Interactions between the endothelium and erythrocytes may contribute to the vascular complications of sickle cell disease (SCD). Endothelium-derived nitric oxide (NO) plays a major role in the regulation of vasomotor tone in response to wall shear stress (WSS) variations and pharmacologic stimuli. However, little is known about endothelial NO production in patients with steady-state SCD. We investigated endothelial NO production in response to flow or vasoactive agonists in 16 homozygous patients with steady-state SCD and 15 controls. Flow-mediated dilation (FMD), arterial diameter changes in response to 100% oxygen inhalation, blood viscosity, and calculated WSS were determined in all patients and controls. At baseline, WSS was higher in SCD patients than in controls, whereas arterial diameter was similar. In patients with SCD, FMD was impaired (1.73% ± 0.44% vs 3.97% ± 0.24% in the controls, P < .001) and vasoconstriction in response to 100% oxygen was abolished. Using venous occlusion plethysmography, forearm blood flow (FBF) was evaluated in response to acetylcholine, nitro-monomethyl-L-arginine (L-NMMA), and sodium nitroprusside (SNP) in subgroups of 9 controls and 7 patients with SCD. Acetylcholine induced a significantly greater FBF increase in the patients (9.7 ± 2.9 mL/min/100 mL of forearm volume vs 2.5 ± 1.5 mL/min/100 mL in the controls,P < .001), whereas responses to L-NMMA and SNP were similar. These results suggest that endothelial dysfunction may prevent the arterial diameter of patients with SCD from adapting to chronic or acute shear stress elevations. This may contribute to the pathophysiology of vaso-occlusive crisis in patients with SCD.
Introduction
Vaso-occlusive crisis is a characteristic manifestation of sickle cell disease (SCD). One current hypothesis suggests that interactions between sickle red blood cells (SSRBCs) and the endothelium may contribute to the pathogenesis of vaso-occlusive crisis.1 Sickle erythrocytes may occlude microvessels directly by adhering to the endothelium or indirectly by altering endothelial functions such as endothelium-dependent vasodilation. Thus, enhanced interactions between SSRBCs and the vascular endothelium on one hand and abnormal vasomotor tone regulation on the other may contribute synergistically to the occurrence of vaso-occlusive crisis. In vivo studies have identified specific hemodynamic conditions in the microcirculation of patients with SCD. During the intervals between crises, there is an increase in peripheral blood flow2 with a periodic microcirculatory flow pattern, which may compensate for the alterations in red blood cell rheology.3 Arteriolar diameter is increased during vaso-occlusive crisis as compared with steady-state phases of the disease.4 Thus, the relationship between endothelial regulation of vascular tone and red blood cell rheology seems to play a key role in SCD.5However, to our knowledge, no studies have investigated the vasoreactive properties of large muscular arteries.
Among endothelial mediators, nitric oxide (NO) plays a central role by contributing to the control of normal vascular tone, cellular adhesion, platelet aggregation, and thrombosis.6 Evidence that SSRBCs may induce endothelial dysfunction has been obtained in vitro7,8 and in animal models.9,10 SSRBCs impair endothelium-dependent relaxation in vitro when applied on vascular strips7 and impair endothelial production of prostanoids.9 The NO pathway may also play a critical role in maintaining cerebral blood flow in experimental animals given intravenous infusions of SSRBCs.11 However, little is known about human endothelial dysfunction in patients with SCD. Evidence supporting a decrease in NO production or release during vaso-occlusive crises has been reported recently: A decrease in NO metabolites was associated with a rise in plasma vascular cell adhesion molecule (VCAM)-1 levels, suggesting an inverse relationship between NO and adhesion molecules in patients with SCD.1
In the present study, we evaluated NO-mediated vasodilation in patients with steady-state SCD. Endothelial NO synthase (NOS) is constitutively expressed by endothelial cells and is stimulated further by receptor-dependent agonists such as acetylcholine or bradykinin.12 Wall shear stress (WSS), which reflects mechanical forces exerted on endothelial cells, is another important physiologic stimulus of endothelial NOS activity and is thought to be responsible for basal NOS activity and for the continuous in vivo release of NO from the endothelium.13 The putative mechanism underlying flow-mediated vasodilation (FMD) is NO release by the endothelium following stimulation by an increase in WSS.14,15 Vascular remodeling has been observed following chronic elevation or diminution of WSS, which induces an increase or a decrease in arterial diameter, respectively.16,17 The long-term regulation of arterial diameter is structural and may counter-regulate variations in WSS. In SCD, the increased cardiac output18 and relative increase in blood viscosity19 contribute to increase WSS.
We designed the study to evaluate in vivo endothelial function and NO-mediated vasodilation in response to both WSS and pharmacologic modulation of NOS activity in patients with steady-state SCD. Using a high-resolution, A-mode, ultrasound and echo-tracking device to monitor brachial artery diameter, we determined WSS at baseline and investigated the vasodilator response to increased forearm blood flow (FBF) generated by reactive hyperemia. Studies were also performed during 100% oxygen inhalation to assess the vasoconstrictor response to decreased FBF. In addition, changes in FBF were explored using venous occlusion plethysmography (VOP) to assess vasodilator responses to acetylcholine and sodium nitroprusside (SNP) as well as the vasoconstrictor response to the selective inhibitor of NO synthesis nitro-monomethyl-L-arginine (L-NMMA).
Patients, materials, and methods
Study population
Sixteen patients with steady-state homozygous (SS) SCD and 15 control (AA) subjects were included in the study. The patients were in a steady-state phase of their disease, with no painful crisis during the last 3 months. None were on a regular transfusion protocol, and none had received a blood transfusion during the last 3 months. Table1 summarizes the characteristics of the subjects, who were all sub-Saharan Africans. The age range was 18 to 40 years; mean age (± SEM) was 28.5 ± 1.1 years in the patients and 29.2 ± 1.7 years in the controls. Exclusion criteria were a history of vaso-occlusive crisis or blood transfusion during the last 3 months; treatment with vasodilators, anti-inflammatory drugs, or hydroxyurea; concomitant systemic disease; and known cardiovascular risk factors (smoking, total cholesterol > 5.5 mM/L, low-density lipoprotein cholesterol > 4 mM/L, diabetes, or blood pressure > 160/90 mmHg). Routine laboratory tests were performed at inclusion.
Population characteristics of control subjects and patients
| . | AA . | SS . | P . |
|---|---|---|---|
| n | 15 | 16 | NS |
| Sex, male/female | 7/8 | 8/8 | NS |
| Age | 29.2 ± 1.7 | 28.5 ± 1.1 | NS |
| Weight, kg | 65 ± 7 | 62 ± 11 | NS |
| Height, cm | 172 ± 6 | 171 ± 8 | NS |
| SAP, mmHg | 120 ± 7.8 | 119 ± 10 | NS |
| MAP, mmHg | 87.3 ± 9.3 | 81.2 ± 9 | NS |
| HR, beats/min | 64 ± 11 | 65 ± 5 | NS |
| Hb, g/L | 140 ± 3 | 86 ± 3 | < .001 |
| Ht | 0.42 ± .01 | 0.27 ± 0.04 | < .001 |
| η, mPa.s | 4.03 ± 0.1 | 3.60 ± 0.1 | < .05 |
| η/Ht | 9.6 ± 0.3 | 13.6 ± 0.6 | < .001 |
| . | AA . | SS . | P . |
|---|---|---|---|
| n | 15 | 16 | NS |
| Sex, male/female | 7/8 | 8/8 | NS |
| Age | 29.2 ± 1.7 | 28.5 ± 1.1 | NS |
| Weight, kg | 65 ± 7 | 62 ± 11 | NS |
| Height, cm | 172 ± 6 | 171 ± 8 | NS |
| SAP, mmHg | 120 ± 7.8 | 119 ± 10 | NS |
| MAP, mmHg | 87.3 ± 9.3 | 81.2 ± 9 | NS |
| HR, beats/min | 64 ± 11 | 65 ± 5 | NS |
| Hb, g/L | 140 ± 3 | 86 ± 3 | < .001 |
| Ht | 0.42 ± .01 | 0.27 ± 0.04 | < .001 |
| η, mPa.s | 4.03 ± 0.1 | 3.60 ± 0.1 | < .05 |
| η/Ht | 9.6 ± 0.3 | 13.6 ± 0.6 | < .001 |
AA indicates controls; SS, patients; NS, not significant; SAP, systolic arterial blood pressure; MAP, mean arterial blood pressure; HR, heart rate; Hb, hemoglobin; Ht, hematocrit; η, viscosity.
All study subjects underwent brachial artery ultrasound scanning to determine the arterial diameter and local blood flow at baseline and under hyperoxia as well as FMD. In subgroups of 9 controls and 7 patients, FBF measurements were obtained using VOP.
The various techniques used in this study to evaluate endothelial function were performed in a random order on different days within the same week. Patients who developed clinical events between evaluations were to be excluded. All subjects gave their written informed consent, and the study protocol was approved by our institutional review board.
Evaluation of flow-mediated dilation
All measurements were performed after a 30-minute rest in bed in a temperature-controlled room (22°C) with continuous blood pressure monitoring (Finapres 2300, Ohmeda, Englewood, CO). FMD was evaluated as previously described.20 A high-resolution ultrasound Wall Track system (Pie Medical, Maastricht, The Netherlands) with a 7.5-Hz linear probe was used to measure the systolic and diastolic internal diameters of the distal brachial artery. This echo-tracking system, which analyzes radiofrequency signals, has a precision for diastolic diameter measurements of 30 μm. FMD was measured as a percentage of the increase in the brachial artery diastolic diameter after 3 minutes of ischemia of the ipsilateral hand induced using an inflatable wrist cuff (hyperemia test). This unusually short duration of ischemia was deliberately chosen because ischemia of the hand can induce complications in patients with SCD. When the wrist cuff is deflated, there is a transient blood flow increase, followed by arterial dilation. The maximum diameter (DM) was defined as the greatest diastolic diameter following deflation of the cuff; measurements were made at deflation and every 30 seconds thereafter for 5 minutes. The measurement at deflation was the minimum diameter (DB, for basal diameter). FMD was calculated as 100 × (DM − DB)/DB.
Hyperoxia
Subjects inhaled 100% oxygen for 10 minutes via an occlusive mask. Brachial artery flow was calculated at baseline and after 5 and 10 minutes of 100% oxygen inhalation, using the formula Q = Vπr2, where V is the mean blood velocity measured over 3 cardiac cycles by pulsed-wave Doppler coupled with high-resolution B-mode ultrasound (Acuson 128 XP 10, Mountain View, CA) and r is the radius of the artery at the flow measurement site.
FBF measurement by VOP
In a first subgroup of patients, FBF was measured at the forearm using strain-gauge VOP as previously described.21 Briefly, a mercury-in-silastic strain gauge was placed around the widest portion of the upper third of the forearm. The strain gauge was electrically coupled to a plethysmograph (Perivein JSI 0539/l, ETNA, Noisy le Gd, France) calibrated to measure normalized changes in volume. The plethysmographic FBF tracings were connected to a Gould chart recorder for analysis (Gould Electrics, Ballainvilliers, France). For each measurement, venous flow was occluded just proximal to the elbow by rapidly inflating a blood pressure cuff to 40 mmHg. A wrist cuff was inflated to suprasystolic pressures starting 1 minute before each measurement to exclude the hand circulation from blood flow determination. FBF measurements are reported in milliliters per minute per 100 milliliters of forearm volume, and each value is the mean of at least 3 determinations. Systolic blood pressure, diastolic blood pressure, mean blood pressure, and heart rate were monitored continuously (Finapres 2300, Ohmeda). All studies were performed in the morning in a quiet room kept at a controlled temperature of 22°C. While the subject was in the supine position, a catheter (Seldicath, diameter 1.0, Teflon ORX, 3P, Plastimed, St Leu la Forêt, France) was inserted after local anesthesia (1% xylocaine) into the brachial artery of the nondominant arm, which was elevated to a level slightly above the right atrium. After insertion of the catheter, a 30-minute rest was observed to ensure hemodynamic stability. To establish resting control FBF values, 0.9% saline was administered for 30 minutes, and blood flow measurements were repeated until a stable baseline condition was obtained. Infusion of vasoactive agents was then started. Between each series of drug injections, FBF was allowed to return to its basal value (this required approximately 20 minutes); during these intervals, 0.9% saline was infused. Three drugs were used to explore endothelial function, as previously described22: (1) acetylcholine (Pharmacie Centrale des Hôpitaux, Paris, France), an M3 muscarinic agonist that stimulates endothelial NO production, as a continuous infusion at rates of 4, 8, and 16 μg/min; (2) SNP (Nitriate, Laboratoires SERB, Paris, France), an NO donor used to study endothelium-independent vasodilation, at rates of 0.5, 0.8, and 1 μg/min; and (3) L-NMMA (Clinalpha, Laufelfingen, Switzerland) at rates of 4, 8, and 16 μM/min. Each concentration of each drug was injected into the brachial artery for 5 minutes. Drugs were administered in random order.
Blood viscosity
Blood viscosity (η) was measured in all patients as previously described.23 Briefly, blood was withdrawn and processed immediately. Blood viscosity was measured using a calibrated coaxial cylinder Couette viscometer (Chaix-Meca, Nancy, France) at shear rates of 0.01 s−1 to 33 s−1 and under atmospheric pressure. Measurements were done according to a well-established protocol, at the native hematocrit, and at a temperature of 37°C. The shear-thinning curve, which is the viscosity decrease plotted against the shear rate, was then analyzed using Quemada's model,24 and viscosity at 100 s−1was derived.
Wall shear stress
WSS was calculated according to Hoeks et al25 from blood viscosity and local blood flow in the brachial artery. Assuming laminar flow, WSS was calculated using the Poiseuille formula: WSS = 4ηQ/(πr3), where WSS is the wall shear stress, η the blood viscosity, Q the local blood flow at rest, and r the arterial radius at the blood flow measurement site.26
Statistical analysis
All data are reported as the means ± SEMs. Analysis of variance (ANOVA) for repeated measurements was used to assess the group effect, order, dose effect, and interaction for the absolute values and percent changes. Paired t tests were then used for pairwise comparisons. Group comparisons were by unpaired t tests.P values of less than .05 were considered statistically significant.
Results
Study population
The patients with SCD and the control subjects were similar regarding age, height, weight, blood pressure, and heart rate at rest (Table 1). Blood hemoglobin and hematocrit levels were significantly lower in the patients than in the controls (hemoglobin: 86 ± 3 vs 140 ± 3 g/L [8.6 ± 0.3 vs 14 ± 0.3 g/dL], respectively,P < .001; and hematocrit: 0.27 ± 0.01 vs 0.42 ± 0.01, [27% ± 1.1% vs 41.9% ± 1%], respectively,P < .001). Blood viscosity was significantly lower in the patients than in the controls (3.6 ± 0.1 vs 4.03 ± 0.15 mPa.s, respectively, P < .05). However, the ratio of viscosity over hematocrit was significantly higher in the patients (13.6 ± 0.6 vs 9.6 ± 0.3 in the controls,P < .001), indicating marked alteration of the physical properties of red blood cells in SCD. No short-term adverse effects were observed in any of our patients after the investigations. In the 3 months following the study, a single patient had a minor vaso-occlusive crisis. This patient did not exhibit any features of note during the study.
Evaluation of endothelial function using brachial arterial diameter monitoring
To evaluate WSS-mediated vasodilation, we measured the percentage increase in arterial diameter following a hyperemia test, as described in “Patients, materials, and methods.” We also monitored changes in brachial artery flow to compare blood flow variations and arterial diameter variations. As shown in Table 2, the brachial artery flow and baseline brachial artery diameter tended to be higher in the patients than in the controls, although the difference was not statistically significant. However, the calculated WSS at baseline was significantly higher in the patients than in the controls.
Baseline and maximal flow, diameter, wall shear stress, and flow-mediated dilation in the brachial artery of control subjects and patients
| . | AA . | SS . | P . |
|---|---|---|---|
| n | 15 | 16 | NS |
| Baseline flow, mL/min | 47 ± 12 | 89 ± 22 | NS |
| Baseline diameter, mm | 4.29 ± 0.12 | 4.58 ± 0.15 | NS |
| Maximal flow, mL/min | 132 ± 23.9 | 218.9 ± 32.3 | < .05 |
| Maximal diameter, mm | 4.46 ± 0.13 | 4.66 ± 0.16 | NS |
| Baseline WSS, dyne/cm2 | 3.86 ± 0.7 | 7.18 ± 1.3 | < .05 |
| Maximal WSS, dyne/cm2 | 11.3 ± 1.3 | 15.2 ± 3 | NS |
| FMD, % | 3.97 ± 0.24 | 1.73 ± 0.44 | < .05 |
| . | AA . | SS . | P . |
|---|---|---|---|
| n | 15 | 16 | NS |
| Baseline flow, mL/min | 47 ± 12 | 89 ± 22 | NS |
| Baseline diameter, mm | 4.29 ± 0.12 | 4.58 ± 0.15 | NS |
| Maximal flow, mL/min | 132 ± 23.9 | 218.9 ± 32.3 | < .05 |
| Maximal diameter, mm | 4.46 ± 0.13 | 4.66 ± 0.16 | NS |
| Baseline WSS, dyne/cm2 | 3.86 ± 0.7 | 7.18 ± 1.3 | < .05 |
| Maximal WSS, dyne/cm2 | 11.3 ± 1.3 | 15.2 ± 3 | NS |
| FMD, % | 3.97 ± 0.24 | 1.73 ± 0.44 | < .05 |
AA indicates controls; SS, patients; NS, not significant; WSS, wall shear stress; FMD, flow-mediated dilation.
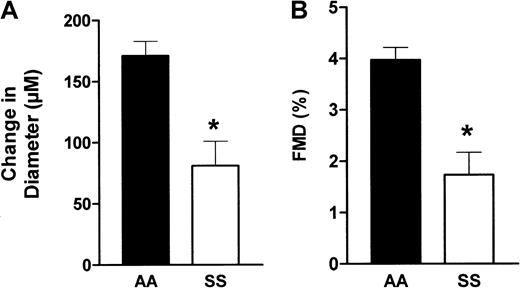
In response to hyperemia, brachial artery flow increased to a greater value in the patients than the controls, but the percentage flow increase was similar in the 2 groups (+193% vs +234% in the patients and controls, respectively, P > .05). However, as shown in Figure 1, FMD was 56% lower in the patients than in the controls (1.73% ± 0.44% vs 3.97% ± 0.24%, P < .001 by t test). This marked FMD decrease in the patients was observed despite the fact that the calculated WSS reached a higher value in the patients following hyperemia, indicating blunted reactivity of the endothelium to an increase in flow.
Brachial artery diameter variations in response to hyperemia.
Evaluation of changes in arterial diameter in response to hyperemia in patients (SS) and controls (AA). Brachial artery diameter was determined using an echo-tracking system before and after a hyperemia test, as described in “Patients, materials, and methods.” (A) The change in diameter, which is the difference (DM − DB) expressed in micrometers, where DM is the maximum diameter following hyperemia and DB the baseline diameter. (B) The FMD, which is calculated as the ratio 100(DM − DB)/DB. *, P < .01.
Brachial artery diameter variations in response to hyperemia.
Evaluation of changes in arterial diameter in response to hyperemia in patients (SS) and controls (AA). Brachial artery diameter was determined using an echo-tracking system before and after a hyperemia test, as described in “Patients, materials, and methods.” (A) The change in diameter, which is the difference (DM − DB) expressed in micrometers, where DM is the maximum diameter following hyperemia and DB the baseline diameter. (B) The FMD, which is calculated as the ratio 100(DM − DB)/DB. *, P < .01.
We then sought to determine whether a decrease in WSS was associated with reduced vasoconstriction in the patients. Hyperoxia is known to reduce cardiac output, mainly via an increase in peripheral resistance. Upon exposure to 100% oxygen, brachial artery blood flow decreased to a similar extent in the 2 groups (−12.3% ± 0.06% of baseline in the patients and −13.2% ± 0.29% of baseline in the controls, nonsignificant). However, after 10 minutes inhaling 100% oxygen, the patients exhibited a significant, 4-fold reduction in constriction as compared with the controls (−1.58% ± 1.4% and −6.48% ± 0.5%, respectively, P = .002 by the Scheffé test). Repeated measures ANOVA revealed a highly significant difference between the 2 groups, withP < .0001 (Figure 2).
Effect of hyperoxia on brachial artery diameter in patients and controls.
Evaluation of changes in arterial diameter (expressed as a percentage from baseline) in response to 100% oxygen inhalation in patients with SCD (▵) and control subjects (▪). Subjects were asked to breathe normally for 10 minutes in a facial mask delivering 100% oxygen. Arterial diameter measurements were performed at baseline and after 5 and 10 minutes of oxygen inhalation. *, P < .01 by ANOVA.
Effect of hyperoxia on brachial artery diameter in patients and controls.
Evaluation of changes in arterial diameter (expressed as a percentage from baseline) in response to 100% oxygen inhalation in patients with SCD (▵) and control subjects (▪). Subjects were asked to breathe normally for 10 minutes in a facial mask delivering 100% oxygen. Arterial diameter measurements were performed at baseline and after 5 and 10 minutes of oxygen inhalation. *, P < .01 by ANOVA.
Pharmacologic evaluation of FBF using VOP
We evaluated endothelial responses to pharmacologic stimulation and inhibition in the patients and controls to explore another major pathway of NOS activation. Acetylcholine is a potent M3 receptor agonist responsible for calcium-dependent enhancement of NO production by endothelial cells.
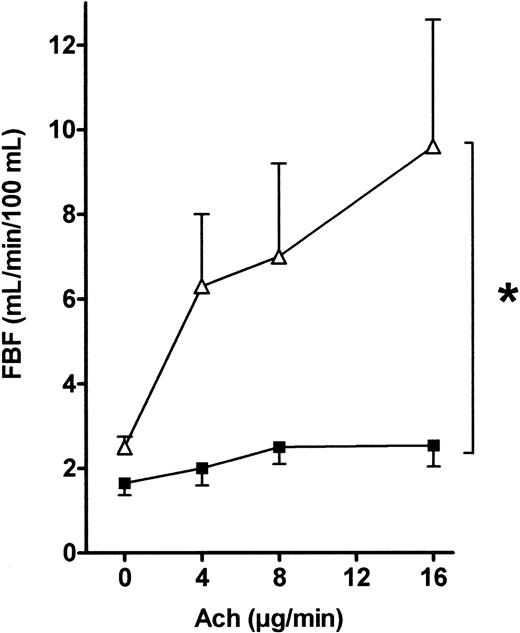
As shown in Figure 3, FBF was significantly higher at baseline in the patients than in the controls (2.5 ± 0.3 vs 1.6 ± 0.2 mL/min/100 mL, respectively,P < .05). This difference was stable throughout the VOP procedure. Figure 3 shows that the FBF increase after a 16 μg/min acetylcholine infusion was significantly greater in the patients than in the controls (9.7 ± 2.9 vs 2.5 ± 1.5 mL/min/100 mL, respectively, P < .001 by the Scheffé test). This increase was dose-dependent, and ANOVA of the effects of 4, 8, and 16 μg/min acetylcholine showed significantly larger FBF increases in the patients than in the controls (P < .001). When FBF values recorded during acetylcholine infusions were normalized for baseline FBF values (to correct for the flow difference at baseline), similar results were obtained (data not shown).
FBF variations in response to acetylcholine infusion into the brachial artery in patients and controls.
Acetylcholine (4, 8, and 16 μg/min) was infused into the brachial artery, and FBF variations expressed in milliliters per minute per 100 milliliters were recorded using VOP. ANOVA detected a significant difference between the 2 groups (*, P = .02). ▵, patients; ▪, controls.
FBF variations in response to acetylcholine infusion into the brachial artery in patients and controls.
Acetylcholine (4, 8, and 16 μg/min) was infused into the brachial artery, and FBF variations expressed in milliliters per minute per 100 milliliters were recorded using VOP. ANOVA detected a significant difference between the 2 groups (*, P = .02). ▵, patients; ▪, controls.
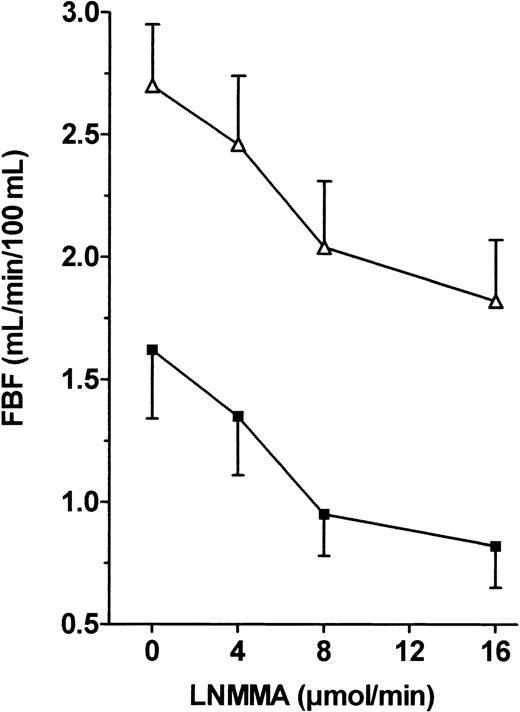
Endothelial NO production is continuously stimulated by shear stress, and NO exerts a continuous and regulated vasodilator effect. We used infusion of the specific NOS inhibitor L-NMMA to evaluate the biologic effect of basal NO on vascular tone. Figure 4 shows that L-NMMA induced significant vasoconstriction in both groups, but ANOVA of FBF variations showed a nonsignificant difference between the 2 groups when FBF values were expressed as raw data. However, the percentage vasoconstriction from baseline in response to 16 μM/min L-NMMA was significantly lower in the patients than in the controls (−34.3% ± 3.6% vs −51.4% ± 5.4%, respectively,P < .05).
FBF variations in response to L-NMMA infusion into the brachial artery in patients and controls.
L-NMMA (0.5, 0.8, and 1 μg/min) was infused into the brachial artery, and FBF variations expressed in milliliters per minute per 100 milliliters were recorded using VOP. ANOVA detected no significant difference between the 2 groups. ▵, patients; ▪, controls.
FBF variations in response to L-NMMA infusion into the brachial artery in patients and controls.
L-NMMA (0.5, 0.8, and 1 μg/min) was infused into the brachial artery, and FBF variations expressed in milliliters per minute per 100 milliliters were recorded using VOP. ANOVA detected no significant difference between the 2 groups. ▵, patients; ▪, controls.
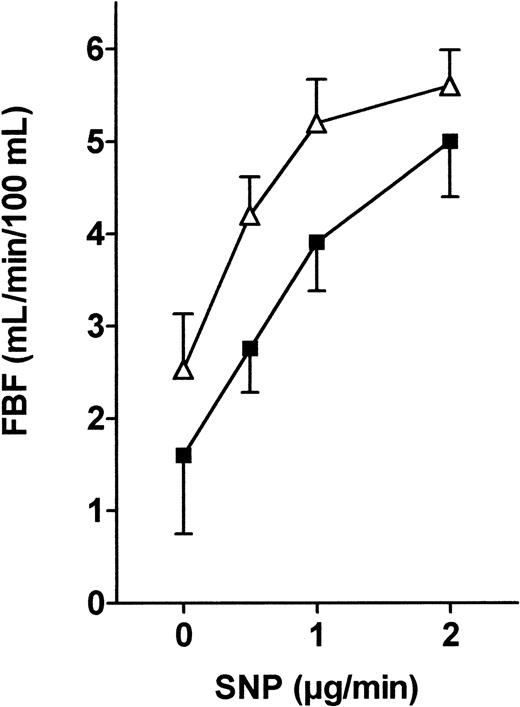
Endogenously produced NO diffuses out of the endothelial cells and promotes relaxation of smooth muscle cells through the production of cyclic guanosine monophosphate by soluble guanylate cyclase. To evaluate endothelium-independent vasodilation, we induced direct smooth muscle cell relaxation by administering the potent nitrovasodilator SNP. As shown in Figure 5, we found a similar dose-dependent SNP-induced vasodilation in both groups (nonsignificant by ANOVA).
FBF variations in response to SNP infusion into the brachial artery in patients and controls.
SNP (4, 8, and 16 μM/min) was infused into the brachial artery, and FBF variations expressed in milliliters per minute per 100 milliliters were recorded using VOP. ANOVA detected no significant difference between the 2 groups. ▵, patients; ▪, controls.
FBF variations in response to SNP infusion into the brachial artery in patients and controls.
SNP (4, 8, and 16 μM/min) was infused into the brachial artery, and FBF variations expressed in milliliters per minute per 100 milliliters were recorded using VOP. ANOVA detected no significant difference between the 2 groups. ▵, patients; ▪, controls.
Discussion
We found evidence of marked alteration in WSS-induced endothelium-dependent vasodilation in patients with SCD. Both the high baseline WSS and the impaired FMD in the patients suggest failure of muscular arteries to adjust their internal diameter in response to mechanical stimulation. The blunted oxygen-induced vasoconstriction also points to a decreased endothelial response to flow variations. The increased acetylcholine-induced vasodilation and unchanged SNP-induced vasodilation argue for a selective alteration in shear stress signal transduction in the vascular wall rather than for a reduction in NOS activity. This is further supported by the similar baseline NO release in the patients and controls as assessed by the response to L-NMMA.
Numerous studies have found evidence that endothelial function plays a critical role in protecting against platelet activation, thrombosis, and vascular remodeling. Endothelial cells respond to circulating vasoactive compounds and to variations in WSS, the dragging frictional force created by blood flow and blood viscosity.13 WSS stimulates the release of vasoactive substances such as NO, modifies gene expression, and alters cell metabolism and morphology.27 One consequence of an acute or chronic increase in WSS in large and small arteries is an adjustment in vessel caliber to restore WSS to its baseline value.16 Both acute and chronic vessel diameter increases in response to shear stress have been shown to involve endothelial release of NO. A sustained decrease in WSS leads to a diminution in arterial caliber, and the capacity for vasodilation and vasoconstriction adjusts to the level corresponding to the new size of the vessel.17,28 Similarly, Tronc et al have shown that a sustained WSS increase induces an NO-dependent increase in arterial diameter that normalizes WSS regardless of blood flow.16 Therefore, failure of vessels to adjust their diameter to WSS changes probably reflects an impairment in the basal or stimulated release of NO.
In the present study, we first investigated baseline brachial artery flow, blood viscosity, and brachial artery diameter in patients with SCD to determine baseline WSS. In accordance with previous studies showing a 50% increase in cardiac output in SCD patients,18 we found that brachial artery flow was about 50% higher in the SCD patients than in the controls. Blood viscosity was slightly lower in the patients, but this difference was due mainly to a lower hematocrit level. When blood viscosity was normalized for the hematocrit using Chien's curves of blood viscosities by cell concentrations,29 viscosity was considerably higher in the patients than in the controls. This observation is consistent with previous data from patients with SCD of similar severity; it reflects the lack of deformability of sickle cells, even during exposure to high oxygen levels.19 Because the SCD patients had a marked FBF increase and a small blood viscosity decrease, their baseline brachial artery WSS was higher than in the controls. Interestingly, baseline brachial artery diameter was similar in the patients and controls, indicating impaired arterial diameter adjustment to the chronically increased WSS in the patients. Moreover, an acute increase in WSS in response to reactive hyperemia induced a smaller increase in brachial artery diameter in the patients than in the controls. This difference occurred although the relative increase in brachial artery flow generated by hyperemia was similar in the 2 groups. The slight increase in arterial diameter in the patients as compared with the controls could suggest a decrease in vasodilation reserve, the artery being closer to maximum dilation. However, the difference between baseline diameters was not statistically significant. Moreover, previous studies have shown that a chronic increase in arterial diameter in response to WSS indicates vascular remodeling rather than vasodilation, and remodeled vessels would be expected to have a normal vasodilation reserve.17 28 Overall, these results strongly suggest that systemic arteries in patients with SCD fail to adjust appropriately to chronic or acute increases in WSS.
A decrease in WSS can be obtained by decreasing the brachial artery flow. This was achieved in our study by inhalation of pure oxygen. An increase in arterial oxygen content is known to induce marked vasoconstriction of the microcirculation, leading to an increase in peripheral resistance. Hyperoxia and increased resistance are responsible for a decrease in cardiac output.30 Following inhalation of 100% oxygen for 10 minutes, we observed similar decreases in brachial artery flow in the patients and controls. However, the decrease in brachial artery diameter was considerably smaller in the patients. This finding is consistent with a previous report of a very small hyperoxia-induced decrease in arteriolar diameter in sickle transgenic mice.31 The authors suggested that the altered microvascular response to oxygen resulted from blood rheologic changes or intrinsic differences in endothelial cell/vascular smooth muscle function. In the light of the present results in humans, the impaired vasoconstrictor response to hyperoxia was probably due, at least in part, to endothelial dysfunction. However, we cannot exclude that other effects of oxygen were involved in the absence of a vasoconstrictive response in our patients with SCD.
As stated above, in vitro and in vivo studies in large and small arteries have indicated that acute or chronic flow-induced dilation is mediated by NO released by the endothelium. Failure of muscular arteries to adjust their diameter in response to changes in WSS could theoretically result from several mechanisms, including impaired synthesis or release of NO, acceleration of NO degradation, or alterations in the endothelial-sensitive signal-transduction pathway leading to endothelial NOS activation. To assess this issue, we used plethysmography to measure FBF during infusion of the endothelium-dependent vasodilator acetylcholine and the NO-donor SNP in the patients and controls. Interestingly, we found that the vasodilator response to acetylcholine was considerably stronger in the SCD patients than in the controls, suggesting enhanced synthesis and/or release of NO induced by pharmacologic stimuli. The vasodilator response to SNP was similar in the patients and controls, indicating that the smooth muscle vasodilator response to NO was normal in patients with SCD. The potentiated endothelium-dependent response to acetylcholine, together with the normal nitrovasodilator (SNP) response, suggests increased production and/or decreased degradation of NO in SCD patients. These findings are consistent with previous animal studies suggesting that endothelial NOS expression and/or activity may be increased in SCD10,32,33 and with other studies showing that endothelial NOS expression is increased by shear stress.34 35Increased endothelium-dependent vasodilation to acetylcholine has also been demonstrated in patients with severe anemia due to various causes. In these patients, the vasoconstrictor response to the selective NO formation inhibitor L-NMMA was potentiated, and correction of anemia was associated with a return of the increased acetylcholine response to the control level. Because the vasoconstrictor response to L-NMMA is thought to be a reliable indicator of basal NO release, this finding was taken as evidence that low hematocrit levels potentiate NO-mediated vasodilation, possibly by increasing the half-life of NO. In our study in SCD patients, the absolute vasoconstrictor response to L-NMMA was similar in the patients and controls, and the percentage response was smaller in the patients. This may indicate that the amount of NO released by the endothelium under basal conditions in SCD patients is similar or lower than in normal subjects. An increase in the basal production of NO would be expected in SCD patients because of their high basal WSS values. Again, this may indicate an inappropriately low level of NO release under basal conditions in patients with steady-state SCD.
These observations suggest that endothelial NO is functionally active in patients with SCD. Possible explanations for the increased vasodilator response to acetylcholine include an increase in the half-life of NO and up-regulation of endothelial NOS activity and/or expression. The main finding from our study is that endothelium-dependent vasodilation mediated by WSS was markedly impaired in patients with SCD. Because WSS is the main physiologic stimulus of NOS, and because variations in WSS lead to adjustments in vessel diameter, this impairment may play a pivotal role in the pathogenesis of vaso-occlusive crisis in patients with SCD. Failure of vessel diameter to adjust to rheologic conditions may favor interactions between SSRBCs and the vessel wall, thereby precipitating vaso-occlusive events. Moreover, insufficient release of NO, which not only induces smooth muscle cell relaxation but also inhibits platelet adhesion and aggregation, decreases the expression of adhesion molecules such as VCAM-1 and may therefore have important pathogenic consequences in SCD.
In conclusion, SCD is characterized by an impairment of the endothelial response to chronic or acute WSS variations in the brachial artery. One likely mechanism of this abnormality is decreased mechanical transduction in endothelial cells secondary to chronic elevation of WSS. Further studies are needed to clarify the complex mechanisms underlying the impaired response to WSS variations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laurent Belhassen, Hôpital Henri Mondor, Service de Physiologie— Explorations Fonctionnelles, 51 rue du Maréchal De Lattre de Tassigny, 94010 CRETEIL, France; e-mail:laurent.belhassen@hmn.ap-hop-paris.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal