Abstract
Plasminogen plays an integral role in the inflammatory response, and this participation is likely to depend on its interaction with cell surfaces. It has previously been reported that isolation of human neutrophils from blood leads to a spontaneous increase in their plasminogen-binding capacity, and the basis for this up-regulation has been explored as a model for mechanisms for modulation of plasminogen receptor expression. Freshly isolated human peripheral blood neutrophils exhibited relatively low plasminogen binding, but when cultured for 20 hours, they increased this capacity dramatically, up to 50-fold. This increase was abolished by soybean trypsin inhibitor and was susceptible to carboxypeptidase B treatment, implicating proteolysis and exposure of carboxy-terminal lysines in the enhanced interaction. In support of this hypothesis, treatment of neutrophils with elastase, cathepsin G, or plasmin increased their plasminogen binding, and specific inhibitors of elastase and cathepsin G suppressed the up-regulation that occurred during neutrophil culture. When neutrophils were stimulated with phorbol ester, their plasminogen binding increased rapidly, but this increase was insensitive to the protease inhibitors. These results indicate that plasminogen binding to neutrophils can be up-regulated by 2 distinct pathways. A major pathway with the propensity to markedly up-regulate plasminogen binding depends upon the proteolytic remodeling of the cell surface. In response to thioglycollate, neutrophils recruited into the peritoneum of mice were shown to bind more plasminogen than those in peripheral blood, suggesting that modulation of plasminogen binding by these or other pathways may also occur in vivo.
Introduction
The migration of leukocytes from the blood to sites of inflammation is facilitated by proteolytic degradation of the extracellular matrix.1-3 In addition, proteolytic enzymes are an important part of the armamentarium of antimicrobial agents brought by recruited inflammatory cells, in particular neutrophils, to sites of infection. Nevertheless, the proteolytic activity associated with inflammatory cells must be tightly regulated to avoid excessive tissue destruction.2 Plasmin, the active serine protease generated from plasminogen, has broad proteolytic capabilities. It can directly degrade multiple matrix proteins,4,5 including fibronectin,6 laminin,7 and thrombospondin,8 as well as the major provisional matrix constituent, fibrin. Moreover, plasmin is an activator of several metalloproteinases, yet another class of matrix-degrading enzymes (reviewed in Lijnen9). The importance of plasminogen in the inflammatory response has been underscored in recent studies of mice in which the plasminogen gene has been inactivated. These animals exhibit decreased recruitment of cells into their peritoneum in response to a model inflammatory stimulus, thioglycollate.10
The binding of plasmin(ogen) to cell surfaces is a central mechanism for mediating its participation in pericellular proteolysis.11-13 Bound plasminogen is more efficiently activated to plasmin, and bound plasmin is partially protected from inactivation by its naturally occurring inhibitors. Several proteins have been identified as candidate plasminogen receptors, and the diversity of these proteins,14 coupled with the extraordinarily high binding capacity of cells for plasminogen, suggests that no single molecule functions as the plasminogen receptor. Several of the candidate plasminogen receptors have carboxy-terminal lysyl residues, which can interact with the lysine-binding sites associated with the kringle domains of plasminogen. This common recognition mechanism leads to a similar affinity (KD ∼ 1 μM) of plasminogen for different receptor proteins and renders these interactions susceptible to inhibition by the basic carboxypeptidases (Cp),15,16 which remove the carboxy-terminal lysines from proteins, and to lysine analogs, such as ε-aminocaproic acid (EACA).17 18
Leukocytes are capable of binding plasminogen,19 and modulation of this interaction provides a potentially important mechanism for regulating the proteolytic functions of plasmin during the inflammatory response. Indeed, regulation of the number of plasminogen-binding sites on leukocytic cells has been documented.11,20-22 Agents that induce cellular adhesion, such as the phorbol ester, phorbol myristate acetate (PMA), increase the number of plasminogen-binding sites on monocytoid cells by 3- to 17-fold.20 Adhesion of such cells to matrix proteins down-regulates the plasminogen-binding capacity of the adherent cells and up-regulates it in the nonadherent cells.20,23Furthermore, Felez et al20 24 reported that short-term (overnight) culturing of neutrophils or monocytes leads to a marked increase in the number of plasminogen-binding sites expressed by these cells. Such modulation of plasminogen-binding capacity may be particularly relevant to neutrophil recruitment as early participants in the inflammatory response. Therefore, in this study, we have sought to understand the mechanisms underlying this up-regulation of plasminogen binding to neutrophils. We also have developed preliminary data to suggest that modulation of plasminogen binding can occur during an inflammatory response in vivo.
Materials and methods
Reagents
Glu-plasminogen was isolated from fresh frozen human plasma by means of affinity chromatography on lysine-Sepharose followed by gel filtration.19 Plasminogen and albumin were labeled with125I with the use of chloramine-T.25Soybean trypsin inhibitor (SBTI, cell culture tested), PMA, bovine serum albumin, cytochrome c, and transferrin were from Sigma Chemical Co (St Louis, MO). Methoxysuccinyl-AAPA-CH2Cl (single-letter amino acid code) (human leukocyte elastase inhibitor, HLE/CMK), methoxysuccinyl-AAPA-CH2Cl (human neutrophil elastase inhibitor, HNE/CMK), and benzylocarbonyl-GLF-CH2Cl (cathepsin-G inhibitor, CG/CMK) were from Enzyme Systems Products (Livermore, CA). Human neutrophil elastase and cathepsin G, 2-guanidinoethylmercaptosuccinic acid (GEMSA), elastatinal, aprotinin, succinyl-AAPF-p-nitroanalide, and phenylmethylsulfonyl fluoride were from Calbiochem (La Jolla, CA). Fluka Chemika (Buchs, Switzerland) supplied o-phenanthroline, and Hank's balanced salt solution (HBSS) was purchased from Gibco (Grand Island, NY). Carboxypeptidase B (CpB) was from Worthington Biochemical Corp (Freehold, NJ); and human plasmin from Enzyme Research Laboratories, Inc (South Bend, IN). Hippuryl-L-arginine was from Bachem (Bubendorf, Switzerland); S-2251 and S-2484 were from Chromogenix Diapharma Group, Inc (Franklin, OH); and recombinant human single-chain tissue plasminogen activator (tPA) Actilyse was from Boehringer Ingelheim (Ingelheim, Germany).
Cell preparations and cell lines
Granulocytes were isolated from human peripheral blood of consenting volunteers drawn into acid citrate dextrose (1/7 vol 145 mM sodium citrate, pH 4.6, and 2% dextrose). Isolation was performed by means of density centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden), followed by dextran sedimentation of erythrocytes and hypotonic lysis of residual erthyrocytes. Granulocytes were washed 3 times with HBSS.19 The remaining cells were 98% granulocytes, of which more than 96% were neutrophils and 2% were eosinophils. Contaminating lymphocytes and monocytes were fewer than 2% as determined by Wright staining. To induce up-regulation of plasminogen binding, the isolated cells were cultured for selected times in Mo-Medium20 (RPMI-1640, containing 100 U/mL penicillin G, 100 μg/mL streptomycin, 1 mM sodium pyruvate, 5 × 10−4% 2-mercaptoethanol, 25 mM HEPES, pH 7.4, and 5% fetal bovine serum [FBS]) in an incubator (humidified atmosphere, 5% CO2) at 37°C.
The human promyelocytic NB4 cell line was provided by Dr M. Lanotte (Hôpital St. Louis, Paris, France). The human monocytoid U937 cell line was from the American Type Culture Collection (ATCC, Rockville, MD). These cell lines were cultured in RPMI-1640 containing 1 mM sodium pyruvate and 5% to 10% FBS.
Ligand binding assays
Binding of 125I-plasminogen to neutrophils was performed as previously described.18-20 Briefly, the cells were washed in HBSS containing 25 mM HEPES and resuspended in HBSS-Hepes containing 0.1% bovine serum albumin (BSA) (HBSS-BSA), supplemented with 1.2 mM CaCl2 and 1.6 mM MgSO4. Cells (1 × 107/mL) were incubated with 100 nM 125I-plasminogen in the presence or absence of 100 mM EACA in a total volume of 200 μL at 37°C for 1 hour. Triplicate 50 μL samples were layered over 300 μL of 20% sucrose in HBSS-BSA and centrifuged for 2.5 minutes in a Beckman microfuge (Beckman Instruments, Inc, Fullerton, CA). The tube tips were amputated and counted in a gamma counter. Specific binding was measured as the difference in radioactivity bound in the absence and presence of EACA. We have previously shown that EACA is equivalent to excess nonlabeled plasminogen in defining the nonspecific binding of125I-plasminogen,19 and approximately 90% of the binding was routinely inhibited by 100 mM EACA. The number of molecules of plasminogen specifically bound were calculated by subtracting the nonspecific binding and using the specific activity of the radiolabeled plasminogen. In selected experiments, a washing step with 50 mM EACA was included to elute plasminogen that may have been bound to the cells in plasma. This procedure led to a slight but statistically insignificant increase in plasminogen binding to cells: 1.61 ± 0.72 vs 2.41 ± 1.05 × 104 plasminogen molecules bound per freshly isolated cell, and 17.79 ± 1.21 vs 18.30 ± 1.50 × 104 plasminogen molecules bound per cultured cell (mean [SEM] of 3 experiments). Therefore, the EACA elution step was not performed routinely.
The up-regulation of plasminogen binding was expressed as the fold increase over the basal binding to freshly isolated neutrophils. The inhibition of up-regulation was calculated according to the following formula:
where Nup-regulated is the number of plasminogen molecules bound per neutrophil (N) that were treated without an inhibitor present, Ninhib. is the plasminogen binding in the presence of the inhibitor, and Nfreshly i. is the plasminogen binding to freshly isolated neutrophils.
Plasminogen activation by tPA on cells
Plasminogen activation studies were carried out in microtiter plates in reaction volumes of 100 μL.26 Briefly, 20 μL tPA (final concentration, 70 pM) was mixed with 40 μL cells (final concentration, 1.5 × 106 cells/mL) and 40 μL of a mixture containing E-plasminogen (final concentration 100 nM) and the chromogenic substrate S-2251 (V-L-K-p-nitroanilide [single-letter amino acid codes], final concentration 0.15 mM). Reactions were performed at 37°C in an assay buffer consisting of Tris-HCl, pH 7.4, containing 1 mg/mL human serum albumin. Absorbance was monitored at 405 nm by means of a Thermomax thermostatted plate reader (Molecular Devices Corp, Stanford, CA). Rates of plasmin generation were calculated as previously described.26
Treatment of neutrophils with enzymes and inhibitors
The effects of various enzymes on plasminogen binding to neutrophils were assessed by the following protocol. Neutrophils, 500 μL at 2 to 4 × 107/mL in HBSS-BSA, were incubated with the test enzymes, plasmin, elastase, or cathepsin G for 30 to 60 minutes at 37°C. An excess of an inhibitor specific for each enzyme was added: aprotinin (200 kallikrein inhibitory units/mL) as a plasmin/kallikrein inhibitor, MeOSuc-Ala-Ala-Pro-Val-CH2Cl (60 μM) as an elastase inhibitor, and Z-Gly-Leu-Phe-CH2Cl (10 μM) as a cathepsin G inhibitor. The cells were washed 3 times by centrifugation in HBSS-HEPES, and then 125I-plasminogen binding was assayed. With plasmin treatment, 200 mM EACA was included in the initial washing step to help remove cell-bound enzyme. In some of the experiments, enzyme-treated cells were subjected to a subsequent treatment with CpB (5 U/mL, 30 minutes, at 37°C). In these experiments with plasmin-treated cells, the EACA washing step was performed after the CpB treatment since EACA can inhibit CpB. With all enzymes used, their activities were verified with the use of synthetic substrates. We determined the activity of CpB using hippuryl-L-arginine27; of plasmin using the chromogenic substrate S-2251; of elastase using S-2484; and of cathepsin G using succinyl-AAPF-p-nitroanalide. To test the effects of protease inhibitors on the up-regulation of plasminogen binding, the various inhibitors were added to the cultured neutrophils overnight. The cells were then washed 3 times by centrifugation, and their plasminogen binding was assessed.
Superoxide anion production by neutrophils
The ability of neutrophils to release superoxide anion was determined by measuring the reduction of cytochrome c.28Neutrophils (2 × 107) were incubated in buffered saline containing 1.3 mg/mL glucose, 0.5 mM CaCl2, 1 mM MgCl2, and 200 μg/mL cytochrome c. Superoxide dismutase (40 μg/mL) was added, and the cells were stimulated with 1 μM PMA for 30 minutes at 37°C in 5% CO2. The neutrophils were removed by centrifugation (at 8,000g for 2 minutes), and activity in the supernatant was measured spectrophotometrically at 550 nm.
Neutrophil stimulation with PMA
For stimulating neutrophils with PMA, a concentration of 0.1 to 0.5 nM was used. At this concentration of PMA, homotypic aggregation and adhesion of the cells to the reaction tube remained minimal for incubation times of less than 30 minutes. At higher concentrations, cell aggregation occurred that precluded accurate measurements of plasminogen binding. The cells (1 × 107) were suspended in 10 mL Mo-Medium for 30 minutes at 37°C. Viability of the stimulated neutrophils was assessed by trypan blue exclusion, and cell recovery by counting in a hemocytometer. The cells were washed once with HBSS-HEPES before plasminogen binding was measured.
Flow cytometry
Plasminogen was labeled with Alexa Fluor 488 (Molecular Probes, Eugene, OR) and purified according to the manufacturer's instructions. Dye incorporation was approximately 4 mol/mol plasminogen. The function of fluorescent plasminogen was assessed in terms of its ability to inhibit 125I-plasminogen binding to cells; it competed as well as nonlabeled plasminogen in these assays. For fluorescence-activated cell sorting (FACS) analyses, 106neutrophils in 500 μL HBSS and 20 μg plasminogen with Alexa Fluor 488 (200 nM), with or without 50 mM EACA, were incubated for 1 hour at 37°C. The binding of the fluorescent plasminogen to these cells was then immediately assessed by means of a FACScan instrument (BD Biosciences, San Jose, CA). FACS data were analyzed by means of the CellQuest software program (version 1.2), and the mean fluorescence intensities derived from this program were used to compare plasminogen binding to the cells.
Murine peritoneal inflammation model
All animal experiments were performed in accordance with institutionally approved protocols. Thioglycollate was used to induce an inflammatory response as previously described.10 Mice of a C57BL/6: 129 (50%/50%) background were injected intraperitoneally with 0.5 mL of a 4% Brewer thioglycollate solution (Difco Laboratories, Detroit, MI). At 6 hours, the mice were killed by isoflurane inhalation (Abbott Laboratories, North Chicago, IL). The peritoneal cavity was exposed, and the exudate was collected by washing the cavity with sterile saline. The peritoneal lavage fluids from 2 mice were pooled and centrifuged at 200g for 10 minutes. To remove erythrocytes, the cell pellet was subjected to hypotonic lysis. Cells were resuspended in HBSS-HEPES and counted. Neutrophils from untreated mice were isolated from blood obtained by cardiac puncture with heparin used as the anticoagulant. The blood was centrifuged for 15 minutes at 100g. The plasma was removed, and the buffy coats were collected. Contaminating erythrocytes in the buffy coat were subjected to hypotonic lysis, and the leukocytes were resuspended in HBSS-HEPES and counted.
Statistical analysis
Results are presented as mean ± SEM. Comparisons were made by means of paired Student t tests. A P < .05 was considered to be statistically significant.
Results
Up-regulation of plasminogen binding to neutrophils
In previous studies, we reported that removal of leukocytes from their blood environment resulted in a marked increase in their plasminogen-binding capacity.20,24 Since delivery of proteolytic potential from the blood to tissues, either as a releasable pool from within the cell or carried on the cell surface, is a major role for neutrophils in the inflammatory response, the basis for this up-regulation has been examined. Human peripheral blood neutrophils were isolated to a purity of greater than 96% under sterile conditions, and these cells were cultured overnight in media containing 5% FBS. Since our previous studies had demonstrated that changes in plasminogen binding to cells arise from increases in receptor number and not affinity,20,23 29 specific binding (defined as that component of binding inhibitable by EACA) was measured by means of 100 nM 125I-plasminogen for 1 hour at 37°C; ie, approximately 1/10 the Kd under equilibrium conditions. As shown in Figure1A, plasminogen binding to the cultured neutrophils was dramatically enhanced relative to the freshly isolated cells from the same donor. The increase was over 50-fold in the experiment shown in Figure 1A. Over the course of 19 such experiments, which used the blood of at least 16 different donors, the increase in plasminogen binding averaged 30.9-fold. Freshly isolated neutrophils bound 1.25 ± 0.24 × 104 plasminogen molecules per cell (range, 0.06-3.91), cultured neutrophils bound 38.64 ± 8.96 × 104 plasminogen molecules per cell (range, 7.10-170.43). This up-regulation was not immediate (see Figure1A); after 6 hours, no increase above the basal level of plasminogen binding to freshly isolated neutrophils was detected. The up-regulation was not associated with a loss in cell viability; trypan blue exclusion of the neutrophils was greater than 95% (range, 95%-100%, n = 19) after overnight culture. Neutrophil functions, as assessed by their capacity to generate O2− (25.2 nmol for fresh vs 17.6 nmol for cultured) or release of cathepsin G (ΔΑ405/minute: 1.6 vs 1.1 × 10−3) upon stimulation with PMA (1 to 5 μM), were only slightly diminished by the 20-hour culture compared with freshly isolated cells. In contrast to the marked increase in plasminogen binding to cultured cells,125I-albumin binding, used as a control protein, remained low (fewer than 1000 molecules bound per cell before or after culture). In Figure 1A, the cells were maintained in 5% FBS. However, as shown in Figure 1B, the augmentation in plasminogen binding of the cultured neutrophils was independent of the FBS concentration used. The increase in plasminogen-binding capacity was observed at all concentrations of FBS tested, including when no FBS was present. In subsequent experiments, the cells were maintained in 5% FBS as this inclusion reduced the tendency of the cells to aggregate spontaneously and to adhere to the culture flasks.
Effect of neutrophil culture on plasminogen-binding capacity.
Freshly isolated human blood neutrophils (1.5 × 107), defined as time 0, were cultured for the indicated times in Mo-Medium at 37°C in a humidified atmosphere, 5% CO2, with 5% FBS or varying amounts of FBS. After washing the cells, specific plasminogen binding was quantitated by means of 125I-plasminogen at 100 nM and a 1-hour incubation. (A) Cultured with 5% FBS. This time-course experiment (means ± SEM of triplicates) is representative of 3 experiments. (B) Cultured with varying amounts of FBS. This FBS dose-titration experiment was done twice.
Effect of neutrophil culture on plasminogen-binding capacity.
Freshly isolated human blood neutrophils (1.5 × 107), defined as time 0, were cultured for the indicated times in Mo-Medium at 37°C in a humidified atmosphere, 5% CO2, with 5% FBS or varying amounts of FBS. After washing the cells, specific plasminogen binding was quantitated by means of 125I-plasminogen at 100 nM and a 1-hour incubation. (A) Cultured with 5% FBS. This time-course experiment (means ± SEM of triplicates) is representative of 3 experiments. (B) Cultured with varying amounts of FBS. This FBS dose-titration experiment was done twice.
Carboxy-terminal lysines and up-regulation of plasminogen binding
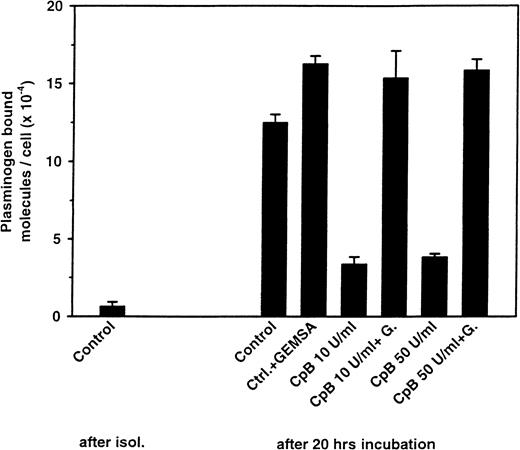
The increase in plasminogen binding to the cultured cells was mediated by its lysine-binding sites since the interaction was inhibited by the carboxy-terminal lysine analog EACA. This recognition specificity raised the possibility that the increase in plasminogen binding depends upon an increased availability of proteins with carboxy-terminal lysine residues on the surface of the cultured cells. To test this hypothesis, the cultured cells were treated with CpB, an enzyme that removes the lysyl and arginyl residues from the carboxy-terminus of proteins and peptides.30,31 As shown in Figure 2, treatment of the cultured cells with CpB decreased their plasminogen binding substantially. The inhibition of up-regulation was greater than 70% at either 10 or 50 U/mL CpB. GEMSA (300 μM), an inhibitor of CpB,32abrogated the effect, an indication of specificity (Figure 2).
Effect of CpB treatment on the up-regulation of plasminogen binding.
Neutrophils were cultured as in Figure 1A for 20 hours. After washing, the cells were treated with 10 (50 μg/mL) and 50 U/mL (250 μg/mL) of CpB for 1 hour at 37°C in the presence or absence of the CpB inhibitor GEMSA (G) at 0.3 mM. Plasminogen binding was then measured. The controls are freshly isolated neutrophils (after isolation) or the untreated, cultured neutrophils. The experiment shown (means ± SEM of the triplicates) is representative of 3 different experiments.
Effect of CpB treatment on the up-regulation of plasminogen binding.
Neutrophils were cultured as in Figure 1A for 20 hours. After washing, the cells were treated with 10 (50 μg/mL) and 50 U/mL (250 μg/mL) of CpB for 1 hour at 37°C in the presence or absence of the CpB inhibitor GEMSA (G) at 0.3 mM. Plasminogen binding was then measured. The controls are freshly isolated neutrophils (after isolation) or the untreated, cultured neutrophils. The experiment shown (means ± SEM of the triplicates) is representative of 3 different experiments.
Implication of proteases in the up-regulation of plasminogen binding
A ready mechanism by which new carboxy-terminal lysines could be generated is proteolysis at the cell surface. In view of the high serine protease activity associated with neutrophils, we tested whether SBTI would influence the up-regulation of plasminogen binding. As shown in Figure 3, concentrations of SBTI of 100 or 1000 μg/mL markedly suppressed the up-regulation of plasminogen binding. Even 1 μg/mL SBTI suppressed up-regulation by 44%. The specificity of this effect was demonstrated; unrelated proteins, transferrin, or albumin, at 100 μg/mL, failed to influence the increase in plasminogen-binding capacity and did not alter the inhibitory activity of SBTI. The addition of SBTI to the cells after overnight culture did not change their expression of increased plasminogen-binding sites, indicating that the effect of the protease inhibitor was on the up-regulation of binding sites and not on the binding per se. We also considered whether SBTI would influence the basal level of plasminogen binding to freshly isolated neutrophils. When SBTI was included in the blood collection tube and maintained at 1000 μg/mL throughout the isolation of the cells, the basal level of plasminogen binding was not significantly affected: 1.91 ± 0.50 × 104 plasminogen molecules bound per neutrophil when isolated without SBTI vs 1.58 ± 0.41 × 104 when isolated with SBTI (P = .52, n = 8).
Effect of SBTI and control proteins, BSA and transferrin (Tf) on the up-regulation of plasminogen binding to neutrophils.
Cells were cultured for 20 hours with or without the indicated concentrations of the test proteins, and then plasminogen binding was measured. The data (means ± SEM of the triplicates) are representative of at least 2 separate experiments.
Effect of SBTI and control proteins, BSA and transferrin (Tf) on the up-regulation of plasminogen binding to neutrophils.
Cells were cultured for 20 hours with or without the indicated concentrations of the test proteins, and then plasminogen binding was measured. The data (means ± SEM of the triplicates) are representative of at least 2 separate experiments.
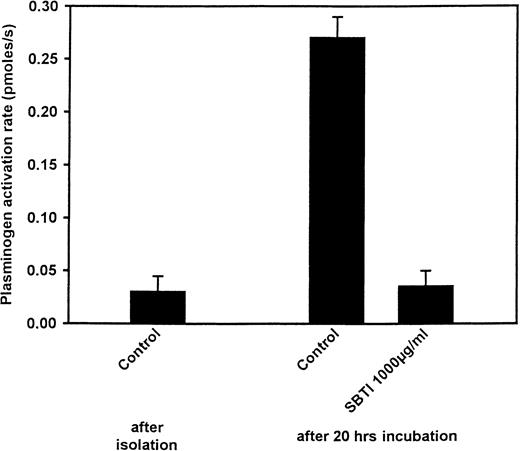
Effect of SBTI on plasminogen activation
In previous studies, plasminogen binding to cells was shown to enhance plasmin generation by tPA.26 33 With a similar approach, we explored the capacity of freshly isolated or cultured neutrophils to promote plasminogen activation by tPA and the effects of SBTI on this activation. In comparison with freshly isolated neutrophils, a 7.7-fold increase in plasminogen activation rate was observed when neutrophils were cultured overnight (Figure4). However, as shown in Figure 4, when neutrophils were cultured in the presence of SBTI (1000 μg/mL), plasminogen activation rates were similar to those observed with freshly isolated neutrophils. Since SBTI can inhibit plasmin, carryover of the inhibitor into the plasminogen activation assay was considered. A promyelocytic cell line (NB4) and a monocytoid cell line (U937) were cultured for 24 hours in the presence or absence of SBTI. In contrast to what happened with neutrophil culturing, plasminogen binding on these cell lines was not modulated; thus, changes in plasminogen activation rates would reflect the carryover of SBTI into the plasmin generation assays. Under the same washing conditions as for neutrophils, the rates of plasminogen activation were not modified by the presence of SBTI in the cultures. The promyelocytic NB4 cells generated 0.72 and 0.78 pmol plasmin per second when cultured in the presence or absence of SBTI, respectively; and the monocytoid U937 cells generated 0.45 and 0.51 pmol plasmin per second. Thus, SBTI suppressed the up-regulation of plasminogen activation as well as of plasminogen binding.
Effect of culture and SBTI on the capacity of neutrophils to promote plasminogen activation by tPA.
Neutrophils (1.5 × 106/mL), either freshly isolated or cultured overnight, with or without SBTI, were washed and tested for their effects on activation of plasminogen (100 nM) by tPA (70 pM) with the use of the chromogenic substrate S-2251 (0.15 mM). Means ± SEM of triplicates are shown.
Effect of culture and SBTI on the capacity of neutrophils to promote plasminogen activation by tPA.
Neutrophils (1.5 × 106/mL), either freshly isolated or cultured overnight, with or without SBTI, were washed and tested for their effects on activation of plasminogen (100 nM) by tPA (70 pM) with the use of the chromogenic substrate S-2251 (0.15 mM). Means ± SEM of triplicates are shown.
Proteases up-regulate plasminogen binding to neutrophils
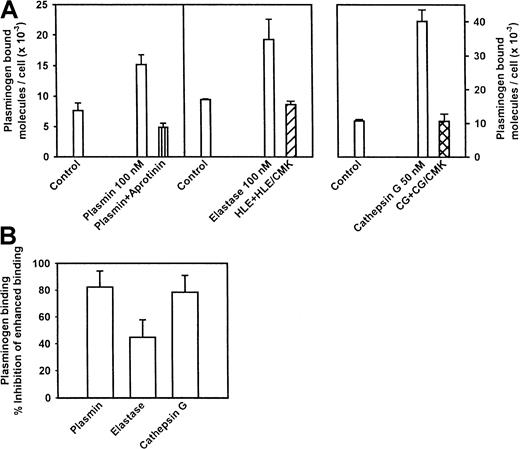
On the basis of the effects of SBTI on both plasminogen binding and activation, we sought to determine if protease treatment of neutrophils could up-regulate their plasminogen-binding capacity. For these experiments, freshly isolated neutrophils were incubated with 50 to 100 nM concentrations of the selected enzyme for 30 to 60 minutes. Inhibitors specific for each enzyme were added, and the cells were washed and then treated or not treated for 1 hour with CpB to determine if changes in plasminogen binding were mediated by exposure of new carboxy-terminal residues. To verify specificity, controls were performed in which each inhibitor was added to the target enzyme prior to its addition to the cells. Incubation of neutrophils with plasmin increased their plasminogen-binding capacity by 2.1-fold (Figure5A). Aprotinin, an inhibitor of plasmin, abrogated the plasmin-induced increase in plasminogen binding. We also tested 2 major serine proteases of neutrophils, elastase, which cleaves at uncharged or nonaromatic residues,34,35 and cathepsin G, which cleaves primarily at aromatic amino acid residues.36 37 Each of these enzymes generated new plasminogen-binding sites on the neutrophils (Figure 5A). At the concentrations used (selected from dose titration experiments as a concentration of each protease that induced a substantial increment in plasminogen binding), the increase in plasminogen binding was 2.1-fold with elastase and 3.8-fold with cathepsin G. For both enzymes, the increase in plasminogen binding was abrogated by pre-incubation of the enzymes with their respective inhibitors (Figure 5A). For elastase and cathepsin G, as well as for plasmin, the increase in plasminogen binding was statistically significant and was partially sensitive to CpB treatment (Figure 5B). The increase in binding induced by plasmin treatment was 82% inhibited by CpB. The increase in binding induced by cathepsin G was also highly susceptible (78%) to CpB, whereas the increase induced by elastase was less sensitive (47%) but still susceptible (see Figure 5B).
Effect of protease treatment and carboxypeptidase treatment on plasminogen-binding capacity induced by neutrophils, plasmin, leukocyte elastase, or cathepsin G.
(A) Effect of protease treatment on the plasminogen-binding capacity of neutrophils. Freshly isolated neutrophils (4 × 107/mL) were incubated for 30 to 60 minutes at 37°C with plasmin (100 nM), leukocyte elastase (100 nM), or leukocyte cathepsin G (50 nM), with or without an excess of a specific inhibitor of the enzyme used. The concentrations of the enzymes were based on dose titration experiments, and the concentrations of the inhibitors produced complete inhibition in chromogenic substrate assays. At the end of the incubation, cells were washed and plasminogen binding was assessed. The results are means ± SEM of triplicates, and the experiments were repeated at least 3 times. For 3 such experiments, the increase in plasminogen binding to cells was statistically significant for each enzyme (P < .05). (B) Effects of carboxypeptidase treatment on the up-regulation of plasminogen binding induced by plasmin, leukocyte elastase, or cathepsin G. Neutrophils, treated as in panel A, were washed and then incubated for 30 minutes with 5 U/mL CpB. Specific plasminogen binding was then measured, and the data are expressed as the inhibition of the increase in plasminogen binding induced by each of the enzymes. The means ± SEM from 3 experiments are shown.
Effect of protease treatment and carboxypeptidase treatment on plasminogen-binding capacity induced by neutrophils, plasmin, leukocyte elastase, or cathepsin G.
(A) Effect of protease treatment on the plasminogen-binding capacity of neutrophils. Freshly isolated neutrophils (4 × 107/mL) were incubated for 30 to 60 minutes at 37°C with plasmin (100 nM), leukocyte elastase (100 nM), or leukocyte cathepsin G (50 nM), with or without an excess of a specific inhibitor of the enzyme used. The concentrations of the enzymes were based on dose titration experiments, and the concentrations of the inhibitors produced complete inhibition in chromogenic substrate assays. At the end of the incubation, cells were washed and plasminogen binding was assessed. The results are means ± SEM of triplicates, and the experiments were repeated at least 3 times. For 3 such experiments, the increase in plasminogen binding to cells was statistically significant for each enzyme (P < .05). (B) Effects of carboxypeptidase treatment on the up-regulation of plasminogen binding induced by plasmin, leukocyte elastase, or cathepsin G. Neutrophils, treated as in panel A, were washed and then incubated for 30 minutes with 5 U/mL CpB. Specific plasminogen binding was then measured, and the data are expressed as the inhibition of the increase in plasminogen binding induced by each of the enzymes. The means ± SEM from 3 experiments are shown.
Effect of protease inhibitors on up-regulation of plasminogen binding
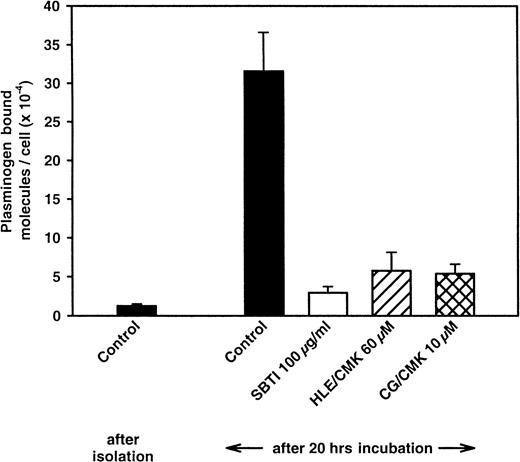
Having shown that relevant enzymes can increase plasminogen binding to neutrophils and that inhibitors of these enzymes ablated their effects, we assessed whether the same inhibitors would influence the up-regulation of plasminogen binding associated with the culturing of neutrophils. As shown in Figure 6, the inhibitors of the major leukocyte proteases were effective in blocking the up-regulation in plasminogen binding. The chloromethylketone inhibitors of elastase and cathepsin G produced 85% and 86% inhibition, respectively, of the up-regulation and were only slightly less effective than SBTI. MeOSuc-Ala-Ala-Pro-Ala-chloromethylketone (HLE/CMK) is an inhibitor not only of neutrophil elastase but also of the closely related neutrophil enzyme, proteinase 3.38Accordingly, 2 additional selective elastase inhibitors, MeOSuc-Ala-Ala-Pro-Ala-chloromethylketone, which does not inhibit proteinase 3, and elastatinal were tested. These compounds produced 22% and 56% inhibition of up-regulation, respectively, suggesting that proteinase 3 may also play a role in the up-regulation of plasminogen binding. Not all protease inhibitors affected the up-regulation of plasminogen-binding capacity. EDTA (10 mM) had a minimal effect, and aprotinin produced less than 50% inhibition. Interestingly, o-phenanthroline (500 μM), which inhibits Cp, increased the extent of plasminogen binding by almost 2-fold, suggesting that the Cp enzymes may dampen the extent of up-regulation. Relevant to this observation, CpM, a basic carboxypeptidase, is associated with neutrophils.39
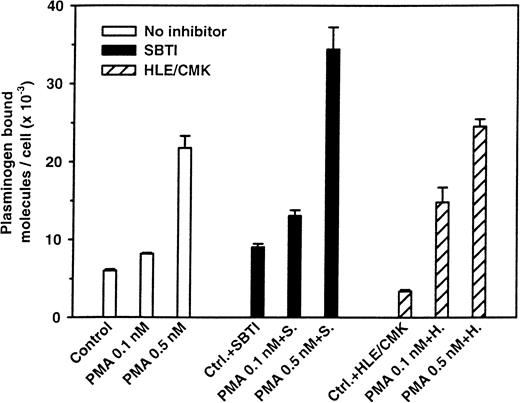
Effect of inhibitors of leukocyte elastase (HLE/CMK) and cathepsin G (CG/CMK) on the up-regulation of plasminogen binding to cultured neutrophils.
Cells (1.5 × 107) were cultured for 20 hours without or in the presence of the indicated enzyme inhibitors: SBTI, 100 μg/mL; HLE/CMK, 60 μM; or CG/CMK, 10 μM. After washing, plasminogen binding was assessed. Means ± SEM are from at least 4 separate experiments.
Effect of inhibitors of leukocyte elastase (HLE/CMK) and cathepsin G (CG/CMK) on the up-regulation of plasminogen binding to cultured neutrophils.
Cells (1.5 × 107) were cultured for 20 hours without or in the presence of the indicated enzyme inhibitors: SBTI, 100 μg/mL; HLE/CMK, 60 μM; or CG/CMK, 10 μM. After washing, plasminogen binding was assessed. Means ± SEM are from at least 4 separate experiments.
Effect of PMA stimulation on up-regulation of plasminogen binding
Stimulation of neutrophils with PMA at concentrations that did not induce homotypic aggregation or adhesion to the culture flask led to a rapid up-regulation of plasminogen binding. As shown in Figure7, after only 20 minutes, a 4-fold increment in plasminogen binding was observed. Addition of the protease inhibitors SBTI, HLE/CMK, or CG/CMK (results of the latter not shown) did not inhibit the up-regulation of plasminogen binding induced by PMA, indicating that this up-regulation is not dependent on protease action. These results indicate that a second pathway, with no apparent dependency on the major leukocyte proteases, can mediate up-regulation of the plasminogen-binding capacity of neutrophils.
Effect of protease inhibitors on the up-regulation of plasminogen binding induced by PMA.
Neutrophils (1 × 107) were stimulated with 0.1 and 0.5 nM PMA for 20 minutes at 37°C in the presence or absence of SBTI (100 μg/mL) or HLE/CMK (10 μM). After washing, specific plasminogen binding was quantified. The results are from one experiment (means ± SEM of the triplicates) and are representative of at least 3 such experiments. In a set of 6 other experiments, the cathepsin G inhibitor CG/CMK (2 to 25 μM) did not inhibit the PMA-induced increase in plasminogen receptor expression (results not shown).
Effect of protease inhibitors on the up-regulation of plasminogen binding induced by PMA.
Neutrophils (1 × 107) were stimulated with 0.1 and 0.5 nM PMA for 20 minutes at 37°C in the presence or absence of SBTI (100 μg/mL) or HLE/CMK (10 μM). After washing, specific plasminogen binding was quantified. The results are from one experiment (means ± SEM of the triplicates) and are representative of at least 3 such experiments. In a set of 6 other experiments, the cathepsin G inhibitor CG/CMK (2 to 25 μM) did not inhibit the PMA-induced increase in plasminogen receptor expression (results not shown).
Up-regulation of plasminogen binding to neutrophils in vivo
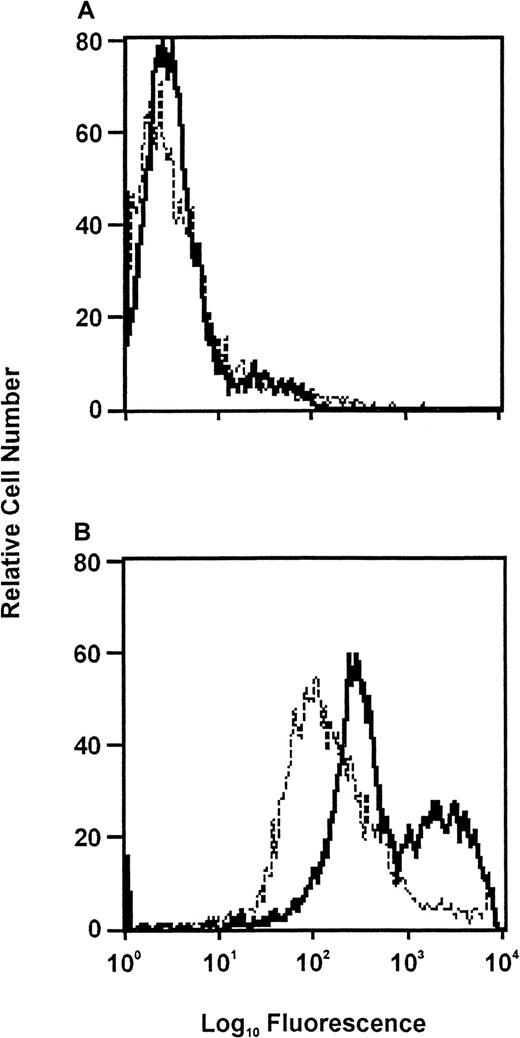
The inflammatory stimulus thioglycollate was used to induce neutrophil recruitment into the peritoneum of mice. As performed in our laboratory10 and consistent with the literature,40 41 neutrophil recruitment into the peritoneum is maximal at 6 hours in this inflammatory model. The peritoneal neutrophil cells were collected at this time by lavage and partially purified, and their plasminogen receptor expression was assessed by FACS with the use of plasminogen labeled with Alexa Fluor 488. The plasminogen binding to these cells was compared with that of neutrophils isolated from blood of mice. As shown in Figure8, there was a substantial difference in plasminogen binding to the peritoneal vs the blood neutrophils. On the basis of the mean fluorescence intensity values, the peritoneal neutrophils bound 4-fold more plasminogen than the blood neutrophils. Similar results were obtained in a second experiment; the difference in plasminogen binding to the peritoneal vs the blood neutrophils was 3-fold. Within the peritoneal neutrophils, plasminogen binding was heterogeneous, and at least 2 subpopulations, one with lower and one with higher plasminogen-binding capacities, could be distinguished. On average, plasminogen binding to both of these subpopulations was higher than to the blood neutrophil (see Figure 8). These results are compatible with the possibility that up-regulation of plasminogen binding to neutrophils may occur in vivo.
FACS analysis of neutrophils from blood of control mice (dashed lines) and from the peritoneal cavity of mice challenged with thioglycollate (solid lines).
Inclusion of EACA in the incubation media decreased plasminogen binding to both the peripheral blood and peritoneal neutrophils by a similar amount. (A) Neutrophils were incubated in buffer followed by FACScan analysis (negative control). (B) Neutrophils were incubated with Alexa Fluor 488–labeled plasminogen for 1 hour at 37°C before FACS.
FACS analysis of neutrophils from blood of control mice (dashed lines) and from the peritoneal cavity of mice challenged with thioglycollate (solid lines).
Inclusion of EACA in the incubation media decreased plasminogen binding to both the peripheral blood and peritoneal neutrophils by a similar amount. (A) Neutrophils were incubated in buffer followed by FACScan analysis (negative control). (B) Neutrophils were incubated with Alexa Fluor 488–labeled plasminogen for 1 hour at 37°C before FACS.
Discussion
The inflammatory reaction is a complex and multifaceted process, and pericellular proteolysis is an important element as a mediator not only of antimicrobial activity but also of the recruitment phase of this response. Studies in knockout mice have implicated components of the plasminogen system,42,43 as well as plasminogen itself,10,44 as important contributors to the inflammatory reaction. The ability of plasminogen to mediate efficient fibrinolysis depends upon its binding to fibrin, an interaction that influences its activation by plasminogen activators and its inhibition by α2-antiplasmin.45 Similarly, the binding of plasminogen to cell surfaces influences the kinetics of its activation17,24,33 and its inhibition.46 47Therefore, modulation of the interaction of plasminogen with phagocytic cells could play a pivotal role in regulating the inflammatory response. Accordingly, in this study, we have examined the mechanisms by which neutrophils alter the plasminogen-binding characteristics of their cell surfaces using the enhancement associated with culture of these cells as a model. Our results provide direct evidence for the existence of a protease-dependent mechanism for up-regulation of the plasminogen-binding capacity of these cells. Moreover, we provide data to suggest that up-regulation of the plasminogen-binding capacity of neutrophils may occur in an in vivo model of the inflammatory response.
Neutrophils cultured overnight in the presence or absence of FBS dramatically up-regulate (by approximately 30-fold) their expression of plasminogen-binding sites. Concomitantly, their capacity to enhance plasminogen activation by tPA also increased substantially (7.7-fold) with culture. The latter parameter depends upon changes in both the Michaelis constant KM and the catalytic constant kcat24,26 which may account, in part, for the disproportion between the changes in binding and kinetic parameters. Also, tPA and plasminogen share binding sites on certain cells including neutrophils, and the binding of both ligands is modulated by cell culture.24 SBTI, a broad-spectrum serine protease inhibitor, or more specific leukocyte protease inhibitors prevented this up-regulation of plasminogen binding. These observations implicate elastase, cathepsin G, and/or related enzymes, such as the elastaselike protease proteinase 3, in this process. Consistent with these interpretations, treatment of neutrophils with elastase, cathepsin G, as well as plasmin did enhance the plasminogen-binding capacity of the cells. The exogenously added proteases were less effective than culture in up-regulating plasminogen binding. Possible explanations for these differences include the time of exposure (1 hour for the added enzymes vs 20 hours of culture); the combination of proteases to which the cultured cells are exposed; and the imposition of other changes that occur at the cell membrane, which may influence the substrates available. Nevertheless, the increase in plasminogen binding induced by these added enzymes and by the culturing of the cells was associated with a common mechanism, the exposure or generation of new carboxy-terminal basic amino acids, probably lysines, as indicated by the sensitivity of the enhanced interaction to CpB. Previous studies have shown that it is the CpB-sensitive plasminogen-binding sites that enhance plasminogen activation on cell surfaces.18 24 Thus, the protease-dependent up-regulation results in an increase in functionally important plasminogen-binding sites. The suppressive effects of SBTI on plasminogen activation by the cultured neutrophils support this conclusion.
The specificity of the enzyme inhibitors used suggests roles for the major granule proteases of neutrophils, elastase, cathepsin G, and possibly proteinase 3 in the up-regulation of plasminogen-binding sites. The question of the role of proteinase 3 arises from the lack of specific inhibitors of this enzyme48 and the uncertainty as to its susceptibility to HLE/CMK.38,49,50 HNE/CMK, a specific elastase inhibitor,48 was considerably less effective than HLE/CMK in blocking up-regulation, compatible with a role for proteinase 3 in the process. All 3 neutrophil enzymes are endopeptidases of the serine protease family: elastase cleaves at uncharged, nonaromatic side chains, A (single-letter amino acid code), V, L, I; proteinase 3 at A, S, V; and cathepsin G at aromatic amino acid residues, with only cathepsin G exhibiting some propensity for the basic amino acids as well.34-37 Therefore, rather than creating new carboxy-terminal lysines directly, the neutrophil enzymes are more likely to influence the exposure and accessibility of pre-existing carboxy-terminal lysines to plasminogen. Thus, we hypothesize that these enzymes may enhance plasminogen binding to the cells by remodeling the cell surface. Numerous cell-surface proteins (eg, tumor necrosis factorα–receptor on neutrophils),51 the protease-activated receptors on endothelial cells,52membrane glycoprotein Ib (GPIb)53 and GPIIb-IIIa,54 and interleukin-8 receptors on neutrophils55 are modified by these enzymes, consistent with their potential to remodel cell surfaces. Elastase, cathepsin G, and proteinase 3 are the most abundant proteases in neutrophils56,57 and are stored primarily in the azurophilic granules of the cells.48 In addition, unstimulated neutrophils have been shown to express elastase and cathepsin G on their cell surface,58 and stimulation of neutrophils can increase the cell-surface expression of elastase, proteinase 3, and cathepsin G.1,58,59 Thus, there is substantial availability of the participating proteases to initiate the postulated remodeling of the cell surface. The entire protease-mediated increment in plasminogen-binding capacity, particularly that induced by elastase, was not susceptible to CpB treatment. Other receptors, such as gangliosides60 or proteins without a carboxy-terminal lysine,61 may become exposed. Also, the penultimate amino acid influences the susceptibility of carboxy-terminal lysines to Cp cleavage,62 so not all carboxy-terminal lysines need to be susceptible to CpB.
Stimulation of neutrophils with PMA enhanced their plasminogen binding substantially and rapidly. Such stimulation is known to increase expression of both elastase and cathepsin G at the cell surface.1,58 However, this up-regulation was insensitive to the proteinase inhibitors (SBTI, HLE/CMK, CG/CMK) that blocked the augmentation induced by culturing of the cells. The lack of involvement of the proteases in this up-regulation may be a reflection of the very short incubation time (20 minutes) required. Because the up-regulation occurred despite the presence of proteinase inhibitors, the existence of a second and mechanistically distinct pathway for up-regulation of plasminogen-binding sites is suggested: a pathway independent of proteases or, at the least, involving distinct PMA-induced proteases. Exposure of neutrophils to PMA and subsequent stimulation of protein kinase C does change the properties of the cell membrane (ruffling) and the cytoskeleton.63 Therefore, the enhanced plasminogen binding induced by PMA may also be a reflection of remodeling of the cell membrane. In previous studies,20 23 we showed that adhesion and de-adhesion of cells influence the expression of plasminogen-binding sites, which may also be an extension of this concept.
While neutrophils have a survival time of 6 to 18 hours in blood, their survival time in tissues can be extended substantially.64,65 Therefore, the overnight culture used in this study to up-regulate plasminogen receptors may have biological and clinical relevance. We found that, compatible with this proposition, neutrophils that have migrated into the peritoneum in response to the inflammatory stimulus thioglycollate exhibited higher plasminogen binding than neutrophils from peripheral blood. We cannot exclude that peritoneal cells represent a selected population of neutrophils with a higher plasminogen-binding capacity, nor can we conclude that the apparent up-regulation of plasminogen binding to the peritoneal neutrophils is due to proteolytic modification of their cell surface. Consistent with these possibilities, Silverstein et al66 reported that the plasminogen was present on the surface of peritoneal macrophages of patients with indwelling catheters and on tissue macrophages from patients with chronic inflammatory lesions under conditions in which its expression on blood monocytes was not detected. Also, in a canine model of ischemia-reperfusion injury, modulation of the surface of the emigrating neutrophils was evidenced by a loss of integrin αMβ2 from the cell surface and appearance of soluble proteolytic fragments of the integrin.67 When neutrophils migrate into tissues, their frequent fate is apoptosis.68-70 Indeed, treatment of neutrophils with proteases can induce their apoptosis.69,71 Up-regulation of plasminogen binding is also a consequence of apoptosis.72 Taken together, our experimental results and these published studies suggest that up-regulation of plasminogen receptors may occur in vivo, and further analyses of the mechanisms, particularly the role of apoptosis, in such modulation are clearly warranted.
Acknowledgments
The authors thank the blood donors from the Primary Laboratory Center at the Cleveland Clinic Foundation; Jane Rein, for expert secretarial assistance; and a facility, established with the support of the W. M. Keck Foundation, at the Cleveland Clinic, for FACS analysis.
Supported by National Institutes of Health grant HL17964 (E.F.P.) and, in part, by the Swiss National Science Foundation and the Schweizerische Stiftung für Medizinisch-Biologische Stipendien (T.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Edward F. Plow, Joseph J. Jacobs Center for Thrombosis and Vascular Biology, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: plowe@ccf.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal