Recent studies indicate that angiogenesis is important in the pathogenesis of leukemias, apart from its well-established role in solid tumors. In this study, the possible role of angiogenesis in acute promyelocytic leukemia (APL) was explored. Bone marrow trephine biopsies from patients with APL showed significantly increased microvessel density and hot spot density compared with normal control bone marrow biopsies. To identify the mediators of angiogenesis in APL, quantitative and functional assays were performed using the NB4 APL cell line as a model system. Conditioned media (CM) from the NB4 cells strongly stimulated endothelial cell migration. CM from the NB4 cells contained high levels of vascular endothelial growth factor (VEGF) but not basic fibroblast growth factor (bFGF). Most important, the addition of neutralizing VEGF antibodies completely inhibited the ability of NB4 CM to stimulate endothelial cell migration, suggesting that APL angiogenesis is mediated by VEGF. The effect of all-transretinoic acid (ATRA) on APL angiogenesis was then studied. ATRA therapy resulted in a decrease in bone marrow microvessel density and hot spot density. CM from ATRA-treated APL cells did not stimulate endothelial cell migration. Finally, quantitative assays showed that ATRA treatment resulted in the abrogation of VEGF production by the NB4 cells. These results show that there is increased angiogenesis and VEGF production in APL and that ATRA therapy inhibits VEGF production and suppresses angiogenesis. The addition of specific antiangiogenic agents to differentiation therapy or chemotherapy should be explored.
Introduction
Angiogenesis is an essential phenotype in growth and development,1 wound healing,2 and reproduction.3-5 An inadequate amount of blood vessel growth contributes to ulcer formation,6 whereas excessive angiogenesis contributes to a number of pathologic conditions including arthritis, psoriasis, and neoplasia.7
In a series of now classical experiments, Folkman and colleagues8 demonstrated that solid tumors cannot grow any larger than 2 to 3 mm in diameter without being able to induce their own blood supply. Recent evidence suggests that angiogenesis is critical in the pathogenesis of numerous different hematologic malignancies, including acute lymphoblastic leukemia9,10acute myelogenous leukemia,11-13 chronic lymphocytic leukemia,14 chronic myeloid leukemia,15 and multiple myeloma.16-18
The purpose of this study was to examine the role of angiogenesis in acute promyelocytic leukemia (APL). APL is distinct from other forms of acute myelogenous leukemia on clinical, morphologic, and molecular bases. The molecular hallmark of this disease is the presence of a balanced reciprocal translocation involving the retinoic acid receptor α (RARα) gene on chromosome 17 in all patients.19-23 This type of leukemia has been extensively studied and is amenable to molecular analysis because of the availability of excellent model systems that recapitulate the disease.24-28
We also wanted to determine the possible effect of all-transretinoic acid (ATRA) on angiogenesis in APL. ATRA therapy has brought about significant improvement in the remission rates of patients with APL29,30 and is effective, at least in part, by inducing differentiation of the abnormal promyelocytes. We had previously shown that ATRA is antiangiogenic in oral squamous cell carcinoma,31 32 and we wanted to assess whether ATRA therapy also has an antiangiogenic effect in APL.
Our results demonstrate that angiogenesis is increased in APL and is mediated by vascular endothelial growth factor (VEGF). In addition, we report that ATRA therapy inhibits VEGF production and is accompanied by a decrease in microvessel density.
Patients, materials, and methods
Patients
Each patient included in this study (n = 12; 8 women, 4 men) had a confirmed diagnosis of APL by either conventional cytogenetics showing the t(15;17) translocation or by polymerase chain reaction for the PML-RARα fusion protein. The mean age was 47.3 years. Of the 12 original patients, bone marrow biopsy specimens were available for 7 patients after ATRA treatment. All 7 patients were in morphologic remission. Of these 7 patients, 4 also underwent concurrent induction chemotherapy. Post-ATRA bone marrow biopsies were taken 23 to 93 days after diagnostic biopsies. Control bone marrow biopsy specimens (n = 12) were obtained from patients undergoing staging biopsy for breast carcinoma and lymphoma and from patients evaluated for anemia and thrombocytopenia. The mean age of the control patients was 52.8 years (8 women, 4 men).
Measurement of microvessel density
The degree of angiogenesis in the APL specimens was quantified by measuring microvessel density in bone marrow trephine sections of patients with newly diagnosed disease (n = 12). Vessel presence in tissue sections was highlighted by the use of standard immunohistochemistry techniques33 using antibodies to CD34 (Immunotech, Westbrook, ME). Any endothelial cell cluster distinct from other endothelial cells, nonendothelial cells, and connective tissue was counted as a microvessel. It was unnecessary for a lumen to be present to define a microvessel. The entire bone marrow core section was examined, and microvessel density was quantified as the average number of microvessels per high-power field (600 ×). Hot spot density was measured by examining the bone marrow core section at low power (100 ×) and identifying the area with the highest microvessel density. Microvessel density in this area was then measured at high magnification (600 ×). In addition, expression of the angiogenic peptide VEGF (Santa Cruz Biotechnology, Santa Cruz, CA) in bone marrow biopsies was assessed using standard immunohistochemistry techniques.33
Cell culture, ATRA treatment, and collection of conditioned media
The NB4 APL cells (obtained as a kind gift from Dr Peter Domer, University of Chicago) were cultured in RPMI 1640 with 10% heat-inactivated fetal calf serum and 1% penicillin-streptomycin and maintained at 37°C and 5% CO2. ATRA (Sigma Pharmaceuticals, St Louis, MO) was prepared in dimethyl sulfoxide and stored as stock solution of 10−1 M at −80°C. ATRA was then added directly to the culture medium of the NB4 cells, with a final concentration of 10−6 M. NB4 cells were cultured in ATRA for 3 days before conditioned media (CM) were generated. Serum-free CM was generated from ATRA-treated and untreated cells by rinsing the cells in RPMI 3 times, incubating the cells in RPMI for 4 hours, refeeding the cells with RPMI, and collecting the CM after 24 hours. Levels of the angiogenic peptides basic fibroblast growth factor (bFGF) and VEGF in the CM were quantified using the Quantikine bFGF and VEGF assays (R&D Systems, Minneapolis, MN), according to the manufacturer's protocols. For endothelial cell migration assays, CM were concentrated using Centriprep-3 concentrators (Amicon, Beverly, MA), and protein concentration was determined using the Coomassie protein assay reagent 23200 (Pierce Biochemical, St Louis, MO).
Endothelial cell migration assays
Endothelial cell migration assay was performed as previously described.31 Briefly, human dermal microvascular endothelial cells (Cell Systems, Kirkland, WA) were starved overnight in endothelial basal media (Clonetics, San Diego, CA) containing 0.1% bovine serum albumin (BSA), harvested, resuspended into endothelial basal media with 0.1% BSA, plated on the bottom side of a modified Boyden chamber (Neuro Probe, Gaithersburg, MD), and allowed to attach in an inverted chamber at 37°C for 2 hours. The chamber was then re-inverted, and the test substances were added to the wells of the upper chamber (1 μg protein/well in a volume of 50 μL). After 4 hours the membranes were stained, and the number of endothelial cells that migrated to the upper chamber were counted per 10 high-power fields. Background migration was detected by using BSA alone, and purified growth factors (VEGF, 100 pg/mL; bFGF, 15 ng/mL) were used a positive controls. Neutralizing antibodies were used at concentrations previously optimized by dose-response experiments (anti-VEGF, 20 μg/mL; anti-bFGF, 20 μg/mL). All growth factors and antibodies were purchased from R&D Systems. The addition of neutralizing antibodies alone did not affect endothelial cell migration. Neutralizing antibodies completely inhibited the ability of the purified growth factors to stimulate migration.
Statistical analyses
Statistical analyses were performed using the Studentt test. Differences were considered statistically significant whenP < .05. Statistical analyses were performed using the Prophet 5.0 program (BBN Systems and Technologies, Cambridge, MA).
Results
Increased angiogenesis in APL bone marrow biopsy specimens
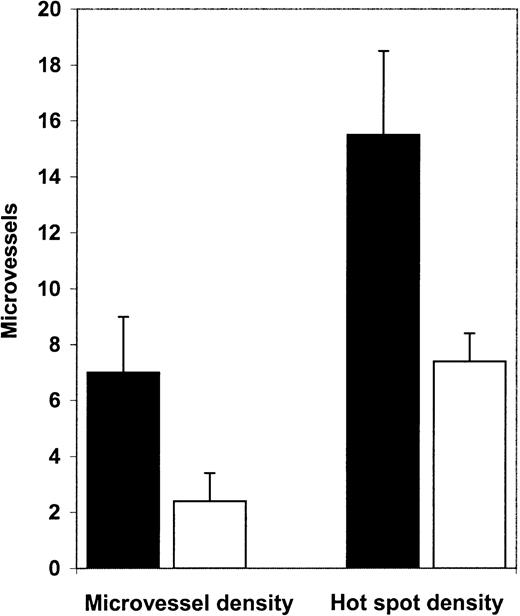
Diagnostic bone marrow biopsy specimens from patients with APL had significantly higher microvessel density than normal control marrows (Figure 1). Mean microvessel density in APL marrows was 7.0/high-power field (hpf), whereas in normal control marrow it was 2.4/hpf (P = .0001, Figure2A). In addition, we examined hot spot density in the APL and control biopsies. Hot spot is defined as the high-power field with the highest microvessel density. An area of high microvessel density, such as a hot spot, may represent the emergence of an angiogenic clone and may be responsible for spread of the disease. Hot spot density was also significantly higher (P = .0007; Figure 2B) in APL marrows (15.5/hot spot) than in normal marrows (7.4/hot spot)
Microvessels in APL and control bone marrow biopsy specimens.
(A) Many microvessels were evident in this diagnostic bone marrow trephine biopsy specimen from a patient with APL. (B) In contrast, this normal control bone marrow biopsy sample had few microvessels. Microvessels were highlighted by immunohistochemistry with the use of antibodies to CD34 (brown). Original magnification, 600 ×.
Microvessels in APL and control bone marrow biopsy specimens.
(A) Many microvessels were evident in this diagnostic bone marrow trephine biopsy specimen from a patient with APL. (B) In contrast, this normal control bone marrow biopsy sample had few microvessels. Microvessels were highlighted by immunohistochemistry with the use of antibodies to CD34 (brown). Original magnification, 600 ×.
Microvessel density and hot spot density in APL and control bone marrow biopsy specimens.
Microvessel density in diagnostic APL bone marrow biopsy specimens (▪, n = 12) was 7.0/hpf, whereas microvessel density in normal control bone marrow biopsy samples (■, n = 12) was 2.4/hpf (P = .0001). Hot spot density in APL bone marrow biopsy specimens (15.5/hot spot) was significantly (P = .0007) higher than in control bone marrow biopsy specimens (7.4/hot spot)
Microvessel density and hot spot density in APL and control bone marrow biopsy specimens.
Microvessel density in diagnostic APL bone marrow biopsy specimens (▪, n = 12) was 7.0/hpf, whereas microvessel density in normal control bone marrow biopsy samples (■, n = 12) was 2.4/hpf (P = .0001). Hot spot density in APL bone marrow biopsy specimens (15.5/hot spot) was significantly (P = .0007) higher than in control bone marrow biopsy specimens (7.4/hot spot)
Angiogenesis in APL is mediated by VEGF
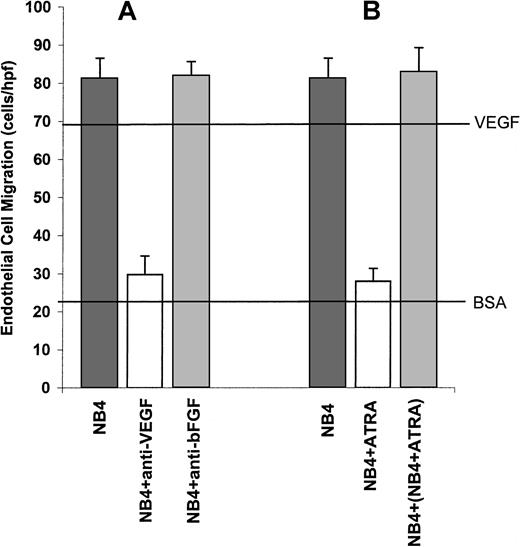
Whether angiogenesis occurs in a particular tissue depends on the balance between the relative amounts of molecules that induce and molecules that inhibit angiogenesis.34 We used the NB4 cell line as a model system to study the possible inducers or suppressors of APL angiogenesis. The NB4 cell line was established from a patient with APL and has all the characteristic features of APL including the t(15;17) translocation.35 To assess the angiogenic potential of the NB4 cells, the conditioned media (CM) from these cells were tested in the endothelial cell migration assay. CM from NB4 cells strongly stimulated endothelial cell migration (Figure3A). To identify the possible mediators of angiogenesis within the NB4 CM, we performed quantitative assays for the angiogenic peptides basic fibroblast growth factor (bFGF) and VEGF. The CM from the NB4 cells contained high levels of VEGF but not bFGF (Table 1). These data suggested that NB4 cells induced endothelial cell migration through the production of VEGF. However, there are at least 18 known inducers of angiogenesis,36 and it is possible that substances other than VEGF are involved in stimulating migration. To test this possibility, we used neutralizing antibodies to VEGF and bFGF in the endothelial cell migration assays. The addition of anti-VEGF neutralizing antibodies completely inhibited endothelial cell migration, indicating that VEGF is a major mediator of angiogenesis (Figure 3A). As was expected, the addition of anti-bFGF antibodies had no effect on endothelial cell migration (Figure 3A).
VEGF-mediated stimulation of endothelial cell migration by NB4 CM.
(A) Conditioned media from NB4 cells strongly stimulated endothelial cell migration. Migration was completely inhibited by the addition of antibodies to VEGF. The addition of anti-bFGF antibodies had no effect on endothelial cell migration. The upper horizontal line is a positive control using purified VEGF. The lower horizontal line is a negative control using bovine serum albumin. (B) In contrast to CM from untreated NB4 cells, CM from NB4 cells treated with ATRA did not stimulate endothelial cell migration. The addition of CM from NB4 cells treated with ATRA to CM from untreated NB4 cells did not result in the inhibition of endothelial cell migration.
VEGF-mediated stimulation of endothelial cell migration by NB4 CM.
(A) Conditioned media from NB4 cells strongly stimulated endothelial cell migration. Migration was completely inhibited by the addition of antibodies to VEGF. The addition of anti-bFGF antibodies had no effect on endothelial cell migration. The upper horizontal line is a positive control using purified VEGF. The lower horizontal line is a negative control using bovine serum albumin. (B) In contrast to CM from untreated NB4 cells, CM from NB4 cells treated with ATRA did not stimulate endothelial cell migration. The addition of CM from NB4 cells treated with ATRA to CM from untreated NB4 cells did not result in the inhibition of endothelial cell migration.
Production of angiogenic peptides by NB4 cells
| . | VEGF (pg/mL) . | bFGF (pg/mL) . |
|---|---|---|
| NB4 | 243.4 ± 28.9 | 1.2 ± 0.5 |
| NB4 + ATRA | <5 | 0.8 ± 0.7 |
| . | VEGF (pg/mL) . | bFGF (pg/mL) . |
|---|---|---|
| NB4 | 243.4 ± 28.9 | 1.2 ± 0.5 |
| NB4 + ATRA | <5 | 0.8 ± 0.7 |
VEGF indicates vascular endothelial growth factor; bFGF, basic fibroblast growth factor; ATRA, all-trans retinoic acid; CM, conditioned media.
CM obtained from NB4 cells contained high concentrations of VEGF but not of bFGF. Treatment of NB4 cells with ATRA resulted in abrogation of VEGF production. Levels of angiogenic peptides in the CM are expressed in terms of pg/mL ± SEM.
ATRA therapy inhibits VEGF production and angiogenesis in vitro and in vivo
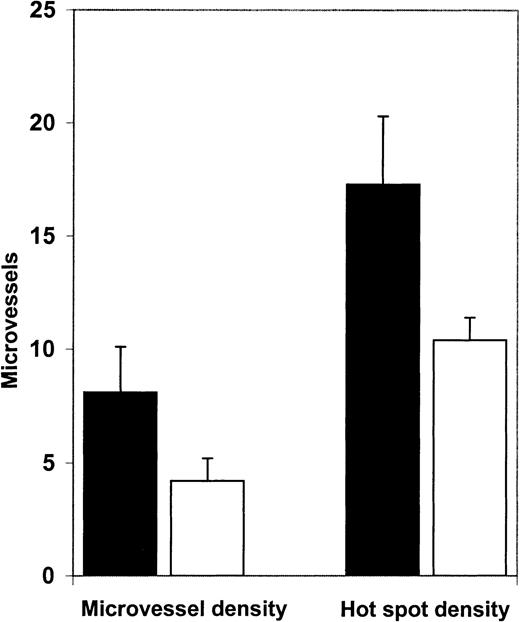
We then examined angiogenesis in bone marrow biopsy samples from patients in morphologic remission after ATRA therapy. ATRA-treated bone marrows had fewer microvessels than the diagnostic bone marrow samples (Figure 4). Quantification (Figure5A) revealed a significant decrease (P = .0063) in microvessel density in bone marrow samples from APL patients treated with ATRA (4.2/hpf) in comparison to their corresponding diagnostic bone marrow biopsy samples (8.1/hpf). Hot spot density (10.4/hot spot) was also lower in the ATRA-treated bone marrow samples than in the corresponding diagnostic bone marrow samples (17.3/hot spot; P = .0159).
Microvessels in APL bone marrows at diagnosis and remission.
Bone marrow trephine section from an APL patient in ATRA-induced remission (A) had fewer microvessels than a diagnostic bone marrow biopsy specimen from the same patient (B). Microvessels were highlighted immunohistochemically with the use of antibodies to CD34 (brown). Original magnification, 600 ×.
Microvessels in APL bone marrows at diagnosis and remission.
Bone marrow trephine section from an APL patient in ATRA-induced remission (A) had fewer microvessels than a diagnostic bone marrow biopsy specimen from the same patient (B). Microvessels were highlighted immunohistochemically with the use of antibodies to CD34 (brown). Original magnification, 600 ×.
Microvessel density and hot spot density in APL bone marrows at diagnosis and remission.
Microvessel density in bone marrow biopsy specimens obtained from patients (n = 7) in ATRA-induced remission (■, 4.2/hpf) was significantly decreased (P = .0063) compared with microvessel density (8.1/hpf) in diagnostic bone marrow biopsy samples from the same patients (▪). Hot spot density in bone marrow biopsy specimens from APL patients in ATRA-induced remission (10.4/hot spot) was also significantly (P = .0159) lower than hot spot density (17.3/hot spot) in their corresponding diagnostic bone marrow biopsy specimens.
Microvessel density and hot spot density in APL bone marrows at diagnosis and remission.
Microvessel density in bone marrow biopsy specimens obtained from patients (n = 7) in ATRA-induced remission (■, 4.2/hpf) was significantly decreased (P = .0063) compared with microvessel density (8.1/hpf) in diagnostic bone marrow biopsy samples from the same patients (▪). Hot spot density in bone marrow biopsy specimens from APL patients in ATRA-induced remission (10.4/hot spot) was also significantly (P = .0159) lower than hot spot density (17.3/hot spot) in their corresponding diagnostic bone marrow biopsy specimens.
To study the possible role of ATRA on APL angiogenesis in our in vitro system, we then treated the NB4 cells with ATRA and assayed its ability to stimulate endothelial cell migration. CM from ATRA-treated APL cells did not stimulate endothelial cell migration (Figure 3B). In addition, mixing CM from NB4 cells and ATRA-treated NB4 cells did not inhibit migration (Figure 3B), indicating that the loss of angiogenic potential by NB4 cells resulted from the loss of production of an inducer of angiogenesis rather than from the production of an inhibitor of angiogenesis. Quantitative assays showed that ATRA treatment resulted in the abrogation of VEGF production by the NB4 cells (Table1).
Finally, to confirm the relevance of our results in vivo, we performed VEGF immunostaining in the APL bone marrow biopsy samples. Figure6 shows that the tumor cells of the diagnostic bone marrow biopsy specimens were positive for VEGF and that ATRA treatment resulted in a marked decrease in VEGF staining.
VEGF staining in APL bone marrows at diagnosis and remission.
(A) Diagnostic bone marrow biopsy sample from a patient with APL showed strong VEGF staining in the tumor cells. (B) Bone marrow biopsy sample from the same patient obtained after remission induced by ATRA therapy showed decreased VEGF staining intensity.
VEGF staining in APL bone marrows at diagnosis and remission.
(A) Diagnostic bone marrow biopsy sample from a patient with APL showed strong VEGF staining in the tumor cells. (B) Bone marrow biopsy sample from the same patient obtained after remission induced by ATRA therapy showed decreased VEGF staining intensity.
Discussion
We have demonstrated increased angiogenesis in the bone marrow of patients with APL. Both microvessel density and hot spot density were higher in diagnostic bone marrow biopsy specimens from patients with APL than in normal control bone marrow. These findings are consistent with increased bone marrow angiogenesis observed in numerous types of acute and chronic leukemia and in multiple myeloma. In APL, abnormal promyelocytes proliferate in the bone marrow, and increased angiogenesis may support this proliferation. Bone marrow angiogenesis may also facilitate the spread of leukemic cells beyond the bone marrow. The experiments presented here suggest that VEGF is a major mediator of angiogenesis in APL. Cultured NB4 APL cells produced VEGF, and APL cells in bone marrow biopsy samples were positive for VEGF. Conditioned media from NB4 cells stimulated endothelial cell migration; this migration was completely inhibited by anti-VEGF antibodies.
In addition to stimulating the production of new blood vessels, VEGF may be involved in complex autocrine and paracrine interactions in the bone marrow microenvironment. One possibility is that there may be paracrine interactions in which VEGF produced by APL cells promotes endothelial cell migration and proliferation and that the endothelial cells produce factors that promote tumor cell growth. This paracrine interaction would result in a positive feedback loop that may enhance both angiogenesis and tumor cell proliferation. Other examples of this type of paracrine loop occur in leukemias. Paracrine pathways involving leukemic cells have been described for tumor necrosis factor-α37 and interleukin (IL)-1.38 In addition, Fiedler et al12 found that VEGF stimulated the production of granulocyte macrophage colony stimulating factor (GM-CSF), and Bellamy et al39 reported that VEGF can stimulate M-CSF, G-CSF, IL-6, and stem cell factor production in human umbilical vein endothelial cells. Each of these cytokines could potentially elicit growth-stimulatory signals on leukemic cells. Alternatively, it is possible that VEGF may act in an autocrine fashion12,40 and have some positive biologic effects on the leukemic cells themselves. In APL, there may also be a link between angiogenesis and the severe coagulopathies that accompany the disease. An important mediator of the coagulation abnormalities in APL is tissue factor.41 VEGF has been shown to increase tissue factor expression42 43 and may, therefore, be responsible, at least in part, for the disordered coagulation by acting in an autocrine fashion on the APL cells.
ATRA therapy results in a decrease in microvessel density, hot spot density, and VEGF reactivity in the bone marrow of patients with APL. NB4 cells treated with ATRA do not stimulate endothelial cell migration because of suppression of VEGF production. Previously, we had demonstrated that retinoic acid can modulate expression of the angiogenic phenotype through 2 different pathways. ATRA induces squamous cell carcinoma tumor cells to produce a retinoic acid-inducible inhibitor of angiogenesis.32 In addition, it can cause endothelial cells to become refractory to inducers of angiogenesis.31 The inhibition of VEGF secretion by ATRA represents a third possible mechanism by which retinoids modulate tumor-induced angiogenesis.
ATRA induces the differentiation of abnormal promyelocytes into neutrophils. This differentiation takes place through multiple mechanisms, including degradation of the PML-RARα protein, transcriptional activation of the wild-type RARα gene, and nuclear relocalization of PML and other proteins.44 The reduction of VEGF production on ATRA treatment could be part of the differentiation process that results in the conversion of the abnormal promyelocytes into mature neutrophils. An alternative explanation is that ATRA directly down-regulates APL tumor cell expression of VEGF, independent of the differentiation pathway. Direct down-regulation of VEGF production after retinoic acid treatment has been observed in human keratinocytes45 and is dependent on the anti–AP-1 activity of retinoic acid.46
Recent improvements in the treatment of APL have led to 4-year disease-free survival rates greater than 70%.47-49However, toxic effects of induction therapy (including retinoic acid syndrome) and relapse remain the major obstacles to cure.48,50 A possible strategy to improve outcomes would be to use retinoids for their differentiating and antiangiogenic activities in conjunction with specific antiangiogenic agents such as anti-VEGF antibodies. The use of multiple inhibitors of angiogenesis to decrease toxicity while maintaining efficacy has been demonstrated in a number of different settings.51-54 Because VEGF stimulates tissue factor production, anti-VEGF therapy may also aid in ameliorating the coagulopathies seen in APL. Although our results suggest that the angiogenesis paradigm may be extended to APL, they do not show that angiogenesis is essential in APL. Prospective clinical trials using specific antiangiogenic drugs, alone or in combination with existing therapeutic modalities, are necessary to assess the importance of angiogenesis in APL and other types of leukemia.
We thank Drs William J. Karpus and James Bartles and members of their laboratories for the use of facilities and assistance with some of the experiments, and we thank Dr Charles Goolsby for helpful discussions.
Supported in part by grants from the National Institutes of Health (CDE 12322) and the Cardinal Bernardin Cancer Center (P20 CA79403).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ameet R. Kini, Department of Pathology, Bldg 110, 2nd Fl, Loyola University Medical Center, 2160 South First Ave, Maywood, IL 60153; e-mail: akini000@md.northwestern.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal