Abstract
In the hematopoietic compartment, the CD13/APN metalloprotease is one of the earliest markers of cells committed to the myeloid lineage where it is expressed exclusively on the surface of myeloid progenitors and their differentiated progeny. CD13/APN is also found in nonhematopoietic tissues, and its novel expression on the endothelial cells of angiogenic, but not normal, vasculature was recently described. Treatment of animals with CD13/APN inhibitors significantly impaired retinal neovascularization, chorioallantoic membrane angiogenesis, and xenograft tumor growth, indicating that CD13/APN plays an important functional role in vasculogenesis and identifying it as a critical regulator of angiogenesis. To investigate the mechanisms of CD13/APN induction in tumor vasculature, the regulation ofCD13/APN by factors contributing to angiogenic progression was studied. In this report, it is shown that endogenous CD13/APN levels in primary cells and cell lines are up-regulated in response to hypoxia, angiogenic growth factors, and signals regulating capillary tube formation during angiogenesis. Transcription of reporter plasmids containing CD13/APNproximal promoter sequences is significantly increased in response to the same angiogenic signals that regulate the expression of the endogenous gene and in human tumor xenografts, indicating that this fragment contains elements essential for the angiogenic induction ofCD13/APN expression. Finally, functional antagonists of CD13/APN interfere with tube formation but not proliferation of primary vascular endothelial cells, suggesting that CD13/APN functions in the control of endothelial cell morphogenesis. These studies clearly establish the CD13/APN metalloprotease as an important regulator of endothelial morphogenesis during angiogenesis.
Introduction
Angiogenesis refers to the formation of new blood vessels from the existing vasculature and occurs at extremely low levels in the adult organism. One notable exception to this paradigm occurs in tumors that have undergone the “angiogenic switch” from a benign to a metastatic phenotype, in which new vessels are actively assembled and directly responsible for the sustained growth and dissemination of the tumor.1 It is clear that the modulation of blood vessel growth is a remarkably effective means to limit or control tumor growth and spread, and therefore, the search for unique targets modulating angiogenesis is of significant importance.
Recent studies designed to identify unique peptides that home specifically to solid tumors in murine breast carcinoma models revealed that the NGR motif binds strictly to the endothelium of angiogenic blood vessels.2 Further investigation identified the CD13/APN cell-surface antigen as the principal receptor for this peptide motif and demonstrated that this protein is expressed exclusively on the endothelial cells of angiogenic but not normal vasculature,3 thereby explaining the tumor-specific destination of the NGR peptide. CD13/APN was originally described in studies seeking to identify lineage-specific markers that would facilitate the classification of human leukemias.4 In this regard, the appearance of CD13 coincides with commitment to the myeloid lineage and is exclusively expressed on the normal and leukemic progeny of myeloid cells within the hematopoietic compartment. The subsequent molecular cloning of the gene encoding this cell surface glycoprotein identified it as the membrane-bound metalloprotease, aminopeptidase N (APN, EC 3.4.11.2), thus extending its known range of expression beyond the hematopoietic system to include fibroblasts and epithelial cells in the liver, intestine, brain, and lung.5
CD13/APN cleaves amino terminal residues from short peptides, and consequently, its specific function in individual tissues (primarily the activation or inactivation of small bioactive molecules) is mandated by available substrates (reviewed in Shipp and Look6). Insights into the function of this unique vascular marker in angiogenesis were gained through experiments in which treatment of animals with CD13/APN functional inhibitors significantly arrested retinal neovascularization, chorioallantoic membrane angiogenesis, and xenograft tumor growth, indicating that CD13/APN plays an important role in the progression of tumor vasculogenesis and identifying it as a critical regulator of angiogenesis.3Therefore, understanding the mechanisms regulating the expression ofCD13/APN is fundamental to the identification of potential therapies aimed at its modulation during angiogenesis.
To investigate these regulatory mechanisms in angiogenic vasculature, we sought to establish the operative basis of CD13/APNinduction in the tumor environment. In early angiogenic stages, hypoxic or ischemic signals alter the expression of numerous and diverse genes important for the angiogenic differentiation program, including angiogenic growth factors.7 These factors in turn activate quiescent endothelial cells of established vessels to proliferate and migrate toward the tumor cell mass (reviewed in Hanahan and Folkman1). In this report, we show thatCD13/APN messenger RNA (mRNA) and protein in primary endothelial cells and cell lines is transcriptionally up-regulated in response to conditions that are characteristic of the tumor microenvironment, such as hypoxia and elevated angiogenic growth factor concentrations, and by signals regulating capillary formation and xenograft tumor growth. Additionally, in experiments that use functional antagonists, we demonstrate a role for CD13/APN in endothelial morphogenesis. Dissection of the transcriptional regulation of CD13/APN during angiogenesis will increase our understanding of the molecular mechanisms responsible for the “angiogenic switch” and identify potential targets for therapeutic antiangiogenic strategies.
Material and methods
Cell culture
All cell lines were maintained in the indicated medium supplemented with 10% fetal bovine serum (FBS), L-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL) unless otherwise indicated. Kaposi sarcoma (KS1767, passage numbers 5 to 12) and murine hemangioendothelioma (EOMA) cells were maintained in MEM containing 10% or 1% FBS, 5 mM L-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, and vitamins (1 ×). HL-60 and KG1a cells (ATCC CRL1593, CRL2168) were maintained in RPMI 1640 medium. NIH3T3 (ATCC CRL 1658) cells were grown in DMEM containing 4.5 g/L glucose. Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics Corp (San Diego, CA) and maintained according to the manufacturer's protocol.
Northern blot analysis
Total RNA was isolated using TRI reagent (Molecular Research Center, Cincinnati, OH). The 30 μg total RNA from each sample was separated by electrophoresis in a 1% agarose gel containing formaldehyde. Membranes were hybridized in NorthernMax Prehyb/Hyb buffer (Ambion, Austin, TX) with EcoRI fragments, 1.8 and 1.6 kilobase (kb), encompassing the full-length CD13 complementary DNA (cDNA) as a probe.5 After stripping, the identical membrane was subsequently hybridized with the control β3-integrin cDNA probe2 and a human 28S ribosomal RNA (rRNA) gene-specific oligonucleotide probe (Clontech Laboratories, Palo Alto, CA) to control for loading and RNA integrity.
Amplification of CD13 complementary DNA fragments using semiquantitative reverse transcriptase–polymerase chain reaction
The cDNA templates for polymerase chain reaction (PCR) were synthesized by reverse transcriptase (RT) (Omniscript RT kit, Qiagen, Valencia, CA) according to the method recommended by the manufacturer. For CD13 mRNA detection, a standard PCR was performed (Taq PCR core kit, Qiagen) using 5′-CCT TCA ACC TGG CCA GTG C-3′ and 5′-CGT CTT CTC CAG GGC TTG CTC CAG-3′ (sense and antisense primers common to murine and human CD13) as primers, producing the 839-base pairs (bp) cDNA fragment. The control RT-PCRs were carried out using either human or mouse β-actin control amplimer sets.
Transfection of recombinant plasmids and reporter gene assays
A 1004-bp BstXI fragment from the intestinal CD13/APN promoter8 was subcloned upstream of the luciferase gene in the promoterless luciferase reporter vector, pGL2 basic (Promega, Madison, WI). Plasmids were transfected into KS1767 cells (3 × 105 cells) using Lipofectamine Reagent (Life Technologies, Rockville, MD), following the manufacturer's protocol. KS1767 cells were seeded in culture medium containing 1% FBS 24 hours before transfection, and triplicate wells were then transfected with 3 μg of the test plasmid and 1 μg β-actin-SEAP or MAP1-SEAP control plasmid. After overnight incubation, fresh growth medium containing growth factors or neutralizing antibodies (human recombinant vascular endothelial growth factor [VEGF] [25 ng/mL], basic fibroblast growth factor [bFGF] [50 ng/mL], tumor necrosis factor alpha [TNFα] [10 ng/mL], insulin-like growth factor-1 [IGF-1] [50 ng/mL]; antihuman VEGF or bFGF [20 μg/mL]; R & D Systems, Minneapolis, MN) were added and cells were harvested 24 hours later. For hypoxia studies, 24 hours after transfection, cells were subjected to either hypoxia (1% oxygen), or cobalt chloride treatment (100 μM) for an additional 24 hours before harvest and assay. The transfection efficiency for each construct was normalized to the control level of secreted alkaline phosphatase (SEAP) activity9; the reported values were calculated as relative light units (RLUs) per unit of SEAP activity. To compare results among reporter constructs, we expressed transcriptional activity as the fold increase over that produced with the promoterless luciferase vector, pGL2basic, determined in parallel transfections. All experiments were carried out at least 3 times in triplicate. Assays to detect chloramphenicol acetyl transferase (CAT) enzymatic activity were performed as described.10 Aliquots of total cellular protein from lysates of cells transfected with each reporter plasmid were analyzed after a 3-hour incubation with 0.25 μg of acetyl Co-A and 0.0185 MBq (0.5 μCi) [14C]chloramphenicol. The acetylated products were separated on silica gel thin-layer chromatography plates in chloroform:methanol (95:5 vol:vol), and chromatograms were analyzed by autoradiography. HUVECs were transfected with Fugene (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer's protocols.
Hypoxia and CoCl2 treatment
KS1767 cells were cultured under hypoxic conditions (5% CO2, 1% O2, and 94% N2) in a BBL 100 Vented GasPak system, (Becton Dickinson, Bedford, MA). Control normoxic conditions (5% CO2, 20% O2, and 75% N2) were maintained in a standard CO2 incubator at 37°C. Cells were seeded onto culture plates 2 days before hypoxic incubation, and serum-free medium was added 24 hours before incubation. Cells were subconfluent at the beginning of hypoxic incubation. Cells were treated with cobalt chloride (100 μM) for 12, 24, and 48 hours before harvest for RNA or 24 hours before flow cytometric analysis with anti-CD13 antibodies. In the retinal neovascularization model,11 7-day-old mice were exposed to 75% oxygen or room air for 5 days, then moved to room air for 5 additional days. Retinas were harvested, and RNA isolated at various time points after exposure to relative hypoxia.
Angiogenesis assays
For in vitro angiogenesis assays, the 1004-bp BstXI fragment of the CD13 proximal promoter was subcloned into the promoterless pEGFP vector (Promega) to produce CD13-pEGFP. Three plasmids (pEGFP [promoterless green fluorescent protein reporter, hereafter referred to as null-GFP], CD13-pEGFP, and pEGFP-N1 [GFP reporter construct containing the cytomegalovirus promoter, hereafter referred to as CMV-GFP]) were each transfected into EOMA cells and selected for neomycin-resistant pools in medium containing 3 mg/mL neomycin (G418, Life Technologies, Rockville, MD). The GFP-high fraction of cells from each pool was isolated by FACS analysis and expanded for in vitro angiogenesis studies. Stably transfected EOMA cells (1 × 106) were plated in 1 mL medium in 6-well tissue culture plates coated with 1 mL basement membrane matrix per well (Matrigel, Becton Dickinson). HUVECs were serum starved overnight in EBM-2 medium and split 2 × 104 cells in 1 mL MEM/2% FBS on 200 μL Matrigel in 24-well plates. After 1 hour incubation at 37°C, growth medium was replaced with medium containing 2% FBS alone or 2% FBS and 100 μg/mL CD13/APN antagonists, MY7 antibodies, amastatin, or bestatin or negative controls, UPC10 isotype-matched control antibody or trypsin inhibitor, and monitored for 18 to 24 hours. Cellular morphology and/or GFP expression was monitored by light and confocal microscopy, respectively (Leica, TCS SP).
For xenograft studies, 107 GFP-containing EOMA cells/flank were injected subcutaneously into the flanks of age-matched female SCID mice, and tumors were harvested when they reached 1 cm in diameter. Tumors were mechanically disrupted and analyzed for GFP expression by flow cytometric analysis. The percentage of GFP-positive cells in test samples (CD13-GFP and CMV-GFP) was determined by comparison to background levels of GFP expression in identically treated null-GFP–derived tumors. Two independent rounds of xenograft tumor production produced identical results.
Human umbilical vein endothelial cell proliferation assay
Cell proliferation was assessed by the AlamarBlue assay (AccuMed International, Westlake, OH). In this assay, the metabolic reduction of the medium by actively growing cells is quantitated by measuring the fluorescent conversion of the AlamarBlue REDOX indicator over time in culture.12 HUVECs were seeded at 1000 cells per well, medium containing inhibitory antibodies or chemical inhibitors was added, and plates were incubated at 37°C. AlamarBlue indicator was added 8 hours later, and accumulated fluorescence was measured every 24 hours over a period of 5 days using the Millipore CytoFluor-2350.
Results
CD13/APN messenger RNA is identical in endothelial and epithelial cells
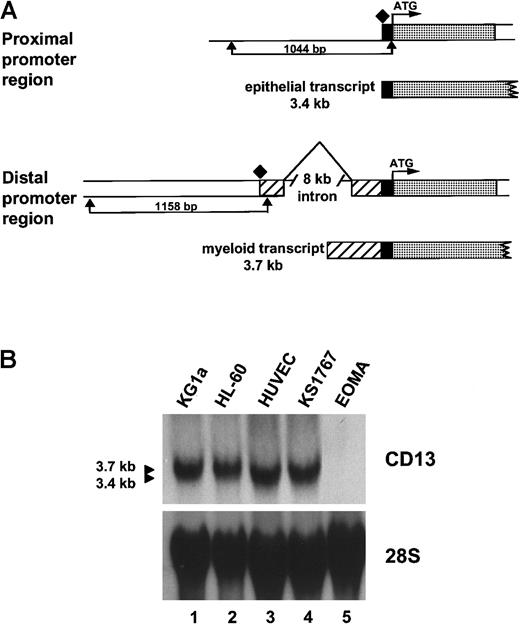
CD13/APN cell-surface expression is regulated by 2 independent, mutually exclusive, promoters separated by an 8-kb intron (Figure 1A and Shapiro et al10). The proximal promoter directs CD13/APNtranscriptional regulation in kidney, intestine, and liver epithelial cells and produces a 3.4-kb transcript, whereas the distal promoter controls CD13/APN expression in myeloid cells and fibroblasts, generating a distinct 3.7-kb transcript. Analysis ofCD13/APN transcripts in primary HUVECs or the Kaposi sarcoma–derived KS1767 cell line by Northern blot demonstrated that the endothelial cells express the shorter 3.4-kb transcript (Figure1B). These data suggest that CD13/APN transcription in endothelial cells initiates from the proximal liver/intestinal epithelial promoter and that the KS1767 cell line faithfully recapitulates CD13/APN transcription initiation in primary cells. Further analysis by S1 nuclease, RT-PCR, and immunoprecipitation confirmed that the CD13/APN transcript in KS1767 cells initiates from the proximal start site, is identical in sequence to that expressed in hepatic epithelial cells, and encodes a protein that is indistinguishable in size and abundance from that expressed in hepatocytes and myeloid cells (data not shown).
Characterization of CD13/APN endothelial cell transcripts.
(A) Schematic diagram showing the CD13/APN promoter regions used by epithelial cells (proximal promoter, generating a 3.4-kb transcript, top) and myeloid cells and fibroblasts (distal promoter, generating a 3.7-kb transcript, bottom). The translation start site (ATG) is identical in transcripts from each cell type and is followed in genomic DNA by the protein coding sequences (░). The proximal (1044-bp) and distal (1158-bp) promoter fragments are delineated by arrows. Additional untranslated sequences found only in transcripts originating from the distal promoter, ▨. Transcriptional start sites for each promoter are shown as diamonds. (B) CD13/APNexpression in various tissues as determined by Northern blot analysis of total cellular RNA. The identical blot is shown after it was stripped and reprobed with a 28S probe as a control for RNA integrity and loading. Lanes are identical in each panel, showing that theCD13/APN transcript in the KS1767 endothelial cell line comigrates with that of the HUVECs (3.4-kb) and is smaller than that found in myeloid cells (HL-60, KG1a, 3.7 kb). CD13/APNtranscripts are undetectable in EOMA cells.
Characterization of CD13/APN endothelial cell transcripts.
(A) Schematic diagram showing the CD13/APN promoter regions used by epithelial cells (proximal promoter, generating a 3.4-kb transcript, top) and myeloid cells and fibroblasts (distal promoter, generating a 3.7-kb transcript, bottom). The translation start site (ATG) is identical in transcripts from each cell type and is followed in genomic DNA by the protein coding sequences (░). The proximal (1044-bp) and distal (1158-bp) promoter fragments are delineated by arrows. Additional untranslated sequences found only in transcripts originating from the distal promoter, ▨. Transcriptional start sites for each promoter are shown as diamonds. (B) CD13/APNexpression in various tissues as determined by Northern blot analysis of total cellular RNA. The identical blot is shown after it was stripped and reprobed with a 28S probe as a control for RNA integrity and loading. Lanes are identical in each panel, showing that theCD13/APN transcript in the KS1767 endothelial cell line comigrates with that of the HUVECs (3.4-kb) and is smaller than that found in myeloid cells (HL-60, KG1a, 3.7 kb). CD13/APNtranscripts are undetectable in EOMA cells.
CD13/APN is regulated by the proximal promoter in human umbilical vein endothelial and KS1767 cells
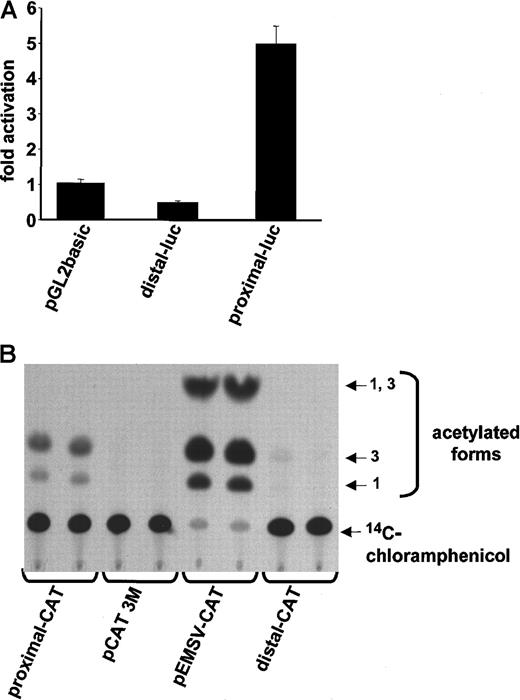
Our Northern blot data suggested that CD13/APN is regulated by the proximal intestinal/liver epithelial promoter in primary endothelial cells and cell lines. To delineate the promoter region regulating endothelial cell expression, we fused approximately 1-kb of upstream sequences flanking the transcriptional start site of either the distal (myeloid) or proximal (epithelial) promoter (Figure1A) upstream of the CAT or luciferase reporter genes and transiently transfected these into HUVECs or KS1767 cells (Figure2A,B, respectively). Significant reporter gene activity was observed in those cells containing sequences from the proximal, but not the distal, promoter, indicating that the information required for CD13/APN expression in both primary endothelial cells and cell lines is contained within this 1-kb fragment.
The proximal promoter controls
CD13/APN transcription in HUVECs and endothelial cell lines. (A) Plasmids containing the proximal or distal promoter fragments immediately upstream of the luciferase reporter gene were transiently transfected into primary HUVECs. Lysates of triplicate dishes were assayed at 24 hours for luciferase activity. pGL2basic refers to the promoterless control luciferase plasmid. (B) The identical experiment in KS1767 cells using CAT reporter plasmids. pEMSV-CAT refers to the positive control plasmid and pCAT3M to the promoterless control plasmid. Equal amounts of lysate (75 μg) were assayed in each lane.
The proximal promoter controls
CD13/APN transcription in HUVECs and endothelial cell lines. (A) Plasmids containing the proximal or distal promoter fragments immediately upstream of the luciferase reporter gene were transiently transfected into primary HUVECs. Lysates of triplicate dishes were assayed at 24 hours for luciferase activity. pGL2basic refers to the promoterless control luciferase plasmid. (B) The identical experiment in KS1767 cells using CAT reporter plasmids. pEMSV-CAT refers to the positive control plasmid and pCAT3M to the promoterless control plasmid. Equal amounts of lysate (75 μg) were assayed in each lane.
CD13/APN expression in primary endothelial cells and the KS1767 cell line is up-regulated by hypoxia
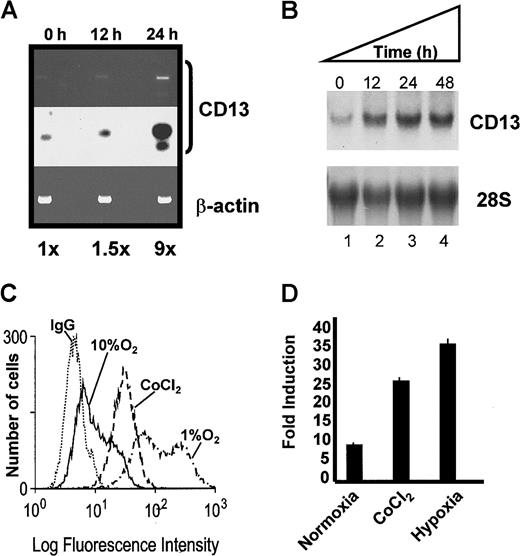
During tumor angiogenesis, oxygen deprivation induces the expression of a heterogeneous group of genes important for tumor vascularization (reviewed in Semenza7). To investigate whether hypoxia also induces CD13/APN expression, we evaluated the response of endogenous CD13/APN mRNA and the proximal promoter constructs to either hypoxic culture conditions or cobalt chloride treatment. RT-PCR analysis indicated that the expression of CD13/APN increases in vivo in mouse retinas undergoing hypoxia-induced neovascularization compared with untreated controls (Figure 3A). In addition, the culture of KS1767 cells with cobalt chloride or in environmental hypoxia induced a time-dependent expression of endogenousCD13/APN mRNA and protein (Figure 3B-C, and data not shown) and transfected CD13/APN reporter gene levels (Figure 3D). Therefore, hypoxia increases CD13/APN expression in a manner analogous to the hypoxic transcriptional up-regulation observed with other angiogenic regulators.1 13-15
Hypoxic conditions induce endogenous
CD13/APN expression and proximal promoter activity. (A) Endogenous CD13/APN mRNA in hypoxia-induced retinal neovessels is up-regulated. RNA isolated from retinas at 0, 12, and 24 hours in relative hypoxia was assayed for CD13/APN and control β-actin levels by RT-PCR. The Southern blot of RT-PCR products (middle panel) was quantitated by phosphorimager analysis. (B) Cobalt chloride treatment induces endogenous CD13/APN in endothelial cell lines. Serum-starved KS1767 cells were treated with cobalt chloride (100 μM) for 12, 24, and 48 hours, and total cellular CD13/APN RNA was assessed. (C) Hypoxia and cobalt chloride treatment induces CD13/APN cell-surface protein levels. Serum-starved KS1767 cells were treated with cobalt chloride (100 μM) or incubated in 1% or 10% oxygen for 24 hours and analyzed for CD13/APN expression with the MY7 monoclonal antibody or negative control immunoglobulin G (IgG) by flow cytometry. (D) Hypoxia induces the CD13/APN proximal promoter. Twenty-four hours after transient transfection of KS1767 cells in 1% serum with 3 μg of proximal promoter plasmid or pGL2basic vector alone and 1 μg of MAP1-SEAP control, cells were subjected to normoxia, hypoxia (1% oxygen), or cobalt chloride (100 μM) treatment for 24 hours before assay for luciferase activity. Results are shown as fold induction over identically treated cells transfected with the promoterless plasmid pGL2basic.
Hypoxic conditions induce endogenous
CD13/APN expression and proximal promoter activity. (A) Endogenous CD13/APN mRNA in hypoxia-induced retinal neovessels is up-regulated. RNA isolated from retinas at 0, 12, and 24 hours in relative hypoxia was assayed for CD13/APN and control β-actin levels by RT-PCR. The Southern blot of RT-PCR products (middle panel) was quantitated by phosphorimager analysis. (B) Cobalt chloride treatment induces endogenous CD13/APN in endothelial cell lines. Serum-starved KS1767 cells were treated with cobalt chloride (100 μM) for 12, 24, and 48 hours, and total cellular CD13/APN RNA was assessed. (C) Hypoxia and cobalt chloride treatment induces CD13/APN cell-surface protein levels. Serum-starved KS1767 cells were treated with cobalt chloride (100 μM) or incubated in 1% or 10% oxygen for 24 hours and analyzed for CD13/APN expression with the MY7 monoclonal antibody or negative control immunoglobulin G (IgG) by flow cytometry. (D) Hypoxia induces the CD13/APN proximal promoter. Twenty-four hours after transient transfection of KS1767 cells in 1% serum with 3 μg of proximal promoter plasmid or pGL2basic vector alone and 1 μg of MAP1-SEAP control, cells were subjected to normoxia, hypoxia (1% oxygen), or cobalt chloride (100 μM) treatment for 24 hours before assay for luciferase activity. Results are shown as fold induction over identically treated cells transfected with the promoterless plasmid pGL2basic.
CD13/APN expression in primary endothelial cells and the KS1767 cell line is induced by serum and growth factor stimulation
Angiogenic growth factors represent one group of genes whose expression is highly induced in response to hypoxic stress and essential for angiogenic progression. Up-regulation of these factors leads to an increase in the expression of numerous proteins that contribute to angiogenesis, including matrix metalloproteases, serine proteases, integrins, and growth factor receptor tyrosine kinases.1 13-15 To address the possibility that the hypoxic induction of CD13/APN is indirectly mediated by hypoxia-induced growth factor up-regulation in vascular endothelium, we cultured primary HUVECs in low- or high-serum concentrations (which contains functional concentrations of many angiogenic factors) or with the individual angiogenic growth factors, bFGF, VEGF, TNFα, or IGF-1 (Figure 4A). CD13/APNexpression was significantly induced on serum stimulation (4-fold) and increased between 1.5- to 2.5-fold on culture with each of the individual angiogenic factors (bFGF > VEGF = TNFα > IGF-1). Similarly, treatment of HUVECs with these same angiogenic factors up-regulated levels of the 3.4-kb CD13/APN transcript to varying degrees (Figure 4B), implying that transcription from the proximal promoter is induced by certain angiogenic growth factors. Therefore, the expression of CD13/APN is activated in primary endothelial cells by growth factors that are produced in the tumor microenvironment.
Angiogenic factors induce CD13/APN cell surface expression in primary endothelial cells.
(A) Primary HUVECs were serum starved for 24 hours, then cultured without serum (none), with the indicated angiogenic growth factors, or with 10% fetal calf serum (FCS), stained for CD13/APN expression with an anti-CD13/APN monoclonal antibody (MY7) and analyzed by flow cytometry. The relative fluorescence index (RFI) and fold induction over untreated cells of each sample are indicated. (B) RNA isolated and probed for CD13/APN or 28S RNA expression shows that treatment of primary HUVECs with certain angiogenic factors inducesCD13/APN mRNA (VEGF [25 ng/mL], bFGF [50 ng/mL], TNFα [10 ng/mL], and IGF-1 [50 ng/mL]).
Angiogenic factors induce CD13/APN cell surface expression in primary endothelial cells.
(A) Primary HUVECs were serum starved for 24 hours, then cultured without serum (none), with the indicated angiogenic growth factors, or with 10% fetal calf serum (FCS), stained for CD13/APN expression with an anti-CD13/APN monoclonal antibody (MY7) and analyzed by flow cytometry. The relative fluorescence index (RFI) and fold induction over untreated cells of each sample are indicated. (B) RNA isolated and probed for CD13/APN or 28S RNA expression shows that treatment of primary HUVECs with certain angiogenic factors inducesCD13/APN mRNA (VEGF [25 ng/mL], bFGF [50 ng/mL], TNFα [10 ng/mL], and IGF-1 [50 ng/mL]).
To confirm that our in vitro model faithfully reflects the induction of CD13/APN in primary vascular endothelium, we assessed whetherCD13/APN expression in the KS1767 cell line was also serum regulated. Flow cytometric analysis of KS1767 cells cultured in the presence of 10% serum for 24 hours demonstrated a 10-fold increase in CD13/APN cell surface expression when compared with cells exposed to low-serum concentrations (Figure 5A). Similarly, Northern analysis of KS1767 cells showed a dose-dependent 3- to 4-fold increase in CD13/APN message levels in response to increasing levels of serum (and consequently, angiogenic factors, Figure 5B), as well as a time-dependent response to the individual factors bFGF and VEGF (Figure 5C), comparable with that of primary vascular endothelium (Figure 4B). Therefore, these data confirmed the validity of the KS1767 cell line as an in vitro model for the study ofCD13/APN regulation in endothelial cells.
Endogenous
CD13/APN is induced in response to serum concentration and angiogenic factors. Expression of CD13/APN protein (A) or mRNA (B) in KS1767 cells is dependent on serum concentration. Serum-starved cells were stimulated with the indicated concentrations of FBS or left untreated (no fetal calf serum [FCS]) for 24 hours. CD13/APN protein expression was measured by flow cytometry; relative transcript levels in total RNA were assayed by Northern blot analysis. (C) bFGF and VEGF induce time-dependent increases inCD13/APN mRNA expression in KS1767 cells similar to that seen with the angiogenic marker, β3 integrin. Total RNA was isolated at the indicated time intervals after addition of bFGF (50 ng/mL) or VEGF (25 ng/mL) to serum-starved cells and hybridized with the indicated probes.
Endogenous
CD13/APN is induced in response to serum concentration and angiogenic factors. Expression of CD13/APN protein (A) or mRNA (B) in KS1767 cells is dependent on serum concentration. Serum-starved cells were stimulated with the indicated concentrations of FBS or left untreated (no fetal calf serum [FCS]) for 24 hours. CD13/APN protein expression was measured by flow cytometry; relative transcript levels in total RNA were assayed by Northern blot analysis. (C) bFGF and VEGF induce time-dependent increases inCD13/APN mRNA expression in KS1767 cells similar to that seen with the angiogenic marker, β3 integrin. Total RNA was isolated at the indicated time intervals after addition of bFGF (50 ng/mL) or VEGF (25 ng/mL) to serum-starved cells and hybridized with the indicated probes.
The CD13/APN proximal promoter regulates the response to serum and angiogenic factors
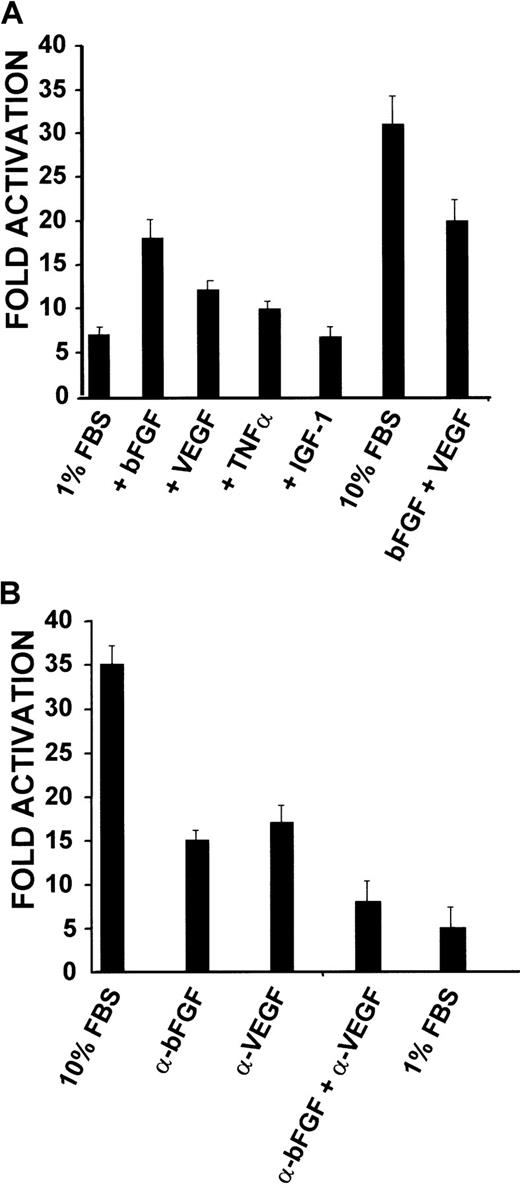
If the proximal promoter controls expression ofCD13/APN during angiogenesis, then reporter plasmids containing this promoter should also be up-regulated in response to serum and growth factor stimulation. KS1767 cells were transiently transfected with a proximal promoter-driven luciferase reporter plasmid and cultured in medium containing 1% serum and either bFGF, VEGF, or IGF-1 (Figure 6A). The bFGF-treated cells consistently showed a 2.5-fold increase in promoter activity, whereas the addition of VEGF or TNFα resulted in more modest 1.5-fold increases over controls. Addition of both bFGF and VEGF together produced a less than additive effect, perhaps suggesting a functional overlap between the stimulatory effects of the individual factors. Importantly, minimal induction was observed with those conditions containing IGF-1, demonstrating that the response is specific for certain angiogenic growth factors. To confirm these observations, neutralizing antibodies for human bFGF, human VEGF, or both factors were added to cultures of KS1767 cells transiently transfected with the proximal promoter constructs in 10% serum; this resulted in a progressive inhibition of CD13 promoter activity (Figure6B). Together, these results indicate that the proximal promoter regulates CD13/APN transcription in response to angiogenic growth factors, and is therefore likely to control CD13/APNexpression during angiogenesis.
CD13/APN promoter constructs are induced in response to serum concentration and angiogenic factors.
(A) KS1767 cells in 1% serum were transiently transfected with 3 μg reporter plasmids containing the proximal promoter or promoterless vector and 1 μg MAP1-SEAP control, followed by stimulation with the indicated angiogenic factors alone or in combination (bFGF, VEGF, TNFα, or IGF-1). Cells were harvested and assayed for luciferase activity at 24 hours. Results are shown as fold activation over the activity of pGL2 basic control plasmid. (B) Anti-bFGF and anti-VEGF neutralizing antibodies inhibit CD13/APN promoter activity in KS1767 cells. Twenty-four hours after transfection, KS1767 cells in 10% serum were incubated with 20 μg/mL of goat IgG, antihuman bFGF, antihuman VEGF, or both anti-bFGF and anti-VEGF for 24 hours and assayed for luciferase activity. Results are shown as fold activation over identical treatment of cells transfected with the promoterless plasmid pGL2basic.
CD13/APN promoter constructs are induced in response to serum concentration and angiogenic factors.
(A) KS1767 cells in 1% serum were transiently transfected with 3 μg reporter plasmids containing the proximal promoter or promoterless vector and 1 μg MAP1-SEAP control, followed by stimulation with the indicated angiogenic factors alone or in combination (bFGF, VEGF, TNFα, or IGF-1). Cells were harvested and assayed for luciferase activity at 24 hours. Results are shown as fold activation over the activity of pGL2 basic control plasmid. (B) Anti-bFGF and anti-VEGF neutralizing antibodies inhibit CD13/APN promoter activity in KS1767 cells. Twenty-four hours after transfection, KS1767 cells in 10% serum were incubated with 20 μg/mL of goat IgG, antihuman bFGF, antihuman VEGF, or both anti-bFGF and anti-VEGF for 24 hours and assayed for luciferase activity. Results are shown as fold activation over identical treatment of cells transfected with the promoterless plasmid pGL2basic.
The proximal promoter regulates CD13/APN expression during capillary tube formation and angiogenesis
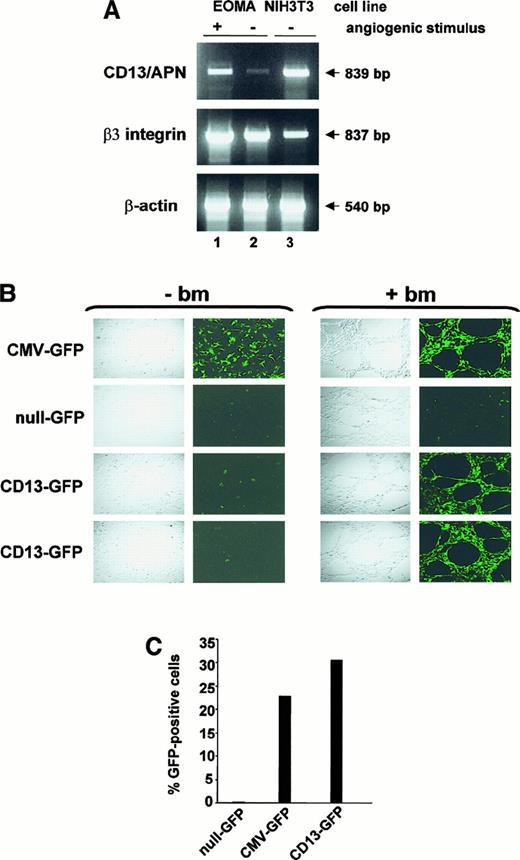
The levels of CD13/APN protein increase dramatically in endothelial cells that form the tumor vasculature.3 We adapted an in vitro model of angiogenesis to determine whether our promoter data obtained in KS1767 cells accurately reflect transcriptional regulation during angiogenesis. In this model, culture of endothelial cells on a basement membrane matrix (Matrigel) leads to the formation of tubular structures resembling the capillary blood vessels characteristic of the angiogenic phenotype.11,16To ensure that this model recapitulated the pattern of selective expression of CD13/APN in tumor endothelium,3we prepared RNA from matrix-stimulated or matrix-unstimulated EOMA cells.17 EOMA cells express very low levels of CD13/APN (Figure 1) and, in this respect, correspond to resting endothelial cells. RT-PCR analysis indicated that the low endogenousCD13/APN mRNA levels in EOMA cells are increased 3- to 4-fold on angiogenic stimulation (+), similar to the levels of the angiogenic regulator β3 integrin, whereas control β-actin levels remain unchanged (Figure 7A).
Expression of CD13/APN is induced during capillary tube formation in vitro and tumor progression in vivo.
(A) RT-PCR analysis of 10 μg total RNA from EOMA cells cultured for 24 hours with (+, lane 1) or without (−, lane 2) reconstituted basement membrane matrix (Matrigel) or NIH3T3 fibroblast positive control cells (lane 3) using primers specific for CD13/APN, β3-integrin and β-actin. (B) A representative experiment showing EOMA cells stably transfected with the indicated plasmid constructs cultured with (+ bm) or without (−bm) basement membrane matrix (Matrigel) to stimulate endothelial morphogenesis. Confocal images were acquired 18 to 24 hours after incubation. CMV-GFP (positive control line with GFP driven by the constitutive CMV promoter); null-GFP (promoterless negative control cell line); CD13-GFP (images of 2 independent cell lines containing the CD13/APN proximal promoter driving GFP expression). (C) Flow cytometric analysis of GFP-expressing cells in single-cell suspensions of EOMA xenografts. Data are expressed as percentage GFP-positive cells and numbers are representative of 2 independent experiments.
Expression of CD13/APN is induced during capillary tube formation in vitro and tumor progression in vivo.
(A) RT-PCR analysis of 10 μg total RNA from EOMA cells cultured for 24 hours with (+, lane 1) or without (−, lane 2) reconstituted basement membrane matrix (Matrigel) or NIH3T3 fibroblast positive control cells (lane 3) using primers specific for CD13/APN, β3-integrin and β-actin. (B) A representative experiment showing EOMA cells stably transfected with the indicated plasmid constructs cultured with (+ bm) or without (−bm) basement membrane matrix (Matrigel) to stimulate endothelial morphogenesis. Confocal images were acquired 18 to 24 hours after incubation. CMV-GFP (positive control line with GFP driven by the constitutive CMV promoter); null-GFP (promoterless negative control cell line); CD13-GFP (images of 2 independent cell lines containing the CD13/APN proximal promoter driving GFP expression). (C) Flow cytometric analysis of GFP-expressing cells in single-cell suspensions of EOMA xenografts. Data are expressed as percentage GFP-positive cells and numbers are representative of 2 independent experiments.
To confirm that the proximal CD13/APN promoter controls its expression during angiogenesis, we stably transfected reporter plasmids containing this promoter upstream of the GFP reporter gene into EOMA cells. Stably transfected pools cultured underunstimulated conditions (−basement membrane [bm]) formed a cobblestone monolayer characteristic of endothelial cells and were negative for CD13-driven GFP expression throughout the culture period, arguing against cell-cell contact inhibition of expression (Figure 7B). By contrast, on angiogenic stimulation on the Matrigel basement membrane (+bm), the CD13-GFP–containing transfectants formed extensive capillary networks that were highly fluorescent, confirming that the factors regulating this promoter are responsible for the induction ofCD13/APN gene expression during tube formation.
Finally, to establish that the proximal promoter responds to angiogenic signals during tumor progression in vivo, we injected the GFP-containing EOMA cell lines subcutaneously into immunocompromised mice to form tumor xenografts. Tumors were harvested, mechanically disrupted to form single-cell suspensions, and assayed for GFP expression by flow cytometric analysis. Tumors derived from the CMV-GFP– and CD13-GFP–containing EOMA lines contained 23% to 30% GFP-positive cells compared with background levels found in the null-GFP–derived tumors (Figure 7C). The relatively low percentage of GFP-positive cells in the xenografts reflects a prominent host-derived stromal component of these tumors. Therefore, transcription from theCD13/APN proximal promoter plasmid faithfully reflects the up-regulation of the endogenous CD13/APN gene that occurs during endothelial cell capillary tube formation in vitro and tumor growth in vivo.
CD13/APN functions in capillary tube formation but not proliferation
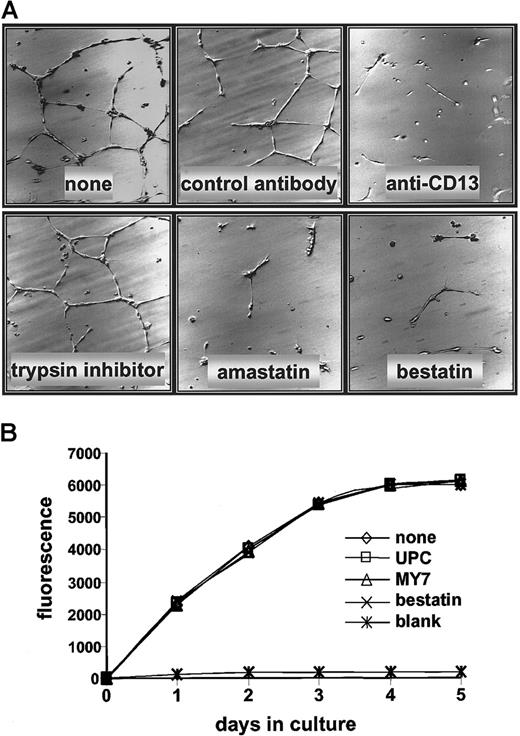
Antagonists of CD13/APN are powerful inhibitors of tumor growth, thus identifying it as a functional regulator of tumor angiogenesis.3 Because CD13/APN transcription is induced in endothelial cells undergoing capillary tube formation, it is possible that its functional role is at the level of control of endothelial morphogenesis. To address this possibility, we cultured primary HUVECs on Matrigel in the presence of the CD13/APN functional antagonists, bestatin or amastatin, or with monoclonal antibodies that interfere with CD13/APN activity (MY7). Each of the inhibitors was extremely effective at abrogating the ability of the cells to organize a capillary network; under control conditions with vehicle alone, a nonspecific protease inhibitor (trypsin inhibitor) or isotype-matched control antibodies (UPC10) formed characteristic, organized networks (Figure 8A). By contrast, treatment of HUVECs with MY7 or bestatin had no effect on proliferation rates as measured by metabolic activity (Figure 8B), implying that CD13/APN does not play a role in endothelial cell proliferation during angiogenesis.
CD13/APN antagonists inhibit HUVEC capillary tube formation but not proliferation.
(A) HUVECs were plated on Matrigel basement membrane preparations and incubated with either the CD13/APN inhibitors amastatin or bestatin, or the anti-CD13/APN monoclonal antibody MY7; or the negative-control trypsin inhibitor or isotype-matched monoclonal antibodies. Plates were incubated for 24 hours before analysis. (B) HUVECs were plated on tissue culture dishes with anti-CD13/APN monoclonal antibody MY7 or isotype-matched control antibodies UPC10, and proliferation was assessed by accumulation of the fluorescent REDOX indicator at the indicated time intervals. The blank condition contained no cells.
CD13/APN antagonists inhibit HUVEC capillary tube formation but not proliferation.
(A) HUVECs were plated on Matrigel basement membrane preparations and incubated with either the CD13/APN inhibitors amastatin or bestatin, or the anti-CD13/APN monoclonal antibody MY7; or the negative-control trypsin inhibitor or isotype-matched monoclonal antibodies. Plates were incubated for 24 hours before analysis. (B) HUVECs were plated on tissue culture dishes with anti-CD13/APN monoclonal antibody MY7 or isotype-matched control antibodies UPC10, and proliferation was assessed by accumulation of the fluorescent REDOX indicator at the indicated time intervals. The blank condition contained no cells.
Discussion
The CD13/APN cell surface protease is specifically expressed on the endothelium of angiogenic, but not normal, vasculature.3 In this report, we demonstrate that CD13/APN expressed in tumor endothelial cell lines is identical to that expressed in epithelial cells. CD13/APNexpression is significantly induced in both primary and tumor endothelial cells on culture with individual angiogenic growth factors, suggesting that the expression of such factors by tumors in situ forms the functional basis for the differential expression ofCD13/APN in tumor versus normal vascular endothelial cells. Molecular characterization of CD13/APN expression indicated that the proximal of 2 CD13/APN promoters regulates gene expression in endothelial cells in response to hypoxia, angiogenic growth factors, signals regulating capillary tube formation, and xenograft tumor growth. Therefore, in addition to its function as a regulator of angiogenesis,3 the transcriptional activation of CD13/APN is also a marker of angiogenic vasculature and establishes CD13/APN and its transcriptional regulatory proteins as potential targets for antiangiogenic therapy.
Consistent with the role of CD13/APN as an angiogenic regulator in vascular endothelial cells, hypoxia markedly induces endogenous CD13/APN mRNA and reporter gene transcription levels in endothelial cells. During the initial stages of angiogenesis, signals generated by hypoxic stimuli alter the expression of many genes contributing to angiogenic differentiation, including those encoding glycolytic enzymes, glucose transporters, and angiogenic and hematopoietic growth factors.7 Expression of these genes can be affected either positively or negatively through transcriptional and posttranscriptional mechanisms.18-22 The elevation ofCD13/APN expression could be a direct response to hypoxia-activated DNA-binding transcription factors, such as hypoxia-inducible factor 1 (HIF-1) or hypoxia-associated factor (HAF), or a secondary consequence of hypoxia-induced autocrine growth factor production (vide infra). Examination of the sequence of the CD13/APN proximal promoter failed to reveal a consensus HIF-123 or EP-1724 binding site; however, numerous other consensus sites for transcription factors important for angiogenesis are present. Identification of the specific transcription factors regulating CD13/APN gene expression in response to angiogenic stimuli will be important for elucidating the molecular mechanisms controlling its angiogenic activation.
Of the specific cytokines tested that are induced by hypoxic conditions, CD13/APN mRNA, protein, and promoter activity levels are increased most strikingly in response to bFGF and VEGF. These factors are coexpressed in a variety of cancers and functionally complement each other during angiogenesis.2Thus, it is reasonable to assume that they would play a similar role in the angiogenic induction of CD13/APN. The regulation of specific genes by bFGF and VEGF has been shown to initiate signaling cascades involving several different intermediates including Ras, phospholipase Cγ, p125FAK, and phosphatidylinositol 3-kinase (reviewed in Tallquist et al25). The molecular mechanisms controlling how these specific signals initiate and maintain the angiogenic phenotype by routing and integration through common signaling pathways is still poorly understood. Information regarding the specific pathway(s) regulated by bFGF and VEGF that induceCD13/APN transcription will be important for future characterization of the mechanisms driving angiogenic modulation of gene expression.
The Kaposi sarcoma–derived KS1767 cell line showed very high constitutive expression of CD13/APN correlating with the highest relative promoter activity among the cell lines tested (S.V.B. and L.H.S., unpublished data, 2000). Assay of the supernatant of cultured KS1767 cells shows that this line secretes 4-fold higher levels of VEGF into the culture medium compared with the CD13/APN-low EOMA cell line (C. McKay, S.V.B., and L.H.S., unpublished data, 2000), suggesting that an autocrine mechanism contributes to the constitutive expression ofCD13/APN in this cell line. Our observation that bFGF and VEGF neutralizing antibodies together inhibit CD13/APNpromoter activity in KS1767 cells more potently than either alone (Figure 6B) indicates that both bFGF and VEGF (and perhaps additional angiogenic factors) may contribute to high levels ofCD13/APN in KS1767 cells. Consistent with this notion, primary cells and cell lines derived from Kaposi sarcoma lesions also secrete high levels of cytokines, including bFGF and VEGF26-29; the autocrine production of bFGF is functionally significant because the addition of neutralizing anti-bFGF antibodies markedly impairs KS1767 cell proliferation.29Additionally, endothelial cells engineered to exogenously express bFGF acquire a Kaposi-like angiogenic phenotype and can activate quiescent endothelial cells when injected into nude mice.30 It is likely therefore that the cells of primary Kaposi lesions also constitutively express CD13/APN31 and that this contributes to the highly vascular nature of this tumor.
How CD13/APN facilitates angiogenesis is not known. Its location on the cell surface mandates that its functional activity is dictated by substrates that are available in the immediate intercellular space. CD13/APN has been implicated in the catabolism of neuroactive peptides,32-34 amino acid scavenging and degradation of regulatory peptides,35,36 cell adhesion alterations,37 tumorinvasion and metastasis,38,39 as well as antigen processing and presentation.40,41 Because the switch from the quiescent to angiogenic endothelial phenotype involves an alteration in the relative levels of angiogenic inhibitors and activators,1it is intriguing to postulate a role for CD13/APN in the processing of small regulatory molecules required to initiate, maintain, or suppress the angiogenic program in tumor vessel endothelium. CD13/APN activity is controlled by its expression; thus, its precise transcriptional regulation is a pivotal factor that potentially controls the switch from quiescence to malignancy.
Acknowledgments
We would like to thank Dr Gopal Murti and Ken Barnes for confocal microscopy, Dr Janet Houghton for xenograft production, Dr Richard Ashmun for flow cytometric data analysis, John Zacher for photomicrography, Drs Catriona McKay, Jingfeng Zhao, and Shrikanth Hegde for technical assistance and helpful discussions, and Drs David Shapiro, Linda Hendershot, Paul Ney, and John Cleveland for critical reading of the manuscript.
Supported by National Institutes of Health (NIH) grant CA-70909 to L.H.S., NIH grant CA-78512 to R.P., National Cancer Institute Cancer Center Support (CORE) grant CA-21765, and the American Lebanese Syrian Associated Charities (ALSAC), St Jude Children's Research Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Linda H. Shapiro, Department of Pathology, St Jude Children's Research Hospital, Memphis, TN 38105; email:linda.shapiro@stjude.org.

![Fig. 4. Angiogenic factors induce CD13/APN cell surface expression in primary endothelial cells. / (A) Primary HUVECs were serum starved for 24 hours, then cultured without serum (none), with the indicated angiogenic growth factors, or with 10% fetal calf serum (FCS), stained for CD13/APN expression with an anti-CD13/APN monoclonal antibody (MY7) and analyzed by flow cytometry. The relative fluorescence index (RFI) and fold induction over untreated cells of each sample are indicated. (B) RNA isolated and probed for CD13/APN or 28S RNA expression shows that treatment of primary HUVECs with certain angiogenic factors inducesCD13/APN mRNA (VEGF [25 ng/mL], bFGF [50 ng/mL], TNFα [10 ng/mL], and IGF-1 [50 ng/mL]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/3/10.1182_blood.v97.3.652/6/m_h80310659004.jpeg?Expires=1765934865&Signature=YrIoSOGgN0SDek9QbnXIOS0KecI90X~Aw5kH3u0rWnuavYVvNT84EFPTOQGL-3tE-VqTt34edYyDPovrc7ojuc7oajWp-~8owe1YdhaWYJ-tyWZgZ5TkiJSQDx2a6t40m8fut1ztOeZBne4BXns2UoGuAj7f7rPLY1BF7Vw-BdzwdNlhoFq9rjyzD1FM6n9TCloid69N6FCpR2r2b8mRIlkY~VshrXYZPNvl8D9jki~~1g8-2ZmJE1ZMcaAwa462Gn5msVdxrfIAwgASDEVGFtyhpqpyyd8kMnVClpoOvPFAabK9EXDfMRJ43uN663Im0iuqVdPgv4jUcG6Gw2D~ng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Endogenous. / CD13/APN is induced in response to serum concentration and angiogenic factors. Expression of CD13/APN protein (A) or mRNA (B) in KS1767 cells is dependent on serum concentration. Serum-starved cells were stimulated with the indicated concentrations of FBS or left untreated (no fetal calf serum [FCS]) for 24 hours. CD13/APN protein expression was measured by flow cytometry; relative transcript levels in total RNA were assayed by Northern blot analysis. (C) bFGF and VEGF induce time-dependent increases inCD13/APN mRNA expression in KS1767 cells similar to that seen with the angiogenic marker, β3 integrin. Total RNA was isolated at the indicated time intervals after addition of bFGF (50 ng/mL) or VEGF (25 ng/mL) to serum-starved cells and hybridized with the indicated probes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/3/10.1182_blood.v97.3.652/6/m_h80310659005.jpeg?Expires=1765934865&Signature=hO5~-~2I2kADrA2R0kAcqA8V4~lHXQANk9-s1-XSUARo5IZ86lDCvOYykU8Ys-ISz2pQ24zpXDBemqY0RBGa9wz7OUxDiiN9XvqyoNr2wH7KMJlB8EO32y3qW-UCmFJsDAcd1WtL1UnoII~dZ677RfqMWjv242h6nQ-jT3t12s21JUqOlgUH8AnURbHe7IqqwUAtbiV~-KEl9t0qSNLD9JPA3TcLKVFAUYVxbNrR6VMv7slfK0xxncniG4Jf07fFjDoYrYC5AwLh3ZqkeS9onEFQzUsmlcUNpETwDdG~s1UZksm1-MTwJy3m~m7aq9oImD-3K~6pBOwGxGcOba~OeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal