The mechanism of lymphomagenesis of hepatitis C virus (HCV)–related B-cell lymphoma is unknown. Recently, it has been suggested that HCV may induce B-cell clonal proliferation and t(14;18) translocation in patients chronically infected with the virus. Thus, this study investigated the effect of antiviral treatment on immunoglobulin heavy-chain gene (IgH) rearrangement and t(14;18) translocation in HCV infected patients. Twenty-nine patients with chronic HCV infection were studied in whom IgH rearrangement and/or t(14;18) translocation were previously detected. The IgH rearrangement (FR3/JH) and t(14;18) translocation (MBR bcl2-JH) were detected in peripheral blood mononuclear cells by polymerase chain reaction. Fifteen of 29 patients (8 with IgH rearrangement, 6 with t(14;18) translocation, and 1 with both) were treated with either interferon-α or by combination therapy with interferon and ribavirin for 6 to 12 months. IgH rearrangement became negative in 7 of 9 treated patients compared with only 1 of 8 of nontreated patients (P < .02). The t(14;18) translocation became negative in 6 of 7 treated patients compared with 1 of 6 nontreated patients (P = .03). Disappearance of IgH rearrangement or t(14;18) translocation was strongly associated with virologic response to treatment. Two t(14;18)+patients developed B-cell lymphoma during follow-up. Antiviral treatment appears to be effective in eliminating the clonal proliferation of B cells in patients with chronic HCV infection and may prevent the subsequent development of lymphoma. The mechanism can be related to a direct effect of interferon-α on the proliferating clone or to an indirect effect by eradicating the antigenic stimulus.
Introduction
Lymphomagenesis is a multistep process in which environmental, genetic, and infectious factors are variably involved. Viral agents have been implicated in the pathogenesis of various types of hematologic malignancies. They may facilitate the appropriate milieu for the development of lymphoma, which may thus constitute one of the opportunistic neoplasias. Examples of this mechanism include the human immunodeficiency virus and B-cell lymphoma. Alternatively, they may act more directly, as in the Epstein-Barr virus with Burkitt's lymphoma, human T-lymphotropic virus I with human T-cell leukemia, or the human herpes 8 virus with body cavity–based non-Hodgkin lymphoma.1 Recently, hepatitis C virus (HCV) has been recognized as an additional agent that may possibly play a role in the multifactorial process of lymphomagenesis.2-4However, the mechanism of malignant transformation is still unknown. In addition to its being hepatotrophic, HCV is also a lymphotrophic virus that is able to infect and replicate within peripheral blood mononuclear cells (PBMCs).5 Chronic infection with HCV has been clearly shown to be associated with mixed cryoglobulinemia type II, which is characterized by clonal expansion of B cells and can be considered a smoldering, low-grade lymphoma that may evolve into an overt or high-grade lymphoma in some patients.6-11 Within this context, a possible mechanism for malignant transformation may involve clonal proliferation of B cell induced by the virus, inhibition of apoptotic cell death of the infected lymphocytes by bcl-2 oncogene product, or both. Recently, an increased rate of clonal proliferation of B cells and bcl-2 translocation and overexpression in PBMCs of patients with HCV infection was demonstrated.12-14 Because chronic antigenic stimulation by HCV has been shown to play a role in the development of B-cell expansion and malignant transformation of immunocytomas,15 it is possible that eradication of the persistent infection by antiviral treatment may possibly lead to regression of the proliferating clone. Thus, this study was conducted to investigate the effect of antiviral treatment on B-cell proliferation and bcl-2 translocation in patients chronically infected by HCV.
Patients, materials, and methods
Twenty-nine patients with chronic HCV infection were studied who are followed at the Liver Clinic at Bnai Zion Medical Center and in whom either immunoglobulin heavy-chain gene (IgH) rearrangement, t(14;18) translocation, or both were detected in PBMCs in our previous study.14 All patients had HCV antibody and detectable HCV RNA in the serum. Excluded were patients who tested positive for antibodies to human immunodeficiency virus or for hepatitis B surface antigen and patients with B-cell malignancy. Sixteen patients had a monoclonal IgH rearrangement, 12 had a reciprocal t(14;18) translocation, and one patient had both. Fifteen of the 29 patients received antiviral therapy; 8 of these 15 patients were IgH+, 6 were t(14;18)+, and one patient was positive for both an IgH rearrangement and t(14;18) translocation. A total of 14 patients received no treatment, including 8 with an IgH rearrangement and 6 with a t(14;18) translocation. Antiviral treatment was avoided in these patients for the following reasons: decompensated cirrhosis or age older than 65 years (9 patients), significant cytopenia (ie, hemoglobin < 100 g/L [< 10 g/dL], leukocytes < 3.0 × 109/L [< 3000/μL], or platelets < 75 × 109/L [< 75 000/μL]) (n = 3), severe depression (n = 1), and refusal of treatment (n = 1). Liver biopsy was performed in all treated patients and in 9 (64%) of the nontreated group. In all patients, a “liver panel” (alanine and aspartate aminotransferases, serum albumin and globulin, bilirubin, alkaline phosphatase, lactic dehydrogenase, and prothrombin time) and detection of anti-HCV antibody, HCV RNA, t(14;18) translocation, and IgH rearrangement were performed at baseline. Evaluation of alanine aminotransferase (ALT), t(14;18) translocation, and IgH rearrangement was also performed at the end of treatment in the treated patients and 12 months after baseline evaluation in the nontreated patients. Testing for HCV RNA was also performed at 6 months and at the end of treatment in the treated group and at 12 months after baseline evaluation in the nontreated group. HCV genotype was determined in 13 of 15 and 8 of 14 treated and nontreated patients, respectively. Patients gave written informed consent, and the study was approved by the Institutional Review Board of the B'nai Zion Medical Center.
Definition of response
Biochemical response was defined as normalization of ALT at the end of treatment. Virologic response was defined as undetectable HCV RNA in the serum at the end of treatment.
Antiviral treatment
Antiviral treatment consisted of (1) monotherapy with recombinant interferon-α, 3 million units thrice weekly for 12 months (6 patients); (2) interferon-α, 3 million units thrice weekly, combined with ribavirin, 1000 to 1200 mg daily, for 12 months (7 patients); and (3) interferon-α, 3 million units thrice weekly, in combination with ribavirin, 1000 to 1200 mg daily, for 6 months (2 patients). We switched treatment protocol from monotherapy with interferon-α to combination therapy in 9 of 15 treated patients because recent studies have clearly demonstrated that ribavirin in combination with interferon-α, in standard doses for 6 to 12 months, significantly improves the sustained biochemical and virologic response rate compared with interferon alone.16-18 According to the recommendations of the European Association for the Study of the Liver International Consensus Conference on hepatitis C,18 we used combination therapy for 12 months in 7 naive patients with genotype 1 and/or a high viral load and for 6 months in another 2 patients.
Assessment of HCV infection
Anti-HCV was detected using a second-generation enzyme-linked immunosorbent assay (ELISA II) (Abbott Laboratories, North Chicago, IL). Blood samples for HCV RNA determination and HCV genotyping were processed and stored at optimal conditions as described by Davis et al.19 HCV RNA in the serum was measured by a reverse transcription–polymerase chain reaction assay (Amplicor HCV test; Roche Molecular Systems, Somerville, NJ) as previously described.20 HCV genotyping was performed on all HCV RNA+ specimens using genotype-specific primers from the HCV core region under conditions previously described.21
Detection of bcl2-JH translocation—t(14;18)
Genomic DNA was extracted from PBMCs according to a commercial kit protocol (Boehringer Mannheim, Indianapolis, IN). Amplification was performed for the actin gene to demonstrate amplifiable DNA. Polymerase chain reaction (PCR) was performed according to Gribben et al22 with a slight modification. Briefly, reaction mixtures contained 0.1 to 0.5 μg DNA, 0.5 units Supertherm DNA polymerase (LPI, London, United Kingdom), and buffer supplied by the manufacturer (containing 1.5 mM magnesium chloride), in a final volume of 25 μL. The first round of amplification was performed using 5′ primer MBRout (CAGCCTTGAAACATTGATGG) and 3′ primer JHout (ACCTGAGGAGACGGTGACCAGGGT). The mixtures were heated at 95°C for 5 minutes followed by 30 cycles of 1 minute at 94°C, 1 minute at 58°C, and 1 minute at 72°C, with a 5-minute final extension at 72°C. The second PCR round was performed under identical conditions using 1 μL of first PCR product, with 5′ primer MBRin (TATGGTGGTTTGACCTTTAG) and 3′ primer JHin (GTGACCAGGGTCCCTTGGCCC CAG). All PCR reactions were performed in a DNA thermal controller (MJ Research, Watertown, MA). Amplification products were analyzed on 2.5% agarose gels stained by ethidium bromide. With each experiment both positive and negative control samples were carried through all the steps with the other samples. This technique is capable of detecting one cell with t(14;18) out of 105normal cells.
Detection of IgH rearrangement
IgH (FR3/JH) rearrangement was detected by a seminested PCR approach. The reaction mixtures were essentially as those for t(14;18) except for the 5′ primer, which was FR3 (ACACGGCTCTGTATTACTCT). Reaction mixtures were heated at 94.5°C for 1 minute, followed by 30 cycles of 40 seconds at 94°C, 40 seconds at 55°C, and 40 seconds at 72°C, with a 5-minute final extension at 72°C. A second round of amplification using 2 μL of 1:500 dilution of the initial reaction was performed under similar conditions using JH in primer for 20 cycles only. PCR products were analyzed on 6% polyacrylamide gels. With each experiment, both positive and negative control samples were carried through all the steps with the other samples.
Cryoglobulin detection and analysis
Venous blood samples were collected into prewarmed tubes after overnight fast and allowed to clot at 37°C. After centrifugation, sera were incubated at 4°C for 3 days. The cryocrit was evaluated by centrifugation of the serum in hematocrit tubes at 4°C. The cryoprecipitate was washed at 4°C and then resolubilized at 37°C. The purified cryoglobulin was further analyzed and characterized by immunofixation electrophoresis (Immunofix Kit; Helena Laboratories, Beaumont, TX).
Statistical analysis
Comparison between continuous parametric groups was performed using the Student t test for unpaired groups. Equality of variances was performed using the Levene test. Associations between categorical groups were analyzed using the chi-square test or Fisher exact test when appropriate. Two-tailed P values of .05 or less were considered to be statistically significant. For statistical analysis of the data, the SPSS package for Windows was used.
Results
Table 1 summarizes the characteristics of the treated and nontreated groups. The patients in the nontreated group were older and had a higher rate of cirrhosis. The 2 groups were comparable with respect to gender, the presence and levels of cryoglobulin, mean ALT levels, and rate of genotype 1. Eight of 15 (53%) treated and 6 of 14 (43%) nontreated patients had risk factors for acquisition of HCV infection. The duration of HCV infection as was determined from the occurrence of a potential risk factor for HCV infection (ie, blood transfusion, injection drug use, or tattooing) to the detection of IgH and/or t(14;18) translocation was similar among patients of both groups. Notably, 4 patients (2 in each group) had a normal ALT; 3 of them had no liver disease. Although 22 patients (13 treated, 9 untreated) had cryoglobulinemia, only 8 of the 13 treated and 3 of the 9 untreated patients had symptoms related to cryoglobulinemia (ie, dermal vasculitis, arthritis, Raynaud phenomenon, peripheral neuropathy, or renal involvement).
Characteristics of treated and nontreated hepatitis C virus–infected patients
| Parameter . | Treated (n = 15) . | Nontreated (n = 14) . | P . |
|---|---|---|---|
| Mean age, y | 51 ± 10 | 65 ± 6 | .0001 |
| Sex (male, female) | 8, 7 | 7, 7 | NS |
| Liver biopsy (%) | 15 (100) | 9 (64) | .01 |
| Cirrhosis (%) | 3 (20) | 9 (64) | .01 |
| Cryoglobulin (%) | 13 (87) | 9 (64) | NS |
| Mean cryocrit, % | 5.8 ± 8.7 | 3.17 ± 3.1 | NS |
| Abnormal ALT (%) | 13 (87) | 12 (86) | NS |
| Mean ALT, IU/L | 101 ± 60 | 86 ± 44 | NS |
| Genotype 1 (%) | 8/13 (66) | 6/8 (75) | NS |
| Mean duration of HCV infection, y* | 16.5 ± 9 | 19.6 ± 6 | NS |
| Parameter . | Treated (n = 15) . | Nontreated (n = 14) . | P . |
|---|---|---|---|
| Mean age, y | 51 ± 10 | 65 ± 6 | .0001 |
| Sex (male, female) | 8, 7 | 7, 7 | NS |
| Liver biopsy (%) | 15 (100) | 9 (64) | .01 |
| Cirrhosis (%) | 3 (20) | 9 (64) | .01 |
| Cryoglobulin (%) | 13 (87) | 9 (64) | NS |
| Mean cryocrit, % | 5.8 ± 8.7 | 3.17 ± 3.1 | NS |
| Abnormal ALT (%) | 13 (87) | 12 (86) | NS |
| Mean ALT, IU/L | 101 ± 60 | 86 ± 44 | NS |
| Genotype 1 (%) | 8/13 (66) | 6/8 (75) | NS |
| Mean duration of HCV infection, y* | 16.5 ± 9 | 19.6 ± 6 | NS |
NS indicates statistically nonsignificant; ALT, alanine aminotransferase; HCV, hepatitis C virus.
Time from the occurrence of potential risk factor for HCV infection to the detection of immunoglobulin heavy-chain gene rearrangement or bcl-2 translocation.
The clinical data and the effect of antiviral treatment in each patient are depicted in Table 2. Two of the treated patients (patients 7 and 13) received antiviral treatment for symptomatic cryoglobulinemia but had no liver disease. Table3 summarizes the effect of antiviral treatment on immunoglobulin heavy-chain gene rearrangement and t(14;18) translocation in treated and nontreated patients.
Clinical data and the effect of antiviral treatment on immunoglobulin heavy-chain gene rearrangement and bcl-2 translocation in patients with chronic hepatitis C virus infection
| . | Sex/Age . | Cirrhosis . | Cryoglobulin (cryocrit) . | MC-related symptoms . | ALT (IU/L) . | HCV genotype . | Treatment or follow-up*(duration) . | IgH† . | Bcl-2 translocation† . | Biochemical response . | Virologic response . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | ||||||||||
| Treated group, no. | |||||||||||||
| 1 | F/47 | Yes | + (2.5%) | Yes | 112 | 1b | IFN (12 m) | + | − | − | − | No | No |
| 2 | M/40 | No | + (7.8%) | Yes | 220 | 2a | IFN (12 m) | + | − | − | − | Yes | Yes |
| 3 | M/62 | Yes | + (2.9%) | Yes | 65 | 1a | IFN (12 m) | + | + | − | − | No | No |
| 4 | F/52 | No | − | 115 | 1b | IFN (12 m) | + | − | − | − | Yes | Yes | |
| 5 | M/48 | No | + (3.5%) | No | 87 | ND | IFN (12 m) | + | − | − | − | Yes | Yes |
| 6 | F/52 | No | + (1.7%) | Yes | 51 | ND | IFN (12 m) | + | − | − | − | Yes | Yes |
| 7 | F/23 | No | + (4.7%) | Yes | 22 | 1b/2a | IFN-RBV (12 m) | + | + | − | − | No | |
| 8 | M/61 | No | + (34%) | Yes | 175 | 1b | IFN-RBV (12 m) | + | − | + | − | Yes | Yes |
| 9 | F/54 | No | + (1.2%) | No | 220 | 4 | IFN-RBV (6 m) | + | − | − | − | Yes | Yes |
| 10 | F/62 | No | + (7.2%) | Yes | 115 | ND | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 11 | F/51 | No | + (1.2%) | No | 74 | 2a | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 12 | M/42 | No | + (1.4%) | No | 55 | 1b | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 13 | M/58 | No | + (2.2%) | Yes | 38 | 1b | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 14 | M/52 | No | + (4.7%) | No | 80 | 1b | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 15 | F/60 | Yes | − | 80 | 2a | IFN-RBV (6 m) | − | − | + | + | No | No | |
| Untreated group, no. | |||||||||||||
| 1 | M/71 | Yes | + (1.7%) | No | 110 | 1b | 12 m | + | + | − | − | No | No |
| 2 | M/64 | No | + (1.7%) | No | 117 | ND | 12 m | + | + | − | − | No | No |
| 3 | M/65 | No | − | 15 | 1b | 12 m | + | + | − | − | No | No | |
| 4 | F/52 | No | + (1.5%) | No | 79 | 2a | 12 m | + | − | − | − | No | No |
| 5 | F/66 | Yes | + (2.2%) | No | 112 | 1b | 12 m | + | + | − | − | No | No |
| 6 | M/57 | Yes | + (3.1%) | Yes | 48 | ND | 12 m | + | + | − | + | No | No |
| 7 | M/59 | Yes | − | 161 | 1b | 12 m | + | + | − | − | No | No | |
| 8 | F/78 | Yes | + (11.4%) | Yes | 49 | 2a | 12 m | + | + | − | + | No | No |
| 9 | F/74 | Yes | + (2.6%) | No | 36 | ND | 12 m | − | − | + | + | No | No |
| 10 | F/65 | Yes | + (3.1%) | Yes | 74 | ND | 12 m | − | − | + | + | No | No |
| 11 | M/58 | Yes | + (1.3%) | No | 115 | 1b | 12 m | − | − | + | + | No | No |
| 12 | F/64 | No | − | 84 | 1b | 12 m | − | − | + | − | No | No | |
| 13 | M/61 | No | − | 49 | UD | 12 m | − | − | + | + | No | No | |
| 14 | F/72 | Yes | − | 151 | ND | 12 m | − | − | + | + | No | No | |
| . | Sex/Age . | Cirrhosis . | Cryoglobulin (cryocrit) . | MC-related symptoms . | ALT (IU/L) . | HCV genotype . | Treatment or follow-up*(duration) . | IgH† . | Bcl-2 translocation† . | Biochemical response . | Virologic response . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre . | Post . | Pre . | Post . | ||||||||||
| Treated group, no. | |||||||||||||
| 1 | F/47 | Yes | + (2.5%) | Yes | 112 | 1b | IFN (12 m) | + | − | − | − | No | No |
| 2 | M/40 | No | + (7.8%) | Yes | 220 | 2a | IFN (12 m) | + | − | − | − | Yes | Yes |
| 3 | M/62 | Yes | + (2.9%) | Yes | 65 | 1a | IFN (12 m) | + | + | − | − | No | No |
| 4 | F/52 | No | − | 115 | 1b | IFN (12 m) | + | − | − | − | Yes | Yes | |
| 5 | M/48 | No | + (3.5%) | No | 87 | ND | IFN (12 m) | + | − | − | − | Yes | Yes |
| 6 | F/52 | No | + (1.7%) | Yes | 51 | ND | IFN (12 m) | + | − | − | − | Yes | Yes |
| 7 | F/23 | No | + (4.7%) | Yes | 22 | 1b/2a | IFN-RBV (12 m) | + | + | − | − | No | |
| 8 | M/61 | No | + (34%) | Yes | 175 | 1b | IFN-RBV (12 m) | + | − | + | − | Yes | Yes |
| 9 | F/54 | No | + (1.2%) | No | 220 | 4 | IFN-RBV (6 m) | + | − | − | − | Yes | Yes |
| 10 | F/62 | No | + (7.2%) | Yes | 115 | ND | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 11 | F/51 | No | + (1.2%) | No | 74 | 2a | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 12 | M/42 | No | + (1.4%) | No | 55 | 1b | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 13 | M/58 | No | + (2.2%) | Yes | 38 | 1b | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 14 | M/52 | No | + (4.7%) | No | 80 | 1b | IFN-RBV (12 m) | − | − | + | − | Yes | Yes |
| 15 | F/60 | Yes | − | 80 | 2a | IFN-RBV (6 m) | − | − | + | + | No | No | |
| Untreated group, no. | |||||||||||||
| 1 | M/71 | Yes | + (1.7%) | No | 110 | 1b | 12 m | + | + | − | − | No | No |
| 2 | M/64 | No | + (1.7%) | No | 117 | ND | 12 m | + | + | − | − | No | No |
| 3 | M/65 | No | − | 15 | 1b | 12 m | + | + | − | − | No | No | |
| 4 | F/52 | No | + (1.5%) | No | 79 | 2a | 12 m | + | − | − | − | No | No |
| 5 | F/66 | Yes | + (2.2%) | No | 112 | 1b | 12 m | + | + | − | − | No | No |
| 6 | M/57 | Yes | + (3.1%) | Yes | 48 | ND | 12 m | + | + | − | + | No | No |
| 7 | M/59 | Yes | − | 161 | 1b | 12 m | + | + | − | − | No | No | |
| 8 | F/78 | Yes | + (11.4%) | Yes | 49 | 2a | 12 m | + | + | − | + | No | No |
| 9 | F/74 | Yes | + (2.6%) | No | 36 | ND | 12 m | − | − | + | + | No | No |
| 10 | F/65 | Yes | + (3.1%) | Yes | 74 | ND | 12 m | − | − | + | + | No | No |
| 11 | M/58 | Yes | + (1.3%) | No | 115 | 1b | 12 m | − | − | + | + | No | No |
| 12 | F/64 | No | − | 84 | 1b | 12 m | − | − | + | − | No | No | |
| 13 | M/61 | No | − | 49 | UD | 12 m | − | − | + | + | No | No | |
| 14 | F/72 | Yes | − | 151 | ND | 12 m | − | − | + | + | No | No | |
MC indicates mixed cryoglobulinemia; ALT, alanine aminotransferase; HCV, hepatitis C virus; IgH, immunoglobulin heavy-chain gene; IFN, interferon α; ND, not done; RBV, ribavirin; UD, undetermined.
Follow-up applies to untreated group.
Pre: at baseline. Post: at the end of the follow-up period (untreated patients) and at the end of treatment (in treated patients). Note that one patient from the treated group (no. 8) was positive for both monoclonal IgH rearrangement and t(14;18) translocation.
The effect of antiviral treatment on immunoglobulin heavy-chain gene rearrangement and t(14;18) translocation in patients with chronic hepatitis C virus infection
| . | Treated group (n = 15) . | Nontreated group (n = 14) . |
|---|---|---|
| Effect on IgH | ||
| IgH+ | 9 | 8 |
| IgH loss | 7 | 1 |
| Virologic response | 6 | 0 |
| Effect on t(14;18) | ||
| t(14;18)+ | 7 | 6 |
| t(14;18) loss | 6 | 1 |
| Virologic response | 5 | 0 |
| . | Treated group (n = 15) . | Nontreated group (n = 14) . |
|---|---|---|
| Effect on IgH | ||
| IgH+ | 9 | 8 |
| IgH loss | 7 | 1 |
| Virologic response | 6 | 0 |
| Effect on t(14;18) | ||
| t(14;18)+ | 7 | 6 |
| t(14;18) loss | 6 | 1 |
| Virologic response | 5 | 0 |
IgH indicates immunoglobulin heavy-chain gene.
At the end of treatment, IgH rearrangement became negative in 7 of 9 treated patients (77%) compared with only 1 of 8 IgH+nontreated patients (12.5%) (P < .02). The loss of IgH in the patients who received antiviral treatment was strongly associated with virologic response: 6 of the 7 (86%) treated patients in whom IgH rearrangement became negative had cleared the virus at the end of therapy (Figure 1). In 1 patient, IgH rearrangement could not be detected despite persistence of HCV infection. In contrast, none of the nontreated patients had lost the HCV RNA, including 1 patient who became IgH−. Treatment with antiviral therapy led to the loss of t(14;18) translocation in 6 of 7 (86%) treated patients. Disappearance of t(14;18) translocation was strongly associated with virologic response to treatment: In 5 of 6 treated patients who lost the translocation, HCV RNA became negative at the end of treatment (Figure 2). In contrast, t(14;18) translocation became negative in only 1 of 6 nontreated patients (17%) (P = .03). As expected, none of the nontreated patients cleared the virus.
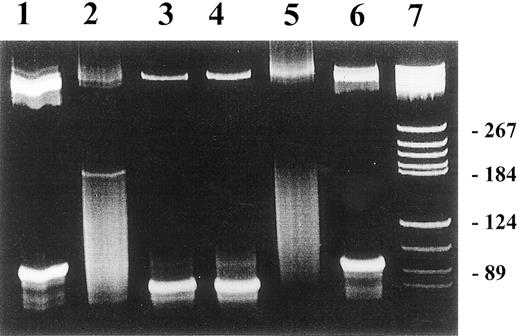
The effect of antiviral treatment on immunoglobulin heavy-chain gene rearrangement in patients with chronic HCV infection.
Detection of IgH rearrangement was performed by FW3/JH seminested PCR in PBMCs from patients with HCV-infected individuals. Lanes 1 and 3: pretreatment bands represent the presence of IgH rearrangement; lane 2: disappearance of the band, indicating the loss of the proliferating B-cell clone upon completion of antiviral therapy. The patient had virologic response with clearance of HCV RNA at the end of treatment. Lane 4: persistence of the proliferative B-cell clone at the end of antiviral treatment. This patient had no virologic response. Lane 5: negative control; lane 6: positive control; lane 7: molecular weight marker. PCR products were separated on 2.5% agarose gel.
The effect of antiviral treatment on immunoglobulin heavy-chain gene rearrangement in patients with chronic HCV infection.
Detection of IgH rearrangement was performed by FW3/JH seminested PCR in PBMCs from patients with HCV-infected individuals. Lanes 1 and 3: pretreatment bands represent the presence of IgH rearrangement; lane 2: disappearance of the band, indicating the loss of the proliferating B-cell clone upon completion of antiviral therapy. The patient had virologic response with clearance of HCV RNA at the end of treatment. Lane 4: persistence of the proliferative B-cell clone at the end of antiviral treatment. This patient had no virologic response. Lane 5: negative control; lane 6: positive control; lane 7: molecular weight marker. PCR products were separated on 2.5% agarose gel.
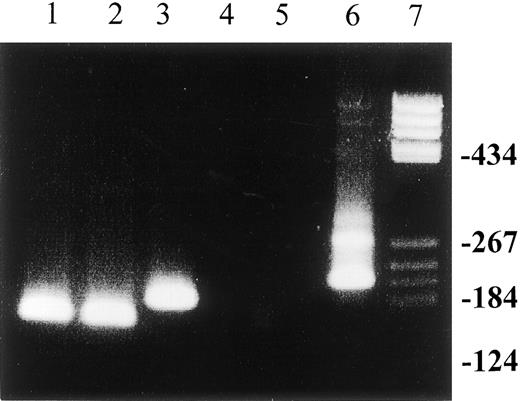
The effect of antiviral treatment on t(14;18) translocation in patients with chronic HCV infection.
Detection of t(14;18) translocation by MBR bcl-2/JH nested PCR in PBMCs from patients with chronic HCV infection before and after antiviral treatment. Lanes 1 and 2: pretreatment t(14;18) translocation in this patient persisted upon completion of antiviral treatment. The patient had no virologic response; lanes 3 and 4: loss of pretreatment t(14;18) translocation in a patient who also had a virologic response to combination treatment with interferon-α and ribavirin; lane 5: negative control; lane 6: positive control (sample from a patient with follicular B-cell lymphoma); lane 7: molecular weight marker. PCR products were separated on 6% acrylamide gel.
The effect of antiviral treatment on t(14;18) translocation in patients with chronic HCV infection.
Detection of t(14;18) translocation by MBR bcl-2/JH nested PCR in PBMCs from patients with chronic HCV infection before and after antiviral treatment. Lanes 1 and 2: pretreatment t(14;18) translocation in this patient persisted upon completion of antiviral treatment. The patient had no virologic response; lanes 3 and 4: loss of pretreatment t(14;18) translocation in a patient who also had a virologic response to combination treatment with interferon-α and ribavirin; lane 5: negative control; lane 6: positive control (sample from a patient with follicular B-cell lymphoma); lane 7: molecular weight marker. PCR products were separated on 6% acrylamide gel.
Two of the 8 nontreated patients who had previous IgH rearrangement but no bcl-2 translocation (patients No. 6 and 8, Table2), acquired a new t(14;18) translocation during the follow-up period (ie, after 12 months). None of these patients had clinical symptoms or signs suggesting lymphoma. One other nontreated patient (patient No. 13, Table 2) with t(14;18) translocation without IgH rearrangement developed extranodal, diffuse large cell, non-Hodgkin lymphoma of the urinary bladder 11 months after enrollment in the study. In a second nontreated patient (patient No. 14, Table 2) with t(14;18) translocation but without IgH rearrangement, diffuse large cell, nodal, non-Hodgkin lymphoma was diagnosed 12 months from the detection of B-cell monoclonality.
Discussion
The data presented in this study show that antiviral treatment may result in regression of t(14;18) translocation and B-cell clonality in patients with chronic HCV infection. Treatment with interferon-α monotherapy or interferon combined with ribavirin resulted in the loss of monoclonal IgH rearrangement and t(14;18) translocation in 77% and 86% of treated patients, respectively. This favorable effect was closely associated with HCV clearance upon the end of treatment. These results may support the role of HCV in inducing clonal expansion of B cells.
Recent studies showed an increased frequency of B-cell clonality and t(14;18) translocation in HCV infected patients,12-14,23suggesting that HCV may induce clonal proliferation of B cells. However, the pathogenetic mechanism of inducing such clonal expansion is not clear. The recent report of somatic hypermutation and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in HCV-associated immunocytomas15 supports the role of chronic antigenic stimulation in inducing B-cell clonality in HCV infected patients. Ivanovski et al have demonstrated substantial intraclonal VH and/or VL gene diversity in each case, consistent with an ongoing somatic hypermutation process in the tumor cells subsequent to the neoplastic transformation.15 A striking similarity regarding V gene repertoire and somatic hypermutation exists between HCV-associated immunocytoma and salivary gland mucosa-associated lymphoid tissue (MALT) lymphoma,24implying a clonal proliferation of a highly selected B-cell population in response to chronic antigenic stimulation. Thus, it is reasonable to hypothesize that eradication of the antigen may result in disappearance of the proliferating B-cell clone. In concordance with this hypothesis is the well-established association of gastric MALTomas with chronic Helicobacter pylori infection. Eradication of the chronic antigenic stimulation (ie, H pylori) by triple antibiotic therapy results in regression of lymphoma in most patients.25,26 Further support to the analogy between HCV-related lymphoma and gastric MALTomas is the report of Mazzaro et al, in which a regression of monoclonal B-cell expansion in HCV-infected patients with mixed cryoglobulinemia (MC) following interferon treatment was demonstrated.27 The favorable effect of interferon therapy in inducing regression of the proliferative B-cell clone or t(14;18) translocation in HCV-infected patients may be achieved through viral clearance, although its direct antiproliferative effect may also play a role. However, the close association between the virologic response and the loss of B-cell monoclonality and t(14;18) in the present study indicates that the more effective the antiviral therapy is, the more likely is loss of B-cell clonality. The thrust of these conclusions is based on the significant percentage of patients with a biochemical, virologic, and clonal proliferative response following treatment, as demonstrated in Table 2. Such a degree of spontaneous responsiveness must be considered highly unlikely to have occurred independently of therapy. Within this context it is recognized that untreated patients with more advanced disease, as manifested by the presence of cirrhosis, may not represent the perfect matched control population (Table 2), although the absence of response in IgH rearrangement or bcl-2 translocation is much more likely to be associated with the fact that such patients received no therapy. Moreover, there are no solid data to support a quantitative increase in cells bearing t(14;18) or IgH rearrangement in patients with more advanced liver disease (ie, cirrhosis) as compared with noncirrhotic patients.
In addition, the older age of the untreated group may contribute to the increased frequency of detection of cells bearing t(14;18) translocation, as suggested by several reports. However, the clonality, as assessed by the presence of t(14;18) translocation, was confirmed by the proper size of the PCR product, and remained unchanged during follow-up.
Clonal B-cell expansion and the presence of t(14;18) translocation in HCV+ patients may reflect a premalignant state, at least in some. In our study, 2 patients developed overt lymphoma within 12 months from the detection of B-cell monoclonality. However, the risk of developing B-cell non-Hodgkin lymphoma in HCV-infected patients is unknown. In a recent report from Japan, the relative risk of developing B-cell lymphoma was 2.1 in patients with chronic HCV infection, after a median of 13 years from the onset of HCV infection.28 Thus, antiviral therapy may also be indicated in HCV-infected patients with evidence of clonal expansion of B cell to decrease the risk for developing lymphoma, even in the absence of liver disease.
One of the clinical implications of the present study relates to the management of HCV-related indolent lymphomas. In respect to this issue, low-grade lymphomas are currently incurable. Traditionally, the management of low-grade, asymptomatic lymphomas involves a watch-and-wait policy. Alternatively, interferon-α can also be used in these patients.29 Hence, it may be reasonable to treat low-grade lymphomas associated with chronic HCV infection with antiviral treatment. Encouraging results emerge from a recent report by Hermine et al, in which most patients with HCV and splenic lymphoma entered complete remission upon treatment with interferon-α.30 Taken together, it may be suggested that low-grade HCV-related lymphomas may be treated with the currently most effective antiviral treatment, including interferon-α and ribavirin.16 17 However, it should be noted that HCV-related lymphomas are a heterogeneous group of diseases including the more common indolent, low-grade lymphoma, preceded by long-standing symptomatic MC type II, along with the “idiopathic,” noncryoglobulinemic, high-grade lymphomas. It is likely that different pathogenetic mechanisms operate in these different types of lymphomas associated with HCV infection. Because antiviral treatment is clearly insufficient for the treatment of intermediate or high-grade lymphomas, it may play a role in maintenance treatment after completion of chemotherapy in HCV-infected individuals.
In conclusion, antiviral therapy for HCV infection may lead to the loss of t(14;18) translocation and regression of B-cell clonal proliferation. The loss of bcl-2 translocation and IgH rearrangement is closely associated with virologic response to antiviral therapy. The mechanism of this response can be related to a direct effect of interferon-α on the proliferating clone or to an indirect effect by eradicating the antigenic stimulus (ie, HCV). This effect may support the role of HCV in inducing clonal B-cell expansion and t(14;18) translocation. Whether antiviral treatment for HCV infection will reduce the risk of developing lymphoma in some patients or should be included in the treatment of HCV-infected patients with low-grade or high-grade lymphomas remains to be elucidated in large, controlled trials with a longer follow-up.
E.Z. and T.Z. made equal contributions to this study and share first authorship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eli Zuckerman, Liver Unit, Department of Internal Medicine A, B'nai Zion Medical Center, 47 Golomb St, PO Box 4940, Haifa 31048 Israel.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal