Abstract
Genetic evidence demonstrates the importance of plasminogen activation in the migration of macrophages to sites of injury and inflammation, their removal of necrotic debris, and their clearance of fibrin. These studies identified the plasminogen binding protein annexin II on the surface of macrophages and determined its role in their ability to degrade and migrate through extracellular matrices. Calcium-dependent binding of annexin II to RAW264.7 macrophages was shown using flow cytometry and Western blot analysis of EGTA eluates. Ligand blots demonstrated that annexin II comigrates with one of several proteins in lysates and membranes derived from RAW264.7 macrophages that bind plasminogen. Preincubation of RAW264.7 macrophages with monoclonal anti–annexin II IgG inhibited (35%) their binding of 125I-Lys-plasminogen. Likewise, plasmin binding to human monocyte-derived macrophages and THP-1 monocytes was inhibited (50% and 35%, respectively) when cells were preincubated with anti–annexin II IgG. Inhibition of plasminogen binding to annexin II on RAW264.7 macrophages significantly impaired their ability to activate plasminogen and degrade [3H]-glucosamine–labeled extracellular matrices. The migration of THP-1 monocytes through a porous membrane, in response to monocyte chemotactic protein-1, was blocked when the membranes were coated with extracellular matrix. The addition of plasminogen to the monocytes restored their ability to migrate through the matrix-coated membrane. Preincubation of THP-1 monocytes with anti–annexin II IgG inhibited (60%) their plasminogen-dependent chemotaxis through the extracellular matrix. These studies identify annexin II as a plasminogen binding site on macrophages and indicate an important role for annexin II in their invasive and degradative phenotype.
Introduction
The recruitment of monocytes to an inflammatory site is a complex process involving their adhesion to endothelial cells, passage into the perivascular connective tissue, and migration toward a chemotactic gradient.1,2 The synthesis and activation of matrix degrading proteinases have been suggested to play an essential role in the migration of monocytes and macrophages through tissue. In this regard, the results of in vitro and in vivo studies have suggested that macrophage expression of urokinase-type plasminogen activator (uPA) and its receptor (uPA-R) are important regulators of migration,3,4 degradation of the fibrin-rich matrix that forms following tissue injury,5 fibrinolysis in the atheroma,6 degradation of extracellular matrices,7,8 and the release of matrix-bound growth factors.9 10
The availability of animals genetically deficient in selected components of the plasminogen/plasminogen activator system has refined our understanding of the role of plasminogen activation in macrophage migration and tissue remodeling following injury. For example, thioglycollate-induced recruitment of monocytes into the peritoneal cavities of mice deficient in plasminogen was diminished when compared to wild-type mice.11 In an experimental model of glomerulonephritis, macrophage infiltration of glomeruli was diminished in mice deficient in uPA.12 Likewise, the recruitment of monocytes to a vascular injury site appears to be critically dependent on uPA-mediated plasminogen activation. In the setting of full-thickness electrical injury of the femoral artery, plasminogen deficiency was associated with reduced infiltration of leukocytes, delayed removal of the necrotic tissue, and reduced formation of a neointima.13 Similar results were observed in mice deficient in uPA but not tissue-type plasminogen activator (tPA).14 The analysis of atherosclerosis in apolipoprotein E–deficient mice demonstrated that superimposed uPA deficiency protects against aortic aneurysm formation, whereas tPA deficiency had no protective effect.15 Lesion infiltration by macrophages was essentially absent in uPA-deficient mice, but appeared normal in tPA-deficient mice.15 In acute myocardial infarction, macrophage infiltration into necrotic tissue was significantly reduced in uPA-deficient mice and these animals were protected from ventricular rupture.16 In contrast, deficiency in uPA-R, tPA, stromelysin-1 (MMP-3), or metalloelastase (MMP-12) did not protect against ventricular rupture. Thus, although neither uPA-R nor tPA seems to be essential for macrophage recruitment to the healing myocardium, uPA appears to play a central role.
The broad substrate specificity of plasmin requires that macrophages localize its activation to the pericellular environment to limit collateral tissue damage. An important mechanism by which macrophages regulate plasminogen activation is the differential expression of uPA and plasminogen activator inhibitor.4,10,17-21 In addition, localized activation is achieved through the expression of uPA-R and binding sites for plasminogen.22-24 Despite direct evidence demonstrating the importance of plasminogen activation in the ability of macrophages to migrate to sites of injury and inflammation, as well as their ability to degrade necrotic debris and fibrin, the identity of plasminogen binding sites remains unclear.
The binding of plasminogen to cells is mediated by plasminogen's kringle domains and is inhibited by the lysine analogue ε-aminocaproic acid (ε-ACA).22,25-27 Several proteins have been identified as plasminogen binding sites on the surface of a number of cell types.28-32 In particular, affinity chromatography of lysates of human U937 monocytes has revealed several plasminogen binding proteins, a major 54-kd band and additional bands at approximately 22, 40, and 96 kd.31 The majority of plasminogen binding activity in these cell lysates appeared to relate to the 54-kd protein identified as α-enolase.31Although α-enolase was subsequently found on the surface of U937 monocytes by flow cytometric analysis,31,32membrane-associated α-enolase accounted for less than 10% of total plasminogen binding.32 Furthermore, anti–α-enolase at concentrations that inhibited plasminogen binding to purified α-enolase had little or no effect on cellular plasminogen binding.32 Consistent with this observation are data demonstrating that increased expression of α-enolase by transformed fibroblasts was not associated with increased plasminogen activation.33 Taken together, these data suggest that although α-enolase accounts for a proportion of plasminogen binding sites on cells of monocyte/macrophage lineage, additional plasminogen binding proteins remain to be identified.
Human umbilical vein endothelial cells express a protein (36 kd) that independently binds both plasminogen and tissue plasminogen activator.30,34 Sequence analysis of internal tryptic peptides indicated that this protein is annexin II.30Annexin II is a member of the annexin family of calcium-dependent phospholipid binding proteins.35 Annexins lack a hydrophobic signal sequence and were initially considered intracellular proteins.35 Nonetheless, surface expression of annexin II by a variety of cells has been demonstrated,30,34,36,37and other annexins have been identified bound to components of the extracellular matrix.37 38 In addition to serving as a receptor for plasminogen and tPA, annexin II has been identified as a high-affinity binding site for tenascin-C. In experiments reported here, we demonstrated that annexin II is a binding site for plasminogen on murine RAW264.7 macrophages, human monocyte-derived macrophages, and THP-1 monocytes. Inhibition of plasminogen binding to annexin II significantly inhibited plasminogen activation and extracellular matrix degradation by RAW264.7 macrophages, as well as the chemotaxis of THP-1 monocytes through the extracellular matrix.
Materials and methods
Cell culture
Murine RAW264.7 macrophages39 and human THP-1 monocytes40 were obtained from American Type Tissue Culture (Rockville, MD) and maintained in culture as previously described.10 Human monocytes were isolated from fresh buffy coat (New York Blood Center, New York, NY) as follows.41 Buffy coat was diluted 1:1 with versine buffer, layered on Histopaque (Sigma Chemical, St Louis, MO) and centrifuged at 400g for 30 minutes at room temperature. Mononuclear cells were recovered and washed 3 times in Dulbecco phosphate-buffered saline (DPBS). The resuspended mononuclear cells were layered on a preformed Percoll gradient (Pharmacia, Piscataway, NJ) and centrifuged at 1300g for 25 minutes. Separated monocytes were recovered and washed 3 times with DPBS. Monocytes were suspended in RPMI medium supplemented with 10% human serum (AB), penicillin (100 U/mL), streptomycin (100 μg/mL), and 4 mM glutamine (Gibco, Grand Island, NY) and cultured in Teflon beakers. Cells were identified as monocytes/macrophages by Wright-Giemsa stain and by immunoperoxidase staining with monoclonal antihuman macrophage (HAM56; Dako, Carpenteria, CA)42 and anti-CD68 (Dako).43
Isolation of a membrane-rich fraction from macrophage lysates
A membrane-rich fraction was isolated from macrophage lysates by differential centrifugation. RAW264.7 cells (2 × 107) were harvested in ice- cold Dounce buffer (VWR, South Plainfield, NJ; 1.5 mM MgCl2, 5 mM KCl, 10 mM HEPES, pH 7.5) containing aprotonin (1 μg/mL) and leupeptin (0.5 μg/mL). Cells were lysed in a Dounce homogenizer. The lysate was centrifuged in an IEC Micromax (Needham Heights, MA; 851 rotor) at 13 000 rpm (16 000g) for 20 minutes to remove unbroken cells, nuclei, mitochondria, and other organelles. The supernatant was recovered and plasma membranes and the microsomal compartment were pelleted by centrifugation in a Beckman TL-100 (Beckman Coulter, Fullerton, CA; TLA-100.3 rotor) at 55 000 rpm (100 000g) for 1 hour. We verified that the membrane preparations were free of intact cells and nuclei by light microscopy. Membrane pellets were dissolved in 1 × sample buffer without β-mercaptoethanol.
Identification of membrane plasminogen binding proteins by ligand blot
Samples and biotinylated molecular weight markers (Amersham Pharmacia Biotech, Piscataway, NJ) were electrophoresed in 4% to 15% polyacrylamide gradient gels. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA). The membrane was blocked in fresh 5.0% dry defatted milk in Tris-buffered saline/0.05% Tween-20 (TTBS) for 1 hour, washed once with TTBS for 15 minutes, and then incubated 1 hour at room temperature with 0.25% dry defatted milk in TTBS with or without Lys-plasminogen (10 μg/mL; American Diagnostica Inc, Greenwich, CT). Following 2 washes with TTBS, the membrane was incubated in 3% dry defatted milk in TTBS containing a monoclonal antibody directed against kringles 1 to 3 of human plasmin(ogen) (2.5 μg/mL; American Diagnostica) for 1 hour. The membrane was washed twice (TTBS), incubated for 1 hour in 3% dry defatted milk in TTBS containing biotinylated rabbit antimouse IgG (1:30 000; Pierce, Rockford, IL), washed twice (TTBS), and incubated 1 hour with preformed avidin-biotin–horseradish peroxidase (HRP) complexes (Pierce) in DPBS/0.1% Tween-20. Bound HRP was visualized using enhanced chemiluminescence (ECL; Amersham Life Science).
Western blot for annexin II
Purified annexin II and samples were electrophoresed in 4% to 15% polyacrylamide gradient gels. Proteins were transferred to a PVDF membrane, following which the membrane was blocked for 1 hour in TTBS containing 5% dry defatted milk. Following 2 washes (TTBS), the membrane was incubated 1 hour with 0.5 μg/mL monoclonal antihuman annexin II IgG (clone 5, IgG1, Transduction Laboratories, Lexington, KY) in TTBS containing 3% dry defatted milk. The membrane was washed twice (TTBS) and incubated 1 hour in 3% dry defatted milk in TTBS containing biotinylated rabbit antimouse IgG (1:30 000; Pierce). The membrane was then washed twice (TTBS) and incubated 1 hour with preformed avidin-biotin-HRP complexes (Pierce) in DPBS/0.1% Tween-20. Bound HRP was visualized using ECL.
Flow cytometric analysis
The RAW264.7 cells were propagated in suspension in Teflon beakers and their reactivity with a monoclonal antibody directed against annexin II (clone Z014, IgG1, Zymed Laboratories Inc, San Francisco, CA) was analyzed by flow cytometry. Cells were washed 3 times with Hepes-buffered saline (HBS; 137 mM NaCl, 4 mM KCl, 11 mM glucose, and 11 mM Hepes, pH 7.4) with or without 10 mM EGTA and resuspended in HBS or HBS/EGTA containing clone Z014 (100 μg/mL). Cell suspensions were incubated on a rocking platform (4°C, 45 minutes), washed in 20 volumes of HBS (or HBS/EGTA), resuspended in HBS (or HBS/EGTA) containing fluorescein isothiocyanate (FITC)–conjugated goat antimouse IgG (20 μg/mL; Cappel/ICN, Costa Mesa, CA) and incubated 45 minutes at 4°C. Following 3 additional washes, cells were resuspended in HBS and analyzed on a Coulter Epics XL flow cytograph (Beckman Coulter).
Iodination of Lys-plasminogen
Human Lys-plasminogen was iodinated according to the method of McFarlane.44 Iodinated proteins were separated from unincorporated 125I by gel filtration on a PD-10 column (Pharmacia) pre-equilibrated with HBS containing 0.5% human albumin. Following iodination, the ability of 125I-Lys-plasminogen to be activated by uPA was determined using the fluorogenic plasmin substrate as described below.
Determination of membrane-bound plasmin activity
Membrane-bound plasmin activity was quantitated as previously described.45 Cells were incubated in macrophage serum-free media (MSFM; Gibco) containing plasminogen for 1 hour at 4°C. Following incubation, media containing unbound plasmin(ogen) were removed and cells were washed (3 ×) with DPBS. MSFM containing the plasmin substrate D-Val-Leu-Lys-amino-4-methylcoumarin (Enzyme Systems Products, Dublin, CA) was added and allowed to incubate 2.5 hours. Cleavage of the plasmin substrate generated a fluorescent product, which was quantitated in a Fluoroscan microplate reader (American Diagnostica) and extrapolated to the fluorescence generated by 0 to 40 ng/mL plasmin prepared in MSFM.
Preparation of [3H]-glucosamine–labeled extracellular matrices
The preparation of [3H]-glucosamine–labeled extracellular matrices has been described previously.8Human aortic smooth muscle cells (SMC) (Clonetics, San Diego, CA) were plated into 12-well plates (105/well) in SMC Growth Medium (Clonetics). On day 3, media were replaced with media containing 5 μCi/well [3H]-glucosamine (Dupont/New England Nuclear, Boston, MA). On day 6, the cell layer was removed by sequential exposure to 0.5% Triton X-100 in PBS (10 minutes) and 0.20 mM NH4OH in PBS (3 minutes). The remaining insoluble extracellular matrices were washed 3 times with sterile DPBS and stored at 4°C.
Matrix invasion assay
Tissue culture inserts (10 mm) with porous (8 μm pore size) polycarbonate membrane (Nunc, Naperville, IL) were coated with Matrigel (Collaborative Biomedical) as follows. Matrigel was thawed on ice and diluted to 0.5 mg/mL with cold sterile water. An aliquot (50 μL/insert) of diluted Matrigel was spread over the surface of the prechilled inserts and allowed to dry overnight. The next day matrices were rehydrated with 300 μL MSFM for 1 hour. THP-1 monocytes were harvested, washed 3 times in DPBS, and suspended in MSFM. Cells (4 × 104/insert) were added to the matrix-coated insert and the insert was placed in a 12-well plate containing MSFM. Cells that crossed the membrane into the lower well were counted by phase contrast microscopy.
Results
Annexin II is expressed on the surface of RAW264.7 macrophages
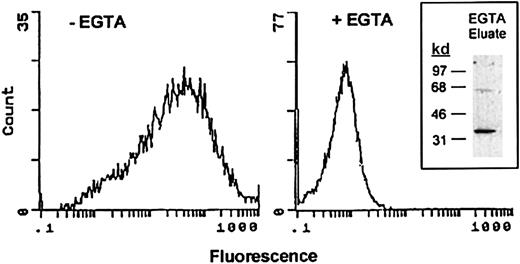
Surface expression of annexin II by RAW264.7 cells was determined by flow cytometry. Macrophages were incubated with monoclonal anti–annexin II IgG followed by FITC-conjugated secondary antibody. The binding of annexin II to cell membranes is calcium dependent.35 46 Therefore, as a control, surface annexin II expression was measured in the presence of the calcium chelator EGTA. As seen in Figure 1, the mean fluorescence intensity of cells treated with EGTA was 0.6. In the absence of the calcium chelator, mean fluorescence was increased 40-fold. The frequency distribution of annexin II–positive cells (ie, numbers of cells plotted as a function of fluorescence level) indicates that the majority of RAW264.7 macrophages express annexin II on their surface. To confirm that EGTA treatment removed annexin II from the cell surface, the eluate was concentrated and tested for the presence of annexin II by Western blot (Figure 1, insert). The EGTA eluate contained an approximate 36-kd protein, which was immunoreactive with monoclonal anti–annexin II IgG.
Demonstration of annexin II surface expression on RAW264.7 macrophages by flow cytometric analysis.
Control and EGTA-treated RAW264.7 macrophages were incubated with anti–annexin IgG (clone Z014, Zymed) in the absence or presence of EGTA followed by FITC-conjugated goat antimouse IgG. Relative fluorescence units are shown for each sample. To verify that annexin II was removed from the cell surface by treatment with EGTA, eluates were examined by Western blot as described in “Materials and methods” (inset).
Demonstration of annexin II surface expression on RAW264.7 macrophages by flow cytometric analysis.
Control and EGTA-treated RAW264.7 macrophages were incubated with anti–annexin IgG (clone Z014, Zymed) in the absence or presence of EGTA followed by FITC-conjugated goat antimouse IgG. Relative fluorescence units are shown for each sample. To verify that annexin II was removed from the cell surface by treatment with EGTA, eluates were examined by Western blot as described in “Materials and methods” (inset).
To quantitate the amount of annexin II on the cell surface, cells were incubated (20 minutes) with 20 mM EGTA. The EGTA eluate was concentrated by ultrafiltration and mixed with 6 × sodium dodecyl sulfate (SDS) sample buffer without β-mercaptoethanol. The presence of annexin II in the EGTA eluate was determined by Western blot. Quantification of annexin II in the eluate was determined by densitometric analysis of annexin II in the sample and a standard curve generated with 10 to 100 ng recombinant annexin II. Densitometric scans were linear to 50 ng annexin II. Based on 2 separate experiments, EGTA eluates contained 2.2 to 3.6 × 105 molecules annexin II/cell.
Annexin II comigrates with a protein that binds plasminogen in cell lysates and membranes derived from RAW264.7 macrophages
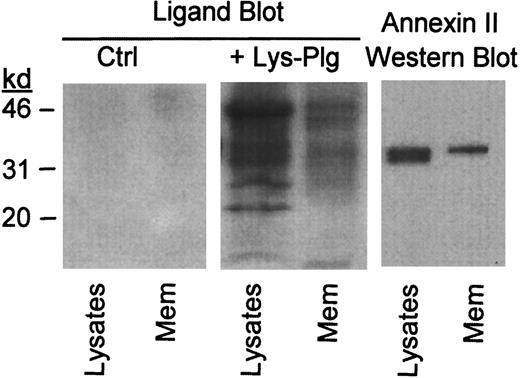
To determine whether annexin II serves as plasminogen binding site on RAW264.7 macrophages, we performed ligand blots on total cell lysates and isolated membranes. Lys-plasminogen was used as a ligand in these studies. Lys-plasminogen, the plasmin-modified form of native Glu-plasminogen, is formed on the cell surface and is preferentially bound by cells.47 48 Cellular proteins to which plasminogen binds were identified using a monoclonal antiplasminogen IgG. Following incubation with Lys-plasminogen, several bands were observed in a blot of cell lysate and isolated membranes (+Lys-Plg, Figure 2). These bands were not visible in blots, which were not incubated with Lys-plasminogen (Ctrl, Figure2). Notably, the relative abundance of plasminogen binding proteins in the membrane preparation was different compared to the cell lysate. As seen in Figure 2, immunoreactive annexin II in RAW264.7 lysate and membranes shares electrophoretic mobility with a plasminogen binding protein observed in the ligand blot. Therefore, annexin II could act as a plasminogen binding site on these cells.
Identification of plasminogen binding proteins in RAW264.7 macrophage membrane preparation.
For ligand blot, cell lysates and isolated membranes (Mem) were electrophoresed in gradient polyacrylamide gels and transferred to a PVDF membrane. PVDF membranes were blocked and subsequently incubated with buffer alone (Ctrl) or buffer containing 10 μg/mL Lys-plasminogen (+Lys-Plg). Bound plasminogen was visualized using monoclonal antiplasminogen IgG as described in “Materials and methods.” The presence of immunoreactive annexin II in macrophage lysates and isolated membranes was determined by Western blot as described in “Materials and methods.”
Identification of plasminogen binding proteins in RAW264.7 macrophage membrane preparation.
For ligand blot, cell lysates and isolated membranes (Mem) were electrophoresed in gradient polyacrylamide gels and transferred to a PVDF membrane. PVDF membranes were blocked and subsequently incubated with buffer alone (Ctrl) or buffer containing 10 μg/mL Lys-plasminogen (+Lys-Plg). Bound plasminogen was visualized using monoclonal antiplasminogen IgG as described in “Materials and methods.” The presence of immunoreactive annexin II in macrophage lysates and isolated membranes was determined by Western blot as described in “Materials and methods.”
Anti–annexin II IgG inhibits binding of125I-Lys-plasminogen to RAW264.7 macrophages
We next determined the ability of monoclonal anti–annexin II IgG to inhibit binding of 125I-Lys-plasminogen to RAW264.7 macrophages. The dose-dependent binding of125I-Lys-plasminogen to RAW264.7 macrophages is shown in Figure 3. Binding of125I-Lys-plasminogen to macrophages was inhibited more than 90% by the lysine analogue ε-ACA. Preincubation of cells with anti–annexin II IgG (clone Z014; Zymed) inhibited their binding of125I-Lys-plasminogen by 35%. Results of experiments in which a greater concentration of anti–annexin II IgG (50 μg/mL) was used to block binding of 125I-Lys-plasminogen to macrophages demonstrated no further inhibition of binding (data not shown). In contrast to anti–annexin II IgG, isotype-matched mouse IgG (25 μg/mL) had no effect on 125I-Lys-plasminogen binding.
Binding of 125I-Lys-plasminogen to RAW264.7 macrophages is inhibited by anti–annexin II.
(Left panel) Adherent cells (105/well) were washed with DPBS (3 ×). The media were replaced with cold MSFM alone or MSFM containing 0.1 to 6 μg/mL 125I-Lys-plasminogen (125Lys-Plg). Following incubation for 1 hour at 4°C with125I-Lys-plasminogen, cells were washed (3 ×) with DPBS and dissolved in 0.5 NaOH. Data represent the mean ± SE of 3 separate samples. (Right panel) Adherent cells (105/well) were washed with DPBS (3 ×). The media were replaced with cold MSFM alone or MSFM containing monoclonal anti–annexin II IgG (10-25 μg/mL; clone Z014, Zymed), mouse IgG (25 μg/mL), or 25 mM ε-ACA. The cells were incubated with the antibodies for 2 hours at room temperature before the addition of 125I-Lys-plasminogen (1.0 μg/mL). Cells were then incubated 1 hour at 4°C. The data represent the mean ± SEM of 6 replicate samples. nIgG indicates normal IgG.
Binding of 125I-Lys-plasminogen to RAW264.7 macrophages is inhibited by anti–annexin II.
(Left panel) Adherent cells (105/well) were washed with DPBS (3 ×). The media were replaced with cold MSFM alone or MSFM containing 0.1 to 6 μg/mL 125I-Lys-plasminogen (125Lys-Plg). Following incubation for 1 hour at 4°C with125I-Lys-plasminogen, cells were washed (3 ×) with DPBS and dissolved in 0.5 NaOH. Data represent the mean ± SE of 3 separate samples. (Right panel) Adherent cells (105/well) were washed with DPBS (3 ×). The media were replaced with cold MSFM alone or MSFM containing monoclonal anti–annexin II IgG (10-25 μg/mL; clone Z014, Zymed), mouse IgG (25 μg/mL), or 25 mM ε-ACA. The cells were incubated with the antibodies for 2 hours at room temperature before the addition of 125I-Lys-plasminogen (1.0 μg/mL). Cells were then incubated 1 hour at 4°C. The data represent the mean ± SEM of 6 replicate samples. nIgG indicates normal IgG.
Because inhibition of 125I-Lys-plasminogen binding to RAW264.7 cells required relatively high concentrations of anti–annexin II, we determined whether the presence of carboxyl terminal lysines in the anti–annexin II IgG might be responsible. First, we determined whether anti–annexin II IgG (clone Z014; Zymed) binds directly to immobilized plasminogen. For this purpose, plasminogen (1 μg) and annexin II (0.1 μg), as a positive control, were run in SDS polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions and transferred to PVDF membrane or blotted directly to nitrocellulose via a slot blot apparatus to rule out an effect of the denaturing conditions of SDS-PAGE. Following blocking, the membrane was incubated with anti–annexin II IgG (1 μg/mL) for 1 hour and probed with biotinylated rabbit antimouse IgG. As expected, anti–annexin II IgG bound strongly to annexin II, whereas we could not detect anti–annexin II IgG bound to the immobilized plasminogen (data not shown). Second, we determined whether the incubation of anti–annexin II IgG with Sepharose-conjugated carboxypeptidase B affected its ability to block plasmin binding to cells. The enzymatic activity of carboxypeptidase B following conjugation to CNBr-Sepharose 4B was 3.8 mU. Incubation of 0.2 nmoles (∼25 μg) of anti–annexin II IgG with carboxypeptidase B-Sepharose (capable of cleaving ∼4 nmol substrate/min) for 1 hour did not affect the ability of anti–annexin II to block plasmin binding (data not shown). Therefore, it is unlikely that anti–annexin II IgG inhibits plasminogen binding to cells due to the presence of carboxyl terminal lysine residues.
EGTA-treated RAW264.7 macrophages bind less plasmin
Annexin II binds to the surface of RAW264.7 macrophages in a calcium-dependent manner (Figure 1). Therefore, we incubated cells with EGTA to remove surface annexin II and then determined their ability to bind plasmin. The dose-dependent binding of plasmin to RAW264.7 macrophages is shown in Figure 4. As demonstrated for 125I-Lys-plasminogen, the binding of plasmin to macrophages was blocked more than 90% by ε-ACA. In the absence of exogenous plasmin, control macrophages had little detectable plasmin activity on their surfaces (< 1 ng plasmin/105cells). Following incubation with exogenous plasmin, membrane-bound plasmin activity increased to 21 ng/105 cells. The binding of plasmin to macrophages preincubated with EGTA was decreased by 30%. Western blots verified that membranes isolated from EGTA-treated macrophages contained markedly less annexin II. The persistent presence of annexin II in these membrane preparations, in contrast to the absence of surface expression following EGTA treatment (Figure 1), may represent annexin II associated with the inner membrane, microsomes, and the cytoskeleton. Importantly, the observed decrease in plasmin bound to the surface of macrophages treated with EGTA is consistent with the inhibition of125I-Lys-plasminogen binding to cells preincubated with anti–annexin II. These data, derived from 2 experimental approaches, demonstrate that annexin II is a plasminogen binding site on macrophages.
Plasmin binding to RAW264.7 macrophages is reduced following incubation with EGTA.
(Left panel) Adherent cells (105/well) were washed with DPBS (3 ×). The media were replaced with cold MSFM alone or MSFM containing 0.2 to 8 μg/mL Lys-plasmin. Following incubation for 1 hour at 4°C, cells were washed (3 ×) with DPBS and membrane-bound plasmin activity determined as described in “Materials and methods.” Data represent the mean ± SE of 3 separate samples. (Right panel) Adherent cells (105/well) were washed with versine buffer (PBS/0.5 mM EDTA) at 4°C and then incubated with versine buffer containing 25 mM EGTA for 20 minutes. The EGTA was removed and cells were washed with versine buffer. The cells were incubated with versine buffer containing 25 mM EGTA, 0.1% bovine serum albumin, and Lys-plasmin (1.0 μg/mL) for 1 hour at 4°C. Membrane-bound plasmin (Pls) activity was determined as described in “Materials and methods.” The data represent the mean ± SEM of 3 replicate samples. The inset is a Western blot for annexin II of membranes isolated from Ctrl (control) and EGTA-treated cells.
Plasmin binding to RAW264.7 macrophages is reduced following incubation with EGTA.
(Left panel) Adherent cells (105/well) were washed with DPBS (3 ×). The media were replaced with cold MSFM alone or MSFM containing 0.2 to 8 μg/mL Lys-plasmin. Following incubation for 1 hour at 4°C, cells were washed (3 ×) with DPBS and membrane-bound plasmin activity determined as described in “Materials and methods.” Data represent the mean ± SE of 3 separate samples. (Right panel) Adherent cells (105/well) were washed with versine buffer (PBS/0.5 mM EDTA) at 4°C and then incubated with versine buffer containing 25 mM EGTA for 20 minutes. The EGTA was removed and cells were washed with versine buffer. The cells were incubated with versine buffer containing 25 mM EGTA, 0.1% bovine serum albumin, and Lys-plasmin (1.0 μg/mL) for 1 hour at 4°C. Membrane-bound plasmin (Pls) activity was determined as described in “Materials and methods.” The data represent the mean ± SEM of 3 replicate samples. The inset is a Western blot for annexin II of membranes isolated from Ctrl (control) and EGTA-treated cells.
Anti–annexin II IgG inhibits activation of plasminogen by RAW264.7 macrophages
The binding of plasminogen to monocyte/macrophages is required for its efficient activation by uPA bound to uPA-R.22,24 49Therefore, we determined the effect of anti–annexin II IgG on plasminogen activation by uPA bound to uPA-R. RAW264.7 cells (105/well) were washed with DPBS (3 ×) and media replaced with MSFM. Cells were incubated 30 minutes at room temperature with 25 μg/mL monoclonal anti–annexin II IgG or mouse IgG prior to adding Lys-plasminogen (1 μg/mL) and the plasmin substrate. Cells were incubated at 37°C for 2.5 hours and the plasmin activity in their conditioned media determined. Media derived from cells incubated with plasminogen or plasminogen and normal mouse IgG contained 21.3 ± 1.3 and 19.3 ± 1.3 ng/mL plasmin (mean ± SE; n = 6), respectively. In contrast, media derived from cells incubated with anti–annexin II IgG and plasminogen contained 7.3 ± 0.8 ng/mL plasmin. The reduced ability of RAW264.7 cells to activate plasminogen in the presence of anti–annexin II IgG was not due to inhibition of uPA because the activation of plasminogen by fluid-phase uPA was unaffected by the same concentration of anti–annexin II IgG. These data indicate that membrane-bound annexin II supports both the binding of plasminogen and its subsequent activation by membrane-bound uPA.
Anti–annexin II IgG inhibits plasmin binding to human monocyte-derived macrophages
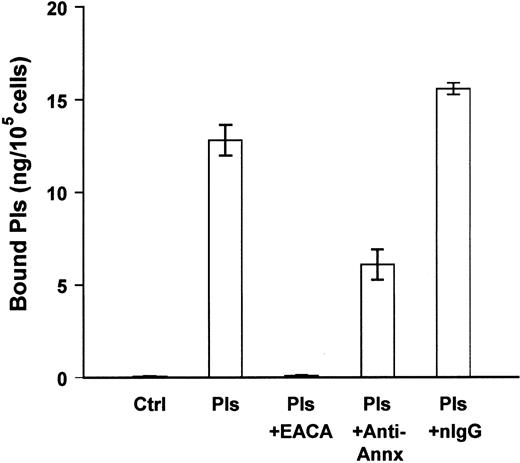
We next determined whether annexin II was a binding site for plasmin on the surface of human monocyte-derived macrophages. Blood monocytes were isolated and cultured in Teflon beakers for 7 days. Cells were aliquoted into 96-well plates and allowed to adhere overnight before the binding studies. In the absence of exogenous plasmin, monocyte-derived macrophages expressed trace amounts of membrane-bound plasmin (Figure 5). Following incubation with plasmin, membrane-bound plasmin activity was 13 ng/105 cells. Preincubation of cells with monoclonal anti–annexin II IgG (25 μg/mL) inhibited about 50% of the binding of plasmin to monocyte-derived macrophages. In other experiments, anti–annexin II IgG inhibited plasmin binding to human THP-1 monocytes by 25% (data not shown). Therefore, in a manner similar to murine RAW264.7 macrophages, cell surface annexin II serves as a binding site for plasmin(ogen) on human monocyte-derived macrophages and THP-1 monocytes.
Anti–annexin II inhibits plasmin binding to human monocyte-derived macrophages.
Cells were aliquoted into 96-well plates (105/well). The next day 2 groups of cells were washed (3 ×) with DPBS. The media were replaced with MSFM containing monoclonal anti–annexin II (clone Z014, Zymed) or normal mouse IgG (nIgG; 25 μg/mL). Following incubation for 30 minutes at 37°C, plasmin (1 μg/mL) was added and cells were incubated for 1 hour at 4°C. The remaining groups of cells were washed and media replaced with plasmin (Pls) alone, plasmin and ε-ACA (25 mM), or plasmin and purified annexin II. Following incubation for 1 hour at 4°C, membrane-bound plasmin was determined as described in “Materials and methods.” The data represent the mean ± SEM of 3 replicate samples. Control (Ctrl) indicates cells in the absence of exogenous plasmin.
Anti–annexin II inhibits plasmin binding to human monocyte-derived macrophages.
Cells were aliquoted into 96-well plates (105/well). The next day 2 groups of cells were washed (3 ×) with DPBS. The media were replaced with MSFM containing monoclonal anti–annexin II (clone Z014, Zymed) or normal mouse IgG (nIgG; 25 μg/mL). Following incubation for 30 minutes at 37°C, plasmin (1 μg/mL) was added and cells were incubated for 1 hour at 4°C. The remaining groups of cells were washed and media replaced with plasmin (Pls) alone, plasmin and ε-ACA (25 mM), or plasmin and purified annexin II. Following incubation for 1 hour at 4°C, membrane-bound plasmin was determined as described in “Materials and methods.” The data represent the mean ± SEM of 3 replicate samples. Control (Ctrl) indicates cells in the absence of exogenous plasmin.
Degradation of extracellular matrix by RAW264.7 macrophages is inhibited by anti–annexin II IgG
Urokinase PA–dependent plasminogen activation plays an important role in tissue remodeling directly via plasmin cleavage of matrix components and indirectly via the activation of metalloproteinases.15,50 Because anti–annexin II IgG was demonstrated to partially inhibit the binding and activation of plasminogen by RAW264.7 macrophages, we determined whether antiannexin IgG would affect the degradation of insoluble [3H]-glucosamine–labeled SMC extracellular matrices. The extracellular matrix consists of 3 principal classes of molecules: proteoglycans, adhesive glycoproteins (ie, fibronectin and laminin), and fibrous proteins (ie, collagen and elastin). [3H]-glucosamine is useful as a tracer in these studies because it labels proteoglycans and adhesive glycoproteins, which are susceptible to plasmin cleavage directly.51 52 When matrices were incubated with media alone, small amounts of labeled matrix were solubilized (Figure 6). The addition of plasminogen to the media controls had no effect, demonstrating the absence of significant plasminogen activator activity in the matrices. When RAW264.7 cells were plated directly on the matrices, solubilization was also unchanged in the absence of plasminogen. However, in the presence of plasminogen, RAW264.7 solubilization of SMC matrices increased 6-fold over media controls. The addition of anti–annexin II IgG (25 μg/mL) inhibited matrix solubilization approximately 40%. Normal mouse IgG had no effect. These data indicate that binding of plasminogen to annexin II plays a significant role in macrophage-mediated matrix degradation.
Extracellular matrix degradation by RAW264.7 macrophages is inhibited by anti–annexin II IgG.
Cells (5 × 105/well) were plated on insoluble [3H]-glucosamine–labeled human smooth muscle cell matrices in MSFM. Following adherence, media were replaced and 25 μg/mL monoclonal anti–annexin II (clone Z014, Zymed) or mouse IgG added. Cells were incubated with the antibodies (30 minutes, 37°C) prior to the addition of plasminogen (Plg, 1 μg/mL). Incubation media were recovered the next day and solubilized [3H]-activity determined as described in “Materials and methods.” The media and media plus plasminogen (Plg) controls depict the levels of [3H]-glucosamine released from insoluble extracellular matrix in the presence of plasminogen but in the absence of macrophages. Data represent the mean ± SEM of 4 replicate samples. nIgG indicates normal IgG.
Extracellular matrix degradation by RAW264.7 macrophages is inhibited by anti–annexin II IgG.
Cells (5 × 105/well) were plated on insoluble [3H]-glucosamine–labeled human smooth muscle cell matrices in MSFM. Following adherence, media were replaced and 25 μg/mL monoclonal anti–annexin II (clone Z014, Zymed) or mouse IgG added. Cells were incubated with the antibodies (30 minutes, 37°C) prior to the addition of plasminogen (Plg, 1 μg/mL). Incubation media were recovered the next day and solubilized [3H]-activity determined as described in “Materials and methods.” The media and media plus plasminogen (Plg) controls depict the levels of [3H]-glucosamine released from insoluble extracellular matrix in the presence of plasminogen but in the absence of macrophages. Data represent the mean ± SEM of 4 replicate samples. nIgG indicates normal IgG.
Invasion of extracellular matrix by THP-1 monocytes is inhibited by anti–annexin II IgG
In these studies, we determined the effect of anti–annexin II IgG on the ability of THP-1 monocytes to migrate through extracellular matrix in response to monocyte chemotactic protein-1 (MCP-1). Cell culture inserts containing THP-1 monocytes suspended on a porous (8 μm) polycarbonate membrane were placed into media alone or media containing MCP-1. Relatively few cells migrated into the lower well in the absence of MCP-1 (Figure 7). The addition of MCP-1 to the lower well resulted in the accumulation of large numbers of monocytes. The addition of MCP-1 to the cells in the upper well did not stimulate migration into the lower well (data not shown). THP-1 monocyte migration induced by MCP-1 was markedly diminished or completely blocked when the polycarbonate membranes were thinly coated with a basement membrane-derived extracellular matrix (Matrigel). However, when plasminogen was added to the cells in the upper well, the number of cells that migrated through the matrix-coated membrane increased 10-fold. If THP-1 monocytes were preincubated with monoclonal anti–annexin II IgG (clone Z014; Zymed), migration was inhibited 60%. Preincubation of cells with control antibodies had no effect. To rule out the possibility that anti–annexin II inhibits THP-1 monocyte migration independent of its effects on plasminogen binding, we determined whether the antibody affected the migration of THP-1 cells through uncoated inserts. Migration induced by MCP-1 was increased 20- ± 10-fold over controls. MCP-1–induced migration of THP-1 cells incubated with anti–annexin II was increased 32- ± 3-fold. Thus, preincubation of THP-1 cells with anti–annexin II did not inhibit MCP-1–induced migration through uncoated inserts. Because the binding of plasminogen to macrophages and its subsequent activation are inhibited by anti–annexin II IgG, we conclude that the migration of THP-1 monocytes through matrix is in part dependent on binding of plasminogen to annexin II.
Matrix invasion by THP-1 monocytes is inhibited by anti–annexin II.
Cells (4 × 104) were suspended in MSFM and preincubated with either 25 μg/mL monoclonal anti–annexin II IgG (clone Z014, Zymed), monoclonal anti-CD16 IgG, or anti-CD11b IgG. Plasminogen (Plg, 10 μg/mL) was added to the cell suspension and cells were aliquoted into an insert containing a porous (8 μm) polycarbonate membrane previously coated with Matrigel. The insert was placed into a well containing MCP-1 (50 ng/mL) and incubated 24 hours at 37°C. Cells that migrated into the lower well were counted using phase-contrast microscopy. The results of 2 independent experiments containing 3 replicate samples for each condition per experiment were pooled. The 2 experiments were identical except for the control IgG used. For the purposes of presentation and analysis the 2 groups were combined. Data are presented as the mean ± SEM. nIgG indicates normal IgG.
Matrix invasion by THP-1 monocytes is inhibited by anti–annexin II.
Cells (4 × 104) were suspended in MSFM and preincubated with either 25 μg/mL monoclonal anti–annexin II IgG (clone Z014, Zymed), monoclonal anti-CD16 IgG, or anti-CD11b IgG. Plasminogen (Plg, 10 μg/mL) was added to the cell suspension and cells were aliquoted into an insert containing a porous (8 μm) polycarbonate membrane previously coated with Matrigel. The insert was placed into a well containing MCP-1 (50 ng/mL) and incubated 24 hours at 37°C. Cells that migrated into the lower well were counted using phase-contrast microscopy. The results of 2 independent experiments containing 3 replicate samples for each condition per experiment were pooled. The 2 experiments were identical except for the control IgG used. For the purposes of presentation and analysis the 2 groups were combined. Data are presented as the mean ± SEM. nIgG indicates normal IgG.
Discussion
Recent genetic evidence has demonstrated the importance of plasminogen activation in the ability of macrophages to migrate to sites of injury and inflammation, and their removal of necrotic debris and fibrin.11,13,15,53 Despite earlier observations that plasminogen binding to the cell surface imparts a kinetic advantage to activation22,24,27,49 and protection of generated plasmin from inhibition,22,47 49 the identities of cell surface binding sites for plasminogen have not been completely elucidated.
In studies reported here, we demonstrate that annexin II, a plasminogen binding protein first described on endothelial cells,54 is present on the surface of RAW264.7 macrophages. The binding of annexin II to macrophages is calcium dependent as evidenced by the loss of surface expression following treatment with EGTA and the identification of annexin II in EGTA eluates. When cell membranes were examined for the presence of plasminogen binding proteins using a ligand blot procedure, plasminogen bound to a membrane protein that comigrates with purified annexin II. Moreover, preincubation of cells with anti–annexin II IgG partially inhibited125I-Lys-plasminogen binding to RAW264.7 macrophages and the binding of plasmin to human monocyte-derived macrophages. Likewise, the removal of membrane-bound annexin II with EGTA inhibited plasmin binding to RAW264.7 macrophages. Based on these data, we conclude that annexin II is a plasminogen binding site on the surface of murine and human macrophages.
Annexin II is a member of a highly conserved family of proteins, which exhibit calcium-dependent binding to membranes and cytoskeleton.35,55 It can exist in a monomeric form or in a heterotetrameric complex with p11, an S-100–like protein.46,56 Although annexin II is primarily an intracellular protein, surface expression has been reported for rat, murine, and human cells.30,36,37 Although the mechanism(s) by which cytoplasmic annexin II is secreted is not known, its binding to the cell surface requires a Ca++-dependent phospholipid binding site located in core repeat 2.57 Annexin II appears to be involved in the trafficking of membrane vesicles,58-60 and is a major protein component of murine J774 macrophage phagosomes61 and vesicles secreted by fibroblasts following cycles of stretch and relaxation.62In addition, calcium-dependent secretion by permeabilized chromaffin cells is stimulated by annexin II.63
The RAW264.7 macrophages and human monocyte-derived macrophages express large numbers (∼106/cell) of binding sites for plasminogen.45,64 The partial inhibition of125I-Lys-plasminogen or plasmin binding to macrophages by anti–annexin II IgG or treatment with EGTA is consistent with the observation that macrophages express several membrane proteins that serve as plasminogen binding sites. In this regard, α-enolase was the first plasminogen binding protein identified on monocytes and monocyte-like cells.31,64,65 Likewise, plasminogen binds to a 45-kd protein expressed by rat neuronal cells,66which is reported to be identical to α-enolase.67 The human cell line MCF7MF also expresses a 55- to 60-kd plasminogen receptor that is partially homologous to α-enolase.68 In addition to α-enolase and related proteins, the gp330 autoantigen of Heymann nephritis has also been identified as a plasminogen receptor.69 The plasminogen receptor in synovial fibroblasts is a 97-kd protein antigenically related to α2-macroglobulin receptor-associated protein.29 A 45-kd protein has been identified in umbilical vein endothelial cells that competitively binds either tissue plasminogen activator or plasminogen and lacks sequence homology with annexin II.28 Finally, cell surface cytokeratin 8 has been identified as a major plasminogen receptor on several breast cancer cell lines.70 71 The significance of this diversity in plasminogen binding sites is not known.
The loss of cell viability is reported to increase cell surface plasminogen binding and activation by U937 monocytes.72Using flow cytometric analysis, the binding of FITC-plasminogen to nonviable cells (5%-10% of the cell population) was 100-fold greater than viable cells. Moreover, the binding of FITC-plasminogen to viable cells was neither lysine dependent nor inhibited by excess plasminogen, whereas the binding of FITC-plasminogen to nonviable cells was lysine dependent and blocked by unlabeled plasminogen.72 Because the mechanism by which cytoplasmic annexin II is secreted is unknown, we determined whether surface expression was limited to apoptotic cells. When adherent cultures of RAW264.7 macrophages were examined for the presence of apoptotic cells via TUNEL staining and the binding of biotinylated annexin V, less than 1% of the macrophages were positive (data not shown). In contrast, when surface annexin II expression was assessed by flow cytometry (Figure 1), the majority of cells were positive ruling out the possibility that annexin II is expressed on the surface of apoptotic cells only. However, these data do not exclude the possibility that the release of intracellular annexin II from injured or dead cells contributes to surface expression by adjacent viable cells.
The essential role of uPA activation of plasminogen in the migration of macrophages and clearance of necrotic debris has been clearly demonstrated using transgenic mice.11-15 However, the role of cell surface plasminogen binding sites in these processes has not been explored. In the present studies, the degradation of [3H]-glucosamine–labeled vascular SMC matrices by macrophages was increased 6-fold in the presence of exogenous plasminogen. Matrix degradation was inhibited about 40% when the binding of plasminogen to macrophages' annexin II was inhibited by antibody. These data likely underestimate the contribution of plasminogen binding to cell surface annexin II, because under the conditions of these experiments plasminogen activation occurs both on the cell surface and the fluid phase. In an assay that is likely to be more sensitive to the localization of plasminogen activation, chemotaxis of THP-1 monocytes through an extracellular matrix-coated membrane was inhibited about 60% by preincubation with anti–annexin II IgG. This is the first demonstration that inhibition of plasminogen binding to a specific protein on the macrophages' surface impairs matrix degradation and migration through the extracellular matrix.
In conclusion, these studies identify surface annexin II as a plasminogen binding site on macrophages. Inhibition of plasminogen binding to annexin II impaired the ability of macrophages to activate plasminogen as well as invade and degrade extracellular matrix. These data indicate an important role for the surface expression of annexin II in the invasive and degradative phenotype of macrophages.
Supported by research grants R01-HL40819, R01-HL42493, and P01-HL46403 from the National Heart, Lung and Blood Institute, and grant P11482 from the Austrian Science Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Domenick J. Falcone, Department of Pathology, Rm A678, Cornell University Weill Medical College, 1300 York Ave, New York, NY 10021; e-mail: dfalcone@mail.med.cornell.edu.



![Fig. 6. Extracellular matrix degradation by RAW264.7 macrophages is inhibited by anti–annexin II IgG. / Cells (5 × 105/well) were plated on insoluble [3H]-glucosamine–labeled human smooth muscle cell matrices in MSFM. Following adherence, media were replaced and 25 μg/mL monoclonal anti–annexin II (clone Z014, Zymed) or mouse IgG added. Cells were incubated with the antibodies (30 minutes, 37°C) prior to the addition of plasminogen (Plg, 1 μg/mL). Incubation media were recovered the next day and solubilized [3H]-activity determined as described in “Materials and methods.” The media and media plus plasminogen (Plg) controls depict the levels of [3H]-glucosamine released from insoluble extracellular matrix in the presence of plasminogen but in the absence of macrophages. Data represent the mean ± SEM of 4 replicate samples. nIgG indicates normal IgG.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/3/10.1182_blood.v97.3.777/6/m_h80310654006.jpeg?Expires=1765891677&Signature=rLuQlvwI5Qgpsg~x3xM2Ulm64Xn8Z4671fXs30-gKzhOPpArgU-~6BVWYyUi9cWG~kSBm589cD2V-v3UP6VOv6iE49wQuhsYfVHc3JU3Pkq3YBFMW~F-Apo3VvmXxH2KeSiRHOpym~jFbZ4x0gyHTZ0eM4HUTzHJwZpOzjz4VzC-Jbmvffr3Gg5EGvl0NCKbJB6FIJjKpenUAszMdTAuHYGxWoX6jfMVY31f3Ad6o6alm~RYOdIIh8YN3CyLbjDBf3JmKgPwJMNPp5daIvPAjiVWI0T9mhW8m~GDRj1ZsGrTXcRALVMNrHasosiJKfkAvEBYJya4bpKMPv-jAVwRYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal