Abstract
To determine the risk factors and outcomes associated with rising cytomegalovirus (CMV) antigenemia levels during preemptive therapy among stem cell allograft recipients, 119 patients with CMV antigenemia were studied. Patients were prospectively monitored for CMV antigenemia weekly; those with positive findings on antigenemia tests were treated with intravenous ganciclovir (5 mg/kg twice daily for 1 week, followed by 5 mg/kg per day for 5-6 d/wk). While on therapy, 47 of 119 (39%) patients demonstrated increases that were 2 or more times greater than their baseline values, whereas 33 of 119 (28%) patients demonstrated increases that were 5 or more times greater. Rising antigenemia was confirmed by polymerase chain reaction for CMV DNA. Multivariate analysis identified corticosteroids as the primary risk factor for increasing antigenemia: for increases greater than or equal to twice the baseline, 1 to 2 mg/kg steroids was associated with an odds ratio (OR) of 4.0. For increases greater than or equal to 2 mg/kg steroids, the OR was 10.1. CMV isolates obtained at the time of rising antigenemia were susceptible to ganciclovir, indicating that resistance was not a major factor. Overall, rising antigenemia levels were not correlated with CMV disease. All 4 patients in whom CMV disease developed during therapy, however, had rising antigenemia levels. Among the 47 patients with antigenemia increases greater than or equal to twice the baseline, 15 were re-induced with antivirals, whereas 32 continued to receive maintenance therapy. All 4 patients in whom CMV disease developed during therapy received maintenance therapy, and 3 died with CMV disease. Thus, host factors such as the receipt of corticosteroids explain increasing viral load during the early phase of preemptive therapy. Continued induction dosing or re-induction may protect against early breakthrough CMV disease and CMV-related death among patients with rising antigenemia on preemptive therapy.
Introduction
Cytomegalovirus (CMV) continues to be a major cause of morbidity and mortality in patients undergoing hematopoietic stem cell transplantation (HSCT). Although antiviral prophylaxis has led to a significant reduction in early CMV disease after bone marrow transplantation,1,2 toxicity associated with the available antiviral agents (ganciclovir, foscarnet, and cidofovir) remains problematic. Furthermore, ganciclovir prophylaxis given at engraftment is associated with increased invasive fungal infections3and delays CMV-specific T-cell reconstitution,4 thereby increasing the risk for late CMV disease.5 Newer, more sensitive methods, such as the pp65 antigenemia assay or plasma polymerase chain reaction (PCR) for CMV DNA, can now detect CMV replication in the bloodstream before the advent of disease. This has allowed “preemptive therapy” to be directed only toward patients at highest risk for CMV disease, sparing many from the toxicity of universally applied antiviral prophylaxis.
The antigenemia assay may have additional benefits because of its quantitative nature. In the absence of antiviral therapy, higher levels of CMV viral load, as measured by the pp65 antigenemia assay or PCR for CMV DNA, have been correlated with increased risk for the development of overt CMV disease.6 Quantitative assays also have the potential for monitoring a patient's virologic response to antiviral therapy. We7 and other investigators,8however, have noted a substantial proportion of patients whose level of pp65 antigenemia increases while they receive preemptive therapy; the underlying mechanism and clinical significance of this finding are unknown. We thus sought to determine the risk factors for increasing antigenemia among recipients of allogeneic HSCT who receive preemptive therapy, and to determine whether increasing antigenemia was associated with poor outcome. Outcomes related to the management of anti-CMV therapy in patients with increasing antigenemia were also investigated to determine whether routine changes in antiviral therapy are warranted.
Patients and methods
Patients
All patients undergoing allogeneic HSCT between August 1, 1995 and June 30, 1997, were eligible for inclusion; all patients received non–T-cell depleted allografts. Patients were prospectively monitored weekly for pp65 antigenemia; patients with positive antigenemia test findings were included in the analysis. Clinical and laboratory data were extracted from a prospectively entered computerized database. Conditioning for stem cell transplantation,9-11prophylaxis,12,13 and treatment12 of graft-versus-host disease (GVHD) was performed according to current protocols. Additional supportive care for herpes simplex virus–seropositive patients included acyclovir 250 mg/m2intravenously twice daily from the start of conditioning until day 30 after transplantation or the resolution of chemotherapy-induced mucositis, whichever occurred first.
Underlying disease was classified as advanced for all patients not in remission, patients with acute nonlymphocytic leukemia in second or later remission, and patients with acute lymphocytic leukemia in third or later remission. Patients with chronic myelogenous leukemia were classified as having advanced disease if they were in blast crisis at time of transplantation. Patients with multiple myeloma, RAEB, or RAEB-T were also classified as having advanced disease. All other patients were classified as not having advanced disease. Assessment and staging of GVHD was made according to the established categories for acute GVHD.14 Approval was obtained from the institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Cytomegalovirus antigenemia monitoring
Weekly heparinized blood samples were fractionated by dextran sedimentation. Slides were prepared in duplicate after cytocentrifugation; 1.5 × 105 cells were fixed with formaldehyde and stained with C10/C11 monoclonal antibodies that target pp65 Ag (CMV Brite; Biotest Diagnostic, Denville, NJ), as described previously.15
Ganciclovir and foscarnet treatment
Ganciclovir treatment was started on the day of the first positive antigenemia finding for all non-neutropenic patients at an induction dose of 5 mg/kg intravenously every 12 hours for 1 week and was continued as maintenance therapy at 5 mg/kg once daily 5 to 6 days per week. Foscarnet was used for neutropenic patients (absolute neutrophil count, less than 750/μL) or at the discretion of the treating physician; induction doses were 60 mg/kg intravenously every 12 hours, with maintenance doses of 90 mg/kg once daily, 5 to 6 days per week. Preemptive antiviral therapy was continued until day 100 after transplantation. Dose reductions according to creatinine clearance were made on the basis of calculated creatinine clearance according to adjusted ideal body weight. Granulocyte–colony-stimulating factor was used at the discretion of the primary physician for patients with neutropenia; ganciclovir dose was not reduced for neutropenia. “Re-induction” with twice-daily doses of ganciclovir or foscarnet for rising antigenemia levels was also performed at the discretion of the treating physician.
Definitions
Antigenemia course was characterized by changes in the pp65 antigenemia level from baseline, which was defined as the antigenemia level recorded at the first test with positive results. Increases of double the baseline (Ag 2× baseline) thus represent increases in the number of positively staining cells of greater than twice the baseline level on subsequent monitoring, while the patient was receiving preemptive antiviral therapy. Increases of 5 times baseline (Ag 5× baseline) were defined in a similar fashion. Cytomegalovirus disease was defined as the demonstration of CMV by biopsy specimen from visceral sites (by culture or histology) or the detection of CMV by culture or direct fluorescent antibody stain in bronchoalveolar lavage fluid in the presence of new or changing pulmonary infiltrates.16 CMV disease was analyzed separately as disease occurring before day 100 after transplantation (early CMV disease) and after day 100 (defined as late CMV disease). CMV-related death was defined as death occurring within 6 weeks of the diagnosis of CMV disease.
CMV DNA testing by quantitative polymerase chain reaction
Plasma samples were tested retrospectively for the presence of CMV DNA using a quantitative fluorescent probe detection method (TaqMan PCR) in a subgroup of 10 consecutive patients whose antigenemia levels rose 5 or more times above baseline. DNA was extracted from 200 μL plasma using QIAamp DNA blood kit (Qiagen) and eluted with 100 μL Tris (10 mM, pH 8.0). Ten microliters DNA was used for each PCR reaction. Primers (CytoF, CCA GTG CCC GCA GTT TTT ATT; CytoR, ACC GGA GAA GAG CCC ATG TC) and probe (CytoG, AAC ATA ACG TGG GAT CTC CAC GCG AAT) were used to amplify and detect 86 bp UL123 region. Reaction conditions and analysis were performed as previously described.17 The TaqMan assay has a dynamic range of 5 log10 copies/mL and a lower limit of detection of 50 copies/mL. Concordance between pp65 antigenemia and the TaqMan assay was 84.5% in a recent prospective study of 1983 blood samples from HSCT recipients; the TaqMan assay appeared to have higher sensitivity and excellent specificity (M.B., unpublished data).
Antiviral susceptibility assays
A standard plaque reduction assay was used to determine susceptibility to antiviral agents.18 Briefly, human foreskin fibroblasts (MRHF cells) were seeded in 24-well plates and infected with HCMV (50-100 plaque-forming units per well). The plates were incubated at 37°C for 3 hours to allow for virus adsorption. Unadsorbed virus was removed and replaced with minimal essential medium containing varying concentrations of ganciclovir and foscarnet. The plates were incubated for 7 days. Cultures were terminated by fixation with 100% methanol. Plaques were stained with Wright-Giemsa stain and counted under low-power microscopy.
All assay points were performed in duplicate, and the data were plotted as percentage inhibition relative to control wells. IC50values were determined manually by graphic extrapolation. Sensitivity cutoff points for ganciclovir and foscarnet were 6 μM and 400 μM, respectively.
Statistical analysis
To determine the risk factors associated with increasing antigenemia, logistic regression models were fit with varied levels of increasing antigenemia (more than twice the baseline, more than 5 times the baseline) as the outcome. Variables were first analyzed for their association with the probability of increasing antigenemia in univariate models and later in multivariate models. Variables were included in multivariate models if they were significantly associated with the probability of increasing antigenemia or if they altered the association of other variables. Cox proportional hazards regression models were fit to examine the association of antigenemia course with the time-to-event outcomes, overall mortality, and CMV disease. For these purposes, antigenemia was modeled as a combination of absolute level and slope of increase between 2 consecutive measurements. Both absolute level and slope were modeled as time-dependent covariates in the regression models. Time zero for these regression models was taken as the date of transplantation, and the data were left truncated so that a patient did not enter the risk set until time of first positive antigenemia finding. All P values from the regression models were 2-sided and were calculated from the Wald test. No adjustments were made for multiple comparisons.
A second model was constructed to study the influence of antigenemia course as a time-dependent covariate on CMV disease and overall survival. Patients in whom CMV disease developed on or before the first positive antigenemia finding were excluded from this analysis.
Results
One hundred nineteen consecutive patients were analyzed; patient characteristics are shown in Table 1. Follow-up data, including cases of CMV disease and cause of death, were available for all patients, with a median follow-up of 465 days (dead, n = 39; alive, n = 80; survival time, 30 to 1137 days) through June 30, 1998. All surviving patients were followed for at least 1 year after transplantation.
Characteristics of 119 recipients of allogeneic HSCT with pp65 antigenemia
| . | n . | % . |
|---|---|---|
| Total | 119 | 100 |
| Age (median, range) (y) | 41 (3-65) | |
| Gender | ||
| Female | 55 | 46 |
| Male | 64 | 54 |
| Underlying disease | ||
| Acute leukemia | 42 | 35 |
| CML | 43 | 36 |
| Myelodysplasia | 13 | 11 |
| Non-Hodgkin lymphoma/Hodgkin disease multiple myeloma | 7 | 6 |
| Nonmalignant diseases | 14 | 12 |
| Disease risk | ||
| Nonadvanced | 65 | 55 |
| Advanced | 54 | 45 |
| CMV serostatus | ||
| D+/R+ | 52 | 44 |
| D+/R− | 62 | 52 |
| D−/R+ | 5 | 4 |
| Conditioning regimen | ||
| Cyclophosphamide + TBI | 67 | 56 |
| Cyclophosphamide + busulfan + TBI | 5 | 42 |
| Other with TBI | 12 | 10 |
| Busulfan + cyclophosphamide | 33 | 28 |
| Other without TBI | 2 | 2 |
| Donor type | ||
| Matched related | 54 | 45 |
| Mismatched related | 14 | 12 |
| Unrelated | 51 | 43 |
| GVHD prophylaxis | ||
| Cyclosporine | 6 | 5 |
| Cyclosporine + MTX | 88 | 74 |
| FK506 + MTX | 14 | 12 |
| Other | 11 | 9 |
| Acute GVHD | ||
| Grades 0-1 | 27 | 23 |
| Grades 2-4 | 92 | 77 |
| . | n . | % . |
|---|---|---|
| Total | 119 | 100 |
| Age (median, range) (y) | 41 (3-65) | |
| Gender | ||
| Female | 55 | 46 |
| Male | 64 | 54 |
| Underlying disease | ||
| Acute leukemia | 42 | 35 |
| CML | 43 | 36 |
| Myelodysplasia | 13 | 11 |
| Non-Hodgkin lymphoma/Hodgkin disease multiple myeloma | 7 | 6 |
| Nonmalignant diseases | 14 | 12 |
| Disease risk | ||
| Nonadvanced | 65 | 55 |
| Advanced | 54 | 45 |
| CMV serostatus | ||
| D+/R+ | 52 | 44 |
| D+/R− | 62 | 52 |
| D−/R+ | 5 | 4 |
| Conditioning regimen | ||
| Cyclophosphamide + TBI | 67 | 56 |
| Cyclophosphamide + busulfan + TBI | 5 | 42 |
| Other with TBI | 12 | 10 |
| Busulfan + cyclophosphamide | 33 | 28 |
| Other without TBI | 2 | 2 |
| Donor type | ||
| Matched related | 54 | 45 |
| Mismatched related | 14 | 12 |
| Unrelated | 51 | 43 |
| GVHD prophylaxis | ||
| Cyclosporine | 6 | 5 |
| Cyclosporine + MTX | 88 | 74 |
| FK506 + MTX | 14 | 12 |
| Other | 11 | 9 |
| Acute GVHD | ||
| Grades 0-1 | 27 | 23 |
| Grades 2-4 | 92 | 77 |
CML, chronic myelocytic leukemia; MTX, methotrexate.
Frequency, time pattern, and course of antigenemia
One hundred nineteen recipients of allogeneic HSCT had CMV antigenemia and were started on antiviral therapy (ganciclovir in 117, foscarnet in 2) during the study period. Time to first positive antigenemia finding did not differ between patients whose antigenemia levels rose on therapy (median, 40 days; range, 15 to 70 days) and patients whose levels declined (median, 42 days; range, 22 to 78 days). After the initiation of therapy, 65 of 119 (55%) patients had declining levels of antigenemia and 54 of 119 (45%) patients had rising antigenemia levels. Overall, 47 of 119 (39%) patients had at least a 2-fold increase from baseline, whereas 33 of 119 (28%) patients had at least a 5-fold increase from baseline. Peak antigenemia levels were similar for those who increased on therapy (median, 5 cells/slide; range, 1 to 5110) versus those who declined (median, 5; range, 0.5 to 1382). Initial antigenemia level was higher for those whose level decreased on therapy (median, 5 cells/slide; range, 0.5 to 1382) than for those who increased (median, 2; range, 0.5 to 88).
Among the 47 patients who saw a 2-fold or higher increase in antigenemia level from baseline, 20 (43%) occurred after 1 week of therapy, 22 (47%) after 2 weeks, 3 (6%) after 3 weeks, and 2 (4%) after 4 or more weeks of therapy.
Correlation of antigenemia assays with quantitative plasma PCR for CMV DNA
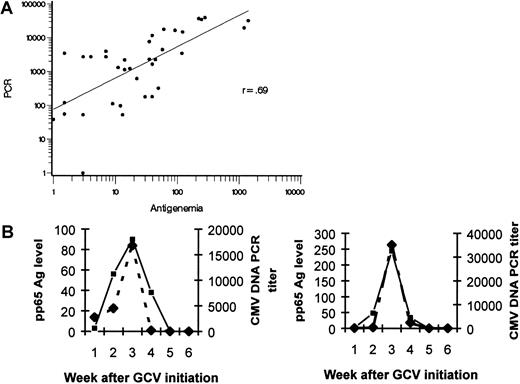
Weekly banked plasma specimens were available for analysis in a subset of 10 consecutive patients who had more than a 5-fold increase in pp65 antigenemia level from baseline. These samples were tested retrospectively by quantitative PCR for the number of copies of CMV DNA present. The log-transformed antigenemia levels were shown to be highly correlated with the log-transformed number of copies of CMV DNA in this analysis (r = 0.69) (Figure1A); the CMV viral load as determined by both methods for 2 representative patients is shown in Figure 1B. This subset analysis indicates that rising antigenemia level was not an assay-specific observation.
Correlation between pp65 antigenemia levels and copies of CMV DNA detected by quantitative TaqMan PCR of plasma.
(A) Banked plasma samples from a subset of 10 consecutive patients with antigenemia increases of greater than 5× baseline were tested for CMV DNA. Log-transformed quantities of the number of pp65 Ag staining cells and number of copies of CMV DNA per milliliter plasma are highly correlated (r = 0.69). (B) In two representative patients, the temporal rise and decline of CMV viral load as measured by pp65 antigenemia and CMV DNA are correlated. ▪ indicates pp65 Ag; ♦, PCR titer.
Correlation between pp65 antigenemia levels and copies of CMV DNA detected by quantitative TaqMan PCR of plasma.
(A) Banked plasma samples from a subset of 10 consecutive patients with antigenemia increases of greater than 5× baseline were tested for CMV DNA. Log-transformed quantities of the number of pp65 Ag staining cells and number of copies of CMV DNA per milliliter plasma are highly correlated (r = 0.69). (B) In two representative patients, the temporal rise and decline of CMV viral load as measured by pp65 antigenemia and CMV DNA are correlated. ▪ indicates pp65 Ag; ♦, PCR titer.
Risk factors for the development of increasing antigenemia on antiviral therapy
In univariate analysis, recipients of CMV-negative marrow, recipients of allografts from unrelated donors, and patients conditioned with total body irradiation (TBI)-containing regimens had a higher risk for rising antigenemia level while on antiviral therapy (Table 2). Furthermore, patients with grades 2-4 acute GVHD or those who received more than 1 mg/kg systemic corticosteroids at the time of first antigenemia finding were at higher risk for increasing antigenemia levels. Age, gender, underlying disease, disease risk, and GVHD prophylaxis were not significant in univariate analysis and, therefore, were not incorporated in the multivariate analysis.
Risk factors for increasing pp65 antigenemia among 119 recipients of allogeneic HSCT
| . | OR . | ↑ Ag > 2× baseline . | OR . | ↑ Ag > 5× baseline . | ||
|---|---|---|---|---|---|---|
| 95% CI . | P . | 95% CI . | P . | |||
| Univariate analysis | ||||||
| Donor CMV serostatus | ||||||
| Positive | 1.0* | 1.0* | ||||
| Negative | 2.5 | 1.2 -5.4 | .02 | 2.9 | 1.3 -6.8 | .01 |
| Donor type | ||||||
| Matched sibling | 1.0* | 1.0* | ||||
| Mismatched related | 0.6 | 0.2 -2.6 | .52 | 0.3 | 0.03 -2.3 | .23 |
| Unrelated | 3.3 | 1.5 -7.5 | .004 | 2.7 | 1.1 -6.4 | .03 |
| Conditioning | ||||||
| Non–TBI-containing | 1.0* | 1.0* | ||||
| TBI-containing | 3.4 | 1.4 -8.0 | .006 | 4.1 | 1.5 -11.8 | .008 |
| Acute GVHD† | ||||||
| Grades 0-1 | 1.0* | 1.0* | ||||
| Grades 2-4 | 3.9 | 1.3-11.1 | .01 | 14.3 | 1.8 -110.7 | .01 |
| Grades 3-4 | 1.6 | 0.7 -3.6 | .25 | 1.7 | 0.7 -4.1 | .22 |
| Steroid use at 1st Ag + ve | ||||||
| None | 1.0* | 1.0* | ||||
| <1 mg/kg | 4.1 | 0.9-17.4 | .06 | 4.3 | 0.4 -51.3 | .25 |
| 12 mg/kg | 4.3 | 1.2-15.3 | .03 | 14.3 | 1.7 -120.2 | .01 |
| ≥2 mg/kg | 10.5 | 3.1-35.8 | .0002 | 28.6 | 3.6 -229.7 | .002 |
| Multivariate analysis‡ | ||||||
| Conditioning | ||||||
| Non–TBI-containing | 1.0* | |||||
| TBI-containing | 2.6 | 1.0 -6.8 | .04 | |||
| Steroid use at 1st Ag + ve | ||||||
| None | 1.0* | 1.0* | ||||
| <1 mg/kg | 5.0 | 1.0-24.5 | .05 | 4.3 | 0.4 -51.3 | .25 |
| 1-2 mg/kg | 4.0 | 1.0-16.8 | .06 | 14.3 | 1.7 -120.2 | .01 |
| ≥2 mg/kg | 10.1 | 2.6-40.2 | .001 | 28.6 | 3.6 -229.7 | .002 |
| . | OR . | ↑ Ag > 2× baseline . | OR . | ↑ Ag > 5× baseline . | ||
|---|---|---|---|---|---|---|
| 95% CI . | P . | 95% CI . | P . | |||
| Univariate analysis | ||||||
| Donor CMV serostatus | ||||||
| Positive | 1.0* | 1.0* | ||||
| Negative | 2.5 | 1.2 -5.4 | .02 | 2.9 | 1.3 -6.8 | .01 |
| Donor type | ||||||
| Matched sibling | 1.0* | 1.0* | ||||
| Mismatched related | 0.6 | 0.2 -2.6 | .52 | 0.3 | 0.03 -2.3 | .23 |
| Unrelated | 3.3 | 1.5 -7.5 | .004 | 2.7 | 1.1 -6.4 | .03 |
| Conditioning | ||||||
| Non–TBI-containing | 1.0* | 1.0* | ||||
| TBI-containing | 3.4 | 1.4 -8.0 | .006 | 4.1 | 1.5 -11.8 | .008 |
| Acute GVHD† | ||||||
| Grades 0-1 | 1.0* | 1.0* | ||||
| Grades 2-4 | 3.9 | 1.3-11.1 | .01 | 14.3 | 1.8 -110.7 | .01 |
| Grades 3-4 | 1.6 | 0.7 -3.6 | .25 | 1.7 | 0.7 -4.1 | .22 |
| Steroid use at 1st Ag + ve | ||||||
| None | 1.0* | 1.0* | ||||
| <1 mg/kg | 4.1 | 0.9-17.4 | .06 | 4.3 | 0.4 -51.3 | .25 |
| 12 mg/kg | 4.3 | 1.2-15.3 | .03 | 14.3 | 1.7 -120.2 | .01 |
| ≥2 mg/kg | 10.5 | 3.1-35.8 | .0002 | 28.6 | 3.6 -229.7 | .002 |
| Multivariate analysis‡ | ||||||
| Conditioning | ||||||
| Non–TBI-containing | 1.0* | |||||
| TBI-containing | 2.6 | 1.0 -6.8 | .04 | |||
| Steroid use at 1st Ag + ve | ||||||
| None | 1.0* | 1.0* | ||||
| <1 mg/kg | 5.0 | 1.0-24.5 | .05 | 4.3 | 0.4 -51.3 | .25 |
| 1-2 mg/kg | 4.0 | 1.0-16.8 | .06 | 14.3 | 1.7 -120.2 | .01 |
| ≥2 mg/kg | 10.1 | 2.6-40.2 | .001 | 28.6 | 3.6 -229.7 | .002 |
Variables found not significant in univariate analysis were age, gender, underlying disease, disease risk, and GVHD prophylaxis.
Reference category.
If GVHD occurred after a rise in antigenemia level, this was regarded as grades 0-1 in the regression model.
For > 2× baseline, the likelihood ratio test for adding donor type to the model above yielded P = .16; for donor CMV serostatus, P = .25; for grades 2-4 GVHD,P = .73. For > 5× baseline, the likelihood ratio test for adding TBI-containing conditioning to the model above yieldedP = .09; for donor type, P = .13; for donor CMV serostatus, P = .09; for grades 2-4 GVHD,P = .23.
In multivariate analysis, receipt of high-dose systemic corticosteroids was the only variable significantly associated with increasing antigenemia levels while patients were on antiviral therapy (Table 2). After adjusting for the receipt of corticosteroids, conditioning with TBI-containing regimens was associated with increases more than twice the baseline but not with increases more than 5 times baseline (though the association with 5 or more times baseline was suggestive). Neither donor type, donor CMV serostatus, nor acute GVHD significantly improved the final model by the likelihood ratio test.
Antiviral susceptibility testing and increasing antigenemia
Twenty-one CMV isolates from 15 patients whose antigenemia levels increased on ganciclovir therapy were available for antiviral resistance testing by plaque reduction assays; these isolates were obtained after a median of 28 days of antiviral therapy (range, 14 to 49 days). Twenty of the 21 isolates were determined to be fully ganciclovir-susceptible. One isolate was determined to be intermediately susceptible to ganciclovir (IC50 of 8.7); this isolate was obtained from blood culture after 4 weeks of ganciclovir therapy. The patient was treated initially with 4 weeks of ganciclovir and had rising antigenemia levels. He was re-induced with 2 weeks of foscarnet, and antigenemia levels declined. Ganciclovir was then restarted and continued until day 100 after transplantation. He was alive without CMV disease or recurrent antigenemia at last contact (540 days after HSCT).
Antigenemia course and CMV disease
CMV disease developed in 20 patients during the study period. In 4 patients it was noted at the same time or before the first positive antigenemia finding, which meant the antigenemia course on therapy could not be assessed as a risk factor for subsequent CMV disease. These patients were excluded from analysis, leaving 16 patients with CMV disease (10 patients with pneumonia, 5 with enteritis, 1 with hepatitis). Disease developed in 12 (75%) of these patients after the completion of preemptive antiviral therapy (more than 100 days after transplantation); the median onset of late CMV disease was at day 212 after HSCT (range, 133-425 days). In 4 (25%) patients, CMV disease developed before day 100, while the patients were receiving preemptive antiviral therapy; median onset was 66 days after transplantation (range, 47-94 days), a median of 21 days after the initiation of antiviral therapy at first positive antigenemia finding (range, 18-40 days).
Among patients whose antigenemia levels at least doubled above baseline during the first 100 days after transplantation, 8 of 47 (17%) developed CMV disease at any time after transplantation, compared with 8 of 68 (12%) of those whose antigenemia levels did not double above baseline (P > .05). Among those whose levels increased at least 5 times above baseline, CMV disease developed in 5 of 33 (15%) compared with 11 of 82 (13%) of those without a 5-fold increase above baseline. Neither initial antigenemia level nor maximum antigenemia level while on antiviral therapy was associated with the development of CMV disease (data not shown). Three patients with early CMV disease had at least a 2-fold rise from baseline, whereas one patient with early CMV disease narrowly missed this definition (increase from 11 cells/slide to 21 cells/slide). Three of 4 of these patients died with CMV disease (2 with pneumonitis, 1 with gastrointestinal disease). Early CMV disease did not occur among patients with less than 2-fold increases from baseline. Rising antigenemia level was not associated with late CMV disease (4 of 47 patients with vs 8 of 68 patients without 2-fold increases); all 3 instances of late CMV-related mortality occurred among patients without rising antigenemia levels.
Antigenemia level was modeled as a time-dependent covariate, as was the change in antigenemia level between successive measurements (the slope was calculated between successive points). Cox regression models were used to assess the association of each with the hazard of CMV disease. Given the relatively low number of cases of CMV disease, we included only the level of antigenemia and the change in the regression model. Neither level nor change was associated with the hazard of CMV disease (P = .46 for level; P = .46 for change).
Antigenemia course and survival
As summarized above for CMV disease, antigenemia level was modeled as a time-dependent covariate, as was the change in level between successive measurements. Neither level nor change showed any suggestion of association with the hazard of overall mortality (P = .58 for level, P = .76 for change) or the hazard of 1-year mortality (P = .64 for level,P = .75 for change).
Effects of antiviral re-induction on clinical outcomes for patients with rising antigenemia levels
Forty-seven patients had at least a 2-fold rise in antigenemia level from baseline, despite ongoing daily antiviral therapy. Daily maintenance therapy with either foscarnet or ganciclovir was continued in 32 of these patients; 15 patients were re-induced with ganciclovir or foscarnet twice daily in a nonrandomized fashion. Patients who were re-induced had significantly higher maximum antigenemia values and greater increases above baseline than those who continued on maintenance therapy (Table 3). After a median 3 weeks (range, 1-5 weeks) of increasing antigenemia, all those who were re-induced had declining values during the following week. Of those continued on maintenance therapy, CMV disease developed in 4 of 32 (13%) before maintenance therapy was discontinued at day 100; 3 of 4 of these patients died with CMV disease (Figure2). No patient acquired early CMV disease after re-induction doses of ganciclovir or foscarnet. Late CMV disease developed in 2 of 32 (6%) patients receiving maintenance therapy, whereas 2 of 15 (13%) acquired late disease after receiving re-induction for increasing antigenemia levels; no one died of late CMV disease. All-cause mortality rates at days 100 and 365 appeared to be higher for recipients of maintenance therapy (19% and 41%, respectively) than for those who were re-induced (7% and 27%); these differences were not statistically significant and could have been strongly influenced by other factors known to be associated with survival. Further breakdown by specific antiviral agent (foscarnet, ganciclovir) yielded groups too small for statistical analysis.
Antiviral therapy and clinical outcome for patients with at least 2× increase from baseline antigenemia level
| . | . | Maintenance GCV or FOS n (%) . | Re-induction with GCV or FOS n (%) . |
|---|---|---|---|
| Patient characteristics | |||
| CMV serostatus | D+/R+ | 12 of 32 (38) | 6 of 15 (40) |
| D+/R− | 19 of 32 (59) | 9 of 15 (60) | |
| D−/R+ | 1 of 32 (3) | 0 of 15 (0) | |
| Donor type | Matched/related | 11 of 32 (34) | 2 of 15 (13) |
| Mismatched/unrelated | 21 of 32 (66) | 13 of 15 (87) | |
| GVHD grade | 0-1 | 1 of 32 (3) | 1 of 15 (7) |
| 2-4 | 31 of 32 (97) | 14 of 15 (93) | |
| Steroids | None | 1 of 32 (3) | 0 of 15 (0) |
| ≤1 mg/kg | 5 of 32 (16) | 1 of 15 (7) | |
| 1-2 mg/kg | 10 of 32 (31) | 4 of 15 (27) | |
| ≥2 mg/kg | 16 of 32 (50) | 10 of 15 (67) | |
| Ag up | >5× baseline | 16 of 32 (50) | 15 of 15 (100) |
| >10× baseline | 10 of 32 (31) | 12 of 15 (80) | |
| Initial Ag | 0.5-2 | 20 of 32 (63) | 5 of 15 (3) |
| 2.5-10 | 6 of 32 (19) | 6 of 15 (40) | |
| 10.5-50 | 3 of 32 (9) | 4 of 15 (27) | |
| >50 | 3 of 32 (9) | 0 of 15 (0) | |
| Maximum Ag | 1-50 | 25 of 32 (78) | 7 of 15 (47) |
| 51-100 | 3 of 32 (9) | 0 of 15 (0) | |
| 101-500 | 3 of 32 (9) | 2 of 15 (13) | |
| >500 | 1 of 32 (3) | 6 of 15 (40) | |
| Outcomes | |||
| CMV disease | ≤Day 100 | 4 of 32 (13) | 0 of 15 (0) |
| ≥Day 100 | 2 of 32 (6) | 2 of 15 (13) | |
| All-cause mortality | Day 100 | 6 of 32 (19) | 1 of 15 (7) |
| Day 365 | 13 of 32 (41) | 4 of 15 (27) | |
| Non-relapse mortality | Day 100 | 6 of 31 (19) | 1 of 14 (7) |
| Day 365 | 10 of 26 (38) | 4 of 14 (29) |
| . | . | Maintenance GCV or FOS n (%) . | Re-induction with GCV or FOS n (%) . |
|---|---|---|---|
| Patient characteristics | |||
| CMV serostatus | D+/R+ | 12 of 32 (38) | 6 of 15 (40) |
| D+/R− | 19 of 32 (59) | 9 of 15 (60) | |
| D−/R+ | 1 of 32 (3) | 0 of 15 (0) | |
| Donor type | Matched/related | 11 of 32 (34) | 2 of 15 (13) |
| Mismatched/unrelated | 21 of 32 (66) | 13 of 15 (87) | |
| GVHD grade | 0-1 | 1 of 32 (3) | 1 of 15 (7) |
| 2-4 | 31 of 32 (97) | 14 of 15 (93) | |
| Steroids | None | 1 of 32 (3) | 0 of 15 (0) |
| ≤1 mg/kg | 5 of 32 (16) | 1 of 15 (7) | |
| 1-2 mg/kg | 10 of 32 (31) | 4 of 15 (27) | |
| ≥2 mg/kg | 16 of 32 (50) | 10 of 15 (67) | |
| Ag up | >5× baseline | 16 of 32 (50) | 15 of 15 (100) |
| >10× baseline | 10 of 32 (31) | 12 of 15 (80) | |
| Initial Ag | 0.5-2 | 20 of 32 (63) | 5 of 15 (3) |
| 2.5-10 | 6 of 32 (19) | 6 of 15 (40) | |
| 10.5-50 | 3 of 32 (9) | 4 of 15 (27) | |
| >50 | 3 of 32 (9) | 0 of 15 (0) | |
| Maximum Ag | 1-50 | 25 of 32 (78) | 7 of 15 (47) |
| 51-100 | 3 of 32 (9) | 0 of 15 (0) | |
| 101-500 | 3 of 32 (9) | 2 of 15 (13) | |
| >500 | 1 of 32 (3) | 6 of 15 (40) | |
| Outcomes | |||
| CMV disease | ≤Day 100 | 4 of 32 (13) | 0 of 15 (0) |
| ≥Day 100 | 2 of 32 (6) | 2 of 15 (13) | |
| All-cause mortality | Day 100 | 6 of 32 (19) | 1 of 15 (7) |
| Day 365 | 13 of 32 (41) | 4 of 15 (27) | |
| Non-relapse mortality | Day 100 | 6 of 31 (19) | 1 of 14 (7) |
| Day 365 | 10 of 26 (38) | 4 of 14 (29) |
GCV, ganciclovir; FOS, foscarnet.
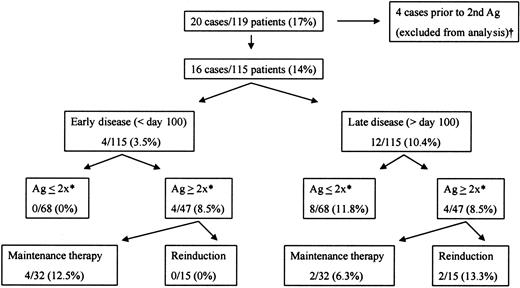
CMV disease among 119 transplant recipients with CMV antigenemia, according to antigenemia trend and antiviral strategy.
CMV disease developed in 16 of 115 evaluable patients with CMV antigenemia. All cases of early CMV disease took place among patients with rising antigenemia levels who were not re-induced with antivirals (ie, continued on maintenance doses); 3 of 4 of these patients died with CMV disease. Antigenemia course or antiviral strategy did not seem to predict the development of late CMV disease. † indicates that antigenemia trend could not be assessed as a risk factor for disease (see text); *, increases in pp65 antigenemia from “baseline” value (for definition, see text).
CMV disease among 119 transplant recipients with CMV antigenemia, according to antigenemia trend and antiviral strategy.
CMV disease developed in 16 of 115 evaluable patients with CMV antigenemia. All cases of early CMV disease took place among patients with rising antigenemia levels who were not re-induced with antivirals (ie, continued on maintenance doses); 3 of 4 of these patients died with CMV disease. Antigenemia course or antiviral strategy did not seem to predict the development of late CMV disease. † indicates that antigenemia trend could not be assessed as a risk factor for disease (see text); *, increases in pp65 antigenemia from “baseline” value (for definition, see text).
Discussion
In the absence of antiviral therapy, the positive predictive value of CMV antigenemia, CMV DNAemia by PCR, or culture-proven viremia for the development of CMV disease has been well documented,6 particularly in patients with no or mild GVHD. In this setting, early antiviral therapy based on systemicviral load can prevent the development of CMV disease. In patients with risk factors such as severe GVHD requiring high-dose steroids, rapid progression from low-grade antigenemia to CMV disease may be more likely.7 At our center, all allogeneic patients are thus treated with ganciclovir at the first positive antigenemia finding, regardless of level. Using this preemptive strategy, we recently reported the incidence of early CMV disease (ie, that occurring before day 100 after transplantation) to be comparable to that of universal prophylaxis at engraftment (3.8% vs 2.7%), with a substantially reduced incidence of invasive fungal disease (5% vs 16%).19 Preemptive therapy, however, still allows some early breakthrough CMV disease to occur. This happens in one of 2 settings: disease develops before the patient tests positive by antigenemia assay (and thus does not receive antiviral therapy), or disease develops despite the application of antivirals for antigenemia. For the former group of patients, more sensitive surveillance tests are needed to allow early detection of viral reactivation. We were interested to determine whether rising antigenemia levels predicted breakthrough disease in the latter group.
Our study indicates that rising antigenemia levels while on antiviral therapy appears “real” (ie, is associated with increasing levels of CMV DNA in plasma) and may be correlated with breakthrough cases of CMV disease and CMV-related death. Furthermore, our data indicate that most patients can be successfully treated by resuming induction doses of the antiviral they are currently receiving.
Overall, 1 week of induction therapy followed by maintenance therapy 5 or 6 days per week for CMV antigenemia was successful in rapidly reducing the viral load for more than 50% of our patients after HSCT. However, 2- and 5-fold rises in antigenemia were observed in 39% and 28% of patients receiving antiviral therapy, respectively. These were early events: of those with 2-fold or higher increases from baseline, 43% reached this endpoint after 1 week of induction doses of ganciclovir, and an additional 47% reached this endpoint after only 1 week of maintenance therapy. While extending the induction period to 2 weeks (as recently reported by Einsele et al20) would likely decrease the incidence of antigenemia rises noted in week 2, this would also result in “over-treatment” of the 61% of patients who had acceptable virologic responses. Thus, 1 week of induction therapy followed by viral load assessments may be used to “tailor” therapy for those at highest risk.
The major factor associated with rising antigenemia level during antiviral therapy was the receipt of high-dose corticosteroids for GVHD. Patients who received 1 to 2 mg/kg steroids daily were 4 times as likely to experience doubling of their antigenemia levels, whereas those receiving 2 mg/kg or more per day were 10 times as likely to reach this endpoint. Rising antigenemia level during therapy did not appear to be an assay-specific phenomenon; indeed, correlation between pp65 Ag and plasma PCR for CMV DNA was high in a subset of these patients (Figure 1). Of interest, the CMV isolates recovered during periods of rising antigenemia levels were still susceptible to ganciclovir in adequate doses, indicating that host factors (rather than resistance to antivirals) explain the phenomenon in this early post-transplantation period. It is important to note, however, that resistance may play a far more significant role in the pathogenesis of late CMV disease, which is often associated with prolonged courses of anti-CMV therapy.
To demonstrate a significant association between outcome (in this case, rising antigenemia level) and steroid use is often difficult, given the potential confounding by donor human leukocyte antigen matching and the presence or absence of GVHD. However, when steroid use was added to logistic regression models that contained various combinations of predictor variables (donor type, presence of GVHD, use of TBI, and donor CMV serostatus), the models were always significantly improved. This suggests that steroid use is associated with the probability of rising antigenemia levels, even after adjusting for combinations of these other variables. On the other hand, once steroid use was included in a regression model, inclusion of these other variables either led to a suggestively improved model (in TBI) or models that were improved little (Table 2). Thus, although donor type and GVHD are associated with rising antigenemia levels in the univariate model, the correlation with rising antigenemia level appears to be primarily driven by the corticosteroids administered to patients with GVHD. The strong dose-response relation (ie, higher probability of rising antigenemia with increasing steroid dose) we have demonstrated supports this assertion, as do previously noted associations between recurrent CMV DNAemia and the cumulative dose of prednisolone.20 It is likely that the “steroid effect” is related to the acute alteration in T-cell–mediated immunity that occurs after the administration of high-dose corticosteroids.
Recent insight into the dynamics of CMV replication in the immunocompromised host has shed further light on the phenomenon of rising viral load on antivirals. Although CMV is perceived as a slowly replicating virus because of the delayed appearance of cytopathic effects when grown in tissue culture, Emery et al21 have recently demonstrated that the virus has a doubling time of approximately 1 day in patients who undergo bone marrow transplantation when frequent measurements are made by quantitative–competitive PCR. Notably, the half-life of CMV in less-immunocompromised recipients of liver transplants appeared to be at least 2 days, highlighting the importance of host immune status in viral control. Our data, which document rapidly rising titers of CMV even while the patient is on antiviral therapy, corroborate the observations of Emery et al. These investigators also suggest that trends in CMV viral load on therapy have the potential to gauge the relative efficacy of different anti-CMV compounds.22 Our data suggest that corticosteroid dose (and other measures of immunosuppression) must be considered if mathematical modeling is applied to assess the activity of these compounds.
In our study, increasing antigenemia level was not associated with a statistically significant increase in the overall incidence of CMV disease. However, our sample size may have been insufficient to demonstrate this association. Interestingly, all 4 patients with early CMV disease that developed while the patient was on preemptive therapy affected patients whose antigenemia level rose significantly from baseline. Although ours was not a randomized trial, it is also important to note that early CMV disease did not develop in any patient who was re-induced with ganciclovir or foscarnet because of increasing antigenemia level, whereas it did develop in 4 patients who were continued on maintenance therapy (and 3 died of CMV disease). As shown in Table 3, imbalances did exist between the groups: patients who were re-induced had higher levels of maximum antigenemia and significantly greater increases from baseline than those who received maintenance. This is to be expected; higher antigenemia levels clearly prompted the selective use of re-induction therapy. However, such poor immunologic control of CMV would be expected to yield a higher incidence of CMV disease rather than the contrary.
The half-life of ganciclovir in plasma averages only 3 to 4 hours, though the intracellular half-life of the active metabolite (ganciclovir-triphosphate) is thought to be 18 hours or longer.23 In patients with risk factors for poor immune responses to CMV, the relative efficacy of antiviral therapy for controlling CMV replication may be reduced. Other investigators have demonstrated that prophylaxis or “low-intensity” maintenance regimens are associated with an unacceptable rate of CMV disease in recipients of marrow from unrelated donors and recipients of T-cell–depleted marrow.24-26 In our study of patients with unmanipulated allogeneic grafts, high-dose corticosteroid therapy also appears to be a risk factor for virologic and clinical failure.
Antiviral resistance testing in our study demonstrated that ganciclovir- or foscarnet-resistant strains were rare and did not explain the phenomenon of increasing pp65 antigenemia. Thus, routine “switches” to alternative antivirals are not needed. Rather, in patients with risk factors for poor immune reconstitution against CMV infection, it appears that the dosing interval for anti-CMV therapy is crucial for CMV control. Quantitation of pp65 antigenemia (or other quantitative assays, such as plasma or PBL PCR for CMV DNA) may be particularly useful for monitoring this population. Prolonged induction courses or re-induction may thus be necessary in certain high-risk populations (such as those with GVHD requiring high-dose corticosteroids) with increasing viral load to achieve viral suppression and to prevent the development of CMV disease. Although the strategy of re-induction has not been widely applied in patients who undergo HSCT, it is worth noting that this principle is well established for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome.27
In conclusion, our study has shown that host factors (such as the receipt of high-dose corticosteroids for GVHD) are the primary predictors of increasing CMV viral load on preemptive antiviral therapy. Although the overall incidence of CMV disease and mortality was not different between those whose levels increased and those whose levels decreased on therapy, more patients with increasing antigenemia levels progressed directly to CMV disease (and CMV-related death) despite maintenance antiviral therapy. Given the results of this study, we have modified our preemptive strategy19 for patients with more than two-fold increases from baseline; these patients now continue to receive induction doses of antivirals or are re-induced until viral control is achieved.
Acknowledgment
We thank Lizette Embuscado for her technical assistance on this paper.
Supported by National Institutes of Health grant CA-18029 (M.B.) and AI 01839-01 (W.G.N.); W.G.N. also supported by the Infectious Disease Society of America fellowship award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Boeckh, Program in Infectious Diseases, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, D3-100, Seattle, WA 98109-1024; e-mail: mboeckh@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal