Abstract
ICL670A (formerly CGP 72 670) or 4-[3,5-bis-(hydroxyphenyl)-1,2,4-triazol-1-yl]- benzoic acid is a tridentate iron-selective synthetic chelator of the bis-hydroxyphenyl-triazole class of compounds. The present studies used selective radioiron probes of hepatocellular and reticuloendothelial (RE) iron stores in hypertransfused rats and iron-loaded heart cells to define the source of iron chelated in vivo by ICL670A and its mode of excretion, to examine its ability to remove iron directly from iron-loaded myocardial cells, and to examine its ability to interact with other chelators through a possible additive or synergistic effect. Results indicate that ICL670A given orally is 4 to 5 times more effective than parenteral deferoxamine (DFO) in promoting the excretion of chelatable iron from hepatocellular iron stores. The pattern of iron excretion produced by ICL670A is quite different from that of DFO and all iron excretion is restricted to the bile regardless of whether it is derived from RE or hepatocellular iron stores. Studies in heart cell cultures have shown a favorable interaction between DFO and ICL670A manifested in improved chelating efficiency of ICL670A, which is most probably explained by an exchange of chelated iron between ICL670A and DFO. These unique chelating properties of ICL670A may have practical implications for current efforts to design better therapeutic strategies for the management of transfusional iron overload.
Introduction
Iron-chelating therapy with deferoxamine (DFO) results in a significant improvement in the life expectancy of patients with transfusional iron overload. This is largely attributed to the prevention of heart disease in well-treated patients and, in a few, to the reversal of existing heart disease by aggressive intravenous DFO therapy.1-4 Nevertheless, there is still an obvious need for the development of alternative drugs that would be convenient for use and available for an increasing number of patients in need of long-term iron-chelating therapy. Despite hopes raised by the introduction of a number of promising compounds, with few exceptions they proved to be either ineffective or too toxic to be of practical value. In the present study we evaluated a new synthetic iron chelator, ICL670A, a member of a new class of tridentate iron-selective synthetic chelators, the bis-hydroxyphenyl-triazoles.5,6The aims of the present study were to: (1) define the source of iron chelated in vivo by ICL670A in hypertransfused rats labeled with selective radioiron probes, using heat-denatured red blood cells (59Fe-DRBC) to introduce radioiron to reticuloendothelial (RE) cells, and 59Fe-ferritin to label hepatic parenchymal cells7; (2) define the ability of ICL670A to interact directly with iron-loaded heart cells using an in vitro system of iron-loaded rat heart cells; and (3) to explore the possibility of improved chelating effect through a putative “shuttle effect,” that is, the removal of iron by one compound and its excretion by another, by combining ICL670A with DFO. The results of these studies may have useful practical implications for designing better strategies of iron-chelating therapy.
Materials and methods
In vivo studies
Female Wistar rats of the Hadassah strain weighing 170 to 200 g were used throughout. Hypertransfusion was performed by 2 intravenous injections of 2 mL packed cells per 100 g body weight on days 4 and 1 before storage iron labeling. The mean hematocrit on the first day of study was 69% ± 1%, the serum iron 459 ± 18 μg/dL, and the unsaturated iron-binding capacity less than 10 μg/dL. The distribution and excretion of radioiron-labeled chelates (10 mg/animal) were assessed after intraperitoneal injection of the chelator mixed with a trace amount of 59Fe-citrate. Prelabeling of iron stores was accomplished via intravenous injection of radioiron labels through a tail vein. Animals were killed under ether anesthesia by exsanguination through the abdominal aorta into heparinized syringes. ICL670A was supplied by Novartis Pharma AG Research (Basel, Switzerland). DFO was purchased as the methane-sulfonate salt (Desferal) produced by Novartis. Prior to use, DFO was dissolved in normal saline and ICL670A in dimethyl sulfoxide (DMSO) diluted in normal saline to a final concentration of 1%.
Counting methods
The radioactivity of spleen, kidney, and weighed portions of the liver, and 1-mL samples of blood was determined in an automatic well-type scintillation counter (Auto-Gamma, Model 5360; Packard Instruments, Downers Grove, IL). Whole body counts were performed in a small-animal counter (Packard Model 446, Armac liquid scintillation detector). To measure the excretion of radioactivity after59Fe labeling, the animals were confined in solitary metabolic cages with stainless steel grid bottoms and urine and stool were collected separately. Radioiron excretion was also determined by whole body counts on the first and last days of the study, and corrections were made for decay and differences in geometry. Recovery of radioactivity in excreta compared with the decrease in whole body radioactivity was over 90%.
In preliminary studies using 51Cr-labeled erythrocytes, the blood volume was found to be 6.4 mL/100 g body weight, and the proportion of blood trapped in the liver was 2.4%. These factors were used in subsequent studies to calculate net hepatic and total blood radioactivity. Residual tissue radioactivity was calculated from total body radioactivity minus the combined radioactivity of liver, spleen, blood, and kidneys.
Preparation of radioiron labels
Heat-damaged erythrocytes (59Fe-DRBC).
In vivo 59Fe-labeled erythrocytes were prepared in rats by injecting 100 to 200 μCi 59Fe-citrate intravenously 5 days or more before harvesting the cells. Repeated injections of 50 to 100 μCi 59Fe were administered as required to maintain a specific activity of 0.05 to 0.1 μCi59Fe/mg hemoglobin. Blood was removed in volumes of 0.5 to 1.0 mL, and after 3 washes of cool normal saline, cells were suspended in 5 volumes of acid-citrate-dextrose (ACD) formula B and incubated at 40°C for 15 minutes. After 2 more washings, the cells were resuspended in saline to a final hemoglobin concentration of 5 mg/mL and injected intravenously without delay in aliquots of 1 mL/animal.8-11
Soluble ferritin (59Fe-ferritin).
In vivo 59Fe-labeled ferritin was prepared by injecting 100 to 200 μCi 59Fe-citrate into rats that had been given 12 mg iron dextran the preceding week. The animals were killed 24 hours later, and purified radioactive ferritin was prepared by the method of Bjorklid and Helgeland.12 Acrylamide gel electrophoresis of the ferritin preparation at pH 8.5 showed a single protein band, and a single precipitin line was obtained on immunodiffusion against antiferritin serum. The specific activity of 59Fe-ferritin was 5 to 7 μCi/mg iron.
Initial processing of storage iron labels.
This has been described in detail in previous communications.8-11 Briefly, 89% ± 1% of soluble ferritin and 81% ± 2% of DRBC were located to the liver and spleen within 1 hour of intravenous injection. After administration of DRBC, the cellular distribution of radioactivity in the liver determined by a quantitative autoradiographic method was 100% in RE cells and 0% in parenchymal cells. In contrast, soluble59Fe-ferritin was located primarily to hepatic parenchymal cells (97%–100%), with little labeling of RE cells (0%–3%). Thus, with one or the other source of radioiron, it was possible to label selectively either RE or parenchymal iron stores. The T1/2in plasma of intravenously injected soluble ferritin was 16 ± 1 minutes and that of DRBC was 2 ± 1 minutes. Within 1 day of injection, 72% of 59Fe-ferritin in the liver could be recovered in the ferritin fraction of tissue homogenates and the rest in nonferritin iron. Hemoglobin in 59Fe-DRBC was completely catabolized within 24 hours of injection. Total nonheme iron in the liver was determined by the method of Torrance and Bothwell.13
Design of animal studies
These studies have been approved by the institutional committee for animal experimentation of the Hebrew University Hadassah Medical School. Except for the initial screening experiments, all studies were performed in hypertransfused animals. Hypertransfused rats were housed in metabolic cages and stool and urine were collected for 7 consecutive days. One hour after intravenous injection of the radioiron-labeled ferritin on day 0, and 30 minutes before the injection of radioiron-labeled heat-damaged RBC, a single 200 mg/kg dose of chelator was administered intraperitoneally (DFO) or orally (ICL670A). In all experiments, each subgroup of control or treated animals consisted of at least 4 (maximum 11) subjects. All animals were killed on day 7 and the organ distribution of radioiron was determined as described above. Whole body radioactivity was determined on day 0 (representing 100% initial radioactivity) and day 7 to determine total radioiron excretion. This measurement was used as an independent confirmation of total radioiron excretion based on the measurement of cumulative fecal and urinary radioiron excretion.
For the statistical evaluation of differences between treatment groups, the Student t test was used.
In vitro studies
Ham F-10 culture medium (Gibco, Grand Island, NY) supplemented with CaCl2/H2O 135 mg/L, penicillin 200 000 U/L, streptomycin 0.2 mg/L, 10% horse serum, and 10% fetal bovine serum (Beth Ha'Emek, Israel) was used as growth medium. For iron loading experiments, heterologous serum was replaced by serum-free medium. For mincing and washing the organs and for trypsinization, H solution (Ham F-10 medium without calcium and magnesium) was used. Trypsin (type III, Sigma Chemical, St Louis, MO) was dissolved in 0.1% wt/vol H solution.
Cell cultures.
Cultures from 1-day-old rats (Hebrew University strain) were obtained by methods described in detail in our previous studies.14-19 Briefly, the ventricles were minced and trypsinized, and the combined fractions resuspended in growth medium into a sterile 250-mL flask (Nunc; Nunclon Delta, Herlev, Denmark) through a sterile mesh, to exclude explants. To reduce the number of fibroblasts and increase the proportion of myoblasts a preplating method was used exploiting the faster attachment of fibroblasts to the dish surface.20 After 1 hour preplating at 37°C in 100-mm diameter Petri dishes, the pooled cells were diluted in growth medium to a density of 9 × 105 to 1 × 106cells/mL and seeded into a 35-mm diameter Petri dish (Falcon 3001; Falcon Labware, Oxnard, CA). This concentration yielded after 24 to 36 hours an almost confluent layer of beating heart cells at a final density of about 2 × 106 cells. Cultures were kept at 37°C in an atmosphere of 5% CO2 and 95% air. Experiments were performed at 5 days of culture when over 80% of the cells were beating myocardial cells. Continued viability of cultured iron-loaded cells was documented by supravital dye exclusion and by the absence of enzyme (lactate dehydrogenase) leakage into the culture medium after 24-hour iron loading. For the chelating studies, DFO was dissolved in serum-free medium and ICL760A in DMSO diluted in serum free medium to a final concentration of 0.25% or less.
Radioactive iron labeling.
59Fe-citrate (specific activity 3–20 μCi/μg; Amersham Radiochemical Centre, Amersham, England) was mixed with sufficient sterile ferric ammonium citrate (BDH Fine Chemicals, Poole, England) to provide a concentration of 100 μg/mL elemental iron. In all studies a final concentration of 20 μg/mL (0.36 mM) iron was used for iron loading and radioiron labeling of heart cell cultures. To terminate incubations the culture plates were washed twice with 1 mL cold culture medium. The cells were scraped and transferred into counting tubes by means of a rubber policeman and resuspended in 0.5 mL culture medium. The 59Fe activity was determined in an automatic well scintillation counter (Auto-Gamma model 5360, Packard Instruments).
Iron shuttle studies.
Fluorescein-DFO (Fl-DFO) was purchased from Molecular Probes, Eugene, OR. Plastic 96-well plates with clear, flat bottoms were F96 Maxisorp from Nunc, Roskilde, Denmark. Hepes-buffered saline (HBS: 150 mM NaCl, 20 mM Hepes pH 7.3) was purchased from Beth Ha'Emek Industr. Kibbutz, Beth Ha'Emek, Israel.
The fluorescence of Fl-DFO was determined in a multiwell plate reader (BMG LabTechnologies, Offenburg, Germany) with excitation/emission filters of 485/538 nm and gain of 20. Complexes of ICL670A-Fe were formed by mixing Fe:NTA (5 mM ferrous ammonium sulfate:35 mM nitrilotriacetate) with ICL670A in HBS to yield solutions containing 12 μM Fe and increasing concentrations of ICL670A, followed by incubation for 1 hour at room temperature. At 0 minute, 20 μL of each chelator-Fe complex was mixed with 100 μL 2.5 μM Fl-DFO in HBS, and fluorescence was monitored over time in a fluorescence plate reader. In previous studies we have shown that DFO and its fluorescein derivative Fl-DFO have similar iron-binding affinities and that Fl-DFO provides a valid model for native DFO.21
Results
Studies in normal rats
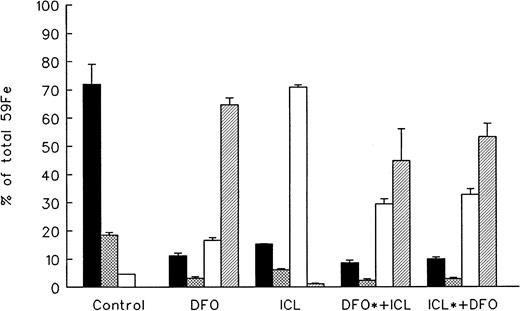
The fate of chelator-bound radioiron following intravenous injection into normal rats of 59Fe-citrate preincubated in vitro with 10 mg of DFO or ICL670A is described in Figure1. Control animals received59Fe-citrate without the iron chelator. Spontaneous radioiron excretion in normal controls was 4.6% ± 0.1% of the injected dose, restricted entirely to the stool. Iron retained in the body was distributed between circulating RBC (72.1% ± 7.0%), the liver (18.5% ± 1.0%), and residual tissues (3.4% ± 0.1%). In contrast to normal control animals showing no urinary iron excretion, 64.7% ± 2.5% of iron bound to DFO was excreted in the urine and 16.6% ± 0.5% in the stool. The pattern of radioiron excretion with prelabelled ICL670A was quite different from DFO: 70.9% ± 0.36% of the excreted radioactivity was in the stool (P < .001) and only 1.2% ± 0.1% was in the urine (P < .001). The proportional organ distribution of iron retained in the body was similar to that in the control groups with the majority of radioiron incorporated into circulating RBC and the rest retained in the liver. The ratio of blood and hepatic radioactivity in all groups was about 4:1.
Distribution of chelated radioiron in normal rats.
Control animals were injected intraperitoneally with59Fe-citrate. In all other groups, 59Fe was first bound in vitro to 10 mg of the chelator indicated and injected intraperitoneally. Measurements are at day 7. DFO indicates deferoxamine; ICL, ICL670A; DFO*+ICL, 59Fe-DFO followed by 10 mg cold ICL670A; ICL*+DFO, 59Fe-ICL670A followed by cold DFO. Each treatment group consisted of 4 animals (mean ± 1 SD). ▪ indicates blood; ⊠, liver; ■, fecal; and ▨, urine.
Distribution of chelated radioiron in normal rats.
Control animals were injected intraperitoneally with59Fe-citrate. In all other groups, 59Fe was first bound in vitro to 10 mg of the chelator indicated and injected intraperitoneally. Measurements are at day 7. DFO indicates deferoxamine; ICL, ICL670A; DFO*+ICL, 59Fe-DFO followed by 10 mg cold ICL670A; ICL*+DFO, 59Fe-ICL670A followed by cold DFO. Each treatment group consisted of 4 animals (mean ± 1 SD). ▪ indicates blood; ⊠, liver; ■, fecal; and ▨, urine.
To examine the possibility of in vivo exchange, or “shuttling” of iron between the 2 chelators, 2 additional groups have been included in the above studies. In the first, (DFO*+ICL670A) radioiron was bound in vitro to 10 mg DFO and then injected, followed by 10 mg ICL670A. In the second and last group (ICL670A*+DFO), radioiron was bound in vitro to ICL670A and injected, followed by DFO. As shown in Figure 1, the subsequent excretion of radioiron was different from the patterns typical of either DFO (urinary excretion) or ICL670A (fecal excretion), and intermediate between the 2, with 29.5% ± 1.7% and 32.8% ± 2.0% excreted in the stool and 44.8% ± 11.3% and 54.3% ± 4.8% in the urine, respectively. The excretion patterns and organ distribution of residual radioiron were almost indistinguishable (P < .1) regardless of whether the radioiron was first bound to DFO or ICL670A, indicating substantial in vivo redistribution of the radioiron between the 2 chelators.
Studies in hypertransfused rats
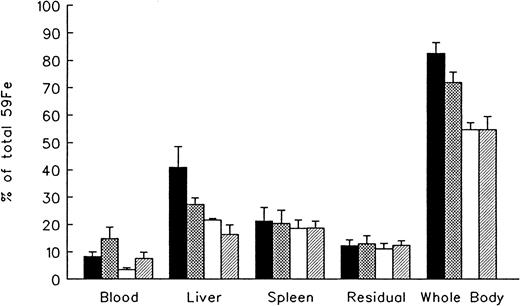
The effect of in vivo iron chelating treatment on hypertransfused rats tagged with the RE label 59Fe-DRBC is described in Figure 2. In untreated controls 7 days after labeling, total body radioactivity was 82.7% ± 3.9% of the injected dose. Of this, 8.2% ± 1.7% was in circulating RBC, 41.0% ± 7.5% in the liver, 21.3% ± 4.9% in the spleen, and 12.2% ± 2.2% in residual tissues (total body radioactivity minus blood, liver, and spleen). As shown in Figure 2, the administration of a single dose of 200 mg/kg DFO or ICL670A resulted in a decrease in whole body radioactivity to 72.1% ± 3.8% and 54.8% ± 2.6%, respectively, with a significantly higher response to ICL670A (P < .001). This was accompanied by a parallel decrease in liver radioactivity from 41.0% ± 7.5% to 27.3% ± 2.4% and 21.7% ± 0.5% in the groups treated by DFO and ICL670A, respectively (P < .001). Variations in blood radioactivity in the chelator-treated groups compared with untreated controls were minimal with the exception of ICL670A, which dropped from 8.2% ± 1.7% to 3.4% ±0.7%. By contrast, the decrease in splenic and residual tissue radioactivity in animals treated with iron chelator was minimal. Combined treatment with 200 mg/kg DFO and the same dose of ICL670A resulted in a further decrease in hepatic radioactivity to 16.3% ± 3.5% (P < .01), and a corresponding increase in combined fecal and urinary radioiron excretion (see below).
Reticuloendothelial iron mobilization in hypertransfused rats.
Measurements at 7 days after the intravenous injection of radioiron-tagged heat-damaged erythrocytes (59Fe-DRBC). Treatment with deferoxamine (DFO), ICL670A (ICL), or both consisted of a single dose of 200 mg/kg, within 1 hour of labeling. Residual radioactivity represents whole body radioactivity minus the combined radioactivity of blood, spleen, and liver. Each treatment group consisted of 4 animals and the control group of 10 animals. Results expressed as percent of injected radioactivity (mean ± 1 SD). ▪ indicates control; ⊠, DFO; ■, ICL; and ▨, ICL + DFO.
Reticuloendothelial iron mobilization in hypertransfused rats.
Measurements at 7 days after the intravenous injection of radioiron-tagged heat-damaged erythrocytes (59Fe-DRBC). Treatment with deferoxamine (DFO), ICL670A (ICL), or both consisted of a single dose of 200 mg/kg, within 1 hour of labeling. Residual radioactivity represents whole body radioactivity minus the combined radioactivity of blood, spleen, and liver. Each treatment group consisted of 4 animals and the control group of 10 animals. Results expressed as percent of injected radioactivity (mean ± 1 SD). ▪ indicates control; ⊠, DFO; ■, ICL; and ▨, ICL + DFO.
Fecal and urinary excretion of radioiron.
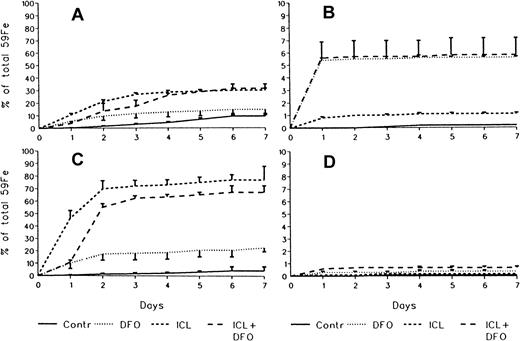
In untreated controls there was no urinary radioiron excretion and spontaneous fecal excretion amounted to 9.4% ± 2.2% of the total initial body radioactivity. In the chelator-treated animals, most of the urinary radioiron excretion was completed within the first day of the study, amounting to 5.6% ± 0.1% with DFO, 1.1% ± 0.1% with ICL670A, and 5.8% ± 1.4% with combined DFO and ICL670A. Fecal excretion of 59Fe-DRBC radioactivity was 14.7% ± 4.3% with DFO and higher at 30.1% ± .6% (P < .001) with ICL670A. Combined DFO and ICL670A treatment failed to enhance fecal excretion beyond that achieved by ICL670A alone (Figure3A), but resulted in an increase in urinary excretion identical with that obtained with DFO alone (Figure 3B).
Cumulative 7-day excretion of radioactivity in stool and urine.
(A) Fecal excretion of the RE label 59Fe-DRBC. (B) Urinary excretion of the RE label 59Fe-DRBC. (C) Fecal excretion of the hepatocellular label 59Fe-ferritin. (D) Urinary excretion of the hepatocellular label 59Fe-ferritin (mean ± 1 SD). Number of experimental animals same as in Figures 2 and 4.
Cumulative 7-day excretion of radioactivity in stool and urine.
(A) Fecal excretion of the RE label 59Fe-DRBC. (B) Urinary excretion of the RE label 59Fe-DRBC. (C) Fecal excretion of the hepatocellular label 59Fe-ferritin. (D) Urinary excretion of the hepatocellular label 59Fe-ferritin (mean ± 1 SD). Number of experimental animals same as in Figures 2 and 4.
The effect of in vivo iron chelating treatment in hypertransfused rats tagged with the hepatic parenchymal cell label59Fe-ferritin is described in Figure4 and Figure 3, panels C and D. The excretion pattern was quite different from that of59Fe-DRBC. In untreated controls, total body radioactivity 7 days after labeling was 88.3% ± 3.4% of the injected dose. Of this, 1.7% ± 1.2% was in circulating RBC, 79.2% ± 9.2% in the liver, 0.3% ± 0.1% in the spleen, and 7.0% ± 0.3% in residual tissues. As shown in Figure 4, the injection of a single dose of 200 mg/kg DFO resulted in a decrease in whole body radioactivity to 68.3% ± 3.2% and a much greater decrease after an identical oral dose of ICL670A to 28.8% ± 10.9% (P < .001). This was accompanied by a decrease in liver radioactivity from 79.2% ± 9.2% to 58.7% ±2.4% following DFO, and to 30.4% ± 18.0% following ICL670A (P < .01). Variations in blood, spleen, and residual radioactivity in the chelator-treated groups compared with untreated controls were minimal. Radioiron measurements in excreta (Figure 3C,D) have shown that urinary excretion of hepatocellular radioiron (59Fe-ferritin) was negligible and that all radioiron excretion was restricted to the stool. The magnitude of fecal radioiron excretion following oral ICL670A was roughly 5 times higher than with parenteral DFO (P < .001).
Hepatocellular iron mobilization in hypertransfused rats.
Measurements at 7 days after the intravneous injection of radioiron-tagged ferritin (59Fe-ferritin). All experimental conditions as in legends to Figure 2. Each treatment group consisted of 4 animals and the control group of 11 animals. ▪ indicates control; ⊠, DFO; ■, ICL; and ▨, ICL + DFO.
Hepatocellular iron mobilization in hypertransfused rats.
Measurements at 7 days after the intravneous injection of radioiron-tagged ferritin (59Fe-ferritin). All experimental conditions as in legends to Figure 2. Each treatment group consisted of 4 animals and the control group of 11 animals. ▪ indicates control; ⊠, DFO; ■, ICL; and ▨, ICL + DFO.
The effect of coadministration of DFO and ICL670A was unimpressive. There was no further decrease in total body radioactivity or increase in fecal radioiron excretion beyond that achieved by ICL670A alone, and only a slight additional decrease in hepatic radioactivity from 30.4% ± 18.0% to 23.4% ± 3.6% could be observed (not significant).
Dose-response studies.
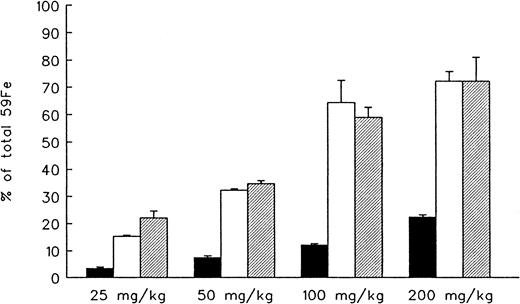
In the last part of our in vivo studies in hypertransfused animals tagged with 59Fe-ferritin, we explored the effect of increasing doses of parenteral DFO and oral ICL670A administered separately or in combination (Figure 5). DFO-induced radioiron excretion was linearly dose-related and ranged from 3.5% ± 0.5% at 25 mg/kg to 22.3% ± 0.1% at 200 mg/kg. Similarly, a linear dose-response relation was observed with ICL670A ranging from 15.3% ± 0.5% at 25 mg/kg to 64.4% ± 8.2% at 100 mg/kg with an efficiency that was 4.3- to 5.3-fold higher than DFO at all drug concentrations (P < .001). However, this response reached a plateau between 100 and 200 mg/kg with no further effect achieved by doubling the dose of ICL670A. The effect of combined DFO and ICL670A, compared with ICL670A alone, was additive at the lower dose range—15.3% ± 0.5% versus 22.1% ± 2.6% (P < .001) at 25 mg/kg and 32.3% ± 0.1% versus 34.7% ± 1.2% (P < .005) at 50 mg/kg—but was no higher than the effect of ICL670A alone at the dose range of 100 to 200 mg/kg.
Effect of drug dose on total hepatocellular59Fe excretion.
Results expressed as net 7-day cumulative excretion (percent of injected radioactivity). Each treatment group consisted of 4 animals (mean ± 1 SD). ▪ indicates DFO; ■, ICL; and ▨, ICL + DFO.
Effect of drug dose on total hepatocellular59Fe excretion.
Results expressed as net 7-day cumulative excretion (percent of injected radioactivity). Each treatment group consisted of 4 animals (mean ± 1 SD). ▪ indicates DFO; ■, ICL; and ▨, ICL + DFO.
Studies in cultured heart cells
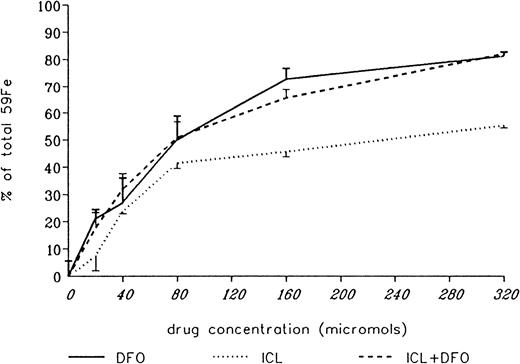
The ability of DFO and ICL670A to remove iron directly from the heart was studied in cultured iron-loaded rat heart cells. Five-day-old rat heart cell cultures were exposed for 24 hours to 360 μM iron supplied as ferric ammonium citrate, washed, and treated subsequently for an additional 24 hours with DFO or ICL670A, or a combination of both, at concentrations ranging from 0 to 320 μM. Washed, untreated control cells had a radioiron content of 29.6% ± 2.6% of the total radioactivity in the iron-loading culture medium. Results are expressed as percent of radioactivity in untreated control cultures. As shown in Figure 6, the ability of DFO to remove heart cell radioiron was substantially better than that of ICL670A, in particular at the higher concentration range, with a percent release of 73.0% ± 3.8% versus 45.8% ± 2.0% (P < .001) after 24 hours in vitro treatment with 160 μM of DFO or ICL670A, respectively, and 81.5% ± 1.7% versus 55.6% ± 1.1% (P < .001) after 24 hours of treatment with 320 μM of each drug.
Effect of drug concentration on radioiron mobilization from cultured, iron-loaded heart cells.
Five-day-old cultures were first incubated with 360 μM59Fe-labeled ferric ammoniun citrate for 24 hours, washed, and then exposed for an additional 24 hours to 20 to 320 μM deferoxamine (DFO), ICL670A (ICL), or both (ICL+DFO). The molar strength of the mixed DFO+ICL670A solution was identical with the corresponding single drug treatment and consisted of a 1:1 molar ratio of the 2 compounds. Results expressed as percent or total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures.
Effect of drug concentration on radioiron mobilization from cultured, iron-loaded heart cells.
Five-day-old cultures were first incubated with 360 μM59Fe-labeled ferric ammoniun citrate for 24 hours, washed, and then exposed for an additional 24 hours to 20 to 320 μM deferoxamine (DFO), ICL670A (ICL), or both (ICL+DFO). The molar strength of the mixed DFO+ICL670A solution was identical with the corresponding single drug treatment and consisted of a 1:1 molar ratio of the 2 compounds. Results expressed as percent or total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures.
In view of the results shown in our initial experiments (Figure 1) indicating considerable in vivo exchange of radioiron between the 2 chelators when administered simultaneously, we have examined the ability of combined chelating therapy to remove iron in vitro from iron-loaded heart cells. In these experiments, the total molar concentration of chelators was identical with that shown for DFO or ICL670A, but instead of single chelators consisted of equal parts of both. As shown in Figure 6, combined chelating treatment resulted in an improved chelating efficiency of ICL670A, and the joint effect of DFO and ICL670A was indistinguishable from that of DFO alone at molar concentrations of 20, 40, 80, and 320 μM (not significant).
To examine the ability of DFO and ICL670A to prevent the uptake of nontransferrin iron by cultured rat heart cells, we have performed an experiment that was similar in design to that described in Figure 6 but used these chelators 10 minutes before and throughout the 24-hour term of iron loading with 160 μM ferric ammonium citrate (Figure7). The inhibitory effect of DFO was directly related to its molar concentration and was complete at 160 μM, that is, when it reached a 1:1 ratio with the iron present in the culture medium. By comparison, the concentrations required with ICL670A to reach an effect similar to DFO were roughly 2-fold, and near-complete inhibition of uptake was only reached at a drug/iron ratio of 2:1.
Effect of drug concentration on radioiron uptake from iron-loading medium.
Five-day-old cultures were first exposed to 0 to 320 μM deferoxamine (DFO) or ICL670A (ICL) and after 10 minutes. incubated with 160 μM59Fe-labeled ferric ammoniun citrate for 24 hours. Results expressed as percent or total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures.
Effect of drug concentration on radioiron uptake from iron-loading medium.
Five-day-old cultures were first exposed to 0 to 320 μM deferoxamine (DFO) or ICL670A (ICL) and after 10 minutes. incubated with 160 μM59Fe-labeled ferric ammoniun citrate for 24 hours. Results expressed as percent or total cellular radioactivity in untreated controls. Each point represents the mean ± 1 SD of 6 cultures.
To document the ability of DFO to remove iron bound to ICL670A (the shuttle effect), we have exploited the phenomenon of fluorescence quenching when iron is bound to Fl-DFO. Fl-DFO (2 μM) was added to preformed ICL670A-Fe complexes containing a fixed Fe concentration (2 μM) with increasing ICL670A/Fe ratios ranging from 1:1 to 64:1. The transfer of iron from ICL670A to DFO at an identical chelator concentration of 2 μM was complete within less than 5 minutes (Figure8). Although at higher molar ratios ICL670A was able to slow down the transfer of Fe to Fl-DFO in a concentration-dependent manner, it was unable to prevent it even when the molar ratio of ICL670A to Fl-DFO was in excess of 32:1.
Effect of ICL670A concentration on iron exchange between ICL670A-Fe and Fl-DFO.
Complexes of ICL670A-Fe were formed by mixing Fe:NTA (5 mM ferrous ammonium sulfate:35 mM nitrilotriacetate) with ICL670A in HBS to yield solutions containing 12 μM Fe and increasing concentrations of ICL670A (0, 12, 96, 192, 384 and 768 μM), followed by incubation for 1 hour at room temperature. At 0 minute, 20 μL of each chelator-Fe complex was mixed with 100 μL 2.5 μM Fl-DFO in HBS, and the fluorescence was monitored over time in a fluorescence plate reader. The final concentrations of both Fe and Fl-DFO were 2 μM in each system, whereas the concentration of ICL670A varied as shown from 0 through 2, 8, 16, 32, 64, to 128 μM.
Effect of ICL670A concentration on iron exchange between ICL670A-Fe and Fl-DFO.
Complexes of ICL670A-Fe were formed by mixing Fe:NTA (5 mM ferrous ammonium sulfate:35 mM nitrilotriacetate) with ICL670A in HBS to yield solutions containing 12 μM Fe and increasing concentrations of ICL670A (0, 12, 96, 192, 384 and 768 μM), followed by incubation for 1 hour at room temperature. At 0 minute, 20 μL of each chelator-Fe complex was mixed with 100 μL 2.5 μM Fl-DFO in HBS, and the fluorescence was monitored over time in a fluorescence plate reader. The final concentrations of both Fe and Fl-DFO were 2 μM in each system, whereas the concentration of ICL670A varied as shown from 0 through 2, 8, 16, 32, 64, to 128 μM.
Discussion
Although DFO has changed the life expectancy of thalassemic patients requiring long-term blood transfusions for survival, the use of DFO involves a number of serious limitations. The need for its parenteral administration by portable pumps and other difficulties make it unacceptable for a substantial proportion of patients and unaffordable for others living in countries with limited resources. These considerations underline the need for the development of new, orally effective iron chelators.
Within recent years more than 1000 candidate compounds have been screened in animal models. These efforts have led to the identification of several interesting compounds, the most outstanding of which are deferiprone (L1), pyridoxal isonicotinoyl hydrazone (PIH), and the polyanionic amines. Of these, only L1 has been used extensively as a substitute for DFO in clinical trials involving hundreds of patients. A recent meta-analysis of 9 clinical trials involving the long-term use of L1 has shown that only 52% of the patients achieved a negative iron balance.22 Significant side effects of L1 include nausea, arthropathy, and zinc deficiency.23-25 L1 is less effective than DFO and its use is associated with a risk of agranulocytosis ranging from 0.6 to 1.8/100 patient-years.26,27 The potential hepatotoxicity of L1 is an issue of intense current controversy.23 28
Pyridoxal isonicotinoyl hydrazone (PIH) was introduced by Ponka and colleagues who recognized its ability to mobilize iron from59Fe-labeled reticulocytes29 and a variety of PIH analogues have been prepared and evaluated by Richardson and Ponka.30,31 The polyanionic amines HBED and dimethyl-HBED are remarkably nontoxic and in hypertransfused rats32 are several times more effective than DFO. Iron balance studies performed in a small number of thalassemic patients treated with HBED33 have shown increased iron excretion following oral treatment both in the urine and stool but this represented only 28% to 48% of the requirements for achieving negative iron balance.
Progress in the development of orally effective iron chelators has been painfully slow. Some of the most promising of these agents (such as PIH or HBED) are not proprietary (patentable) and therefore their attractiveness for drug industry is severely limited. Moreover, interest in recent years in L1 has left limited space for further development of other, alternative orally efficient chelators.
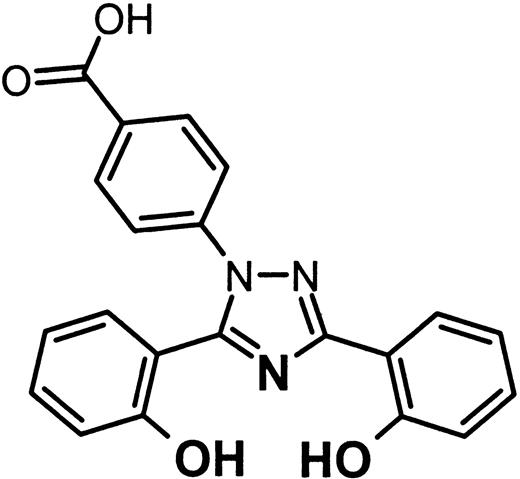
ICL670A (formerly CGP 72 670) or 4-[3,5-bis-(hydroxyphenyl)-1,2,4-triazol-1-yl]-benzoic acid is a tridentate iron-selective synthetic chelator of the bis-hydroxyphenyl-triazole class of compounds.6,34 35 Its structure is given in Figure 9.
Two molecules of the chelator are required to form a complete complex with ferric iron. ICL670A was selected as the most promising drug from over 700 compounds of potential usefulness as iron chelators for clinical use. Previous studies have shown that at pH 7.4 and a ligand concentration of 10 μM and iron concentration 1 μM the pFe+++ value of ICL670A is 22.5, compared with 19.5 for L1 and 26.6 for DFO6,36; that the ligand is stable both in vivo and in vitro; that in iron-loaded rats and marmosets oral ICL670A is over twice as effective as subcutaneous DFO; and that in these animals iron excretion is predominantly fecal.5,6 In view of the low toxicity of ICL670 in iron-loaded animals and its high oral effectiveness, studies are currently underway to explore its pharmacokinetics, safety, and efficacy in thalassemic patients.37
The objectives of the present studies were to define the source of iron chelated by ICL670A and its mode of excretion in hypertransfused rats, examine its ability to remove iron directly from iron-loaded myocardial cells, and examine its ability to interact with other chelators through a possible additive or synergistic effect. In all of these studies we have used DFO as the standard reference compound.
Previous studies have shown that DFO has a dual mechanism of action.38,39 First, it is able to chelate iron released by the RE system following the catabolism of senescent RBC. Such iron, once bound firmly to DFO, is not taken up by any organ but is excreted unaltered through the kidneys. Second, unbound DFO is internalized by hepatic parenchymal cells, interacts with the chelatable intracellular pool, and is subsequently excreted through the biliary system. DFO is also able to remove iron directly from myocardial cells,14,40 41 an important effect because myocardial siderosis is the most important cause of mortality in transfusional iron overload, but quantitatively this iron pool contributes little to overall iron excretion.
The objective of our initial studies using radioiron injected after in vitro binding to the chelator was to demonstrate the route of iron excretion following its chelation. These preliminary studies have demonstrated a fundamental difference between DFO and ICL670A excretory pathways. In contrast to circulating ferrioxamine (Fe-DFO), which is eliminated almost entirely by the kidneys, the excretion of Fe-ICL670A is limited exclusively to the bile. Thus, regardless of whether the iron chelated by ICL670A is derived from RE cells or other extrahepatic organs, once released to the plasma, all of the chelated iron is cleared by the liver and excreted through the bile. These studies, performed in normal animals, have also shown that some of the iron bound by DFO or ICL670A is reused for incorporation into newly formed RBC or stored in the liver, representing most likely a limited in vivo exchange of chelated iron with transferrin. In these preliminary studies we have also examined the ability of one chelator to remove iron from the other (exchange or “shuttling”) by first binding iron in vitro to either DFO or ICL670A, and then administering the alternative chelator at an identical dose. Our data indicate that regardless of whether iron is bound initially to one chelator or the other, a new equilibrium is reached resulting in an iron excretion pattern that is intermediate between that achieved by pure DFO or ICL670A, indicating considerable iron exchange between the 2 chelators.
In the next phase of our studies we wished to establish the origin of iron chelated in vivo. Selective radioiron probes were used for labeling hepatocellular iron stores with 59Fe-ferritin, and RE iron stores, located mainly to the spleen and liver, with59Fe-DRBC. By using hypertransfusion we have minimized the reutilization of storage iron through transferrin uptake. These studies comparing the in vivo effect of DFO and ICL670A allow the following conclusions to be drawn: (1) ICL670 given orally is 4 to 5 times more effective than parenteral DFO in promoting the excretion of storage iron from parenchymal iron stores and 2 to 3 times more effective in promoting RE iron excretion. (2) The pattern of iron excretion produced by ICL670A is quite different from that of DFO. Whereas with DFO a substantial proportion of iron derived from RE cells is excreted in the urine, with ICL670 all iron excretion is restricted to the bile regardless of whether it is derived from RE or hepatocellular stores. These findings are in keeping with those predicted from our initial observations on prebound radioiron. (3) The combined effect of DFO and ICL670A on storage iron excretion in the present study was additive at the lower dose range of 25 to 50 mg/kg, but negligible at 100 to 200 mg/kg where the response of chelatable iron to ICL670A (the more powerful of the 2 chelators) has already been exhausted. Significantly, the effective oral dose of ICL670A in marmosets and in humans is estimated to be 22 to 50 mg/kg,6 that is, in the same dose range that appears to be optimal in our own studies, for combined chelating treatment.
In the last part of our studies we wished to examine the ability of ICL670A to prevent myocardial cell iron uptake, remove iron directly from heart cells, and exchange iron with DFO. Both ICL670A and DFO were effective in preventing the accumulation of nontransferrin iron in heart cells. As anticipated with a tridentate chelator requiring 2 molecules to form a stable 2:1 complex with iron, twice higher molar concentrations were required with ICL670A to achieve the same effect as the hexadentate chelator DFO. Similarly, at identical molar strength ICL670A was less effective than DFO in removing iron from iron-loaded heart cells over a wide range of concentrations. Correction of this defect by the coadministration of ICL670A and DFO at a 1:1 molar ratio and at final molar concentrations identical with DFO alone indicates that the favorable interaction of the 2 chelators is most probably explained by an exchange of iron between DFO and ICL670A. The design of the present studies does not permit a distinction between 2 possible mechanisms of interaction: (1) primary intracellular chelation by ICL670A and subsequent transfer of chelated iron to extracellular DFO or (2) primary intracellular chelation by ICL670A and subsequent intracellular transfer to DFO, which is more effective in its egress to the extracellular compartment. The latter mechanism is in line with the hypothesis of Liu and Hider36 predicting intracellular trapping of the 2:1 triazole-iron complex caused by its hydrophobicity and high molecular weight. The studies described in Figure 8 clearly show that ICL670A readily yields iron to DFO, a finding that could be predicted from the higher pFe+++ value of DFO.6 36
Judged by its in vivo effectiveness in promoting the excretion of parenchymal and RE iron stores in hypertransfused rats, ICL670A is undoubtedly one of the most potent oral iron chelators identified. Comparing the present data with the results of our previous studies of L1,10,42 PIH,43 the polyanionic amine HBED,32 and the substituted polyaza compound IRCO11,11 44 all of which have been studied in the same experimental models, ICL670A appears to be the most effective and promising new oral chelator described so far. Although available data on its limited toxicity in experimental animals are encouraging, documentation of its chelating efficacy and possible toxic side effects in the clinical setting is still required.
A new orally effective iron chelator should not necessarily be regarded as one displacing the presently accepted and highly effective parenteral drug DFO. Rather, it could be used to extend the scope of iron chelating strategies in a manner analogous with the combined use of medications in the management of other conditions such as hypertension or diabetes. Coadministration or alternating use of DFO and a suitable oral chelator may allow a decrease in dosage of both drugs and improve compliance by decreasing the demand on tedious parenteral drug administration. Combined use of DFO and L1 has already been shown to result in successful depletion of iron stores in patients previously failing to respond to single drug therapy45 and to lead to improved compliance with treatment.46 It may also result in a “shuttle effect” between weak intracellular chelators and powerful extracellular chelators or exploit the enterohepatic cycle to promote fecal iron excretion.47 All of these innovative ways of chelator usage are now awaiting evaluation in experimental models and in the clinical setting.
Supported by grant DK54199 of the National Institute of Diabetes and Digestive and Kidney Diseases.
One of the authors (H.N.) is employed by Novartis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chaim Hershko, Department of Medicine, Shaare Zedek Medical Center, PO Box 3235, Jerusalem, Israel; e-mail:hershko@szmc.org.il.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal