The SH2-containing inositol-5′-phosphatase, SHIP, restrains bone marrow–derived mast cell (BMMC) degranulation, at least in part, by hydrolyzing phosphatidylinositol (PI)-3-kinase generated PI-3,4,5-P3 (PIP3) to PI-3,4-P2. To determine which domains within SHIP influence its ability to hydrolyze PIP3, bone marrow from SHIP−/− mice was retrovirally infected with various SHIP constructs. Introduction of wild-type SHIP into SHIP−/− BMMCs reverted the Steel factor (SF)-induced increases in PIP3, calcium entry, and degranulation to those observed in SHIP+/+ BMMCs. A 5′-phosphatase dead SHIP, however, could not revert the SHIP−/− response, whereas a SHIP mutant in which the 2 NPXY motifs were converted to NPXFs (2NPXF) could partially revert the SHIP−/− response. SF stimulation of BMMCs expressing the 2NPXF, which could not bind Shc, led to the same level of mitogen-activated protein kinase (MAPK) phosphorylation as that seen in BMMCs expressing the other constructs. Surprisingly, C-terminally truncated forms of SHIP, lacking different amounts of the proline rich C-terminus, could not revert the SHIP−/− response at all. These results suggest that the C-terminus plays a critical role in enabling SHIP to hydrolyze PIP3 and inhibit BMMC degranulation.
Introduction
Mast cells have a critical role in initiating acute inflammatory responses against invading bacteria, helminthic parasites, and harmless allergens.1 On exposure to multivalent antigens or allergens, these cells rapidly degranulate, releasing preformed inflammatory mediators that act on the vasculature, smooth muscles, connective tissue, mucous glands, and inflammatory cells to cause immediate-type hypersensitivity reactions.2Understanding how this degranulation process is regulated is key to our being able to effectively treat inflammatory disorders such as allergies, which affect as many as 20% of the Western population. We recently demonstrated that the hemopoietic-specific Src-homology 2 containing inositol phosphatase, SHIP, is a key negative regulator or “gatekeeper” of normal mast cell degranulation, setting the threshold for and limiting IgE-induced degranulation3 as well as preventing Steel factor (SF)-induced intracellular signaling from progressing to degranulation.4 This protein was originally identified as a 145-kd protein that became both tyrosine phosphorylated and associated with the adaptor protein Shc in response to multiple cytokines and to B- and T-cell antigen receptor engagement in hemopoietic cells.5,6 Subsequent cloning revealed that it possessed an amino terminal SH2 domain, a central inositol polyphosphate 5′-phosphatase catalytic domain that could hydrolyze phosphatidylinositol-3,4,5-trisphosphate (PIP3) in vivo, 2 NPXY sequences that, when phosphorylated, could bind phosphotyrosine-binding (PTB) domains, and a proline-rich C-terminus that was theoretically capable of binding to many SH3 domain-containing proteins7 (Figure 1). Initial studies showing that its enzymatic activity did not change following cytokine stimulation,7 coupled with subsequent studies indicating that SHIP exerted its effects in mast cells and B cells by translocating to the plasma membrane,8 suggested that it acts in large part, if not exclusively, by breaking down antigen-, IgE-, or SF-induced PIP3 at the plasma membrane to PI-3,4-P2. This reduces the ability of certain pleckstrin homology (PH)-containing proteins (eg, protein kinase B [PKB/Akt], phosphoinositide-dependent protein kinase 1 [PDK1], Bruton's tyrosine kinase [Btk]) to target to the plasma membrane and be activated.9-13 There is a good deal of support for this mode of action. For example, it has been shown that SHIP breaks down both cytokine- and antigen-induced PIP3 to PI-3,4-P2 in mast cells and B cells4,10 and this leads to a reduction in extracellular calcium entry4and a decrease in the activation of the survival enhancing serine/threonine kinase, PKB/akt.10-12 It has also been shown in both mast cells and B cells that colocalization of the inhibitory coreceptor, FcγRIIB, with the activated Fc[epislon]R1- and B-cell receptor (BCR), respectively, inhibits their activation by attracting SHIP to its intracellular tyrosine phosphorylated immunoreceptor tyrosine-based inhibitory motif (ITIM). This then enables SHIP to reduce the FcεR1- and BCR-induced PIP3levels and this, in turn, inhibits calcium influx sufficiently to curtail degranulation and proliferation, respectively.8 13-16
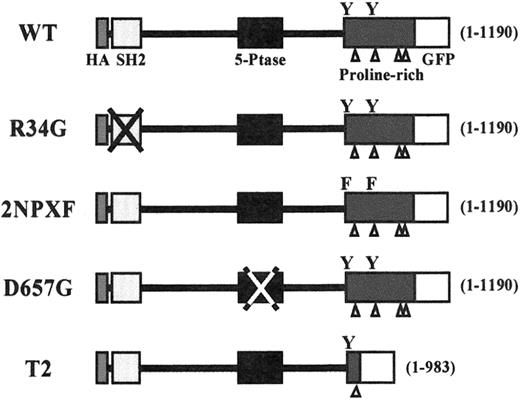
A schematic diagram of the initial SHIP constructs used in this study.
All constructs were HA-tagged at the N-terminus (black rectangle) and GFP-tagged at the C-terminus (open square). The SHIP lengths (in amino acids) are given in brackets on the right side of each construct. The open triangles indicate the proline-rich sequences within the C-terminus and the Ys indicate the positions of the 2 NPXY motifs.
A schematic diagram of the initial SHIP constructs used in this study.
All constructs were HA-tagged at the N-terminus (black rectangle) and GFP-tagged at the C-terminus (open square). The SHIP lengths (in amino acids) are given in brackets on the right side of each construct. The open triangles indicate the proline-rich sequences within the C-terminus and the Ys indicate the positions of the 2 NPXY motifs.
However, although there is much support for SHIP exerting its biologic effects through its ability to hydrolyze PIP3, other data show that SHIP acts in part by competing with Grb2 for Shc and thereby reducing Ras activation.16,17 Because the Ras pathway has been shown to be important for survival in interleukin (IL)-3–stimulated cells,18 this competition may be responsible, at least in part, for the reduced viability we observed in SHIP overexpression studies in DA-ER cells.19 In fact, strong evidence for this competition playing a role in FcγRIIB-mediated inhibition of BCR activation has been reported.16
Given SHIP's many protein-binding domains, it has the potential to interact with many signal transduction intermediates and a number of these have already been identified.5,6 For example, it has been shown to bind to Shc via SHIP's SH2 and tyrosine phosphorylated NPXY motifs,19 SHP-2 via SHIP's SH2 domain20and Grb2 in some cells (eg, B cells),21 but not in others (eg myeloid cells)17 via SHIP's proline-rich regions. SHIP also binds via its SH2 domain (which binds preferentially to pY(Y/D)X(L/I/V))22 to the tyrosine phosphorylated ITIM of certain inhibitory coreceptors (ie, the FcγRIIB23 and gp49B124) and the tyrosine phosphorylated “ITIM-like” motif of the adhesion receptor, PECAM-1.25 SHIP's SH2 domain also binds in vitro to the tyrosine phosphorylated CDw150,26 and to the tyrosine phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) within the β27 and γ22 subunits of the high affinity IgE receptor (FcεRI) and the (subunit of the T-cell receptor (TCR).22
To gain some insight into the role that SHIP's various domains play in preventing SF-induced signaling from progressing to degranulation in mast cells, we retrovirally infected bone marrow from SHIP−/− mice with either wild-type (WT) or various mutant forms of SHIP. SHIP−/− bone marrow–derived mast cells (BMMCs) expressing these different constructs were then stimulated with SF and the PIP3, intracellular calcium, and degranulation levels compared.
Materials and methods
Construction of SHIP mutants
The hemagglutinin (HA)-tagged murine SHIP complementary DNA (cDNA),19 in a BSKS+ vector, was the starting material for all polymerase chain reaction (PCR)-based point mutations using the QuickChang site-directed mutagenesis kit (Stratagene, La Jolla, CA). Construction of the R34G and D675G mutants was described previously.19,28 To construct the 2NPXF mutant the first INPNY917 was point mutated using the primers 5′ ATGATCAATCCAAACTTCATTGGTATGGGG 3′ and 3′ TACTAGTTAGGTTTGAAGTAACCATACCCC 5′ as per the protocol outlined in the Stratagene kit. This mutant was confirmed by sequencing and then used as the template for the second, ENPLY1020 point mutant using the primers 5′ TTTGAGAACCCACTGTTAGGATCCGTGAGT 3′ and 3′AAACTCTTGGGTGACAAACCTAGGCACTCA 5′. To tag SHIP at its C-terminus with green fluorescence protein (GFP) we removed the stop codon and replaced the tail of the HA-tagged SHIP cDNA's with a PCR product that eliminated the stop codon and put the SHIP construct in frame with the GFP from the plasmid pGFPN1. The truncated mutants were constructed similarly except that the PCR primers were designed to eliminate various amounts of the proline-rich tail as indicated. Once in the GFP vector all mutants were subcloned into the viral vector MSCVpac.29
Mast cell isolation and FACS analysis
Bone marrow cells were aspirated from 4- to 8-week-old SHIP+/+ and SHIP−/− littermates and infected or not with the empty (GFP-expressing) MSCVpac vector. In addition, the SHIP−/− cells were infected with the various SHIP constructs. For the infections, viral supernatants, in the presence of 5 μg/mL protamine sulfate, were added 3 times over a 2-day period and all the cells were plated in methylcellulose (StemCell Technologies, Methocult M3234, Vancouver, BC) supplemented with 30 ng/mL murine IL-3, 50 ng/mL murine SF, and 10 ng/mL human IL-6. In the case of the infected cells, 2 μg/mL puromycin was added for 10 days. Colonies were then harvested, pooled, and grown in suspension in Iscove modified Dulbecco medium (IMDM) containing 15% fetal calf serum (FCS) (Stemcell Technologies, Oakville, ON), 150 μM monothioglycerol (MTG) (Sigma), and 30 ng/mL IL-3, for 8 weeks. FACS analysis was then carried out to assess IgE receptor expression using fluorescein isothiocyanate (FITC)-conjugated IgE. To analyze cells for surface expression of c-kit, cells were first washed with HFN buffer (Hanks buffered saline, 2% FCS, 0.5% Na azide) resuspended in 100 μL HFN, and incubated on ice for 30 minutes with or without 2 μL phycoerythrin (PE)-tagged antimouse CD117 (0.5 mg/mL, Pharmingen, Mississauga, ON). Cells were then washed twice and analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
Northern Blot analysis
The BMMCs of all SHIP-infected populations were washed once with phosphate-buffered saline (PBS) and then lysed at 1 × 107 cells/mL using TRIzol (Gibco BRL, Burlington, ON). Total RNA was isolated and separated on a 1% formaldehyde/agarose gel, transferred to a nylon membrane (Zeta-Probe, Bio-Rad, Mississauga, ON), prehybridized, hybridized, and washed as previously described.30 Probes used for hybridization were a 250-bp fragment of the most 5′ part of the first exon of SHIP and the entire puromycin cDNA removed from the MSCVpac viral vector.
Immunoprecipitation and immunoblotting
Cells were starved overnight in IMDM, with 10% FCS and 150 μM MTG, washed, and stimulated at 37°C for various times with 400 ng/mL SF. The cells were then washed with ice-cold PBS and solubilized either by boiling 3 minutes with sodium dodecyl sulfate (SDS) sample buffer for total cell lysates or with 0.5% NP-40 in 4°C phosphorylation solubilization buffer17 at 4 × 107 cells/mL and subjected to immunoprecipitation as indicated. Western blotting was carried out as described previously.17 The anti-SHIP antibody (anti-N) was generated in rabbits using a glutathione S-transferase (GST) fusion protein containing the SH2 domain of murine SHIP (amino acids 7-133), the anti-GFP monoclonal antibody was from Clontech (catalogue no. 8362, Palo Alto, CA), the anti-Shc antibody was from Transduction Laboratories (catalogue no.14630, Lexington, KY), the anti-phosphoMAPK antibody was from New England BioLabs (Beverly, MA), the anti-MAPK antibody (Erk 1 CT) was a generous gift from Dr Steven Pelech and the antiphosphotyrosine antibody, 4G10, was purchased from Upstate Biotechnology Inc (Lake Placid, NY).
PIP3 measurements
Determination of PIP3 levels were performed essentially as described by Huber and coworkers.4
Calcium measurements
SHIP+/+ and SHIP−/− BMMCs, as well as SHIP−/− BMMCs expressing various SHIP constructs (5 × 106 cells/mL), were incubated with 2 μM fura-2/am (Molecular Probes, Eugene, OR) in Tyrode buffer31 at 23°C for 45 minutes. The cells were then washed, resuspended in 1 mL of the same buffer at 5 × 105 cell/mL in a stirring cuvette. Following stimulation by addition of 400 ng/mL SF, cytosolic calcium was measured by monitoring fluorescence intensity at 510 nm, after excitation of the sample with 2 different wavelengths (340 and 380 nm) using an MC200 monochromator from SLM AMINCO (Rochester, NY) with a 8100 V3.0 software program.
Degranulation assay
Cells were washed with and resuspended in Tyrode buffer and then treated for 15 minutes with or without 400 ng/mL SF. The degree of degranulation was determined by measuring the release of β- hexosaminidase.32
Plasma membrane preparation
The SHIP+/+ BMMCs, as well as SHIP−/−BMMCs expressing WT and T1 SHIP constructs, were starved overnight in IMDM with 1% FCS and 150 μM MTG, washed, and treated with and without 200 ng/mL SF for 3 minutes at 37°C. Plasma membrane–enriched membrane fractions were prepared as described by Huber and colleagues.33 The final NP40 solubilized membrane fraction, which was highly enriched for plasma membranes (as assessed by biotinylating the cell surface of intact BMMCs (Hughes and Krystal, manuscript in preparation), was then subjected either to Western blot analysis with anti-SHIP or anti-HA antibodies and the blot reprobed with anti-c-kit as a loading control.
Results
Introduction of SHIP mutants into SHIP−/−BMMCs
In previous studies we demonstrated that a 2-minute stimulation of SHIP−/− BMMCs with SF or IgE results in substantially more PIP3 and significantly less PI-3,4-P2 than in SHIP+/+ BMMCs.5 To identify the domains within SHIP that play a role in regulating both PIP3 levels and degranulation, we initially constructed the N-terminal-HA and C-terminal GFP-tagged SHIP constructs shown in Figure 1. Specifically, in addition to the WT construct containing the full-length 1190 amino acids of murine SHIP, we generated an R34G mutant, lacking a functional SH2 domain, a D675G mutant in which a critical aspartic acid in its catalytic domain was replaced with a glycine, rendering this enzyme 90% inactive (Ravichandran, personal communication, May 2000), a 2NPXF mutant in which the tyrosines within the 2 PTB consensus motifs, INPXY and ENPXY, were converted to phenylalanines, and a C-terminally truncated form of SHIP containing 1 to 983 amino acids of murine SHIP (T2).
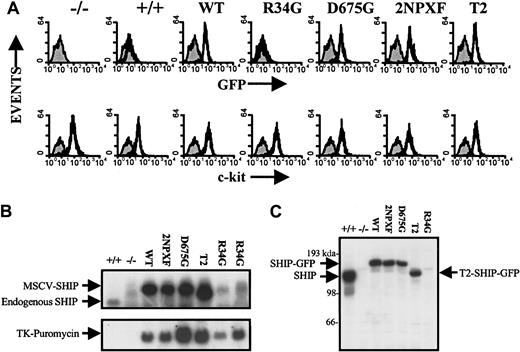
In these initial studies, bone marrow from SHIP−/− mice was infected with WT, R34G, D675G, 2NPXF, and T2 SHIP constructs in MSCVpac for 2 days and infected cells selected by growth in methylcellulose containing puromycin for 10 days, as described in “Materials and methods.” Mast cell colonies were then pooled and placed into suspension culture for 8 weeks, by which time all the SHIP−/− and SHIP+/+ uninfected and infected cells were 100% IgE- and SF-receptor positive. Expression of SHIP protein was then examined by FACS analysis using the GFP tag. As can be seen in the top panel of Figure 2A, all infected cultures, with the exception of the R34G mutant, expressed GFP to the same extent. The lower panel demonstrates that all the cells at this time expressed about the same level of c-kit on their surfaces. Northern blot analysis revealed that all infected cells, again with the exception of the R34G mutant (assessed at both 2 and 4 months of suspension culture), expressed a similar, high level of the proviral 7.3-kb SHIP message (Figure 2B, upper panel). Reblotting with a puromycin probe showed that expression of this drug marker was readily apparent in all infected cells, indicating that the SHIP−/− bone marrow had indeed been infected with the R34G construct (Figure 2B, lower panel).
Expression pattern of the various SHIP constructs in SHIP−/− BMMCs.
Uninfected SHIP−/− and SHIP+/+ BMMCs are shown in the first 2 panels/lanes in each figure. (A) FACS analysis of the BMMCs expressing the various SHIP constructs following 2 months in suspension culture. GFP was used as a marker for SHIP protein expression (top panel) and c-kit (using PE-tagged anti-c-kit) for SF receptor levels (bottom panel). (B) Northern blot analysis of the BMMCs expressing the various SHIP constructs following 2 months in suspension culture. SHIP messenger RNA (mRNA) levels were determined using a 250-bp fragment of the most 5′ part of the first exon of SHIP as a probe (top panel). The blot was reprobed with the entire puromycin cDNA removed from the MSCVpac viral vector as an independent measure of infection (bottom panel). The last lane (R34G) was from a 4-month suspension culture. (C) Western blot analysis of total cell lysates from SDS-sample buffer lysed BMMCs expressing the various SHIP constructs following 2 months in suspension culture to measure SHIP protein levels using anti-SHIP antibodies.
Expression pattern of the various SHIP constructs in SHIP−/− BMMCs.
Uninfected SHIP−/− and SHIP+/+ BMMCs are shown in the first 2 panels/lanes in each figure. (A) FACS analysis of the BMMCs expressing the various SHIP constructs following 2 months in suspension culture. GFP was used as a marker for SHIP protein expression (top panel) and c-kit (using PE-tagged anti-c-kit) for SF receptor levels (bottom panel). (B) Northern blot analysis of the BMMCs expressing the various SHIP constructs following 2 months in suspension culture. SHIP messenger RNA (mRNA) levels were determined using a 250-bp fragment of the most 5′ part of the first exon of SHIP as a probe (top panel). The blot was reprobed with the entire puromycin cDNA removed from the MSCVpac viral vector as an independent measure of infection (bottom panel). The last lane (R34G) was from a 4-month suspension culture. (C) Western blot analysis of total cell lysates from SDS-sample buffer lysed BMMCs expressing the various SHIP constructs following 2 months in suspension culture to measure SHIP protein levels using anti-SHIP antibodies.
Western blot analysis of SDS-lysed total cell lysates using anti-SHIP antibodies (Figure 2C) confirmed the GFP FACS results shown in Figure2A, specifically, that the expression of all but the R34G construct was similar at the protein level, and also showed that their levels were about half that of the endogenous SHIP levels in SHIP+/+BMMCs. Interestingly, in this regard, GFP FACS analysis of BMMC progenitors containing the R34G SHIP mutant revealed a low but detectable level of this protein at 3 weeks of suspension. However, it became totally undetectable by 6 weeks (data not shown). This is consistent with progenitors expressing high levels of this construct being selected against during BMMC maturation (as assessed by IgE R expression) and precluded our determining the role that SHIP's SH2 domain plays in regulating PIP3 levels and degranulation in this study.
Expression of WT, but not D675G nor T2 SHIP, in SHIP−/−BMMCs reverts SF-induced PIP3levels to that seen in SHIP+/+BMMCs
Following preliminary time-course studies, which established that a 2-minute stimulation with an optimal concentration of SF yielded maximal PIP3 levels in SHIP−/− BMMCs (data not shown), we used this time point to determine if introducing WT-SHIP into SHIP−/− BMMCs reduced the SF-induced increase in PIP3 levels to that observed in SHIP+/+ cells. As can be seen in Figure 3A, the results of several experiments revealed that although there was considerable variation in the SF-induced PIP3 levels generated in uninfected or vector control–infected SHIP−/− BMMCs, they were all significantly higher than those achieved in SHIP+/+ BMMCs. Expressing the WT-SHIP construct in SHIP−/− BMMCs reduced the SF-induced PIP3levels to very near the levels found in SHIP+/+ BMMCs. The effect of the various SHIP mutants on SF-induced PIP3levels was then determined and, as shown in Figure 3B, the D675G-SHIP was found incapable of reverting the PIP3 levels to those seen in SHIP+/+ cells, as would be expected for a mutant in which the enzymatic activity is severely compromised. The 2NPXF mutant on the other hand was partially capable of reducing the PIP3 levels to those observed in SHIP+/+ cells. In fact, with longer time in suspension culture (approximately 5 months), cells expressing this mutant were capable of hydrolyzing PIP3 as well as WT-expressing cells (data not shown). Intriguingly, the C-terminally truncated SHIP mutant, T2, was completely unable to revert the PIP3 levels to SHIP+/+ cell levels. This is especially interesting given that Aman and coworkers very recently found that a C-terminally truncated SHIP similar to T2 possessed full in vitro enzymatic activity.34
The effect of the SHIP constructs on SF-induced PIP3 levels in SHIP−/− BMMCs.
(A) PIP3 levels were determined, as described in “Materials and methods,” following a 2-minute stimulation of SHIP−/−, SHIP+/+ and SHIP−/−BMMCs expressing the WT-SHIP construct with 400 ng/mL SF. (B) PIP3 levels were determined after 2 minutes of stimulation with 400 ng/mL SF in SHIP−/− BMMCs expressing the indicated SHIP constructs. Because the PI-4,5-P2 levels were unaffected by either the presence or absence of SHIP or by SF stimulation, they were used as a loading control for the Partisol 10 SAX high-performance liquid chromatography column runs. To reflect this standardization the results are expressed as PIP3/PI-4,5-P2 ratios. Each point is the mean ± SE of a minimum of 4 determinations.
The effect of the SHIP constructs on SF-induced PIP3 levels in SHIP−/− BMMCs.
(A) PIP3 levels were determined, as described in “Materials and methods,” following a 2-minute stimulation of SHIP−/−, SHIP+/+ and SHIP−/−BMMCs expressing the WT-SHIP construct with 400 ng/mL SF. (B) PIP3 levels were determined after 2 minutes of stimulation with 400 ng/mL SF in SHIP−/− BMMCs expressing the indicated SHIP constructs. Because the PI-4,5-P2 levels were unaffected by either the presence or absence of SHIP or by SF stimulation, they were used as a loading control for the Partisol 10 SAX high-performance liquid chromatography column runs. To reflect this standardization the results are expressed as PIP3/PI-4,5-P2 ratios. Each point is the mean ± SE of a minimum of 4 determinations.
T2-SHIP does not become tyrosine phosphorylated in response to SF
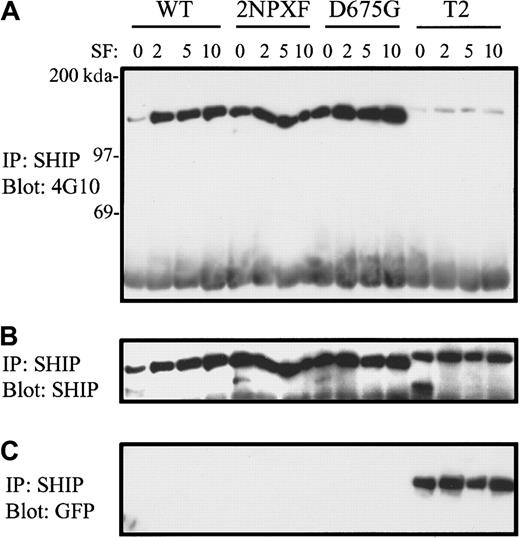
To gain some insight, at the signaling level, into why the T2 SHIP mutant was incapable of hydrolyzing PIP3, we first compared the tyrosine phosphorylation patterns of the various SHIPs in response to SF. Specifically, SHIP−/− BMMCs expressing the various SHIPs were stimulated with SF for 0, 2, 5, and 10 minutes and then subjected to anti-SHIP immunoprecipitation and Western blot analysis with antiphosphotyrosine antibodies. As can be seen in Figure4A, the tyrosine phosphorylation levels of WT, 2NPXF, and D675G SHIP were similar following SF stimulation. However, the T2 mutant was negligibly phosphorylated. Reprobing with an anti-SHIP antibody demonstrated equal loading of the gel (Figure 4B). These results suggest that SHIP's proline rich C-terminus is required for its SF-induced tyrosine phosphorylation. Also, because the tyrosine phosphorylation level of the 2NPXF mutant, following SF stimulation, was close to that observed with the WT and D675G forms of SHIP, it suggests that the tyrosines in the NPXYs are not the major phosphorylation sites in SF-stimulated BMMCs.
T2-SHIP in SHIP−/− BMMCs does not become tyrosine phosphorylated in response to SF.
(A) SHIP−/− BMMCs expressing the indicated SHIP constructs were stimulated for the indicated times (in minutes) with 400 ng/mL SF and the NP40 cell lysates subjected to immunoprecipitation with anti-SHIP antibodies and Western analysis with 4G10. (B) A reprobe of this blot with anti-SHIP antibodies demonstrates equal loading. (C) A reprobe of the same blot with anti-GFP antibodies.
T2-SHIP in SHIP−/− BMMCs does not become tyrosine phosphorylated in response to SF.
(A) SHIP−/− BMMCs expressing the indicated SHIP constructs were stimulated for the indicated times (in minutes) with 400 ng/mL SF and the NP40 cell lysates subjected to immunoprecipitation with anti-SHIP antibodies and Western analysis with 4G10. (B) A reprobe of this blot with anti-SHIP antibodies demonstrates equal loading. (C) A reprobe of the same blot with anti-GFP antibodies.
In the present study we found that extraction of endogenous SHIP from SHIP+/+ BMMCs or exogenously expressed full-length SHIPs from SHIP−/− BMMCs using an NP40 or TX100 solubilization procedure (for immunoprecipitation studies) resulted in the major species of SHIP being the C-terminally truncated 135 kd.35As a result, the GFP tag at the C-terminus was cleaved off as shown in the GFP Western blot in Figure 4C. However, this did not occur with the T2 SHIP mutant (Figure 4C) and it appears slightly larger than the full-length SHIP constructs (Figure 4A,B). This suggests that the protease(s) responsible for cleaving the C-terminus of SHIP recognizes specific amino acid sequences that have been deleted in the T2 mutant.
To further elucidate the signaling capacity of T2 SHIP and the other mutants we determined their capacity to associate, following SF stimulation, with Shc. As a first step, we asked if Shc was tyrosine phosphorylated in response to SF to the same degree in SHIP−/− BMMCs expressing these constructs. As shown in Figure 5A, top panel, this appears to be the case. Reprobing with anti-Shc antibodies confirmed equal loading (Figure 5A, bottom panel). As shown in Figure 5B (upper panel), we also carried out antiphosphoMAPK immunoblots with total cell lysates from this same experiment and found no significant difference in their phosphorylation levels on SF stimulation (compare the SF-induced WT and 2NPXF lanes).
The ability of SHIP to bind Shc does not affect MAPK phosphorylation.
(A) SHIP+/+ and SHIP−/− BMMCs (4 × 107) as well as SHIP−/− BMMCs expressing the indicated SHIP constructs were treated with or without 400 ng/mL SF for 2 minutes and NP40 cell lysates subjected to immunoprecipitation with anti-Shc antibodies and Western analysis with 4G10. The lower panel is a reprobe with anti-Shc antibodies to show equal loading. (B) At the same time as panel A was carried out, 1 × 106 cells/sample were lysed with SDS-sample buffer and subjected directly to Western analysis with anti-phosphoMAPK antibodies. The lower panel shows a reprobe of this blot with anti-MAPK antibodies to demonstrate equal loading. (C) SHIP−/−BMMCs expressing the indicated constructs were treated with or without 400 ng/mL SF for 2 minutes and the NP40 cell lysates subjected to immunoprecipitation with anti-Shc antibodies and Western analysis with anti-HA antibodies (top panel). The bottom panel shows a reprobing of this blot with anti-Shc antibodies to demonstrate equal loading. The NP40 cell lysates used in panel C were from the 2-minute time point in Figure 4 and thus contained equivalent levels of SHIP (Figure4B).
The ability of SHIP to bind Shc does not affect MAPK phosphorylation.
(A) SHIP+/+ and SHIP−/− BMMCs (4 × 107) as well as SHIP−/− BMMCs expressing the indicated SHIP constructs were treated with or without 400 ng/mL SF for 2 minutes and NP40 cell lysates subjected to immunoprecipitation with anti-Shc antibodies and Western analysis with 4G10. The lower panel is a reprobe with anti-Shc antibodies to show equal loading. (B) At the same time as panel A was carried out, 1 × 106 cells/sample were lysed with SDS-sample buffer and subjected directly to Western analysis with anti-phosphoMAPK antibodies. The lower panel shows a reprobe of this blot with anti-MAPK antibodies to demonstrate equal loading. (C) SHIP−/−BMMCs expressing the indicated constructs were treated with or without 400 ng/mL SF for 2 minutes and the NP40 cell lysates subjected to immunoprecipitation with anti-Shc antibodies and Western analysis with anti-HA antibodies (top panel). The bottom panel shows a reprobing of this blot with anti-Shc antibodies to demonstrate equal loading. The NP40 cell lysates used in panel C were from the 2-minute time point in Figure 4 and thus contained equivalent levels of SHIP (Figure4B).
Having established that Shc was tyrosine phosphorylated to the same degree, following SF stimulation, in the various SHIP−/−BMMCs, we then immunoprecipitated Shc from SHIP−/− BMMCs expressing either WT, 2NPXF, D675G, or T2, following SF stimulation, and found that SHIP coprecipitated with Shc in SHIP−/−BMMCs expressing the WT and D675G SHIP constructs but not the 2NPXF or T2 mutants (Figure 5C, top panel). Reprobing with anti-Shc antibodies established equal loading (Figure 5C, lower panel). These results indicate that one or both NPXY motifs are critical for Shc association, as was found previously following TCR activation of BYDP cells.36 However, the fact that the 2NPXF SHIP mutant was still substantially phosphorylated on tyrosines and was at least partially capable of hydrolyzing PIP3 in BMMCs suggests that Shc is not essential for these 2 events in BMMCs.
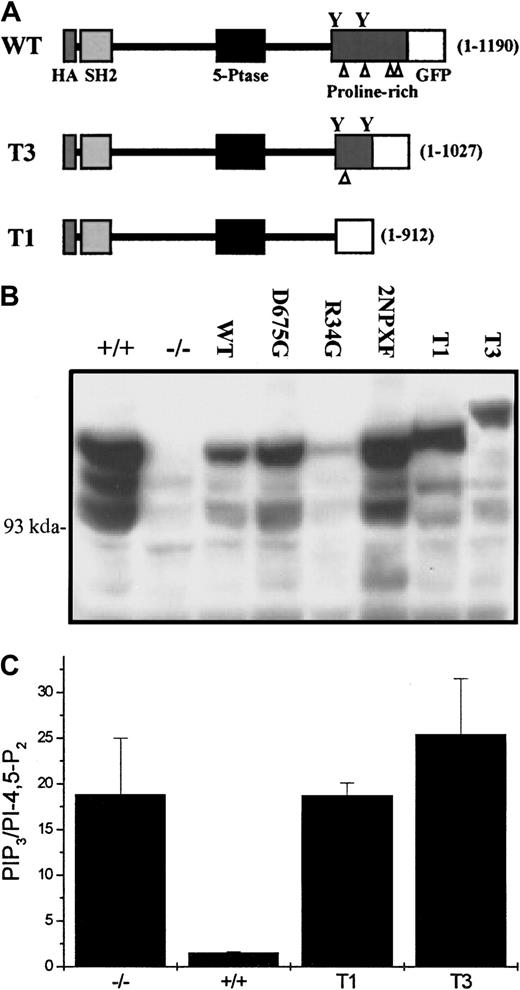
Because, on some occasions we observed a low but detectable level of T2 SHIP in our anti-Shc immunoprecipitates, we became concerned at this point that the one remaining NPXY motif in our T2 mutant might confound the interpretation of our results. We therefore constructed 2 more truncated mutants, T1 (1-912 amino acids), which lacked both NPXY motifs, and T3 (1-1027 amino acids), which contained both NPXY motifs (Figure 6A) and generated a second set of SHIP−/− BMMCs expressing either an empty vector, these 2 truncated mutants, and the WT, R34G, D675G, and 2NPXF constructs used in the first series of bone marrow infections. As can be seen in Figure6B, anti-SHIP Western blots revealed that, with the exception of R34G, expression of the various constructs was similar at the protein level. We also asked if these 2 new truncated constructs could hydrolyze PIP3 and, as can be seen in Figure 6C, they could not.
The T1 and T3 SHIP constructs do not revert the SF-induced PIP3 levels to that seen in SHIP+/+BMMCs.
(A) A schematic diagram of the second set of SHIP constructs used in this study. The SHIP lengths (in amino acids) are indicated in brackets to the right of each construct, the triangles refer to the proline-rich sequences in the C-terminus and the Ys indicate the position of the 2 NPXY motifs. (B) Western blot analysis using anti-SHIP antibodies of total cell lysates from NP40 lysed SHIP+/+ and SHIP−/− BMMCs expressing empty vector as well as SHIP−/− BMMCs expressing the indicated constructs, following 2 months in suspension culture. (C) PIP3determinations of SHIP+/+ and SHIP−/− BMMCs expressing empty vector as well as SHIP−/− BMMCs expressing the T1 and T3 SHIP constructs following a 2-minute stimulation with 400 ng/mL SF. Each point is the mean ± SE of 2 determinations.
The T1 and T3 SHIP constructs do not revert the SF-induced PIP3 levels to that seen in SHIP+/+BMMCs.
(A) A schematic diagram of the second set of SHIP constructs used in this study. The SHIP lengths (in amino acids) are indicated in brackets to the right of each construct, the triangles refer to the proline-rich sequences in the C-terminus and the Ys indicate the position of the 2 NPXY motifs. (B) Western blot analysis using anti-SHIP antibodies of total cell lysates from NP40 lysed SHIP+/+ and SHIP−/− BMMCs expressing empty vector as well as SHIP−/− BMMCs expressing the indicated constructs, following 2 months in suspension culture. (C) PIP3determinations of SHIP+/+ and SHIP−/− BMMCs expressing empty vector as well as SHIP−/− BMMCs expressing the T1 and T3 SHIP constructs following a 2-minute stimulation with 400 ng/mL SF. Each point is the mean ± SE of 2 determinations.
C-terminally truncated SHIPs do not inhibit SF-induced calcium entry
We had shown previously that SF stimulation of SHIP−/− BMMCs leads to a far higher entry of extracellular calcium than in SHIP+/+ BMMCs.4We therefore compared the abilities of our various SHIP constructs to revert the SF-induced intracellular calcium concentrations to that observed in SHIP+/+ BMMCs. As shown in Figure7, WT-SHIP effectively reduced the calcium levels to that observed in SHIP+/+ cells, reflecting its effects on PIP3 levels (Figure 3A). The D675G, on the other hand, was totally incapable of lowering the SF-induced calcium levels, showing that the phosphatase activity of SHIP is critical to this function in these cells (as was shown in chicken DT40 B cells following BCR/FcγRIIB stimulation8). Of interest, although the 2NPXF SHIP mutant was also totally incapable of lowering calcium levels, it did partially hydrolyze PIP3, suggesting that these 2 events may be uncoupled to some extent. Also of interest, the T1 and T3 truncated mutants were not only incapable of lowering the calcium concentrations following SF stimulation to those in SHIP+/+BMMCs, they consistently raised them above that seen in the SHIP−/− cells (Figure 7).
The WT, but not the D675G, 2NPXF, T1, or T3, SHIP construct reverts the SF-induced intracellular calcium concentration to that seen in SHIP+/+ BMMCs.
SHIP+/+ ( · · · · · · ) and SHIP−/− (–-) BMMCs expressing empty vector as well as SHIP−/− BMMCs expressing the indicated constructs (—) were preloaded with 2 μM fura-2/am and then stimulated with 400 ng/mL SF. Cytosolic calcium was measured by monitoring fluorescence intensity at 510 nm, after excitation of the sample with 2 different wavelengths (340 and 380 nm).
The WT, but not the D675G, 2NPXF, T1, or T3, SHIP construct reverts the SF-induced intracellular calcium concentration to that seen in SHIP+/+ BMMCs.
SHIP+/+ ( · · · · · · ) and SHIP−/− (–-) BMMCs expressing empty vector as well as SHIP−/− BMMCs expressing the indicated constructs (—) were preloaded with 2 μM fura-2/am and then stimulated with 400 ng/mL SF. Cytosolic calcium was measured by monitoring fluorescence intensity at 510 nm, after excitation of the sample with 2 different wavelengths (340 and 380 nm).
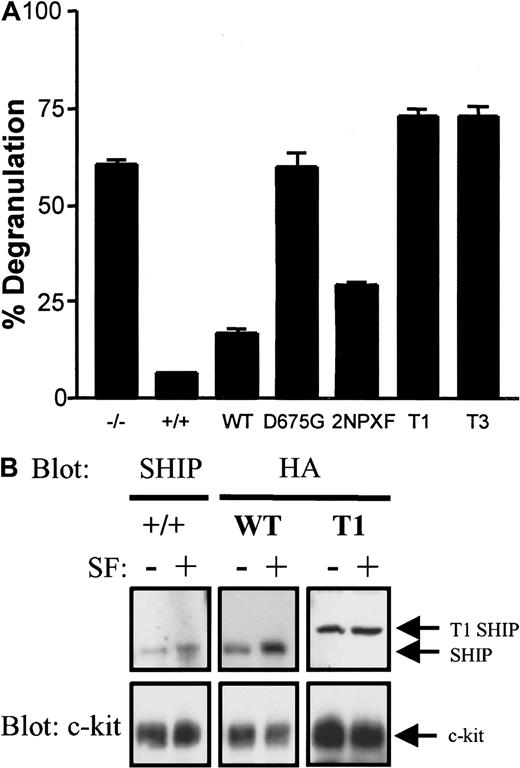
C-terminally truncated SHIPs are ineffective at reducing SF-induced degranulation
SHIP prevents SF from inducing the degranulation of normal BMMCs.4 We therefore compared the abilities of our SHIP constructs to revert the SF-induced SHIP−/− to a SHIP+/+ BMMC degranulation phenotype. As can be seen in Figure 8A, GFP-infected SHIP−/− BMMCs degranulated to a far greater degree than GFP-infected SHIP+/+ BMMCs, as expected (no differences were observed between uninfected and empty (GFP) MSCVpac-infected SHIP+/+ or SHIP−/− BMMCs in terms of PIP3, Ca++ or degranulation levels). As well, SHIP−/− BMMCs expressing WT-SHIP almost completely reverted the degranulation phenotype to SHIP+/+ BMMCs, whereas the D675G did not. The 2NPXY construct was partially capable of reducing the SF-induced degranulation, but both truncated mutants were totally ineffective. This degranulation pattern was directly proportional to the PIP3 levels exhibited in these cells (Figures 3B and 6C).
The WT, but not the D675G, T1, or T3, SHIP construct reverts the SF-induced degranulation to that seen in SHIP+/+ BMMCs.
(A) SHIP+/+ and SHIP−/− BMMCs expressing empty vector as well as SHIP−/− BMMCs expressing the indicated constructs were treated with or without 400 ng/mL SF for 15 minutes and the degree of degranulation determined by measuring the release of β-hexosaminidase. Unstimulated values were subtracted for each cell type (typically < 10% degranulation) and the mean ± SE of duplicate determinations are shown. (B) SHIP+/+ BMMCs and SHIP−/− BMMCs expressing WT and T1 SHIP were treated with and without 200 ng/mL SF for 3 minutes and plasma membranes prepared as described in “Materials and methods.” Western analysis of the NP40 solubilized membranes with anti-SHIP (for SHIP+/+ cells) or anti-HA (for SHIP−/− BMMCs expressing the constructs) antibodies (upper panel) was followed by reprobing with anti-c-kit antibodies (lower panel) to confirm equal loading of each set of unstimulated and SF-stimulated lanes. Similar results were obtained in 2 separate experiments.
The WT, but not the D675G, T1, or T3, SHIP construct reverts the SF-induced degranulation to that seen in SHIP+/+ BMMCs.
(A) SHIP+/+ and SHIP−/− BMMCs expressing empty vector as well as SHIP−/− BMMCs expressing the indicated constructs were treated with or without 400 ng/mL SF for 15 minutes and the degree of degranulation determined by measuring the release of β-hexosaminidase. Unstimulated values were subtracted for each cell type (typically < 10% degranulation) and the mean ± SE of duplicate determinations are shown. (B) SHIP+/+ BMMCs and SHIP−/− BMMCs expressing WT and T1 SHIP were treated with and without 200 ng/mL SF for 3 minutes and plasma membranes prepared as described in “Materials and methods.” Western analysis of the NP40 solubilized membranes with anti-SHIP (for SHIP+/+ cells) or anti-HA (for SHIP−/− BMMCs expressing the constructs) antibodies (upper panel) was followed by reprobing with anti-c-kit antibodies (lower panel) to confirm equal loading of each set of unstimulated and SF-stimulated lanes. Similar results were obtained in 2 separate experiments.
T1-SHIP does not translocate to the plasma membrane on SF stimulation
To gain some insight into why the C-terminally truncated SHIPs were ineffective at reducing SF-induced degranulation, we compared the ability of T1, WT, and endogenous SHIP to translocate, in response to SF stimulation, to the plasma membrane fraction of BMMCs. As can be seen in Figure 8B, WT-SHIP in SHIP−/− BMMCs translocated to the plasma membrane in a similar fashion to endogenous SHIP in SHIP+/+ BMMCs. However, no increased levels of T1-SHIP were apparent on SF-stimulation of SHIP−/− BMMCs expressing this construct. Reprobing with anti-c-kit established equal loading of the stimulated and unstimulated lanes.
Discussion
We show herein that introduction of WT-SHIP into SHIP−/− BMMCs reduces the SF-induced PIP3, intracellular Ca++, and degranulation levels to those observed with SHIP+/+ BMMCs. This confirms that the absence of SHIP is responsible for the aberrant behavior of the SHIP−/− BMMCs. Moreover, we found that WT-SHIP behaved similarly to endogenous SHIP, becoming both tyrosine phosphorylated and associated with Shc following SF stimulation. This enabled us to assess the contribution of SHIP's various domains to its biochemical and biologic functions by introducing various mutants into SHIP−/− BMMCs. Our finding that the phosphatase dead mutant, D675G, could not revert SF-induced degranulation to that observed with SHIP+/+ BMMCs confirmed that SHIP's ability to hydrolyze PIP3 was critical to its inhibition of degranulation. The 2NPXF mutant, on the other hand, partially restored proper PIP3 hydrolysis and degranulation but could not revert the calcium influx. Perhaps most intriguingly, we found that SHIP's C-terminus also plays a critical role in enabling SHIP to function properly. In particular, the inability of our T3 construct, which contains both NPXY motifs, to re-establish a SHIP+/+phenotype reveals that the last 163 amino acids of SHIP are vital to these activities.
What is it that makes these last 163 amino acids of SHIP so crucial? It is possible that one or more of the 3 resident PXXP sequences in this region may participate in the recruitment of SHIP to a specific location within the plasma membrane and/or submembranous cytoskeleton, affecting both access to its substrate and its association with other phosphotyrosine-binding proteins. The fact that we see the truncated SHIP mutant T1 at the membrane, even though it does not translocate, suggests that this mutant may not translocate to a specific subcellular compartment where PI-3-kinase and the substrate PIP3 are available. In support of this Petrie and coworkers38have recently shown that SHIP and PI-3-kinase translocate into a low-density insoluble fraction, lipid rafts, within 2 minutes following BCR stimulation. It is possible therefore, that SHIP recycling to lipid rafts may be compromised. Aman and colleagues34 have also found incomplete restoration of SHIP function in a SHIP deletion mutant (1-900 amino acids) fused to the FcγRIIB receptor, suggesting a more complex role for the C-terminal proline-rich tail. In this regard, a yeast 2-hybrid screen with SHIP's C-terminus has identified 3 in vitro binding partners: protein inhibitor of activated Stat1 (PIAS1), Grb2, and a novel SH3-containing protein of undefined function.6Furthermore, PIAS1 was shown to interact with SHIP in unstimulated FD/Fms cells, then dissociate following stimulation with macrophage colony-stimulating factor (M-CSF), and we and others have seen SHIP constitutively bound to Grb2 in B cells, though we have yet to observe this interaction in BMMCs. It remains to be seen what role, if any, these binding partners may play in SHIP localization or enzymatic regulation.
Previous studies have suggested that SHIP's SH2 domain is integral to the association of SHIP with the plasma membrane where it can hydrolyze its membrane-associated substrate, PIP3. Our early overexpression studies with WT and R34G SHIP demonstrated that SHIP's SH2 domain was essential for SHIP's IL-3–induced tyrosine phosphorylation, its association with Shc, and its ability to increase the apoptosis of confluent DA-ER cells.19 As well, SHIP's SH2 domain has been shown to bind to several phosphorylated ITAM motifs in vitro,22,27 the ITIM of FcγRIIB both in vitro23,37 and in vivo,8 and the PH domain-containing scaffold binding protein Gab1.6Unfortunately, we were unable to assess the effect of expressing an SH2-defective SHIP (ie, R34G) in SHIP−/− BMMCs. However, our data do suggest that its expression may be detrimental to the development of BMMCs, perhaps because it can still bind up signaling molecules critical for their proliferation/survival. Because the tyrosine phosphorylation of the SHIP C-terminal truncation mutants is dramatically impaired (Figure 4 and data not shown) despite the presence of functional SH2 domains, our data suggest that both an intact SH2 domain and C-terminal tail may be required for SHIP phosphorylation. This observed reduction in the tyrosine phosphorylation of the truncated mutants is unlikely to be the result of a loss of heavily phosphorylated tyrosines in the C-terminus because only one very C-terminal tyrosine is not present (Y1164) in the T3 mutant and this tyrosine is most likely clipped off the WT-SHIP during the generation of the heavily phosphorylated 135-kd form (Figure 4).
Our finding that the tyrosine phosphorylation levels of WT and 2NPXF are similar after SF stimulation (Figure 4) was unexpected given that the tyrosines within the 2 NPXY motifs have been reported to be the major tyrosine phosphorylation sites in SHIP, at least in the TCR-stimulated murine T-cell hybridoma cell line, BYDP.36Interestingly, in COS cells this 2NPXF mutant was only slightly less tyrosine phosphorylated than WT-SHIP when the Src kinase, Lck, was coexpressed36 and our results may therefore reflect a greater contribution of Lyn to SHIP's overall tyrosine phosphorylation in BMMCs than in BYDP cells. Furthermore, in keeping with the very recent results in BCR/FcγRIIB-engaged DT40 cells,34 our 2NPXF mutant was incapable of reverting the SF-induced Ca++influx to that seen in SHIP+/+ BMMCs, although it could still partially reduce PIP3 and degranulation levels. This indicates that Shc association is not essential for the latter 2 processes and that Ca++ influx and degranulation can be partially uncoupled, perhaps because the 2NPXF mutant does not bind to Shc, and perhaps not to PI-3K,39 p62Dok,40and Dok341 either.
Our finding that Shc is tyrosine phosphorylated in response to SF to almost the same degree in SHIP−/− and SHIP+/+BMMCs is in stark contrast to IgE stimulation of these cells where we found Shc phosphorylation was barely detectable in SHIP−/− BMMCs.3 This difference probably reflects the ability of Shc to be phosphorylated via c-kit46 and thus not be dependent on SHIP recruiting it to the plasma membrane for phosphorylation. This similarity in SF-induced Shc tyrosine phosphorylation found in both SHIP−/− and SHIP+/+ BMMCs allowed us to ask if the sequestration of Shc by SHIP had any effect on MAPK activation. As shown in Figure 5B, this does not appear to be the case. However, it should be borne in mind that Shc is substantially more tyrosine phosphorylated in response to SF than to IgE in SHIP+/+ BMMCs and it is likely that the ability of SHIP to compete with Grb2 for Shc, and thus reduce MAPK activation, is highly dependent on the relative accessible concentrations of SHIP, Grb2, and Shc.
The fact that SHIP's C-terminus is critical to its function has an impact on the roles of the C-terminally truncated forms of SHIP that have been detected.35,42 Mounting evidence suggests that SHIP may localize to different cellular compartments38 and this localization may be regulated by the C-terminal PXXP motifs. Typically, when we SDS-lyse normal BMMCs we obtain more full-length SHIP than when solubilizing at 4°C with NP40 or TX100 (which yields primarily the 135-kd SHIP species). This is consistent with there being a pool of SHIP that is not accessible using nonionic lysis procedures. If we solubilize the NP40-insoluble, cytoskeletal fraction from these BMMCs with SDS sample buffer we also obtain full-length SHIP. Additionally, it has been shown in platelets that thrombin stimulates the tyrosine phosphorylation and translocation of SHIP to the actin cytoskeleton43 and synaptojanin 2, another inositol polyphosphate 5-phosphatase containing a proline-rich C-terminus, is located predominantly within the particulate fraction.44This latter observation is of particular interest because a second isoform, synaptojanin 1, differs markedly in its proline-rich tail and resides primarily in the cytosol.44
In summary, we show herein that SHIP's C-terminal 163 amino acids are essential for SHIP to reduce SF-induced PIP3, intracellular Ca++, and degranulation levels in BMMCs. Although much remains to be done to elucidate how it regulates these effects, it is worthy of note that SHIP's C-terminus is very different from that of SHIP244 and this may allow for differential regulation. Incidentally, the type I 5-phosphatases and the type II INPP5P, which do not contain proline-rich tails, have C-terminal prenylation sites that mediate their membrane association similar to those found for the Rho/Rac family of signaling intermediates.44 It is tempting to speculate that SHIP and SHIP2 may use their proline-rich tails in lieu of prenylation to assist in their recruitment to a specific location within the plasma membrane, in a cooperative effort with their SH2 domains.
We thank Christine Kelly for typing the manuscript and Vivian Lam for excellent technical support.
Supported by the NCI-C and the MRC-C with core support from the BC Cancer Foundation and the BC Cancer Agency. J.K. and M.R.H. hold NSERC studentships. G.K. is a Terry Fox Cancer Research Scientist of the NCI-C and supported by funds from the Canadian Cancer Society and the Terry Fox Run.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gerald Krystal, Terry Fox Laboratory, 601 West 10th Ave, Vancouver, British Columbia, Canada, V5Z 1L3; e-mail:gerryk@terryfox.ubc.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal