The Philadelphia (Ph) chromosome is found in approximately 3% of pediatric patients with acute lymphoblastic leukemia (ALL) and the percentage markedly increases in adult patients. The prognosis for this class of patients is poor, and no standard chemotherapy combination so far has demonstrated long-term efficacy. The Ph-translocation joins theBCR and ABL genes and leads to expression of a chimeric Bcr/Abl protein with enhanced tyrosine kinase activity. This increase in activity leads to malignant transformation by interference with basic cellular functions such as the control of proliferation, adherence to stroma and extracellular matrix, and apoptosis. One important pathway activated by Bcr/Abl is the Ras pathway. Ras proteins have to undergo a series of posttranslational modifications to become biologically active. The first modification is the farnesylation of the C-terminus catalyzed by farnesyl transferase. We studied the effect of the farnesyl transferase inhibitor SCH66336 in an in vivo murine model of Bcr/Abl-positive acute lymphoblastic leukemia. In the early leukemic phase, mice were randomly assigned to a treatment, a vehicle, and a nontreatment group. The treatment was well tolerated without any detectable side effects. All animals of the control groups died of leukemia/lymphoma within 103 days (range, 18-103 days). In contrast, 80% of the drug-receiving group survived without any signs of leukemia or lymphoma until termination of treatment, after a median treatment period of 200 days (range, 179-232 days). We conclude that farnesyl transferase inhibitor SCH66336 is able to revert early signs of leukemia and significantly prolongs survival in a murine ALL model.
Introduction
The Philadelphia (Ph) chromosome is the usual finding in chronic myeloid leukemia (CML). In acute lymphoblastic leukemia (ALL), it is detectable only in a subgroup of patients, and it confers a poor prognosis. It is found in approximately 1% to 3% of pediatric ALL patients, and the percentage markedly increases in adult patients, among whom up to 25% show this reciprocal translocation. Although these patients can achieve remission with almost the same frequency as Ph-chromosome–negative patients, no standard chemotherapy combination so far has demonstrated long-term efficacy.1Allogeneic stem cell transplantation is the only postremission therapy with demonstrated long-term disease-free survival. Thirty percent of patients who have an HLA-matched donor show prolonged disease-free survival after allogeneic stem cell transplantation.2
The Ph-translocation fuses the 5′-exons of the BCRgene to 3′-exons of the ABL gene. Depending on the localization of the breakpoint within the BCR gene, a chimeric P210 or P190 Bcr/Abl fusion protein is generated. Most Ph-positive-ALL patients express a P190 protein, whereas the P210 is commonly found in CML. In comparison with the normal 145-kd c-Abl product, these fusion proteins show enhanced tyrosine kinase activity, which leads to malignant transformation by interference with basic cellular processes, such as control of proliferation, adherence, and apoptosis. One important pathway activated by Bcr/Abl is the Ras pathway. It has been shown that inhibition of Ras activation in cells transfected with BCR/ABL prevents transformation.3-5
To become biologically active, Ras proteins have to undergo a series of posttranslational modifications. These modifications are necessary for the correct subcellular localization of Ras to the plasma membrane.6 First, a 15-carbon isoprenyl (farnesyl) group is added to a conserved cysteine residue in the carboxyterminal CAAX motive (C, cysteine; A, aliphatic amino acid; X, any other amino acid). The formation of a stable thioether bond is catalyzed by the enzyme farnesyl-protein transferase (FPT). Prenylated proteins associate with the endomembrane and are subsequently transported to the plasma membrane.7 The farnesylation is essential for the transforming ability of Ras. Unfarnesylated Ras remains in the cytosol and does not exhibit transforming activities.8
During the last decade, selective inhibitors of the farnesyl transferase (FTIs) have become available. They have demonstrated antitumor effects in several in vivo and in vitro systems and some are currently being tested in phase I and II studies in human patients with solid tumors.9 Initially, it was thought that the antiproliferative ability of FTIs was solely due to the reduced farnesylation of Ras and, consequently, prevention of its membrane localization,10 but it now appears that other mechanisms, such as inhibition of farnesylation of currently unknown proteins or the gain of geranylgeranylated RhoB are also involved.11
A line of mice transgenic for BCR/ABL P190 has been previously generated, which consistently develop pre–B-cell leukemia.12-14 The disease in these mice closely resembles human Ph-positive ALL, making the mouse model suitable for testing new treatment strategies.15 In this study, we have analyzed the effects of treatment with farnesyl transferase inhibitor SCH6633616,17 on disease evolution in this line of transgenic mice.
Materials and methods
Mice and treatment
All animal studies were carried out at the Animal Care Facility of the Research Institute of Childrens Hospital Los Angeles in accordance with institutional guidelines. Animals were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Blood samples of 10 μL were withdrawn from the tail artery of each mouse every 14 days. At the first detection ofBCR/ABL expression in peripheral blood (PB), the mice were randomly assigned to an untreated control group, a vehicle control group, or the SCH66336 treatment group. SCH66336 is an orally available, nonpeptidic tricyclic FTI. It displays excellent pharmacokinetics and is specific for the inhibition of farnesyl transferase. A phase I trial in 20 patients with advanced solid tumors established a tolerable toxicity profile at a dose of 350 mg twice a day and gave first evidence of clinical activity of this drug.18 Phase II trials are currently in progress.
Drug treatment with 40 mg SCH66336 per kilogram body weight twice a day was initiated on day 1. This regimen was based on pharmacokinetic studies in mice16 and the phase I trial conducted with this compound.18 SCH66336 was dissolved in 20% hydroxypropyl-β-cyclodextrin (wt/vol). Vehicle controls received 20% hydroxypropyl-β-cyclodextrin. The 100 μL vehicle or drug solution was administered by oral gavage19 twice a day. A nontreatment control was always included with the vehicle control to evaluate the influence of the gavage treatment. The animals were monitored daily for general health and signs of lymphoma. Animals were killed when terminally ill. An autopsy was carried out, and macroscopic as well as microscopic analysis of pathologic findings performed. Tissues were fixed in buffered formalin, sectioned, and stained with hematoxylin and eosin. Peripheral blood films were stained with Wright-Giemsa.
Reverse transcriptase-polymerase chain reaction forBCR/ABL and bcr in peripheral blood
To detect BCR/ABL expression in peripheral blood, reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as described previously12 on a small sample of blood withdrawn from the tail artery. To ensure all RNAs were suitable for RT-PCR, we used primers (forward primer: 5′ CGC ATG TTC CGG GAC AAA AGC, reverse primer: 5′ CCA TTT TCT CAT CTC CAA GCC) to amplify a segment of the endogenous bcr messenger RNA (mRNA). This is an appropriate control because the sizes of bcr mRNA and P190 BCR/ABL are similar (around 7 kilobases [kb]) and both are rare mRNAs. In addition, all samples were tested for suitability for RT-PCR using actin amplimers. Samples were run on agarose gels.
Quantitative polymerase chain reaction
To generate a competitor, a 350-base pair (bp) RT-PCR product from PB of a leukemic P190 mouse made with oligonucleotides ALL-E and ALL-F was subcloned in pSK. The PCR product spans theBCR/ABL junction in the mRNA encoding P190. This plasmid was digested with Bsu36l and NcoI, the small fragment removed and the plasmid religated. ALL-E and ALL-F primers generate a 260-bp fragment from this plasmid. The DNA concentration of the plasmid was determined by measurement of OD260 and by electrophoresis of aliquots of DNA on an agarose gel, together with samples of known concentration. Serial dilutions were made so that 2 μL solution would contain an absolute number of competitor molecules in a logarithmic range from 104 to 108molecules. Reverse transcriptase and polymerase chain reactions were performed as described previously13 by using 0.5 μg total bone marrow RNA per reaction. Competitor was added before the PCR step. Total RNA was isolated with TRIazol, (Life Technologies, Grand Island, NY) according to the manufacturer′s recommendations.
FACS analysis
We isolated bone marrow from the tibia and femur. The cells were analyzed with double-color flow cytometry on a FACScan (Becton Dickinson) as described.18 Monoclonal antibodies were anti-CD45R, anti-Thy1.2, anti-GR1 from PharMingen, San Diego, CA, and anti-Mac1 from Caltag, Burlingame, CA.
Statistical analysis
For statistical analysis, we compared the days from the start of treatment ( = detection of expression of BCR/ABL) until death or until the end of treatment for the vehicle control group and the SCH66336 receiving group, respectively. In survival analysis, aP value under .05 was designated significant in the log-rank test.
Results
Start of treatment
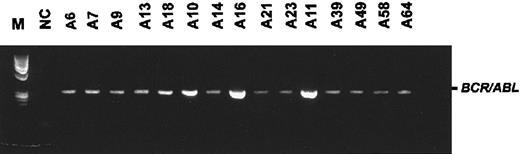
All mice included in this study were homozygous carriers of a P190-type BCR/ABL transgene12 and were derived from a single line of mice, no. 623, which has been characterized extensively.13,14,20,21 Mice initially have no detectableBCR/ABL-expressing cells in their PB. As they grow older, such cells can be detected in the PB, and this heralds the onset of overt disease.13 In the bone marrow, BCR/ABLexpression is always detectable with RT-PCR. Fifteen mice transgenic for BCR/ABL were included in the study on the day expression of BCR/ABL was detected for the first time in PB. As determined by RT-PCR (Figure 1), all animals started expressing BCR/ABL in PB between 128 and 262 days. We randomly assigned the mice to the 3 treatment modalities. The average age in each group (n = 5 mice) was 165 days for the nontreatment (range, 128-262 days) and gavage treatment (range, 131-207 days) and 157 days for the SCH66336 treatment group (range, 134-184 days). At this time, the animals had normal white blood cell (WBC) counts and no signs of lymphoma.
First detection of
BCR/ABL transcripts in PB by using RT-PCR.Animals were detected as positive at an average age of 163 days (range, 128-262 days). The figure shows the first positive PCR for each mouse included in the study. M, φX/HaeIII marker; NC, negative control. The numbers above the lanes designate an individual animal.
First detection of
BCR/ABL transcripts in PB by using RT-PCR.Animals were detected as positive at an average age of 163 days (range, 128-262 days). The figure shows the first positive PCR for each mouse included in the study. M, φX/HaeIII marker; NC, negative control. The numbers above the lanes designate an individual animal.
No apparent side effects of treatment with SCH66336
The gavage treatment given twice a day with 40 mg SCH66336 per kilogram body weight or with the vehicle was well tolerated, and no difference in appearance or behavior between control and treated mice was apparent. The mice receiving gavage treatment twice a day showed a 10% weight loss in comparison to the untreated mice during the first 14 days, but later no difference in weight gain among the 3 groups was seen.
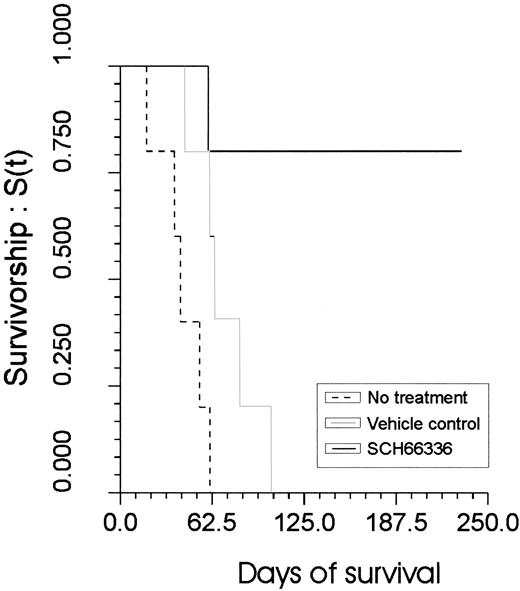
SCH66336 prolonged survival
The survival in the drug-receiving group was significantly better (P = .03), when compared with the gavage control group (Figure 2). All control animals had leukemia/lymphoma develop in a time span between 18 to 103 days (average, 56 days). Necropsy revealed enlarged lymph nodes and spleen and infiltration of the bone marrow (Figure3), surrounding tissues, and various organs with leukemic blasts. Leukemic cells were also detectable in PB of all control animals. One SCH66336-treated mouse also had leukemia/lymphoma develop and died after 60 days of treatment. The other 4 mice of the treatment group survived without any signs of disease.
SCH66336-treated mice survive significantly longer in comparison to the control groups.
On the y-axis, the relative survivorship is shown. On thex-axis, the time of survival is indicated, with the first day of treatment designated as day 0.
SCH66336-treated mice survive significantly longer in comparison to the control groups.
On the y-axis, the relative survivorship is shown. On thex-axis, the time of survival is indicated, with the first day of treatment designated as day 0.
Leukemia/lymphoma in control group.
A sagittal section is shown of the sternum of mouse A21. This mouse was treated for 64 days with the vehicle and then killed when terminally ill. A homogeneous infiltration of the bone marrow and the surrounding muscular tissue is seen with intermediate-sized lymphoid cells with round nuclei, large central nucleolus, immature chromatin pattern, and a scant rim of cytoplasm representing pre-B leukemic cells.
Leukemia/lymphoma in control group.
A sagittal section is shown of the sternum of mouse A21. This mouse was treated for 64 days with the vehicle and then killed when terminally ill. A homogeneous infiltration of the bone marrow and the surrounding muscular tissue is seen with intermediate-sized lymphoid cells with round nuclei, large central nucleolus, immature chromatin pattern, and a scant rim of cytoplasm representing pre-B leukemic cells.
After termination of treatment, no evidence of leukemia/lymphoma was found in surviving SCH66336-treated mice
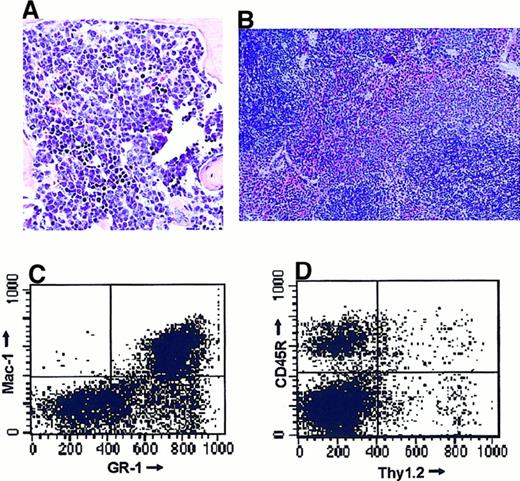
We treated 2 mice for 179, one for 220, and one for 232 days. On the last day of treatment, we repeated the RT-PCR of PB and at this time no fusion transcripts were found (Figure4, bottom panel), whereas the control RT-PCR for bcr showed positive signals for each sample (Figure 4, top panel). All animals had normal WBC counts. We randomly assigned one mouse (A39) of the 4 surviving mice on the last day of treatment and killed it to determine the composition of hematopoietic and lymphopoietic tissues and to detect possible side effects of the treatment in internal organs. At the autopsy, we found normal-looking internal organs and no enlargement of lymphatic tissues. Histologic sections of the bone marrow (Figure 5A) showed normal erythropoiesis, thrombopoiesis, and lymphopoiesis with a slight increase of normal maturing myelopoiesis. No signs of leukemic blasts were found. This was confirmed by FACS analysis of the bone marrow cells. We found 20% lymphocytes (normal, 20%-24%), 14.3% B-lineage, 3.5% T-lineage (Figure 5C), 54% granulocytes, macrophages, and dendritic cells (normal, 40%-47%) (Figure 5D). Histologic sections of all internal organs were normal, except for a slight granulocytic infiltration in a basal area of the right lung. The spleen showed a normal architecture on a histologic level (Figure 5B). To determine the expression level of BCR/ABL transcripts in the bone marrow of this animal, we performed semiquantitative competitive RT-PCR. The bone marrow of the treated animal A39 showed the same level of expression as a young healthy P190 BCR/ABL transgenic mouse without any signs of leukemia, whereas the bone marrow of a P190BCR/ABL leukemic mouse exhibited an expression level that was 2 logarithmic levels higher (Figure6).
The 4 surviving SCH66336-treated mice did not express
BCR/ABL in PB on the last day of treatment. RNA was subjected to RT-PCR using either control bcr primers (top panel) or BCR/ABL P190 primers (bottom panel). NC, negative control; PC, positive control. The numbers above the lanes refer to the individual animals in the treatment group.
The 4 surviving SCH66336-treated mice did not express
BCR/ABL in PB on the last day of treatment. RNA was subjected to RT-PCR using either control bcr primers (top panel) or BCR/ABL P190 primers (bottom panel). NC, negative control; PC, positive control. The numbers above the lanes refer to the individual animals in the treatment group.
Surviving SCH66336-treated mice showed no signs of leukemia/lymphoma at the end of the treatment period.
After 220 days of treatment with SCH66336, one mouse (A39) was killed. (A) Sagittal section of the sternum. Note the normal maturation in the myeloid and the lymphoid lineage, a slight increase in the presence of mature granulocytes and the lack of signs of leukemic cells. (B) Transverse section of the spleen. Note the normal architecture of red and white pulp. Original magnification: panel A ×40; panel B, ×20. (C, D) FACS analysis of bone marrow cells. Bone marrow cells were isolated and stained with fluorescence-labeled antibodies against B-cell (CD45R), T-cell (Thy1.2), and myeloid (GR1, Mac1) markers. We detected a slight increase in myeloid cells, but no increase in B-lineage cells, which could indicate developing B-cell leukemia.
Surviving SCH66336-treated mice showed no signs of leukemia/lymphoma at the end of the treatment period.
After 220 days of treatment with SCH66336, one mouse (A39) was killed. (A) Sagittal section of the sternum. Note the normal maturation in the myeloid and the lymphoid lineage, a slight increase in the presence of mature granulocytes and the lack of signs of leukemic cells. (B) Transverse section of the spleen. Note the normal architecture of red and white pulp. Original magnification: panel A ×40; panel B, ×20. (C, D) FACS analysis of bone marrow cells. Bone marrow cells were isolated and stained with fluorescence-labeled antibodies against B-cell (CD45R), T-cell (Thy1.2), and myeloid (GR1, Mac1) markers. We detected a slight increase in myeloid cells, but no increase in B-lineage cells, which could indicate developing B-cell leukemia.
Treatment group mouse A39 shows
BCR/ABL transcript levels similar to those of a young preleukemic P190 transgenic mouse. Total RNA was isolated from the bone marrow of BCR/ABL transgenics, including A39, a terminally ill leukemic mouse, and a young healthy mouse. Competitive RT-PCR was performed as described in “Materials and methods.” The numbers below the lanes indicate the number of competitor molecules added to the PCR reaction. The equivalence interval was determined by comparing the intensity of the band resulting from the testerBCR/ABL with the band resulting from the competitor sample. For the leukemic mouse, the interval is between 106 and 106.5, for A39 between 104 and 104.5, and for the preleukemic mouse between 104.5 and 105.
Treatment group mouse A39 shows
BCR/ABL transcript levels similar to those of a young preleukemic P190 transgenic mouse. Total RNA was isolated from the bone marrow of BCR/ABL transgenics, including A39, a terminally ill leukemic mouse, and a young healthy mouse. Competitive RT-PCR was performed as described in “Materials and methods.” The numbers below the lanes indicate the number of competitor molecules added to the PCR reaction. The equivalence interval was determined by comparing the intensity of the band resulting from the testerBCR/ABL with the band resulting from the competitor sample. For the leukemic mouse, the interval is between 106 and 106.5, for A39 between 104 and 104.5, and for the preleukemic mouse between 104.5 and 105.
Monitoring of surviving mice after termination of treatment
Of the remaining 3 mice that had obtained prior treatment with SCH66336, 2 died of leukemia 71 and 111 days after termination of treatment. The third mouse died 136 days after termination of treatment of a huge benign ovarian tumor, which obstructed the ureters and led to kidney failure. No leukemic blasts were detected in PB and bone marrow.
Discussion
Ph+ALL is incurable using standard chemotherapy. However, the underlying genetic abnormality is known, so new treatment strategies can be based on the knowledge obtained concerning the signal transduction pathways disturbed by the constitutively active Bcr/Abl tyrosine kinase. To inhibit Bcr/Abl signaling, we have administered an FTI to mice transgenic for P190 Bcr/Abl, which develop pre–B-cell leukemia/lymphoma closely resembling human Ph+ALL. The FTI SCH66336 is a small nonpeptidic molecule with excellent oral bioavailability and pharmacokinetics. It has demonstrated potent antitumor activity on human cancer cell lines, human tumor xenograft models in nude mice, and RAS transgenic mice.16In our treatment study, we found that 80% of the mice treated with SCH66336 not only survived longer than the control mice, but they did so without any signs of leukemia and lymphoma. Therefore, the drug was very effective in our murine model and demonstrated a specific and selective effect on Bcr/Abl leukemic cells, whereas nonmalignant hematopoiesis remains unaffected.
We saw no side effects during the treatment with the drug, in particular no hematologic or gastrointestinal toxicity. An increased number of mature granulocytes was observed in the bone marrow of mouse A39, which was killed as representative for the surviving treatment group mice after a treatment period of 210 days with SCH66336. This might be due to the observed pneumonic infiltration of the right lung, perhaps as a side effect of the long gavage treatment in the sense of an aspiration pneumonia and not a direct effect of the FTI.
In an inducible transgenic mouse model for P210BCR/ABL, acute B-cell leukemia developed after induction of Bcr/Abl expression, but the leukemia was completely reversible on inhibition of oncogene expression.22 It was shown that termination of Bcr/Abl expression led to apoptosis of the leukemic cells. In our model, the disease is also completely reversible at an early stage of leukemia, indicating that inhibition of one signal transduction pathway is sufficient to tip the balance toward the reestablishment of normal hematopoiesis.
We chose to start treatment at an early phase of the disease, corresponding to an early stage of human Ph-positive ALL, at which time point the bone marrow is clearly affected but peripheral blood parameters are still normal (similar to found in early relapse). At this phase, secondary genetic changes are likely to have accumulated.14 However, only approximately 30% of Ph+ALL patients show the Ph chromosome as their sole chromosomal defect at the time of diagnosis, whereas others have already acquired additional visible genetic abnormalities. Mouse A11 died of leukemia/lymphoma after 60 days of treatment with SCH66336, in the same period the control mice developed leukemia and died. It is possible that the leukemic cells in this mouse had already acquired secondary mutations, which led to resistance against the FTI. Treatment of cancer cells with one single drug frequently results in the development of resistance. Treatment of the leukemia cell line LAMA84 with the Abl tyrosine kinase inhibitor STI571, for example, resulted in the development of cells that were resistant to the apoptosis-inducing effects of the drug. This resistance was mediated through gene amplification.23 Therefore, it has to be evaluated whether presumed inhibitors of a single signal transduction pathway, such as FTIs, are still effective in advanced forms of malignancies, and whether combination treatment would be able to overcome mechanisms of resistance development. Preliminary data suggest that the FTI may be active at least against a subset of cases of advanced disease. We recently treated one set of P190 transgenics that had developed large lymphomas with vehicle or with vehicle plus SCH66336 for 8 days as described before, after which both animals were killed. The mouse treated with vehicle was terminally ill at that point, whereas the mouse treated with the FTI showed a spectacular reduction in the size of the lymphomas.
FTIs have been shown to induce tumor regression by multiple mechanisms, including activation of suppressed apoptotic pathways.16 24 After terminating the treatment, we monitored the mice for recurrence of the leukemia. Two mice died 71 days (A49) and 111 days (A58) after the end of treatment of leukemia/lymphoma and one (A64) after 136 days of a benign ovarian tumor. This long disease-free period after termination of the treatment strongly suggests that the original malignant clone disappeared and that mice A49 and A58 had new leukemic clones develop. Therefore, the FTI seems not only to suppress the proliferation, but also may have led to the death of the leukemic cells.
Taken together, our results suggest that FTI SCH66336 can revert early signs of leukemia, inhibit leukemogenesis, and prolong survival in a murine model for Ph+ALL. In a phase I trail, SCH66336 showed an acceptable toxicity profile, and was used in one patient for long-term treatment, up to 14 months.18 Because this FTI has already undergone such phase I trials in humans, its possible use against Ph-positive ALL should be facilitated, and could constitute a valuable addition to the treatment of this high-risk type of leukemia.
We thank Leena Haataja for providing the mice, Stijn DeLanghe for assistance with computer graphics, and Harold Soucier for the FACS analysis.
Supported by National Institutes of Health grants CA47456 (J.G.) and CA50248 (N.H.), and the Kenneth T. and Eileen L. Norris Foundation for Leukemia Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John Groffen, Division of Hematology/Oncology, Ms #54, Section of Molecular Carcinogenesis, Childrens Hospital of Los Angeles Research Institute, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail: jgroffen@hsc.usc.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal