Abstract
The mechanism whereby HIV-infected cells transit from the bloodstream into tissues is not well defined. This phenomenon was addressed by studying the effects of HIV-1 Tat, a protein secreted by infected cells, on human lung microvascular endothelial cells (HMVEC-Ls). It was found that monocyte chemoattractant protein-1 (MCP-1) was released from HMVEC-Ls in a dose- and time-dependent manner after Tat treatment. MCP-1 is a potent β-chemokine that recruits monocytes and T cells and promotes cell adhesion and transmigration across an endothelial monolayer. It was also observed that MCP-1 and the culture medium from Tat-treated HMVEC-Ls were chemotactic for CD14+ monocytes from human peripheral blood and for THP-1, a promonocytic cell line used as a model system. To characterize the signaling pathways underlying the observed induction of MCP-1, HMVEC-Ls were treated with 2 different protein kinase inhibitors: PD98059, a MAP kinase inhibitor, and GF109203X, a protein kinase C (PKC) inhibitor. MCP-1 release was significantly reduced when PKC was inhibited, and slightly decreased when PI3 kinase was blocked; no effect on MCP-1 release was observed on MAP kinase inhibition. Similarly, transmigration of THP-1 cells was significantly impaired by the PKC inhibitor, but not by the other tested inhibitors. These data indicate that the HIV-1 Tat protein may act as a protocytokine by causing the release of MCP-1 from the endothelial monolayer, and thereby facilitating monocyte transmigration into tissues via a PKC signaling pathway.
Introduction
Although both CD4+ T cells and monocytes/macrophages are infected by HIV-1, it is generally believed that monocytes/macrophages serve as reservoirs for virus replication and act as the primary source of transmission of virus to various tissues.1 Indeed, most of the virus found in the extravascular tissues of AIDS patients has been shown to reside in macrophages.2,3 In the lung, alveolar macrophages are the predominant resident immune cells in the alveolar airspace and are targets for HIV-1 infection.4 Direct infection of HIV-1 into these macrophages impairs monocyte/macrophage effector cell functions, thereby inviting opportunistic infections. Thus, HIV-1–infected monocytes/macrophages in peripheral tissues are an important therapeutic target in AIDS treatment, particularly in relation to restoring host defenses.
Localization of monocytes to the peripheral tissues begins with interactions between the adhesion molecules LFA-1, -2, and -3, expressed on the monocyte surface, and the vascular endothelium.5,6 The monocytes then traverse the endothelial barrier by producing extracellular matrix-degrading metalloproteinases, which allow passage through the vascular basement membrane.7 In the process of monocytic infiltration, it is believed that monocyte chemoattractant protein-1 (MCP-1), a member of the beta (C-C) subfamily of chemokines, plays an important role. It is known that mechanical shear stress increases MCP-1 secretion in cultured human endothelial cells8-11 and in astrocytes, the most abundant cell type in the brain,12,13 resulting in increased monocyte adhesion to the endothelium. Further, MCP-1 is the most potent of the various monocyte chemoattractants, which include RANTES, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and MCP-1, -2, and -3.14
HIV-1 Tat protein is released from HIV-1–infected T cells and monocytes/macrophages15 as a protocytokine. It interacts specifically with vascular endothelial growth factor (VEGF) and the beta-integrin receptor molecules of endothelial cells.16-19 Tat ligation of these specific surface molecules activates several cellular protein kinases,18,20-22 including Flk-1/KDR, Flt-1, and MAP kinase. HIV-1 Tat induces MCP-1 secretion from astrocytes23 and is thought to enhance monocyte-macrophage trafficking into the central nervous system. We postulated that MCP-1 could induce the transmigration of HIV-1–infected monocytes across an endothelial monolayer. This transmigration would increase viral transmission to extravascular compartments and play a role in HIV-1–associated immune dysfunction. To address this hypothesis, we assessed a model of Tat-mediated MCP-1 secretion from primary human lung microvascular endothelial cells (HMVEC-Ls) and characterized downstream signaling substrates involved in MCP-1 secretion and the transmigration of monocytes. We identified protein kinase C (PKC) as a key signaling molecule that mediates Tat effects in such microvascular endothelium.
Materials and methods
Antibodies
The anti–Flk-1, anti–Flt-1, and antiphosphotyrosine (PY99) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and the antihuman CCR2 monoclonal antibody was purchased from R & D Systems (Minneapolis, MN).
Cell cultures
HMVEC-Ls, obtained from Clonetics Inc (San Diego, CA), were cultured in EGM-2M medium containing microvascular endothelial cell growth factors, antimicrobials, and 5% fetal bovine serum (FBS). To avoid phenotypic drift associated with decreasing expression of surface receptor molecules, HMVEC-Ls were not used beyond passage 4. The THP-1 monocytic cells (ATCC TIB 202), obtained from the American Type Culture Collection (Rockville, MD), were cultured in DMEM supplemented with 10% FBS. The CD14+ monocytes were isolated from human peripheral blood mononuclear cells (PBMCs) by using CD14 MicroBeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol. Briefly, human PBMCs were isolated by density gradient centrifugation with Ficoll-Hypaque and then mixed with MACS CD14 MicroBeads. After 15 minutes incubation at 4°C, CD14+cells were separated by using a positive selection column, type MS+/RS+. Flow cytometric analysis showed that the purity of the CD14+ cells was more than 93%.
Tat protein
HIV-1 IIIB Tat protein was purified, lyophylized, and reconstituted in Tat buffer (phosphate-buffered saline [PBS] containing 1 mg of bovine serum albumin [BSA] and 0.1 mM dithiothreitol [DTT]), as described.18 The purified protein was endotoxin free, as judged from the timed gel formation assay with the Limulus amoebocyte lysate reagent (Sigma). This protein was biologically active, as assessed by the rescuability of tat-defective provirus replication in HLM-1 cells with the purified Tat protein.18
Tat treatment of cells
At 80% confluence, cells were serum-starved overnight by replacing culture medium with the endothelial basal medium, EBM-2 (Clonetics), supplemented with 0.5% FBS. The cells were then treated with HIV-1 Tat protein (at the indicated concentrations) plus 10 U/mL heparin, or with 10 U/mL of heparin alone, in fresh EBM-2 medium containing 0.5% FBS for the indicated periods. Heparin was used in these experiments, as it is known to augment the biologic activities of Tat, such as the induction of endothelial cell growth, migration, and invasion in vitro.24 To examine the effect of inhibition of intracellular kinases on Tat-induced MCP-1 secretion, starved HMVEC-Ls were incubated with the indicated amounts of inhibitors or with diluent controls in EBM-2 medium 1 hour before treatment with Tat.
Quantitation of MCP-1, MIP-1α, and MIP-1β in culture supernatants
After Tat stimulation, culture supernatants were collected, and cell debris removed by low-speed centrifugation at 1500 rpm for 10 minutes. MCP-1, MIP-1α, and MIP-1β levels in the clarified supernatants were measured by using a commercial MCP-1, MIP-1α, or MIP-1β antigen capture enzyme-linked immunosorbent assay (ELISA) kit (R & D Systems) according to the manufacturer's instructions. All results are the average of 2 or more experiments.
Flow cytometric analyses of CCR2 expression
CCR2 is a major chemokine receptor for MCP-1. To investigate MCP-1–mediated chemotaxis, we first examined the expression of CCR2 on the surface of THP-1 cells and HMVEC-Ls by flow cytometric analysis. Briefly, THP-1 cells were washed once with PBS and incubated with antihuman CCR2 MAB150 (5 μg/mL) (R & D Systems) or with isotype IgG as a control in 1 mL of PBS with 10% normal goat serum for 1 hour on ice, followed by treatment with FITC-conjugated goat antimouse secondary antibody (1:500 dilution) (Boehringer-Mannheim, Indianapolis, IN) for 20 minutes on ice. Surface expression of each sample was then analyzed on an Epics Profile Flow Cytometer (Coulter Electronics, Hialeah, FL). To evaluate CCR2 expression in HMVEC-Ls, cells were detached, washed once, and then incubated with the primary antibody, as described previously.
Chemotaxis assay
Chemotaxis was assayed in 24-well plates (CoStar Corp, Cambridge, MA) carrying transwell inserts of 5 μm pore size. Briefly, CD14+ monocytes from human peripheral blood or THP-1 cells were washed twice, resuspended, and then loaded onto the inserts at 3 × 105 cells/200 μL for each assay. Chemotaxis medium containing the indicated chemoattractant was placed in the bottom chamber. After 3 hours of incubation at 37°C with 5% CO2, cells that migrated to the lower chamber were collected; the percentage of viable cells that migrated from the top to the bottom compartment was measured by counting cells with the trypan blue dye exclusion method. To investigate the effects of the inhibitors involved in the signal transduction or of the concentration gradients of the chemoattractants on the migration of CD14+ monocytes or THP-1 cells, the cells were incubated for 1 hour with the indicated inhibitors or chemoattractants before their loading onto transwell inserts.
MAP kinase assay
The in vitro MAP kinase assay was performed as described previously.18 Briefly, after the indicated treatments, cells were lysed by the addition of radioimmunoprecipitation assay (RIPA) buffer,18 and the lysates were immunoprecipitated with the indicated monoclonal antibodies. The precipitates were washed twice with deoxycholate-free RIPA buffer, followed by one wash with kinase assay buffer (20 mM HEPES, pH 7.4, 50 mM NaCl, 5 mM MgCl2, 5 mM MnCl2, 100 mM Na3VO4). The washed precipitates were then incubated at room temperature for 30 minutes in a kinase assay buffer containing 0.18 MBq (5 μCi) [γ32P]-ATP along with: (1) myelin basic protein (MBP) (Upstate Biotechnology, Lake Placid, NY) for the p44/42 MAP (Erk1/2) or p38 kinase assay, or (2) c-Jun for the Jun kinase assay. The reaction was stopped by adding 4 times sodium dodecylsulfate (SDS) sample buffer. The kinase substrate proteins were analyzed on 12% SDS-polyacrylamide gels (PAGE).
Protein kinase C assay
PKC activity was assayed by quantification of 32P incorporation from [γ32P]-ATP into the substrate, MARCKSPSD peptide (Calbiochem, Inc, La Jolla, CA). Assays were performed at room temperature in a total volume of 20 μL containing 20 mM HEPES-NaOH (pH 7.5), 10 mM MgCl2, 10 mM DTT, 10 μg/mL leupeptin, 0.1 mM CaCl2, 0.1 mM [γ32P]-ATP, 0.03% Triton X-100, and 0.5 μg MARCKSPSD peptide,25 5 μg cell lysate, 0.3 mg/mL phosphatidylserine (PS), and 62 μg/mL 1,2-diolein. Reactions were initiated by the addition of [γ32P]-ATP and terminated after 15 minutes by adding 1 mL 25% trichloroacetic acid (TCA) and 10 μL of BSA (50 mg/mL). Samples were then precipitated by centrifugation at 12 000 × g for 2 minutes, the pellet rinsed twice with 0.5 mL of 25% TCA, and air-dried. Incorporation of32P into the substrate was then quantitated by Cherenkov counting.
Immunoprecipitation and Western blot analysis
For the immunoprecipitation studies, an equivalent amount of protein from each sample was clarified by incubation with protein A-Sepharose for 1 hour at 4°C, and the antigen in the clarified solution was then immunoprecipitated with the indicated primary antibody, as described.18 The precipitates were next separated by SDS-PAGE, followed by transfer to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk protein and probed with the indicated primary antibody at 4°C overnight. Immunoreactive bands were visualized by using horseradish peroxidase (HRP)–conjugated secondary antibody and the enhanced chemiluminescent (ECL) system (Amersham Pharmacia Biotech, Piscataway, NJ).
Results
Tat effect on MCP-1 secretion
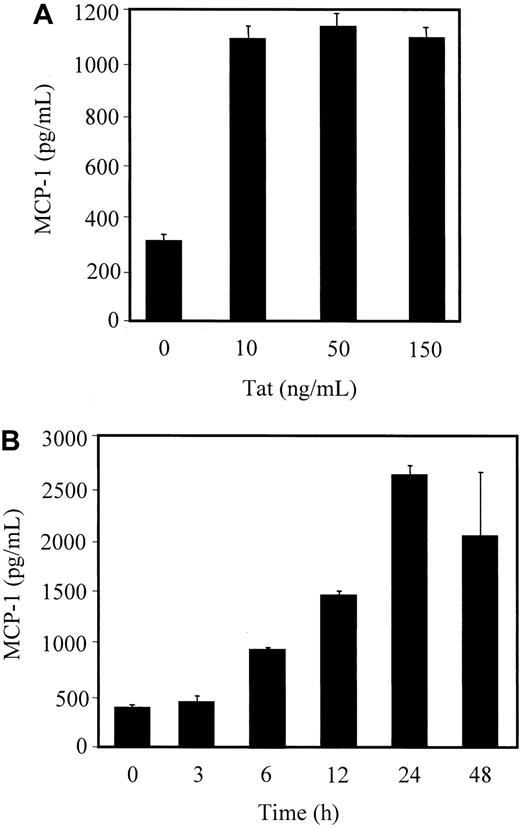
To examine modulation of MCP-1 secretion from endothelial cells on HIV-1 Tat treatment, HMVEC-Ls at 80% confluence were stimulated with Tat protein plus heparin or with heparin alone in culture medium for the indicated periods. MCP-1 concentrations in the clarified culture supernatants were then measured by an ELISA.
Treatment of HMVEC-L with Tat significantly induced MCP-1 secretion into the culture supernatants. This induction of secretion peaked at 24 hours after Tat stimulation (Figure 1B). We further assessed whether the Tat effect on MCP-1 release was dose-dependent. HMVEC-Ls were stimulated over 24 hours with different concentrations of Tat in culture media, and the amount of MCP-1 in the supernatant was quantitated by ELISA. MCP-1 secretion was significantly augmented by Tat, at concentrations as low as 10 ng/mL (Figure 1A). A significant amount of MCP-1 was secreted from HMVEC-Ls treated with 6.4 ng/mL of Tat, whereas MCP-1 secretion was barely detectable at 1.6 ng/mL of Tat (data not shown). Because MIP-1α and MIP-1β are also known as potent chemoattractants for the migration of monocytes, we examined the secretion of these chemokines into the culture supernatants. MIP-1β, but not MIP-1α, secretion was detected at a concentration of approximately 300 pg/mL after 24 hours treatment of HMVEC-Ls with 50 ng/mL of Tat (data not shown). Taken together, these data indicate that Tat protein enhances MCP-1 release from HMVEC-Ls and that this enhancement of MCP-1 is time- and dose-dependent. Because MCP-1, and not MIP-1α or MIP-1β, is the major chemokine secreted from HMVEC-Ls, we focused on the signaling cascade leading to the Tat-mediated secretion of MCP-1 and examined its effect on the migration of CD14+ monocytes and THP-1 cells.
Tat-induced MCP-1 secretion from HMVEC-Ls.
Serum-starved cells were stimulated with different doses of HIV-1 Tat protein (A) or with 50 ng/mL of Tat plus 10 U/mL of heparin or 10 U/mL heparin alone (0) for the indicated times (B). After stimulation, the levels of MCP-1 in the culture supernatants were titrated, as described in “Materials and methods.”
Tat-induced MCP-1 secretion from HMVEC-Ls.
Serum-starved cells were stimulated with different doses of HIV-1 Tat protein (A) or with 50 ng/mL of Tat plus 10 U/mL of heparin or 10 U/mL heparin alone (0) for the indicated times (B). After stimulation, the levels of MCP-1 in the culture supernatants were titrated, as described in “Materials and methods.”
Activation of protein kinase C activity by Tat
We then examined which signaling molecules were activated by Tat in HMVEC-Ls. Changes in PKC activity were sought. Cell lysates from Tat-treated HMVEC-Ls were incubated with the substrate, MARCKSPSD,25 and incorporations of 32P into the substrate were quantitated. Figure 2shows that radioisotope incorporation increased steadily with the increasing incubation time of HMVEC-Ls with Tat. When the cells were treated for 20 minutes with the positive control phorbol 12-myristate 13 acetate (PMA), a potent activator of PKC, approximately 3 times higher PKC activity was observed than was seen with Tat treatment (data not shown). These results indicate that Tat activates PKC activity in HMVEC-Ls.
PKC assay.
PKC activity was assayed by quantitation of 32P incorporation from [γ32P]-ATP into the substrate, MARCKSPSD peptide, by using lysates from HMVEC-Ls treated with Tat for the indicated times. The 32P-incorporated substrates were precipitated with TCA plus BSA, and the amount of radioactivity in the precipitates was measured by Cherenkov counting.
PKC assay.
PKC activity was assayed by quantitation of 32P incorporation from [γ32P]-ATP into the substrate, MARCKSPSD peptide, by using lysates from HMVEC-Ls treated with Tat for the indicated times. The 32P-incorporated substrates were precipitated with TCA plus BSA, and the amount of radioactivity in the precipitates was measured by Cherenkov counting.
Activation of surface receptor molecules on HMVEC-Ls
It is known that HIV-1 Tat protein interacts specifically with VEGF and the beta-integrin receptor molecules of endothelial cells.16-19 Tat ligation of these specific surface molecules activates several cellular protein kinases,18 20-22 including Flk-1 and Flt-1, which are important for endothelial cell proliferation and migration. Thus, we examined whether these surface molecules are activated by Tat protein. To this end, tyrosine phosphorylations of Flk-1 and Flt-1 in Tat-treated HMVEC-Ls were investigated by immunoprecipitation, followed by Western blot analysis. The data showed that both Flk-1 and Flt-1 were tyrosine-phosphorylated on Tat stimulation (Figure3A,B, respectively). The tyrosine phosphorylation of Flk-1 was sustained for up to 20 minutes after stimulation, whereas that of Flt-1 was rapid and transient, with decreased amounts of phosphorylated Flt-1 observed beginning 2 minutes after stimulation. The amount of Flt-1 or Flk-1 protein loaded at the different time points was equivalent, indicating that the observed differences in the amount of phosphorylated protein did not reflect the quantitative variance of each protein. Taken together, these data suggest that the signaling cascade leading to the Tat-mediated modulation of chemokine secretion might be initiated on the ligation of Tat by Flk-1 and/or Flt-1.
Effect of Tat on tyrosine phosphorylation.
Tat effect on tyrosine phosphorylation of Flk-1 (A) and Flt-1 (B), and on MAP kinase activity (C). (A and B) Total cell lysates were immunoprecipitated with the indicated antibodies, then subjected to Western blot analysis with antiphosphotyrosine antibody (PY99) (upper panels) or with the indicated antibodies (lower panels). (C) Lysates from HMVEC-Ls stimulated for the indicated times were immunoprecipitated with monoclonal antibodies against Erk 1/2, and the precipitates were incubated at room temperature for 30 minutes in kinase assay buffer containing [γ32P]-ATP, along with MBP. Incorporation of 32P into MBP was analyzed on 12% SDS-PAGE and visualized by autoradiography.
Effect of Tat on tyrosine phosphorylation.
Tat effect on tyrosine phosphorylation of Flk-1 (A) and Flt-1 (B), and on MAP kinase activity (C). (A and B) Total cell lysates were immunoprecipitated with the indicated antibodies, then subjected to Western blot analysis with antiphosphotyrosine antibody (PY99) (upper panels) or with the indicated antibodies (lower panels). (C) Lysates from HMVEC-Ls stimulated for the indicated times were immunoprecipitated with monoclonal antibodies against Erk 1/2, and the precipitates were incubated at room temperature for 30 minutes in kinase assay buffer containing [γ32P]-ATP, along with MBP. Incorporation of 32P into MBP was analyzed on 12% SDS-PAGE and visualized by autoradiography.
Activation of MAP kinase activity
The MAP kinase pathway is known to be a major conduit for the transmission of downstream signals regulating cell proliferation,26-28 cell cycle control,26,29,30 and chemotaxis31-33 in different cell types. To investigate whether MAP kinase is involved in Tat-induced MCP-1 release, 200 μg of lysates generated from Tat-treated HMVEC-Ls were precipitated with Erk ½ antibodies, and then the kinase activity in the precipitates was determined by using MBP as a substrate. As shown in Figure 3C, strong Erk ½ kinase activity was observed; this activity increased with time, peaking at 20 minutes. By contrast, activity of the other major MAP kinases, p38 and c-Jun kinase, was not enhanced in the presence of Tat (data not shown). These results indicate that Erk ½ was activated by Tat in HMVEC-Ls and suggest that signals for the modulation of MCP-1 release could be mediated through the MAP kinase and/or PKC pathway.
Effect of protein kinase inhibitors on MCP-1 secretion
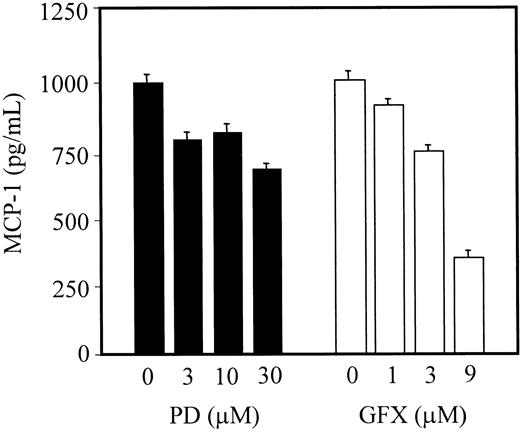
We further tested whether p44/42 MAP (Erk ½) and/or PKC were necessary for the induction of MCP-1 release by Tat protein. Serum-starved HMVEC-Ls were treated with various concentrations of protein kinase inhibitors 1 hour before Tat stimulation. The MAP kinase specific inhibitor, PD98059, had no significant effect on MCP-1 secretion (Figure 4). By contrast, the PKC inhibitor, GF109203X, significantly decreased MCP-1 secretion induced by Tat treatment (Figure 4). These data indicate that the signaling cascade for the secretion of MCP-1 acts primarily through PKC, but not through the MAP kinase.
Effect of kinase inhibitors on Tat-induced MCP-1 secretion.
Starved cells were incubated with the indicated amounts of kinase inhibitors in EBM-2 medium 1 hour before stimulation with Tat. MCP-1 levels in the clarified culture supernatants were then quantitated as described in “Materials and methods.” PD and GFX indicate PD98059 (MAP kinase inhibitor) and GF109203X (protein kinase C inhibitor), respectively.
Effect of kinase inhibitors on Tat-induced MCP-1 secretion.
Starved cells were incubated with the indicated amounts of kinase inhibitors in EBM-2 medium 1 hour before stimulation with Tat. MCP-1 levels in the clarified culture supernatants were then quantitated as described in “Materials and methods.” PD and GFX indicate PD98059 (MAP kinase inhibitor) and GF109203X (protein kinase C inhibitor), respectively.
MCP-1–dependent transwell migration of CD14+ monocytes and THP-1 cells
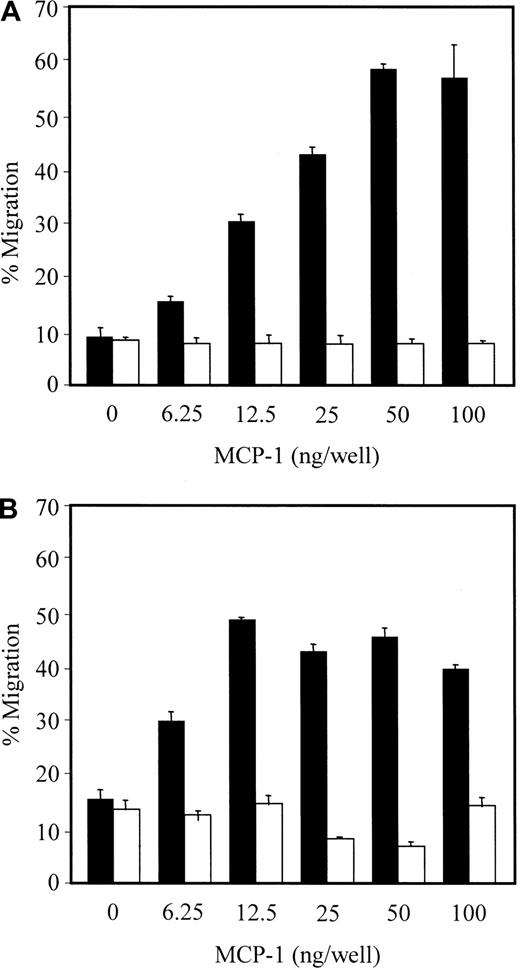
Because MCP-1 is one of the most potent chemoattractants for monocytes/macrophages,14 we assessed the levels of MCP-1–dependent transwell migration of CD14+ monocytes and model THP-1 monocytic cells. To this end, we first examined whether THP-1 cells and HMVEC-Ls express CCR2, the major chemokine receptor for MCP-1, using flow cytometric analysis. The data show that significant amounts of CCR2 were expressed on the surface of THP-1 cells but not on HMVEC-Ls (data not shown), suggesting that MCP-1 secreted from HMVEC-Ls could function to chemoattract THP-1 cells. Expression of CCR2 on the surface of peripheral blood monocytes has already been reported.34 35 We then assayed for possible MCP-1–dependent transwell migration of THP-1 cells by quantitating cell migration from the upper to lower compartment, which contained various concentrations of MCP-1. Migration of THP-1 cells was concentration dependent, peaking at 50 ng/mL and was abrogated by the removal of the concentration gradient (Figure5A). In contrast, in controls treated with anti-CCR2 antibody alone, there was no chemoattraction for THP-1, even at high concentrations of antibody (100 ng/mL) (data not shown). Transmigration of CD14+ monocytes was also concentration dependent up to 12.5 ng/mL of MCP-1 and was saturated beyond that concentration (Figure 5B). Similar to the migration of THP-1 cells, the removal of concentration gradient completely abrogated the migration of CD14+ monocytes (Figure 5B). These data indicate that the observed transwell migrations of CD14+ monocytes and THP-1 cells were specifically chemoattractive for MCP-1, rather than the result of a simple MCP-1 receptor/ligand interaction.
Migration of monocytic THP-1 cells and CD14+monocytes.
MCP-1 effect on transwell migration of monocytic THP-1 cells (A) and CD14+ monocytes (B). After a 3-hour incubation at 37°C with 5% CO2, cells that migrated from the upper to lower compartments were collected. The percentage migration was derived from counting cells with the trypan blue dye exclusion method (closed bars). To remove concentration gradient, the cells were incubated for 1 hour at room temperature with the indicated concentrations of MCP-1 before loading onto inserts (open bars).
Migration of monocytic THP-1 cells and CD14+monocytes.
MCP-1 effect on transwell migration of monocytic THP-1 cells (A) and CD14+ monocytes (B). After a 3-hour incubation at 37°C with 5% CO2, cells that migrated from the upper to lower compartments were collected. The percentage migration was derived from counting cells with the trypan blue dye exclusion method (closed bars). To remove concentration gradient, the cells were incubated for 1 hour at room temperature with the indicated concentrations of MCP-1 before loading onto inserts (open bars).
Chemotaxic properties of culture supernatants from Tat-treated HMVEC-Ls
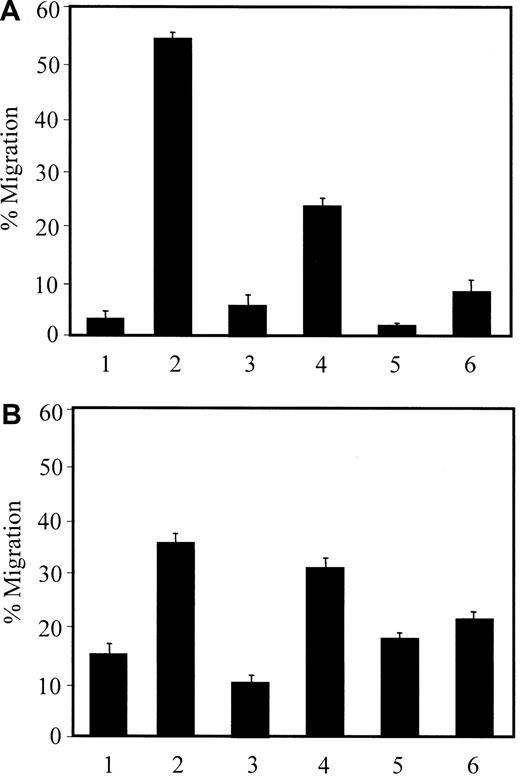
The above data indicate that treatment of HMVEC-Ls with HIV-1 Tat induces MCP-1 secretion and that MCP-1 is a strong chemoattractant for CD14+ monocytes and THP-1 cells. Thus, we investigated whether culture supernatants of HMVEC-Ls treated with Tat were chemotactic. Consistent with the above data, MCP-1 (50 ng/mL) showed strong chemotactic activity (Figure 6A, lane 2). When Tat-treated culture supernatants (containing 1 ng of MCP-1 as determined by an ELISA) were placed in the lower chamber, more than 20% of the THP-1 cells migrated (Figure 6A, lane 4), which is a higher percentage of migration compared with a gradient of 6.25 ng/mL of MCP-1 (Figure 5A). This result indicated that culture supernatants from Tat-treated HMVEC-Ls were chemotactic and implied that these supernatants contain other chemoattractants for the migration of monocytes in addition to MCP-1. As previously addressed, the small amount of MIP-1β secretion observed in our earlier experiment might partially account for the THP-1 cell migration. The resultant chemotactic activity was abrogated by pretreatment of the THP-1 cells with either MCP-1 (20 ng/mL) (Figure 6A, lane 5) or supernatants from Tat-treated HMVEC-Ls (Figure 6A, lane 6). Similar data were obtained with CD14+ monocytes (Figure 6B), even though significant portions of the monocytes were nonspecifically transmigrated; that is, approximately 15% of the monocytes migrated in the absence of chemokines (Figure 6B, lane 1). These data demonstrate that the culture supernatants induced the migration of CD14+ monocytes and THP-1 cells, and that this migration was dependent on a concentration gradient.
Chemotactic activity of culture supernatants from Tat-treated HMVEC-Ls.
Migration of the THP-1 cells (A) and CD14+ monocytes (B) was determined as described in “Materials and methods.” The chemoattractants placed onto the upper/bottom chambers were as follows: (1) EBM-2/EBM-2; (2) EBM-2/MCP-1; (3) EBM-2/untreated culture supernatant; (4) EBM-2/Tat-treated culture supernatant; (5) MCP-1/Tat-treated culture supernatant; and (6) Tat-treated culture supernatant/Tat-treated culture supernatant.
Chemotactic activity of culture supernatants from Tat-treated HMVEC-Ls.
Migration of the THP-1 cells (A) and CD14+ monocytes (B) was determined as described in “Materials and methods.” The chemoattractants placed onto the upper/bottom chambers were as follows: (1) EBM-2/EBM-2; (2) EBM-2/MCP-1; (3) EBM-2/untreated culture supernatant; (4) EBM-2/Tat-treated culture supernatant; (5) MCP-1/Tat-treated culture supernatant; and (6) Tat-treated culture supernatant/Tat-treated culture supernatant.
Discussion
HIV-1 infection of monocytes increases their interaction with human microvascular endothelial cells, thereby disrupting the integrity of the endothelial cell monolayer.36-38 This perturbation of the endothelium may promote extravasation of HIV-1–infected cells into peripheral tissues. Our results demonstrate that HIV-1 Tat up-regulates MCP-1 secretion and transmigration of monocytic THP-1 cells, an effect that would enhance the passage of virus and virus-infected cells through the endothelium. This induction of MCP-1 secretion and the migration of THP-1 cells was dependent on PKC activation in HMVEC-Ls.
MCP-1, a member of the beta subfamily of chemokines, attracts blood monocytes and T cells and facilitates their transendothelial migration.39,40 MCP-1 messenger RNA (mRNA) or protein has been detected at high levels in several diseases, including pulmonary fibrosis,41,42 arthritis,43 and various tumors,44 45 strongly suggesting that the chemokine recruits monocytes in these settings. Our data suggest that Tat modulates MCP-1 secretion from lung microvascular endothelium. This would enhance the infiltration of virus-infected cells from the pulmonary microcirculation into the extravascular spaces, ie, the alveoli.
The MCP-1 secretion pathway may be initiated by activation of the surface molecules Flk-1 and/or Flt-1. We found that although HIV-1 Tat treatment activated several signaling molecules, including p44/42 MAP kinases and PKC, only inhibition of PKC resulted in significantly diminished MCP-1 secretion and THP-1 monocyte chemotaxis. This result indicated that regulation of MCP-1 secretion is primarily mediated through this specific protein kinase signaling cascade. These data are consistent with a previous report, wherein a general protein kinase inhibitor blocked tumor necrosis factor alpha (TNFα)–mediated MCP-1 expression.46 In that prior study, the specific kinase pathway was not further characterized.
Culture supernatants from Tat-treated HMVEC-Ls displayed chemotactic activity for THP-1 cells. When the THP-1 cells and culture supernatants containing 1 ng of MCP-1 were placed onto upper and lower compartments, respectively, more than 20% of the THP-1 cells migrated from the top to the bottom chamber (Figure 6). This is a higher proportion of cells migrating compared with a gradient of 6.25 ng/mL of MCP-1 (Figure 5). The percentage inhibition of MCP-1 secretion by the PKC inhibitor did not parallel that of the THP-1 migration; that is, the reduced level of migration induced by the PKC inhibitor (25% decrease with 9 μM of GF109203X compared with the control) was less than the level of inhibition of MCP-1 secretion (80% decrease with the same concentration of inhibitor). These data imply that the culture supernatants from the Tat-treated HMVEC-Ls contain several chemoattractants, including MCP-1, for the migration of monocytes. We observed higher random transmigration of the CD14+monocytes compared with the THP-1 cells. However, transmigration of the CD14+ monocytes was significantly increased in response to either MCP-1 or Tat-treated culture supernatants, and this increased transmigration was eliminated on removal of the concentration gradient. These data indicate that the observed transmigration was specific to MCP-1.
Taken together, our data demonstrate that HIV-1 Tat induces secretion of MCP-1 and other chemoattractants that facilitates the transendothelial migration of monocytes from HMVEC-Ls; this occurs via a PKC-dependent pathway. The other chemoattractants released from HMVEC-Ls in the Tat-mediated PKC signaling pathway remain to be elucidated. Additional studies to characterize this signaling pathway should provide insight into the passage of HIV-1–infected monocytes across the microvascular endothelium to the tissue spaces, a signature event in the pathogenesis of reduced host defense in AIDS.
Acknowledgments
We thank Janet Delahanty for editing this manuscript and Nancy DesRosiers for preparation of the figures.
Supported by NIH grants HL61940 and HL53745.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jerome E. Groopman, Division of Experimental Medicine, Harvard Institutes of Medicine/BIDMC, 4 Blackfan Circle, Rm 351, Boston, MA 02115; e-mail: jgroopma@caregroup.harvard.edu.

![Fig. 2. PKC assay. / PKC activity was assayed by quantitation of 32P incorporation from [γ32P]-ATP into the substrate, MARCKSPSD peptide, by using lysates from HMVEC-Ls treated with Tat for the indicated times. The 32P-incorporated substrates were precipitated with TCA plus BSA, and the amount of radioactivity in the precipitates was measured by Cherenkov counting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.352/5/m_h80210602002.jpeg?Expires=1771009067&Signature=K8-u25evcZrLmKjBWPgNZLYLEh0eDwLO8aoO1p0zTMltm-t~jGCU7lo2V6svvvUh-Tgx3X5T5u67D2TivlGZovuGw33-aI1iLISV8gRvo5KUYtOfmTreIMHKeJmJNHPK7thwCNRsFKC6IaLeQKyuCDO7hzz1LaCWG9oPAAKA3vRhphxGWyueMbfm2anIowU3kXmeQsaRl5BhvEZshnQDsMflw5hBFzdjw2rE3qS96RbxSx9dqBTAk7G-J46imJ2VbORQFQYffKvyDzQv35RBAPfRR-KMKllNvPKywEgEF8dyjSme1-NkfQlxcR1zbdgnuZqEY7Tc4TstfU7tq8IrBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of Tat on tyrosine phosphorylation. / Tat effect on tyrosine phosphorylation of Flk-1 (A) and Flt-1 (B), and on MAP kinase activity (C). (A and B) Total cell lysates were immunoprecipitated with the indicated antibodies, then subjected to Western blot analysis with antiphosphotyrosine antibody (PY99) (upper panels) or with the indicated antibodies (lower panels). (C) Lysates from HMVEC-Ls stimulated for the indicated times were immunoprecipitated with monoclonal antibodies against Erk 1/2, and the precipitates were incubated at room temperature for 30 minutes in kinase assay buffer containing [γ32P]-ATP, along with MBP. Incorporation of 32P into MBP was analyzed on 12% SDS-PAGE and visualized by autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/2/10.1182_blood.v97.2.352/5/m_h80210602003.jpeg?Expires=1771009067&Signature=2OL03JhZWaXFXhU2r9l3LkGRD3ysLCkvrhWnW-4nO5nPMBEHmT43rsku8SaOzaMCiIW9VFHIZvvkpVfSPrGJpkUfdTFbKabHl3Z3fQH4mwip19FxRLdK6QJlTbmALIF6Q1NP5tT4vbTZMQi3lF8pDARwAmZKD9Jdd9DgimXvWoOgcEI04lDKBMDpfpOtdMEM1vdMblRDUu~DgYEpwTyx25Sy2hg46QiAutwr6bkPGPPyB9LxqmTPBAVojWH9oOeLpUxW5XpGHKTckwdckAIkl-s4qPKtsswRFWoATbDpgetP9BoUAMoMZS95pdjlbRw6mrgZ-ezefMJ4rz6uHwj4Yw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal