence of acetaminophen sulfate
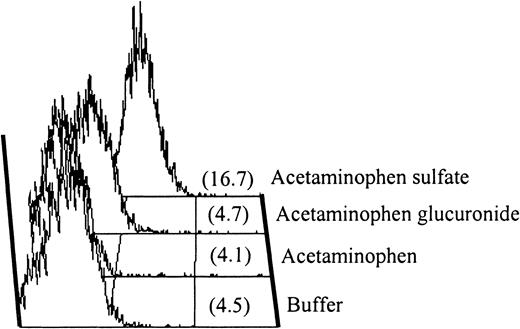
Negative reactions were obtained with patients 4 and 5 with the use of acetaminophen, undiluted urine from an individual taking 2 g acetaminophen daily, and metabolites extracted from this urine as the source of “drug.” However, as shown in Figure4 for patient 5, both sera contained immunoglobulins that reacted with normal platelets in the presence of acetaminophen sulfate, 1 of the 2 major metabolites of acetaminophen (Figure 2, bottom), at a concentration of 1.0 mg/mL (0.004 M) (Figure4). Essentially identical results were obtained with serum from patient 4 (not shown). With immunoglobulin class–specific secondary probes, the antibodies from patients 4 and 5 were found to be of the IgG and IgA subclass, respectively. We attribute failure of the urinary extract to give positive reactions with these sera to the fact that the extraction column used was designed to concentrate relatively hydrophobic compounds and failed to extract highly water-soluble acetaminophen sulfate efficiently. Neither serum gave positive reactions with acetaminophen glucuronide, the other major metabolite of acetaminophen. Antibodies reactive with platelets in the presence of acetaminophen sulfate were not detected in sera from 8 normal subjects or 2 persons taking acetaminophen who had normal platelet counts.
IgA antibody in serum from patient 5.
Serum from patient 5 contained an IgA antibody that recognized normal platelets in the presence of acetaminophen sulfate. Negative reactions were obtained with acetaminophen and acetaminophen glucuronide.
IgA antibody in serum from patient 5.
Serum from patient 5 contained an IgA antibody that recognized normal platelets in the presence of acetaminophen sulfate. Negative reactions were obtained with acetaminophen and acetaminophen glucuronide.
Characterization of target glycoproteins
The quantity of sera available from patients 1 through 3 with naproxen-associated thrombocytopenia was too small to perform immunoprecipitation studies to identify the GP for which they were specific. However, the GPIIb/IIIa complex was tentatively identified as their target on the basis of negative reactions obtained with platelets from a patient with type I GT (lacking GPIIb/IIIa) and normal platelets treated with EDTA at 37°C to dissociate the GPIIb/IIIa complex3 (Table 1).
Drug-dependent reactions of sera from patients 1 through 5 against different target platelet preparations (flow cytometry)
| Sample . | Normal donor platelets . | GT platelets . | BSS platelets . | EDTA-treated normal platelets . |
|---|---|---|---|---|
| Normal | Neg | Neg | Neg | Neg |
| Patient 1 | Pos | Neg | NT | Neg |
| Patient 2 | Pos | Neg | NT | Neg |
| Patient 3 | Pos | Neg | NT | Neg |
| Patient 4 | Pos | Neg | Pos | NT |
| Patient 5 | Pos | Pos | Neg | NT |
| Sample . | Normal donor platelets . | GT platelets . | BSS platelets . | EDTA-treated normal platelets . |
|---|---|---|---|---|
| Normal | Neg | Neg | Neg | Neg |
| Patient 1 | Pos | Neg | NT | Neg |
| Patient 2 | Pos | Neg | NT | Neg |
| Patient 3 | Pos | Neg | NT | Neg |
| Patient 4 | Pos | Neg | Pos | NT |
| Patient 5 | Pos | Pos | Neg | NT |
GT indicates Glanzmann thrombasthenia (type I); BSS, Bernard-Soulier syndrome; EDTA, ethylenediaminetetraacetic acid; neg, negative; pos, positive; NT, not tested.
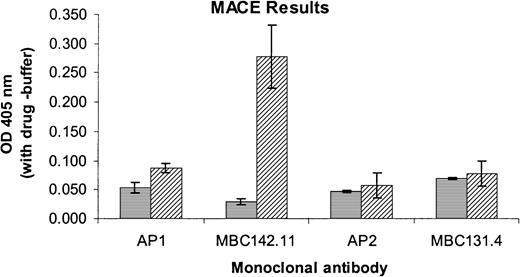
The acetaminophen-induced antibody of patient 4 also failed to react with GT platelets but reacted with those from a patient with BSS lacking GPIb/IX/V (Table 1), providing evidence that it too was specific for GPIIb/IIIa. In contrast, the antibody from patient 5 reacted with GT but not BSS platelets, indicating probable specificity for the GPIb/IX/V complex (Table 1). This was confirmed by an immunoprecipitation assay using mAb MBC 142.11 specific for GPIb (Figure 5). However, negative reactions were obtained with mAb AP-1, specific for the glycocalicin fragment of GPIb.
Antibody specificity in patient 5 for the GPIb/IX/V complex.
Specificity of the antibody in patient 5 for the GPIb/IX/V complex was demonstrated by MACE. Values shown are the differences of optical density values obtained in the presence and absence of drug. Positive reactions were obtained with mAb MBC 142.11 specific for the GPIb/IX complex, but not with AP2 (anti-GPIIb/IIIa), MBC131.4 (anti-GPIV), or AP1 specific for the glycocalicin domain of GPIb. ▤, normal serum; ▨, patient 5. Brackets indicate mean ± 1 SD of a study done in triplicate.
Antibody specificity in patient 5 for the GPIb/IX/V complex.
Specificity of the antibody in patient 5 for the GPIb/IX/V complex was demonstrated by MACE. Values shown are the differences of optical density values obtained in the presence and absence of drug. Positive reactions were obtained with mAb MBC 142.11 specific for the GPIb/IX complex, but not with AP2 (anti-GPIIb/IIIa), MBC131.4 (anti-GPIV), or AP1 specific for the glycocalicin domain of GPIb. ▤, normal serum; ▨, patient 5. Brackets indicate mean ± 1 SD of a study done in triplicate.
Discussion
Sporadic cases of acute thrombocytopenia of possible immune etiology have been described previously in patients treated with naproxen17,18 and acetaminophen,19-24 but testing for drug-dependent antibodies either was not done or was described in insufficient detail to show conclusively that they were present. The 5 patients described in this report experienced thrombocytopenia while taking naproxen or acetaminophen and regained a normal platelet count when the drug was discontinued. Although the clinical presentations were suggestive of drug-induced immune thrombocytopenia, antibodies reactive with normal platelets in the presence of the implicated drugs could not be demonstrated.
Sera from patients 1 through 3, who were taking naproxen did, however, contain IgG antibodies that reacted with platelets in the presence of a major naproxen metabolite, the glucuronide conjugate. On the basis of their failure to react with platelets from a patient with type 1 GT or with normal platelets treated to dissociate the GPIIb/IIIa complex, the 3 naproxen-induced antibodies were assigned probable specificity for GPIIb/IIIa.
Sera from patients 4 and 5, who were taking acetaminophen when they became thrombocytopenic, contained IgG (patient 4) and IgA (patient 5) that reacted with normal platelets in the presence of acetaminophen sulfate, 1 of the 2 major metabolites of acetaminophen. On the basis of their reactions with selected target platelets (Table 1), these antibodies were assigned probable specificity for GPIIb/IIIa (patient 4) and GPIb/IX/V (patient 5). This was confirmed for patient 5 by immunoprecipitation with the GPIb-specific mAb MBC 142.11 (Figure 5). The negative reaction obtained with mAb AP-1, specific for the glycocalicin domain of GPIb (Figure 5) suggests this antibody may recognize a site on GPIb close to the AP-1 binding site.
Drug metabolites have been identified as sensitizing agents in patients suspected of having immune thrombocytopenia in only a few previous instances. The best documented example is a study by Eisner and Shahidi,25 who described a young man who developed profound thrombocytopenia after taking large quantities of an analgesic mixture containing acetaminophen and other substances. The patient's serum contained mainly IgA, but also IgG antibodies that activated normal platelets (measured by shortening of the Russell's viper venom clotting time of platelet-rich plasma) and fixed complement in the presence of acetaminophen sulfate, but not acetaminophen itself. It is of interest that the antibody in our patient 5 was also of the IgA class. Three other reports of individual patients with immune thrombocytopenia possibly related to sensitization by a drug metabolite are summarized in Table 2.
Previous reports of immune thrombocytopenia caused by sensitivity to a drug metabolite
| Reference . | Implicated drug . | Ig class . | Sensitizing metabolite . |
|---|---|---|---|
| Eisner and Shahidi,251972 | Acetaminophen | IgA > IgG | Acetaminophen sulfate |
| Eisner and Kasper,26 1972 | PAS | IgG, IgA | Glycine conjugate of PAS? |
| Meyer et al,27 1993 | Ibuprofen | IgG, IgM | Unidentified urinary metabolite |
| Kiefel et al,71987 | Sulfamethoxazole | IgG | N4-acetyl sulfamethoxazole |
| Reference . | Implicated drug . | Ig class . | Sensitizing metabolite . |
|---|---|---|---|
| Eisner and Shahidi,251972 | Acetaminophen | IgA > IgG | Acetaminophen sulfate |
| Eisner and Kasper,26 1972 | PAS | IgG, IgA | Glycine conjugate of PAS? |
| Meyer et al,27 1993 | Ibuprofen | IgG, IgM | Unidentified urinary metabolite |
| Kiefel et al,71987 | Sulfamethoxazole | IgG | N4-acetyl sulfamethoxazole |
Ig indicates immunoglobulin; PAS, para-aminosalicylic acid.
How low-molecular-weight compounds or their metabolites induce a remarkable class of antibodies that recognize specific targets on the platelet membrane only in the presence of soluble drug is unknown. Acetaminophen is oxidized by microsomal enzymes to an electrophilic intermediate, N-acetyl-p-benzoquinoneimine, that reacts irreversibly with free sulfhydryl groups on proteins.28 Glucuronide-conjugated NSAIDs, modified by spontaneous intramolecular rearrangements, can irreversibly bind to free amino groups on protein,29,30 and these conjugates can induce hapten-specific antibodies in animals.31Various other metabolic pathways also convert drugs to reactive intermediates capable of binding covalently to proteins.32-34 Drug-protein conjugates created by these mechanisms should, in theory, be capable of triggering a hapten-dependent immune response in ways that are fairly well understood.35-37 However, hapten-induced antibodies can generally be inhibited by soluble hapten at high concentrations, whereas antibodies typical of drug-induced immune cytopenia recognize their targets even when drug is present at the highest concentrations achievable in vitro.4 38 How soluble drug promotes tight binding of such antibodies to a specific cell membrane target is unresolved.
Our findings indicate that the glucuronide conjugate of naproxen should be added to the list of drug metabolites capable of causing immune cytopenia and confirm the previous report by Eisner and Shahidi25 that immune thrombocytopenia can be provoked by acetaminophen sulfate. In patients with a clinical picture strongly suggestive of drug-induced immune thrombocytopenia but who test negative for antibodies with the primary drug, sensitivity to a drug metabolite should be considered. Further studies of this possibility may bear out the suggestion made a decade ago by Mueller-Eckhardt and Salama8 that involvement of metabolites in immune cytopenias may be “a general rule rather than the exception.”
Supported by grant HL13629 from the National Heart, Lung, and Blood Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Daniel Bougie, Blood Research Institute, The Blood Center of Southeastern Wisconsin, PO Box 2178, Milwaukee, WI 53201-2178; e-mail: dwbougie@bcsew.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal