In 1997, a chimeric anti-CD20 monoclonal antibody (mAb) (Rituxan) was approved for the treatment of low-grade/follicular B-cell lymphoma. Rituxan has a long half-life and low immunogenicity, and it mediates effector function. Rituxan induces apoptosis in some tumor cell lines in vitro. Previous studies with mAbs that react with neoplastic B cells have demonstrated that homodimers of immunoglobulin G ([IgG]2) often inhibit cell growth more effectively than their monomeric (IgG)1counterparts. In this study, the ability of IgG or F(ab′)2 homodimers vs monomers of Rituxan were compared for their ability to inhibit the growth of several different B-lymphoma cell lines in vitro. It was found that homodimers of Rituxan had superior antigrowth activity in vitro and that F(ab′)2 homodimers were the most active. Homodimers, but not monomers, of Rituxan induced both apoptosis and necrosis of several B-cell lymphoma lines in vitro; the inhibition of cell growth was not dependent upon the presence of Fc receptors or upon 10-fold or greater differences in the density of CD20 on the target cells. Rituxan homodimers, compared with monomers, also rendered drug-resistant CD20+ B-lymphoma cells more sensitive to chemotherapeutic agents and synergized with an anti-CD22 immunotoxin in vitro.
Introduction
In a previous study, we demonstrated that monoclonal antibodies (mAbs) (ie, anti-CD19, anti-CD20, anti-CD21, and anti-CD22) that have little or no antigrowth activity on neoplastic B-cells can become potent antitumor agents when they are converted into homodimers.1 These homodimers exert their antigrowth activity either by signaling G0/G1arrest or by inducing apoptosis, depending upon the cell surface molecule to which they bind. Our results confirmed and extended previous reports in which a chimeric mAb (Chi BR96) homodimer killed tumor cells 10-fold more effectively than its monomeric counterpart.2
The chimeric anti-CD20 mAb (Rituxan/Rituximab; Genentech, San Francisco, CA) has recently been approved by the Federal Drug Administration in the United States for the treatment of patients with relapsed or refractory, low-grade or follicular, B-cell non-Hodgkin lymphoma, and in Europe for patients with relapsed stage III/IV follicular lymphoma (reviewed in Grillo-Lopez et al3 and Gopal and Press4). The overall response was 50% when it was used as a single agent,4,5 and response rates were significantly higher when it was used in combination with chemotherapy.6 It is not yet known whether Rituxan will improve the long-term survival of patients with low-grade lymphoma, although early results look encouraging.3 4
In vitro studies, as well as studies in both animals and humans, suggest that the antitumor activity of Rituxan can be mediated by antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC).3,4,7 It has also been shown that Rituxan can induce apoptosis7-10 in DHL-4 or DHL-4–derived cell lines with bcl2 gene rearrangements.9,10 However, in accord with studies using other anti-CD20 mAbs such as 1F5 and B1,11 we could induce only modest levels of apoptosis with Rituxan (even in DHL-4 cells) unless we hypercrosslinked the bound Rituxan with rabbit antihuman immunoglobulin (Ig)–G (RAHIg) or goat antimouse immunoglobulin (GAMIg). In accord with these observations, it has recently been reported that Rituxan can induce apoptosis in lymphoma cells from patients only when GAMIg is added.10
Because hypercrosslinking with a secondary antibody would be impractical in vivo, in this study we evaluated tetravalent F(ab′)2 or IgG homodimers of Rituxan for their ability to exert antigrowth activity and to induce apoptosis in B-lymphoma cell lines. We found that homodimers had superior antigrowth activity and induced apoptosis in several neoplastic B-cell lines in vitro. Apoptotic activity did not require the participation of Fc receptors (FcRs) on target cells and was caspase dependent. Treatment with homodimers, compared with monomers, also rendered tumor cell lines more sensitive to chemotherapeutic agents and synergized with an anti–CD22-immunotoxin (IT) in vitro.
Materials and methods
Cells
Five human Burkitt lymphoma cell lines, including Daudi, Raji, Ramos, Namalwa (ATCC, Rockville, MD); the P-glycoprotein (P-gp)–transfected drug-resistant Namalwa/multidrug-resistant 1 (MDR1)12 (a gift from Dr Rosemary O'Connor, Immuno Gen, Cambridge, MA); 1 diffuse histiocytic cell line (DHL-4); and 2 T-leukemia cell lines, MOLT-4 and Jurkat (ATCC), were maintained in culture by serial passage in RPMI-1640 containing 25 mM Hepes, 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin (complete medium [CM]), and 100 mM glutamine. The cells were grown in a humidified atmosphere of 5% CO2 and air. Cell viability was determined by trypan blue exclusion. Cell lines were maintained in culture for 6 weeks and were then replaced with frozen stock.
Antibodies and reagents
The chimeric anti-CD20 mAb (Rituxan) developed by IDEC Pharmaceuticals13 (San Diego, CA) was purchased from Genentech (San Francisco, CA). We used 2 controls: (1) an isotype-matched mouse IgG1 of irrelevant specificity (3F12), prepared in our laboratory by purification of hybridoma cell supernatants on a protein A–sepharose column; and (2) a human IgG (Sigma, St Louis, MO), purified by chromatography on protein G–sepharose. GAMIg and GAHIg were affinity-purified in our laboratory. Fluorescein isothiocyanate (FITC)–GAMIg (H + L) was purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD). Rabbit antiserum against human IgG Fc was purchased from Cappel, Biochemical Division (Aurora, OH). Goat antihuman IgM (GAHIgM) was purchased from Chemicon (Temecula, CA). FITC-labeled annexin V apoptosis detection kits were purchased from BioVision Research Products (Palo Alto, CA). Caspase inhibitors (Z-VAD-FMK, Ac-YVAD-CHO, and Z-DEVD-FMK) were purchased from Calbiochem (San Diego, CA).
Flow cytometric assays
Indirect immunofluorescence assays were carried out by means of the panel of mAbs described above and FITC-GAMIg. The mAbs (monomers and homodimers) were labeled with FITC as described elsewhere14 and were used to determine the cell-binding and cell-dissociation rates of mAbs. Cells were suspended at 106 cells per milliliter in CM and were treated with 0.1 to 1.0 μg/mL mAbs for 30 minutes on ice, washed twice with CM containing 0.1% sodium azide, resuspended in 100 μL CM, and treated with 2 to 3 μL FITC-GAMIg. The direct immunofluorescence assay was carried out by means of FITC-mAbs. To determine the dissociation rate of FITC-mAbs (monomers vs homodimers), cells were treated as described above with saturating concentrations of FITC-mAbs. The excess mAbs were washed out, and the cells were incubated at 37°C for 2, 4, 6, 24, 48, and 72 hours in CM. At the indicated intervals, samples were taken, washed, and analyzed on a FACScan (Becton Dickinson, Franklin Lakes, NJ) to determine the percentage of the remaining cells that were still stained.
Preparation of homodimers of mAbs
Homodimers of Rituxan and the mouse IgG1 control mAb 3F12 were prepared and purified as previously described.1Briefly, we used 2 hetero-bifunctional cross-linkers, succinimidyl 4-(maleimidemethyl) cyclohexane-1-carboxylate (SMCC) and N-succinimidyl S-acethylthio-acetate (SATA) (Pierce, Rockford, IL) to bind 2 IgG molecules through a stable thioether bond. The final product was a mixture of polymers, trimers, dimers, and monomers, which were further separated on a preparative 21.5 mm inner diameter (ID) × 60 cm TSKG3000SW (Tosohaas, Montgomeryville, PA) high-performance liquid chromatography (HPLC) column. The protein fractions obtained by HPLC were concentrated to 5 to 10 mg/mL, filter-sterilized, and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and HPLC. The purity of the homodimer fraction was 80% to 90%.
Preparation of F(ab′)2 homodimers
HPLC-purified homodimers were digested with insoluble pepsin (Sigma) as previously described.15 The F(ab′)2fraction was affinity-purified on a protein G–Sepharose column.
Preparation of an immunotoxin using good laboratory practice
The mAb, RFB4, which recognizes human CD22, was coupled to deglycosylated (dg) ricin toxin A chain (RTA). RFB4-dgRTA was prepared in our good laboratory practice (GLP) scale-up laboratory as described.16 RFB4-dgRTA, has been evaluated in severe combined immunodeficient (SCID)/Daudi mice (reviewed in Ghetie et al17) and in patients with B-cell lymphoma (reviewed in Sausville and Vitetta18 and Engert et al19).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Proteins were analyzed under both reducing and nonreducing conditions by SDS-PAGE on 4% to 15% gels by means of the Pharmacia PhastSystem Separation Unit.20 Protein bands were visualized by staining the gels with Coomassie blue.
Molecular size determination by analytical HPLC
The molecular weights of the dimers were determined by analytical HPLC with the use of a 7.5 mm ID × 60 cm TSKG4000SW column (Tosohaas). A standard protein mixture of apoferritin (450 kd), IgA (300 kd), and IgG (150 kd) was fractionated on the same HPLC column to establish the retention time of known molecular weight standards.
Measurement of cell growth
We incubated CD20+ human cell lines [Ramos, DHL-4, Daudi, Raji] and the CD20−MOLT-4 cells at 2 × 105 cells per milliliter in CM at 37°C for different time periods in the presence and absence of different concentrations of anti-CD20 (1, 10, and 100 μg/mL). The irrelevant IgG1 isotype–matched mAb 3F12 and a human IgG (100 μg/mL) were used as a controls. Cells were stained with trypan blue and counted. Cell growth rates were then determined.
3H-thymidine assay
The antiproliferative activity of different mAb monomers and homodimers was determined by means of a 3H-thymidine incorporation assay on Ramos, DHL-4, Daudi, Raji, Namalwa, Namalwa/MDR1, and MOLT-4 cells. Briefly, 5 × 104 cells in 100 μL CM were plated in 96-well plates (Costar, Corning, NY) and incubated for 24 to 48 hours with 100 μL of different concentrations (0.01 to 1.0 mg/mL) of mAbs (monomers and homodimers) diluted in CM. To determine the cytotoxic effect of the immunotoxin (IT), the cells were incubated with 6 serial dilutions (10−8 to 10−13 M) of either IT alone or IT in combination with a constant amount (20 μg/mL) of Rituxan monomers or homodimers. Doxorubicin was used in a range of 1 to 70 nM, either alone or in the presence of Rituxan as described above. After incubating the cells at 37°C, the plates were pulsed for 4 hours with 1 μCi3H-thymidine (Amersham Pharmacia Biotech, Piscataway, NJ), harvested, and counted in a liquid scintillation spectrometer. The percentage reduction in 3H-thymidine incorporation, compared with untreated controls, was used to quantitate the cytotoxic effect.
FITC–Annexin V assay
We treated 2 × 105 cells with different concentrations (1 to 100 μg/mL) of Rituxan or irrelevant mAbs (monomers and homodimers); with Rituxan monomers (10 μg/mL) and GAMIg (50 μg/mL) or rabbit antihuman immunoglobulin (RAHIg) (10 μg/mL); or with GAMIg or RAHIg alone for 2, 4, 8, and 24 hours at 37°C. Cells were washed, treated with FITC–Annexin V and propidium iodide (PI), washed again, and analyzed within 1 hour on a FACScan. FL1-positive cells (stained with FITC-Annexin) were considered apoptotic and FL2-positive cells (permeable to PI) were considered necrotic. NaN3 (5 to 10 mg/mL) was used as a positive control for the induction of apoptosis. The caspase inhibitors (Z-VAD-FMK, Ac-YVAD-CHO, and Z-DEVD-FMK) were used at 50 μM.
Results
Characterization of Rituxan multimers
Rituxan multimers were separated on an HPLC column and were characterized by SDS-PAGE and by binding to, and dissociation from, cells.
HPLC-purification and SDS-PAGE.
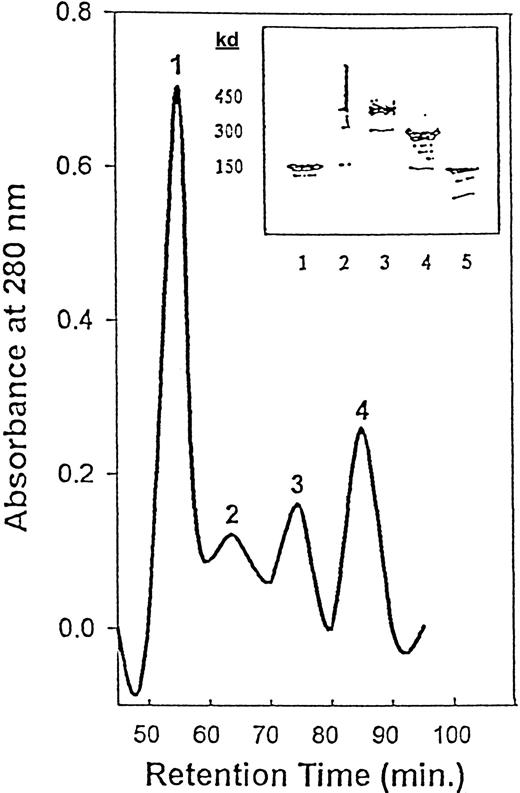
The initial Rituxan contained approximately 100% monomers (150 kd). Homodimers of Rituxan were purified by preparative HPLC (Figure1). We obtained 4 fractions and analyzed them by SDS-PAGE. Fraction 1 consisted of high molecular weight polymers (greater than 450 kd); fraction 2 consisted of approximately 80% trimers (450 kd); fraction 3 consisted of 80% to 90% dimers (300 kd); and fraction 4 consisted of greater than 90% monomer (150 kd).
HPLC purification of crude Rituxan homodimers.
Peak 1 = polymers; peak 2 = trimers; peak 3 = homodimers; peak 4 = monomers. The inset shows SDS-PAGE (4% to 15%) of the Rituxan monomers and homodimers separated by HPLC. Lane 1, Rituxan (monomer); lane 2, polymer (greater than 450 kd), HPLC fraction 1; lane 3, trimer (450 kd), HPLC fraction 2; lane 4, dimer (300 kd), HPLC fraction 3; lane 5, monomer (150 kd), HPLC fraction 4.
HPLC purification of crude Rituxan homodimers.
Peak 1 = polymers; peak 2 = trimers; peak 3 = homodimers; peak 4 = monomers. The inset shows SDS-PAGE (4% to 15%) of the Rituxan monomers and homodimers separated by HPLC. Lane 1, Rituxan (monomer); lane 2, polymer (greater than 450 kd), HPLC fraction 1; lane 3, trimer (450 kd), HPLC fraction 2; lane 4, dimer (300 kd), HPLC fraction 3; lane 5, monomer (150 kd), HPLC fraction 4.
Binding of Rituxan to cells.
The 6 CD20+ B-cell lines and the CD20− T-cell line were stained with FITC-Rituxan to determine their levels of expression of CD20. As Table 1 shows, 4 of the 6 B-cell lines were more than 90% positive and expressed varying densities of CD20. Raji cells expressed the highest density and DHL-4 cells the lowest density of CD20. Namalwa and Namalwa/MDR1 were less positive (Figure 2A), and Jurkat cells were negative. When the relative binding affinities to all the cell lines were determined in the same experiment (by plotting the percentage of positive cells vs concentration and calculating the concentration required to reach 50% saturation), Rituxan showed the highest relative binding affinity on DHL-4 cells and the lowest on Namalwa and Namalwa/MDR1 cells. The relative binding affinities on Daudi, Ramos, and Raji cells were very similar (Figure 2A and Table 1). The binding of Rituxan monomers, dimers, and trimers to cells was also compared by means of direct immunofluorescence on Daudi, Raji, Namalwa, Namalwa/MDR1, Ramos, DHL-4, and Jurkat cells. When the relative binding affinity to Ramos cells was determined, it was found to be similar for monomers, homodimers, and trimers, indicating that increasing the valency of the mAb did not change its relative binding affinity for CD20 (Figure 2B and Table 1).
Binding of FITC-Rituxan (monomers and homodimers) to different cell lines
| Cells . | % Positive . | MFI . | Relative binding affinity (×10−10 M) . | |||
|---|---|---|---|---|---|---|
| Monomer . | Dimer . | Monomer . | Dimer . | Monomer . | Dimer . | |
| Daudi | 93.7 | 91.5 | 750 | 452 | 4.3 | 1.5 |
| DHL-4 | 99.8 | 99.6 | 467 | 545 | 0.5 | 1.5 |
| Ramos | 97.7 | 99.1 | 617 | 341 | 2.8 | 2.1 |
| Raji | 99.1 | 99.2 | 1358 | 670 | 1.7 | 2.4 |
| Namalwa | 54.0 | 47.4 | 93 | 121 | 400 | 430 |
| Namalwa/MDR1 | 65.3 | 39.3 | 108 | 99 | 670 | 730 |
| Jurkat | 5.3 | 4.5 | 16 | 37 | — | — |
| Cells . | % Positive . | MFI . | Relative binding affinity (×10−10 M) . | |||
|---|---|---|---|---|---|---|
| Monomer . | Dimer . | Monomer . | Dimer . | Monomer . | Dimer . | |
| Daudi | 93.7 | 91.5 | 750 | 452 | 4.3 | 1.5 |
| DHL-4 | 99.8 | 99.6 | 467 | 545 | 0.5 | 1.5 |
| Ramos | 97.7 | 99.1 | 617 | 341 | 2.8 | 2.1 |
| Raji | 99.1 | 99.2 | 1358 | 670 | 1.7 | 2.4 |
| Namalwa | 54.0 | 47.4 | 93 | 121 | 400 | 430 |
| Namalwa/MDR1 | 65.3 | 39.3 | 108 | 99 | 670 | 730 |
| Jurkat | 5.3 | 4.5 | 16 | 37 | — | — |
Data are based on 1 experiment of 2. Cells were stained with 10 μg/mL per 106 cells fluorescein isothiocyanate (FITC)-Rituxan (ie, saturating conditions) and analyzed by FACScan. Both FITC-Rituxan monomers and homodimers were titrated on different cell lines, and then the percentage of positive cells was plotted vs the monoclonal antibody concentration to determine the concentration necessary to reach 50% saturation of cells. This value represents the relative binding affinity.
MFI indicates mean fluorescence intensity.
Binding of Rituxan and FITC-Rituxan monomers, homodimers, and trimers to various cell lines and their dissociation from cell lines.
(A) Binding of FITC-Rituxan monomers to different cell lines: Daudi (●); DHL-4 (○); Ramos (▾); Raji (▿); Namalwa (▪); and Namalwa/MDR1 (■). (B) Binding of Rituxan monomers (●), homodimers (○), and trimers (▾) to Ramos cells. In this procedure, 1 × 106 cells per milliliter were incubated with different concentrations of mAbs (0.1 to 100 μg/mL) for 30 minutes on ice; then excess mAbs were washed out, and cells were treated with FITC-GAMIg for 20 minutes on ice, washed, and analyzed in FACScan. The percentage of positive cells was determined for each mAb, and values were plotted against the concentration of mAbs. The amount of mAb needed to saturate 50% of cells was determined and used to compare the relative avidity of monomers, homodimers, and trimers. (C) Dissociation of FITC-Rituxan monomers (●), homodimers (○), and trimers (▾) from Ramos cells. Cells at 1 × 106/mL were treated with 10 μg/mL (a saturating concentration) of FITC-Rituxan monomers, homodimers, or trimers on ice for 30 minutes, then washed, and analyzed by FACScan. The percentage of positive cells was determined at this point, which was considered time 0. Cells were incubated at 37°C for 2, 4, 6, 24, 48, and 72 hours, and at each time point, samples of cells were taken, washed, and analyzed by FACScan for FITC-positive cells. The results were plotted against time. This is 1 of 2 experiments carried out.
Binding of Rituxan and FITC-Rituxan monomers, homodimers, and trimers to various cell lines and their dissociation from cell lines.
(A) Binding of FITC-Rituxan monomers to different cell lines: Daudi (●); DHL-4 (○); Ramos (▾); Raji (▿); Namalwa (▪); and Namalwa/MDR1 (■). (B) Binding of Rituxan monomers (●), homodimers (○), and trimers (▾) to Ramos cells. In this procedure, 1 × 106 cells per milliliter were incubated with different concentrations of mAbs (0.1 to 100 μg/mL) for 30 minutes on ice; then excess mAbs were washed out, and cells were treated with FITC-GAMIg for 20 minutes on ice, washed, and analyzed in FACScan. The percentage of positive cells was determined for each mAb, and values were plotted against the concentration of mAbs. The amount of mAb needed to saturate 50% of cells was determined and used to compare the relative avidity of monomers, homodimers, and trimers. (C) Dissociation of FITC-Rituxan monomers (●), homodimers (○), and trimers (▾) from Ramos cells. Cells at 1 × 106/mL were treated with 10 μg/mL (a saturating concentration) of FITC-Rituxan monomers, homodimers, or trimers on ice for 30 minutes, then washed, and analyzed by FACScan. The percentage of positive cells was determined at this point, which was considered time 0. Cells were incubated at 37°C for 2, 4, 6, 24, 48, and 72 hours, and at each time point, samples of cells were taken, washed, and analyzed by FACScan for FITC-positive cells. The results were plotted against time. This is 1 of 2 experiments carried out.
Dissociation of Rituxan from cells.
Dissociation experiments were performed by means of a direct immunofluorescence assay in which the DHL-4 or Ramos cells were saturated with FITC-mAbs and the positively stained cells were quantified after different time intervals in culture. As shown in Figure 2C, the monomers dissociated much more rapidly than the homodimers (T1/2 of 2 vs more than 72 hours), and the trimers showed no dissociation even after 72 hours. The increased rate of dissociation as the valency of the mAb increased indicates a stronger and more prolonged interaction with the target cells. These results support the previously reported finding that CD20 does not internalize.21 22
Taken together, these experiments demonstrate that homodimers and trimers have similar binding affinity but a slower rate of dissociation than monomers.
Growth inhibition by monomers, homodimers, and trimers
Growth index.
Several different lymphoma cell lines were cultured with different amounts of Rituxan monomers or homodimers for 6 days. At different time intervals, the cells were counted, and their growth indexes and viabilities were compared with those of untreated cells. As shown in Figure 3 the growth indexes of cells treated with either monomers or homodimers (either intact IgG or as F(ab′)2) were lower than those of untreated cells. DHL-4 and Ramos cells treated with homodimers grew more slowly than those treated with monomers, demonstrating that the antigrowth activity of Rituxan was increased by using it in homodimeric form. The F(ab′)2 homodimers also had significant antigrowth activity, suggesting that interaction with cellular FcγRII (CD32) is not involved in this growth inhibition (Figure 3). In addition, since Ramos cells lack CD3223 and their growth was inhibited, the presence of CD32 on target cells is not required for growth inhibition in vitro. We also demonstrated that the binding of FITC-Rituxan (monomer or dimer) to CD20+ cells (eg, Ramos, DHL-4) was not impaired by blocking CD32 with specific antibodies prior to adding Rituxan (data not shown). Rituxan had no effect on the growth of CD20−MOLT-4 or Jurkat cells (data not shown).
The inhibition of cell growth by Rituxan monomers and homodimers.
DHL-4 cells (2 × 105/mL) were cultured in CM in the presence of different concentrations (1 to 100 μg/mL) of Rituxan monomers (●), IgG homodimers (○), or F(ab′)2 homodimers (▾) or in CM (▿) (control) for 6 days, and the growth was compared with that of untreated cells. Samples of cells were taken daily and counted. The viability was determined by trypan blue exclusion. Cell concentrations were plotted against time in culture, and the growth index was determined. This is 1 of 2 experiments carried out.
The inhibition of cell growth by Rituxan monomers and homodimers.
DHL-4 cells (2 × 105/mL) were cultured in CM in the presence of different concentrations (1 to 100 μg/mL) of Rituxan monomers (●), IgG homodimers (○), or F(ab′)2 homodimers (▾) or in CM (▿) (control) for 6 days, and the growth was compared with that of untreated cells. Samples of cells were taken daily and counted. The viability was determined by trypan blue exclusion. Cell concentrations were plotted against time in culture, and the growth index was determined. This is 1 of 2 experiments carried out.
Early apoptotic events induced by homodimers versus monomers.
The FITC–Annexin V assay demonstrated that monomers of Rituxan did not induce apoptosis unless cell-bound mAbs were cross-linked with a secondary antibody (GAMIg) (Table 2). Annexin V positivity was achieved after incubating cells with Rituxan monomers cross-linked with GAMIg at 37°C for 24 hours. A comparable effect was observed when Ramos cells were treated for 24 hours with homodimers or with monomers and GAMIgM (Table 2). The positive control, sodium azide, induced a very profound apoptotic effect after 24 hours. The apoptotic activity of Rituxan homodimers on different B-cell lines is shown in Table 3. Apoptosis was completely inhibited by the irreversible caspase inhibitors Z-VAD-FMK and Z-DEVD-FMK and partially inhibited by the reversible inhibitor (Ac-YVAD-CHO) of PARP cleavage (Figure4).
The apoptotic effect of Rituxan on Ramos cells
| Treatment . | Apoptosis (%)* . | Necrosis (%)† . |
|---|---|---|
| Control | 3.5 ± 2.5 | 5.0 ± 2.3 |
| Rituxan monomer | 6.2 ± 1.5 | 6.0 ± 2.2 |
| Rituxan monomer + GAMIg | 32.2 ± 6.8 | 9.5 ± 0.7 |
| Rituxan homodimer | 33.5 ± 3.9 | 8.2 ± 0.3 |
| GAHIgM | 33.7 ± 7.8 | 9.6 ± 3.2 |
| NaN3 | 85.4 ± 6.4 | 10.4 ± 5.6 |
| Treatment . | Apoptosis (%)* . | Necrosis (%)† . |
|---|---|---|
| Control | 3.5 ± 2.5 | 5.0 ± 2.3 |
| Rituxan monomer | 6.2 ± 1.5 | 6.0 ± 2.2 |
| Rituxan monomer + GAMIg | 32.2 ± 6.8 | 9.5 ± 0.7 |
| Rituxan homodimer | 33.5 ± 3.9 | 8.2 ± 0.3 |
| GAHIgM | 33.7 ± 7.8 | 9.6 ± 3.2 |
| NaN3 | 85.4 ± 6.4 | 10.4 ± 5.6 |
Cells (2 × 105/mL) were incubated overnight at 37°C with Rituxan monomer (10 μg/mL) alone or cross-linked by 50 μg/mL GAMIg, Rituxan homodimers (10 μg/mL), GAMIgM (20 μg/mL), or NaN3 (5 mg/mL), and then evaluated in the annexin V assay.
GAMIg indicates goat antimouse immunoglobulin; GAHIg, goat antihuman immunoglobulin M.
As determined by means of the Annexin V staining assay in 3 to 5 experiments.
As determined by propidium iodide staining in 3 to 5 experiments.
Cytotoxic vs apoptotic effect of Rituxan homodimers on different cell lines
| Cells . | IC50 (×10−7 M)3-150 . | % Apoptotic cells3-151 . |
|---|---|---|
| Daudi | 6.0 ± 2.7 | 32.2 |
| Raji | 3.4 ± 2.0 | 19.5 |
| Ramos | 0.23 ± 0.2 | 32.3 |
| DHL-4 | 4.8 ± 1.0 | 30.3 |
| Namalwa | 2.2 ± 1.0 | 23.9 |
| Namalwa/MDR-1 | 3.0 ± 1.0 | 25.7 |
| Jurkat | >10 | 0.6 |
| Cells . | IC50 (×10−7 M)3-150 . | % Apoptotic cells3-151 . |
|---|---|---|
| Daudi | 6.0 ± 2.7 | 32.2 |
| Raji | 3.4 ± 2.0 | 19.5 |
| Ramos | 0.23 ± 0.2 | 32.3 |
| DHL-4 | 4.8 ± 1.0 | 30.3 |
| Namalwa | 2.2 ± 1.0 | 23.9 |
| Namalwa/MDR-1 | 3.0 ± 1.0 | 25.7 |
| Jurkat | >10 | 0.6 |
Cells were incubated for 24 hours at 37° with 1 to 100 μg Rituxan and pulsed with 3H-thymidine for 4 hours. IC50 values represent the concentration of mAbs necessary to kill 50% of cells (3 to 4 experiments carried out).
Cells were incubated for 24 hours at 37° with 10 μg Rituxan and then stained with FITC–Annexin V and propridium iodide. Data represent 1 experiment of 2 carried out.
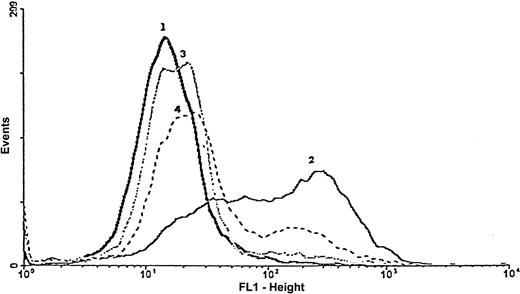
The inhibition of FITC–Annexin V staining by caspase inhibitors.
The apoptotic effect induced by Rituxan homodimers (peak 2) and inhibition by caspase inhibitors Z-VAD-FMK (peak 3) and Ac-YVAD-CHO (peak 4) were determined by means of the FITC–Annexin V assay. Cells at 2 × 105/mL were treated at 37°C with Rituxan homodimers (10 μg/mL) for 24 hours in the presence or absence of caspase inhibitors (50 μM), washed, and then treated with FITC–Annexin V for 15 minutes. Cells were washed again; PI was added; and the cells were then analyzed by FACScan. This is 1 representative experiment of 3.
The inhibition of FITC–Annexin V staining by caspase inhibitors.
The apoptotic effect induced by Rituxan homodimers (peak 2) and inhibition by caspase inhibitors Z-VAD-FMK (peak 3) and Ac-YVAD-CHO (peak 4) were determined by means of the FITC–Annexin V assay. Cells at 2 × 105/mL were treated at 37°C with Rituxan homodimers (10 μg/mL) for 24 hours in the presence or absence of caspase inhibitors (50 μM), washed, and then treated with FITC–Annexin V for 15 minutes. Cells were washed again; PI was added; and the cells were then analyzed by FACScan. This is 1 representative experiment of 3.
Inhibition of 3H-thymidine incorporation.
The Rituxan monomers, homodimers, and trimers were incubated for different intervals of time with Ramos, DHL-4, Daudi, Raji, Namalwa, and Namalwa/MDR1 cells, and the levels of 3H-thymidine incorporation were measured and compared with those of untreated cells (Table 3). CD20−MOLT-4 cells were used as negative control cells. The Rituxan monomers did not inhibit 3H-thymidine incorporation in any of the cell lines tested, even at high concentrations (greater than 10−6 M). Both the homodimers and trimers reduced 3H-thymidine incorporation in a dose-dependent manner. Neither the irrelevant 3F12 mAb nor the human IgG had cytotoxic activity in any form (data not shown). The same assay was used to determine the cytotoxic activity of Rituxan in combination with doxorubicin as described below.
Cytotoxic effect of combinations of mAbs and doxorubicin
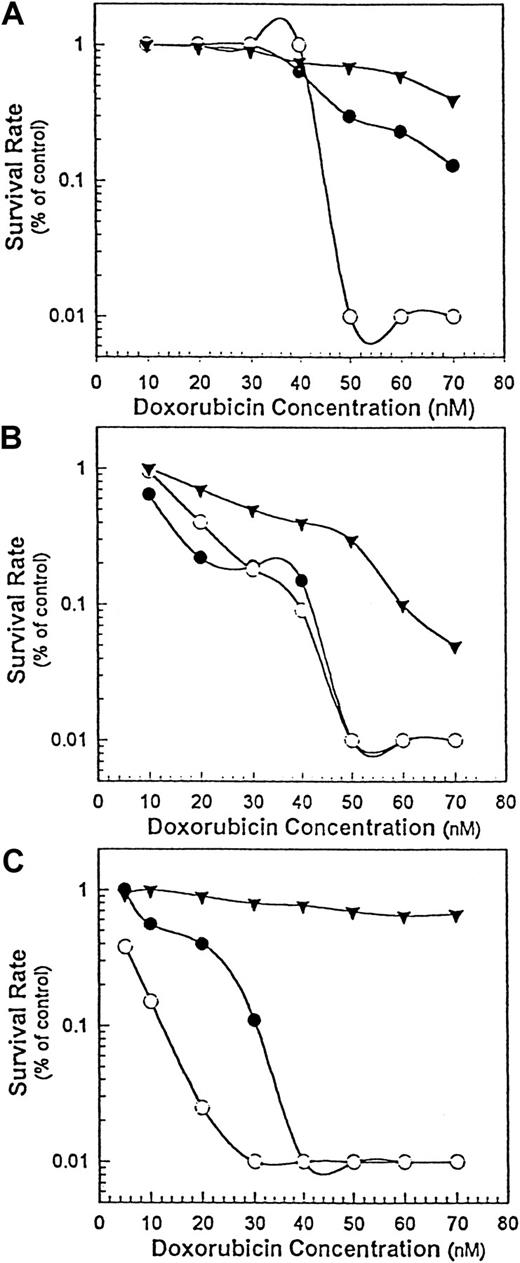
We used 3 cell lines with different sensitivities to doxorubicin: DHL-4, Namalwa, and Namalwa/MDR1. When treated with doxorubicin alone, the IC50 for drug-sensitive Namalwa cells was 3- to 4-fold lower than that of either drug-resistant Namalwa/MDR1 cells (Figure5C) or the less drug-sensitive DHL-4 cells (Figure 5B). When Rituxan monomers (20 μg/mL) were added to different concentrations of doxorubicin (1 to 70 nM), their IC50 on DHL-4 (Figure 5A) or Namalwa/MDR1 (Figure 5C) cells decreased in a dose-dependent manner, suggesting that both the drug-resistant Namalwa/MDR1 cells and the DHL-4 cells became more sensitive to the cytotoxic effect of doxorubicin. When added to the same amount of homodimer (at a noncytotoxic concentration), doxorubicin became even more toxic for DHL-4 (Figure 5A) and Namalwa/MDR1 (Figure5C) cells. In contrast, both homodimers and monomers increased the sensitivity of Namalwa cells to doxorubicin to the same extent (Figure5B), suggesting that the Rituxan homodimers are most advantageous when cells are insensitive to doxorubicin.
The chemosensitization of B-lymphoma cells by Rituxan homodimers.
The cytotoxic effect of doxorubicin alone (▾) or doxorubicin in combination with Rituxan monomers (●) or homodimers (○) at 20 μg/mL was determined by means of 3 cell lines: DHL-4 (panel A), Namalwa (panel B), and Namalwa/MDR1 (panel C). Cells at 5 × 104 cells per well in 100 μL were plated in 96-well plates, and various concentrations of doxorubicin (1 to 70 nM) alone or in combination with a constant noncytotoxic concentration of Rituxan (20 μg/mL) were added in 100 μL media in triplicate wells. The plates were incubated for 24 hours, pulsed with3H-thymidine, and then harvested. Rituxan alone (data not shown) had no effect. This is 1 of 2 experiments carried out.
The chemosensitization of B-lymphoma cells by Rituxan homodimers.
The cytotoxic effect of doxorubicin alone (▾) or doxorubicin in combination with Rituxan monomers (●) or homodimers (○) at 20 μg/mL was determined by means of 3 cell lines: DHL-4 (panel A), Namalwa (panel B), and Namalwa/MDR1 (panel C). Cells at 5 × 104 cells per well in 100 μL were plated in 96-well plates, and various concentrations of doxorubicin (1 to 70 nM) alone or in combination with a constant noncytotoxic concentration of Rituxan (20 μg/mL) were added in 100 μL media in triplicate wells. The plates were incubated for 24 hours, pulsed with3H-thymidine, and then harvested. Rituxan alone (data not shown) had no effect. This is 1 of 2 experiments carried out.
Synergistic effect of Rituxan and RFB4-dgA
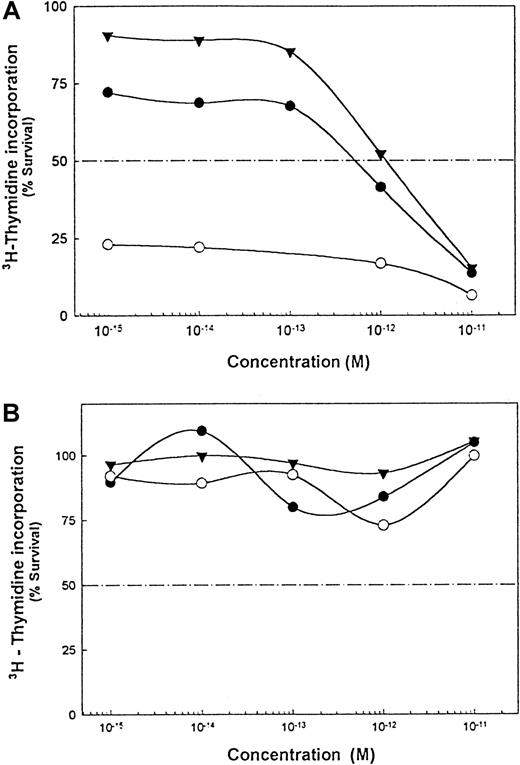
The IT RFB4-dgRTA kills human B-lymphoma cells in vitro and in vivo.24 25 In the presence of a noncytotoxic concentration of Rituxan monomers or homodimers, the IC50 of the IT decreased by 3-fold and greater than 1000-fold, respectively (Figure6A). RFB4-mAbs plus either Rituxan monomers or homodimers had no effect on the cells (Figure 6B).
Effect of Rituxan homodimers on sensitivity of DHL-4 and Namalwa/MDR1 cells to RFB4-dgRTA.
(A) Cytotoxic effect of RFB4-dgRTA alone (▾) or IT in combination with Rituxan monomers (●) or homodimers (○). (B) Noncytotoxic effect of RFB4 antibody alone (▾) or in combination with Rituxan monomers (●) or homodimers (○). The protocol described in Figure 5was used here. This is 1 of 2 experiments carried out.
Effect of Rituxan homodimers on sensitivity of DHL-4 and Namalwa/MDR1 cells to RFB4-dgRTA.
(A) Cytotoxic effect of RFB4-dgRTA alone (▾) or IT in combination with Rituxan monomers (●) or homodimers (○). (B) Noncytotoxic effect of RFB4 antibody alone (▾) or in combination with Rituxan monomers (●) or homodimers (○). The protocol described in Figure 5was used here. This is 1 of 2 experiments carried out.
Discussion
Monoclonal antibodies that exert antitumor activity may do so by virtue of their effector function (CDC and ADCC)26-28 and/or of their ability to signal growth arrest or cell death.29 In this study, we have evaluated the negative signaling activity of the mAb Rituxan, a chimeric antihuman CD20 mAb that has excellent activity in humans with lymphoma. Previous in vitro studies have shown that Rituxan arrests cell growth most effectively in vitro following cross-linking with a secondary anti-immunoglobulin antibody or with CD32-expressing cells.11 To take advantage of these observations, we have generated mAb homodimers (tetravalent antibody molecules) of Rituxan in order to optimize its ability to hypercrosslink. In general, homodimers have higher avidity, cross-link their target antigen more efficiently, and dissociate from cells more slowly.1,2 Previously, Wolff et al2 and Caron et al30 reported that homodimers had more potent antitumor activity than monomers, and we reported that mAbs that have little or no negative signaling activity can become potent antitumor agents when they are converted to homodimers.1 In vitro, some homodimers can exert antigrowth activity by signaling Go/G1 arrest and/or apoptosis, depending upon which cell surface molecule they bind.1 In some cases F(ab′)2 homodimers work as well as, or better than, IgG homodimers in vitro.1
Rituxan has exhibited in vitro antigrowth activity on Ramos, DHL-4, and Raji cells, but apoptosis has been demonstrated only in Ramos and DHL-4 cells.7-11 It has also been shown that exposure of some, but not all, cell lines to anti-CD20 mAbs induces tyrosine phosphorylation.7
Our studies were designed to determine whether homodimers of Rituxan have better antigrowth activity than monomers. The major findings to emerge are the following: (1) homodimers are far more effective than monomers (and as effective as monomers plus GAMIg) in inducing growth arrest; (2) homodimers but not monomers induce both apoptosis and necrosis, and these effects are not dependent upon the presence of CD32 on the tumor cells; (3) the apoptotic effect of homodimers is inhibited by the caspase inhibitors (Z-VAD-FMK and Z-DEVD-FMK); (4) as compared with monomers, homodimers of Rituxan show decreased dissociation from, CD20+ cells; (5) the inhibitory activity of Rituxan on different cell lines does not correlate with their density of CD20; (6) Rituxan homodimers show marked synergy with an anti-CD22 IT in vitro; (7) Rituxan homodimers chemosensitize doxorubicin-resistant or insensitive tumor cells more effectively than monomers in vitro.
Taken together with the results of other studies, the results presented here suggest that Rituxan homodimers might have better antitumor activity in vivo by virtue of enhanced negative signaling. This may, of course, be the case only if homodimers cross-link better in vivo where FcR+ cells are present.
With regard to the mechanism of action of Rituxan in vivo, it has been clearly demonstrated that its FC portion and effector functions play major roles in its therapeutic efficacy in genetically engineered mice.30 Whether the effector function of homodimers will be enhanced in vivo remains to be determined. Hence, some mAb homodimers show improved ADCC and CMC30 whereas others do not.2 In an in vivo setting, an improvement may depend upon half-life and the binding of homodimers vs monomers of a particular mAb to effector cells.
Regardless of whether homodimers will outperform monomers in vivo by virtue of enhanced negative signaling, effector function, or both, another way to improve the activity of Rituxan is to use it in combination with other antitumor agents. For this reason, we also tested Rituxan in combination with ITs and chemotherapy in vitro. With regard to ITs, we have previously reported that an anti-CD19 mAb (HD37) acts synergistically with the anti-CD22 IT RFB4-dgRTA in SCID mice with human lymphoma.24 In this study, we demonstrate that both Rituxan monomers and homodimers can increase the cytotoxic effect of RFB4-dgRTA but not an RFB4 mAb in vitro and that homodimers were more effective than monomers. This finding suggests that Rituxan homodimers plus ITs should be considered for further in vivo development. Our preliminary data in SCID mice with human lymphomas support this hypothesis.
Combination therapy with Rituxan and chemotherapeutic agents (eg, CHOP) has been evaluated in patients with indolent B-cell lymphoma; a 95% overall response (55%, complete remissions; 40%, partial remissions) was achieved6; it is too early to predict what impact this will have on long-term survival. In a previous in vitro study, it was shown that Rituxan sensitized a B-lymphoma cell line to several cytotoxic drugs.8 In the present study, we demonstrate that both monomers and homodimers of Rituxan can potentiate the in vitro effect of doxorubicin on partially resistant or MDR+tumor cells, but that homodimers are much more effective. The fact that Rituxan can render MDR+ cells sensitive to chemotherapy is in accord with our previous finding that an anti-CD19 mAb rendered MDR+ cells (P-gp+) sensitive to chemotherapeutic drugs in vitro.31 Taken together, these findings suggest exploring a strategy using homodimers followed by chemotherapy.
In conclusion, Rituxan homodimers are in all respects superior to monomers in the in vitro killing of the CD20+ cell lines used in these studies, and this superiority can be attributed to enhanced negative signaling by Rituxan. Homodimers might also have improved effector function, but this remains to be tested. Finally, homodimers were also highly effective in synergizing with an IT and in chemosensitizing cells to doxorubicin, suggesting that they might be useful for the treatment of patients with MDR+ lymphomas. What remains to be determined is whether homodimers will also show improved antitumor activity in vivo.
The authors thank Drs J. Uhr and V. Ghetie for their critical reviews of this work, and Ms S. Flowers, Ms T. Bowdler, and Ms R. Lewis for secretarial assistance.
Supported by National Institutes of Health grant CA-64679.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ellen S. Vitetta, Cancer Immunobiology Center, UT Southwestern Medical Center at Dallas, 6000 Harry Hines Blvd, NB9.210, Dallas, TX 75390-8576; e-mail: ellen.vitetta@utsouthwestern.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal