A recent clinical trial of gene therapy for X-linked severe combined immunodeficiency (XSCID) has shown that retroviral-mediated gene correction of bone marrow stem cells can lead to the development of normal immune function. These exciting results have been preceded by successful immune reconstitution in several XSCID mouse models, all carrying null mutations of the common gamma chain (γc). One question not formally addressed by these previous studies is that of possible dominant-negative effects of the endogenous mutant γc protein on the activity of the wild-type transferred gene product. The present work was therefore undertaken to study whether corrective gene transfer was applicable to an XSCID murine model with preserved expression of a truncated γc molecule (Δγc+-XSCID). Gene correction of Δγc+-XSCID mice resulted in the reconstitution of lymphoid development, and preferential repopulation of lymphoid organs by gene-corrected cells demonstrated the selective advantage of γc-expressing cells in vivo. Newly developed B cells showed normalization of lipopolysaccharide-mediated proliferation and interleukin-4 (IL-4)–induced immunoglobulin G1 isotype switching. Splenic T cells and thymocytes of treated animals proliferated normally to mitogens and responded to the addition of IL-2, IL-4, and IL-7, indicating functional reconstitution of γc-sharing receptors. Repopulated thymi showed a clear increase of CD4−/CD8− and CD8+fractions, both dramatically reduced in untreated Δγc+-XSCID mice. These improvements were associated with the restoration of Bcl-2 expression levels and enhanced cell survival. These data indicate that residual expression of the endogenous truncated γc did not lead to dominant-negative effects in this murine model and suggest that patient selection may not be strictly necessary for gene therapy of XSCID.
Introduction
X-linked severe combined immunodeficiency (XSCID), the most common form of severe combined immunodeficiency, is characterized by profound defects of humoral and cellular immunity.1 Affected boys succumb during infancy to severe infections, unless allogeneic bone marrow transplantation (BMT) is successfully performed. Genetic defects of expression and function of the γc of cytokine receptors have been shown to be responsible for this disease.2,3 The involvement of γc in multiple cytokine receptors, including those for interleukin-2 (IL-2), IL-4, IL-7, IL-9, and IL-15, explains the severe impairment of lymphoid development and function in XSCID patients,4,5 as has also been illustrated by the generation of a series of γc-knockout mice,6-9 which manifest a severe immunodeficient phenotype.
Since its first application in 1968,10 BMT has been performed as a treatment for XSCID with great success.11,12 However, potential severe complications, such as graft-versus-host disease, and incomplete reconstitution of B-cell immunity make BMT imperfect and leave room for improvement. Genetic correction of autologous hematopoietic stem cells (HSCs) has been proposed as a beneficial alternative therapeutic approach for this disease.13-17
Recently, we and others have demonstrated the feasibility of stem cell gene therapy for XSCID using murine models.18-20 Soon thereafter, the results of the first human clinical trial for XSCID patients were reported and showed clear evidence of a clinical benefit associated with the gene correction procedures.21
Mutated proteins have been demonstrated to act as transdominant inhibitors of wild-type protein functions in various systems,22,23 and dominant-negative effects of endogenous mutant proteins may hinder the efficacy of gene therapy attempts.23 Mutation analysis efforts have shown that a significant number of XSCID patients carry genetic aberrations compatible with residual γc protein expression.24 These mutant receptor chains may show dominant-negative effects against the wild-type γc introduced in the patients' cells by gene transduction, thus limiting the efficacy and potential applications of gene therapy for XSCID. We have used a murine model of XSCID carrying a truncated γc to formally examine whether retroviral-mediated gene therapy is affected by the presence of residual endogenous γc expression. Treated mice showed restored lymphoid development with evidence of coexpression of mutant and ectopic normal γc, suggesting that the presence of the truncated γc did not hinder the therapeutic effects of gene transfer. Moreover, we demonstrated that the functional reconstitution of γc-mediated signaling led to a correction of immunoglobulin (Ig) isotype switching in B cells and to the up-regulation of Bcl-2 and enhanced cell survival in gene-corrected thymocytes.
Materials and methods
Mice
Generation and characterization of Δγc+-XSCID mice were previously described.8 As a result of gene targeting, these mice express a truncated γc protein with conserved extracellular and transmembrane domains. We used affected male mice and normal littermates that had been backcrossed onto a C57BL/6 background for at least 15 generations. All animal experiments were approved by the National Human Genome Research Institute Animal Care and Use Committee.
Retroviral-mediated stem cell gene correction procedures
The retroviral producing cell line and transduction procedures were previously described.19 Briefly, the retroviral vector MNDmγc, carrying the murine γc (mγc) cDNA, was constructed using the MND-X-IRES-EGFP vector (gift of Dr D. B. Kohn, Children's Hospital, Los Angeles, CA) after removal of the IRES-EGFP fragment and packaged using the ecotropic GP+E-86 cells.25Bone marrow (BM) cells obtained from 5-fluorouracil (5-FU)–treated Δγc+-XSCID mice (4-14 weeks old) were prestimulated for 48 hours with 10 ng/mL murine IL-3 (Peprotech, Rocky Hill, NJ), 100 ng/mL human IL-6 (Amgen, Thousand Oaks, CA), and 100 ng/mL rat stem cell factor (Amgen). After 48 hours of co-cultivation with producing cells, 1 to 4 × 106 nonadherent cells were injected intravenously into lethally irradiated (9 Gy) 8-week-old Δγc+-XSCID mice (referred to as mγc-BMT mice). As control, BM cells harvested from either normal or Δγc+-XSCID mice after 5-FU treatment were mock transduced and transplanted into Δγc+-XSCID mice. The animals receiving normal and Δγc+-XSCID BM cells were designated control-BMT and mock-BMT mice, respectively.
Polymerase chain reaction–based estimation of transgene copy numbers
The polymerase chain reaction (PCR) method used to assess transgene copy numbers in vector-transduced cells was previously described.19 Briefly, genomic DNA was prepared from tissue samples using the SNAP genomic DNA isolation kit (Invitrogen, Carlsbad, CA). The integrated provirus and β-globin sequences were co-amplified for 29 and 25 cycles, respectively, from 20 ng test DNA and reference standards. Amplified products were then analyzed using the BIT Image software (Aladdin Systems, Watsonville, CA) and the NIH Image software (http://rsb.info.nih.gov/nih-image) as described.19
Reverse transcription–polymerase chain reaction analysis of γc expression
Total RNA samples were prepared from BM cells with the QIAamp RNA Blood Mini Kit (Qiagen, Valencia, CA), followed by first-strand DNA synthesis using the Retroscript kit (Ambion, Austin, TX). The following primers were used for PCR amplification of obtained cDNA: P1, 5′-CTGCTCAGAATGCCTCCAATTCC-3′; P2, 5′-CCTGCGTGCAATCCATCTTGTTCAAT-3′; P3, 5′-TCTGCAGCCAGACTACAGTG-3′; P4, 5′-GATCCAGATTGCCAAGGTGAGTAG-3′; P5, 5′-GCTCGTACTCTATAGGCTTC-3′. The cyclophilin primers were from the QuantumRNA kit (Ambion). Equal amounts of first-strand cDNA were added to a mixture of 0.2 μM dNTP, 1.5 mM MgCl2, 2.5 U AmpliTaq DNA polymerase, 1× PCR buffer (PE Biosystems, Foster City, CA), and 0.4 μM each primer. PCR reactions were performed using 34 amplification cycles (94°C for 30 seconds, 58°C for 30 seconds), and the amplified products were separated on a 2% agarose gel and then visualized by ultraviolet light.
Flow cytometry analysis of peripheral blood lymphocytes
Peripheral blood (PB) samples were collected from retro-orbital sinus 8 weeks after BMT, and white blood cells were enumerated using a Multisizer (Coulter Electronics, Hialeah, FL). PB samples were stained with combinations of monoclonal antibodies (mAbs) described below. The percentage of each lymphoid fraction was determined by flow cytometry using a FACSCalibur and the CellQuest software (Becton Dickinson, San Jose, CA), and the obtained value was used to estimate the absolute cell count of each PB lymphocyte subset. The following mAbs were used: fluorescein isothiocyanate (FITC)–anti-CD8a (Ly-53-6.7), phycoerythrin (PE)–anti-CD62L (L-selectin; MEL-14), CyChrome (Cy)–anti-CD4 (GK1.5), PE–anti-IgMb (Igh-6b), Cy–anti-CD45R/B220 (RA3-6B2), PE–anti–natural killer (NK) 1.1 (Ly-55), and Cy–anti-CD3e (145-2C11). All flow cytometry reagents were purchased from Pharmingen (San Diego, CA).
In vitro immunoglobulin isotype switching assay
Splenocytes obtained from euthanized mice at 18 to 22 weeks after BMT were plated at 1 × 106 cells/mL in R-10 medium (RPMI 1640 containing 10% fetal bovine serum, and 50 μM 2-mercaptoethanol). For the induction of IgG3 isotype switching, lipopolysaccharide (LPS; 20 μg/mL; Sigma, St Louis, MO) was added to the cultures. To induce IgG1 or IgE isotype switching, LPS plus IL-4 (25 ng/mL; Peprotech) were used with daily addition of 500 ng/mL anti–interferon γ neutralizing Ab (R4-6A2) or 500 ng/mL rat IgG1 (R3-34). After 6 days of culture, cells were collected and stained with either biotin-conjugated anti-IgG1 (A85-1), anti-IgG3 (R40-82), anti-IgE (R35-72), or rat IgG1. Cells were then stained with Cy-B220 and streptavidin-PE, and analyzed by flow cytometry. All flow cytometry reagents were from Pharmingen.
Cell proliferation assay
Splenocytes obtained from euthanized mice at 18 to 22 weeks after BMT were seeded in triplicate in 96-well flat-bottom plates (1 × 105 cells/well) in R-10 medium. Thymocytes were cultured in the same medium at 5 × 104 cells/well in 96-well U-bottom plates. Cells were cultured for 48 hours with or without the addition of the mitogens listed below and were pulsed with 0.5 μCi/well of [3H]-thymidine (NEN-Dupont, Boston, MA) for the final 16 hours. Cells were then harvested, and incorporated radioactivity was determined by using a scintillation counter. The stimulants used were as follows: LPS (20 μg/mL; Sigma), phorbol myristate acetate (PMA; 10 ng/mL; Sigma), ionomycin (500 ng/mL; Sigma), soluble anti-CD3e (145-2C11; 20 μg/mL; Pharmingen), soluble anti-CD28 (37.51; 2 μg/mL; Pharmingen), concanavalin A (ConA; 2 μg/mL; Sigma), human IL-2 (500 U/mL), murine IL-4 (50 ng/mL; R&D Systems, Minneapolis, MN), and murine IL-7 (50 ng/mL; Peprotech).
Immunophenotypic and functional analyses of thymocytes
Thymocytes were collected from euthanized animals at 18 to 22 weeks after BMT and then analyzed by flow cytometry. For immunophenotype determination, cells were stained with FITC-CD8a and Cy-CD4 mAbs. To assess mγc expression, cells were stained with either purified rat IgG2a (R35-95) or anti-mγc mAbs [4G3 or TUGm3],26 followed by biotin–antirat immunoglobulin and streptavidin-PE. Bcl-2 expression levels were determined by intracellular staining according to previously described procedures27 with minor modifications. Briefly, cells were first stained with FITC-CD8a and Cy-CD4 mAbs, then permeabilized using the Cytofix/Cytoperm kit. This was followed by intracellular staining with PE-isotype control or PE–anti–Bcl-2 mAb 3F11. To assess the spontaneous death of thymocyte subsets, cells were cultured for 24 hours in R-10 medium, then stained with FITC-CD8a and PE-CD4 mAbs and followed by the addition of 7-amino-actinomycin D (7-AAD). Dead cells were defined as 7-AAD–positive cells in each fraction determined by CD4/CD8 staining. All reagents, except for TUGm3, were purchased from Pharmingen.
Results
Successful mγc gene transfer and expression in hematopoietic stem cells of Δγc+-XSCID mice
BM cells collected from Δγc+-XSCID mice were transduced with the retroviral vector MNDmγc and then infused into irradiated recipient animals. At 8 weeks after BMT, 8 mγc-BMT mice, 3 control-BMT mice, and 4 mock-BMT mice were available for analysis.
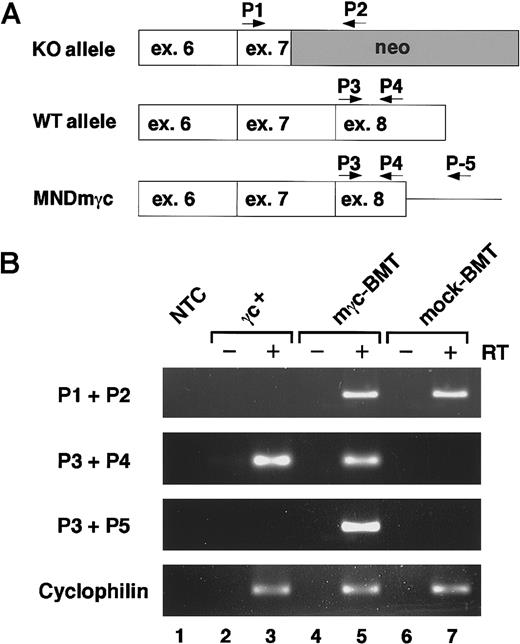
To assess the mγc transgene expression in treated mice, we performed reverse transcription (RT)–PCR analysis on BM samples. According to the particular experimental group, mice were expected to express one or more γc transcripts (Figure 1A). Amplification reactions using the P1 and P2 primers specific for the targeted il-2rg allele (knockout allele) generated similar levels of amplicons in samples from mγc-BMT and mock-BMT mice (Figure1B, lanes 5 and 7), whereas no product was obtained in untreated normal mice (lane 3). Using the primers specific for exon 8 that has been targeted in Δγc+-XSCID mice (P3 + P4), amplicons were generated only in samples from untreated normal mice reflecting endogenous γc-mRNA and mγc-BMT mice representing retroviral vector-mediated transgene expression (Figure 1B, lanes 3 and 5). When amplified with vector-specific primers (P3 + P5), γc transcripts were demonstrable only in the sample originated from mγc-BMT mice (Figure 1B, lane 5), confirming retroviral vector-mediated transgene expression in treated mice. Similar intensity of internal control cyclophilin signals in all samples (lanes 3, 5, 7) and absence of amplification products in RT minus reactions (lanes 2, 4, 6) confirmed the accuracy of this study. Collectively, retroviral-mediated γc gene transfer into BM cells of Δγc+-XSCID mice resulted in significant levels of expression of the ectopic, normal γc while in the presence of the truncation mutant γc gene. Because the truncated γc is expressed on the surface of Δγc+-XSCID cells8, flow cytometry analysis of γc expression could not be easily used in this model as a measure of successful gene transfer.
Analysis of retroviral-mediated expression of γc.
(A) Schematic representation of the relative location of primers used for RT-PCR analysis of mγc expression. Primers P1 and P2 are specific for the transcripts derived from the targeted il-2rg allele in Δγc+-XSCID mice (knockout allele, KO). Primers P3 and P4 amplify equal fragments from the endogenous wild-type (WT allele) and retroviral-expressed mγc (MNDmγc). Primers P3 and P5 were used to specifically amplify the MNDmγc-derived transcripts. ex, exon; neo, neomycin-resistant gene cassette. (B) RT-PCR analysis of mγc transcripts. Expression levels of γc transcripts originated by RT-PCR amplification of BM samples of wild-type γc+, gene-corrected (mγc-BMT), and mock-treated (mock-BMT) mice are shown. Amplicons were not detected in control samples without template (NTC, lane 1) or in samples amplified in the absence of the reverse transcriptase step (RT−). Similar amplification of cyclophilin sequences demonstrated equal loading of each sample.
Analysis of retroviral-mediated expression of γc.
(A) Schematic representation of the relative location of primers used for RT-PCR analysis of mγc expression. Primers P1 and P2 are specific for the transcripts derived from the targeted il-2rg allele in Δγc+-XSCID mice (knockout allele, KO). Primers P3 and P4 amplify equal fragments from the endogenous wild-type (WT allele) and retroviral-expressed mγc (MNDmγc). Primers P3 and P5 were used to specifically amplify the MNDmγc-derived transcripts. ex, exon; neo, neomycin-resistant gene cassette. (B) RT-PCR analysis of mγc transcripts. Expression levels of γc transcripts originated by RT-PCR amplification of BM samples of wild-type γc+, gene-corrected (mγc-BMT), and mock-treated (mock-BMT) mice are shown. Amplicons were not detected in control samples without template (NTC, lane 1) or in samples amplified in the absence of the reverse transcriptase step (RT−). Similar amplification of cyclophilin sequences demonstrated equal loading of each sample.
Restored lymphocyte development by stem cell gene correction
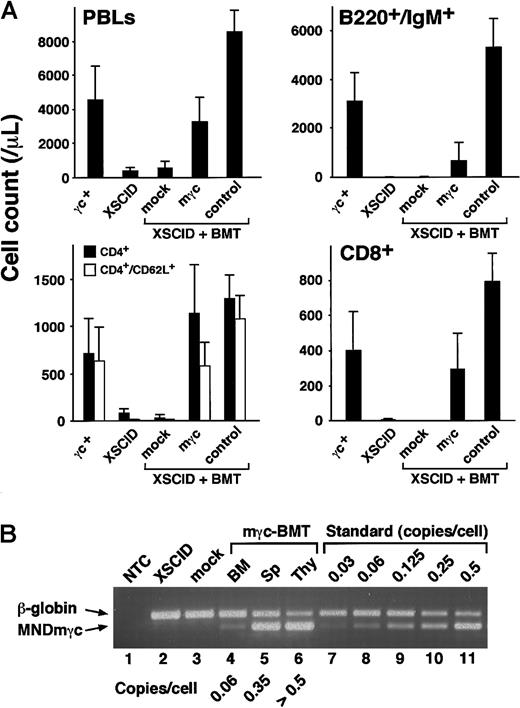
To assess whether γc transgene expression in the presence of mutant γc leads to the restoration of lymphoid development in Δγc+-XSCID mice, we analyzed PB samples at 8 weeks after BMT. Absolute PB lymphocyte counts showed an approximately 8-fold increase in mγc-BMT mice compared to untreated Δγc+-XSCID mice, reaching levels comparable to those of normal mice (Figure 2A, PBLs). FACS analysis of PB lymphocytes demonstrated the reconstitution of all lymphoid fractions, including mature B cells (B220+/IgM+), naive CD4+ T cells, CD8+ T cells, and mature NK cells (CD3−/NK1.1bright) (Figure 2A and data not shown). Mature B cells increased by approximately 130-fold, though the absolute values were still significantly lower than normal. CD4+ T cells showed significant increases in total and naive (CD62L+) cell counts (approximately 14- and 60-fold, respectively), and CD8+ T cells increased by approximately 70-fold. No significant changes in any lymphocyte compartment were observed in mock-BMT control mice (Figure 2A, mock), indicating that the BMT procedure itself had no positive effects on lymphoid reconstitution in transplanted animals. NK cells appeared in 7 of 8 treated mice at frequencies ranging between 0.1% and 0.9% of lymphoid cells, whereas none of the untreated Δγc+-XSCID or mock-BMT mice showed detectable NK1.1+ cells (data not shown). Altogether, retroviral-mediated mγc gene transfer into HSCs resulted in the efficient restoration of lymphoid development in transplanted Δγc+-XSCID animals.
Assessment of lymphoid development and transgene copy numbers in treated animals.
(A) Lymphoid development after stem cell gene therapy (8 weeks after BMT). Presented are absolute counts of PB lymphocytes (PBLs), mature B cells (B220+/IgM+), CD4+ T cells (total and CD62L+), and CD8+ T cells. Shown are the mean (±SD) values obtained from 6 wild-type mice (γc+), 9 untreated Δγc+-XSCID mice (XSCID), 4 mock-BMT mice (mock), 8 mγc-BMT mice (mγc), and 3 control-BMT mice (control). (B) Transgene copy numbers in mouse tissues. PCR products derived from the mγc-provirus (326 bp) and control β-globin sequences (401 bp) were separated on agarose gel and quantified as described in “Materials and methods.” Copy numbers estimated by interpolation with the reference standard are indicated. Sp; splenocytes, Thy; thymocytes. As expected, MNDmγc-bands are absent in BM samples obtained from untreated Δγc+-XSCID mice (XSCID, lane 2) and mock-BMT mice (mock, lane 3).
Assessment of lymphoid development and transgene copy numbers in treated animals.
(A) Lymphoid development after stem cell gene therapy (8 weeks after BMT). Presented are absolute counts of PB lymphocytes (PBLs), mature B cells (B220+/IgM+), CD4+ T cells (total and CD62L+), and CD8+ T cells. Shown are the mean (±SD) values obtained from 6 wild-type mice (γc+), 9 untreated Δγc+-XSCID mice (XSCID), 4 mock-BMT mice (mock), 8 mγc-BMT mice (mγc), and 3 control-BMT mice (control). (B) Transgene copy numbers in mouse tissues. PCR products derived from the mγc-provirus (326 bp) and control β-globin sequences (401 bp) were separated on agarose gel and quantified as described in “Materials and methods.” Copy numbers estimated by interpolation with the reference standard are indicated. Sp; splenocytes, Thy; thymocytes. As expected, MNDmγc-bands are absent in BM samples obtained from untreated Δγc+-XSCID mice (XSCID, lane 2) and mock-BMT mice (mock, lane 3).
Copy number assessment of mγc transgene in treated mice
We next set out to quantify the mγc transgene in lymphohematopoietic tissues obtained from treated animals. As shown in Figure 2B, transgene-specific signals were detected in BM, spleen, and thymus of mγc-BMT mice (lanes 4-6), but not in BM samples obtained from untreated Δγc+-XSCID or mock-BMT mice (lanes 2-3). Estimation of average transgene copy numbers demonstrated that the BM samples had relatively low copies of the provirus (approximately 0.06 copies/cell), whereas peripheral lymphoid tissues contained much higher copy numbers of the mγc-transgene (spleen, approximately 0.35; thymus, more than 0.5 copies/cell). Analysis of a second mγc-BMT mouse led to similar results (BM, approximately 0.10; spleen, approximately 0.38; thymus, more than 0.5 copies/cell; data not shown). These results indicate that although relatively low numbers of transduced HSCs engrafted in the BM, gene-corrected cells accumulated and repopulated peripheral lymphoid tissues, especially the thymus, suggesting a selective advantage over noncorrected cells.
Assessment of immunoglobulin isotype switching in B lymphocytes
We and others have previously demonstrated that retroviral-mediated gene correction of XSCID mice results in normal immunoglobulin levels and in the development of humoral immune responses to foreign antigens.18-20 To confirm and extend these findings, we performed in vitro immunoglobulin isotype switching assay on splenic B lymphocytes. DiSanto et al6 have shown that splenic B cells obtained from γc-deficient mice were able to produce IgG3 on LPS stimulation but failed to switch to IgG1-producing cells in response to LPS plus IL-4, indicating an indispensable role of γc in IL-4–mediated immunoglobulin isotype switching. Consistent with this observation, we found that LPS stimulation induced IgG3 surface-positive B cells regardless of the presence or the absence of normal γc (Figure 3A). In contrast, the addition of IL-4 to LPS-stimulated B cells resulted in the appearance of IgG1 surface-positive cells in wild-type (γc+) and mγc-BMT animals, but not in untreated Δγc+-XSCID mice (XSCID), suggesting that transduced γc allowed the IL-4–mediated signaling required for IgG1 class switching (Figure 3B). Similar experiments assessing IgE class switching demonstrated that IgE surface-positive cells could be induced only in B cells from wild-type mice and Δγc+-XSCID mice after gene correction (data not shown). We conclude that stem cell gene correction of Δγc+-XSCID mice resulted in the reconstitution of IL-4 receptor–mediated signaling and the restoration of proper immunoglobulin isotype switching.
Analysis of in vitro immunoglobulin isotype switching in splenic B cells.
(A) LPS stimulation induced IgG3 expression on B220+ B cells obtained from normal γc+, untreated Δγc+-XSCID (XSCID), and gene-corrected Δγc+-XSCID (mγc-BMT) mice. (B) LPS alone failed to induce IgG1 expression, whereas in combination with IL-4, IgG1-expressing B220+ cells developed in samples from normal and mγc-BMT mice but not from untreated Δγc+-XSCID mice. Shown are representative results of 2 independent experiments.
Analysis of in vitro immunoglobulin isotype switching in splenic B cells.
(A) LPS stimulation induced IgG3 expression on B220+ B cells obtained from normal γc+, untreated Δγc+-XSCID (XSCID), and gene-corrected Δγc+-XSCID (mγc-BMT) mice. (B) LPS alone failed to induce IgG1 expression, whereas in combination with IL-4, IgG1-expressing B220+ cells developed in samples from normal and mγc-BMT mice but not from untreated Δγc+-XSCID mice. Shown are representative results of 2 independent experiments.
Proliferative responses of lymphocytes
We next examined whether lymphoid cells developed in mγc-BMT mice proliferated in response to mitogens. When stimulated with LPS, splenocytes obtained from treated animals proliferated similarly to normal splenocytes, whereas cells from untreated Δγc+-XSCID mice showed extremely poor responses (Table1). Proliferation of T cells was tested by stimulating splenocytes with PMA plus ionomycin or plate-bound anti-CD3 plus soluble anti-CD28 mAbs. As shown in Table 1, splenocytes of untreated Δγc+-XSCID mice showed only marginal responses, whereas splenocytes of mγc-BMT mice exhibited significant proliferative responses to both stimuli at levels comparable to normal.
Mitogen-induced proliferative responses of splenocytes
| Experiment . | . | n . | Medium . | LPS . | CD3 + CD28 . | PMA + ionomycin . |
|---|---|---|---|---|---|---|
| XSCID | 1 | 323 ± 51 | 10 021 ± 1504 | 1203 ± 118 | 3564 ± 446 | |
| 1 | γc+ | 1 | 664 ± 3 | 135 343 ± 6844 | 67 522 ± 6813 | 47 717 ± 3485 |
| mγc-BMT | 1 | 594 ± 53 | 105 925 ± 808 | 28 072 ± 1086 | 27 651 ± 812 | |
| XSCID | 4 | 547 ± 138 | 3560 ± 933 | 1226 ± 179 | 2176 ± 712 | |
| 2 | γc+ | 1 | 225 ± 41 | 120 746 ± 4263 | 5183 ± 1432 | 33 840 ± 1864 |
| mγc-BMT | 2 | 638 ± 117 | 67 360 ± 31 299 | 14 795 ± 262 | 18 617 ± 4974 |
| Experiment . | . | n . | Medium . | LPS . | CD3 + CD28 . | PMA + ionomycin . |
|---|---|---|---|---|---|---|
| XSCID | 1 | 323 ± 51 | 10 021 ± 1504 | 1203 ± 118 | 3564 ± 446 | |
| 1 | γc+ | 1 | 664 ± 3 | 135 343 ± 6844 | 67 522 ± 6813 | 47 717 ± 3485 |
| mγc-BMT | 1 | 594 ± 53 | 105 925 ± 808 | 28 072 ± 1086 | 27 651 ± 812 | |
| XSCID | 4 | 547 ± 138 | 3560 ± 933 | 1226 ± 179 | 2176 ± 712 | |
| 2 | γc+ | 1 | 225 ± 41 | 120 746 ± 4263 | 5183 ± 1432 | 33 840 ± 1864 |
| mγc-BMT | 2 | 638 ± 117 | 67 360 ± 31 299 | 14 795 ± 262 | 18 617 ± 4974 |
Shown are the mean values ± SEM of incorporated [3H]-thymidine (cpm) obtained from triplicate cultures.
LPS indicates lipopolysaccharide; PMA, phorbol myristate acetate; XSCID, untreated γc+ X-linked severe combined immunodeficiency mice; γc+, untreated normal mice; mγc-BMT, Δγc+-XSCID mice transplanted with mγc-transduced bone marrow cells.
To examine whether the transduced γc subunit could allow for cytokine responses in T cells, we investigated thymocyte proliferation to ConA stimulation with or without IL-2, IL-4, and IL-7. Consistent with previous observations,6 8 thymocytes obtained from untreated Δγc+-XSCID animals did not show significant proliferation in response to ConA, nor was the response enhanced by the addition of cytokines (Table 2). In contrast, thymocytes developed in mγc-BMT mice showed ConA-mediated proliferation at levels similar to normal, and the addition of cytokines further increased cell proliferation, though to a lesser degree than that observed in normal thymocytes (Table 2). These results indicate the reconstitution of functional γc-containing cytokine receptors in T cells derived from treated animals.
Thymocyte proliferation to concanavalin A stimulation with or without cytokines
| Experiment . | . | n . | Medium . | ConA . | ConA + IL-2 . | ConA + IL-4 . | ConA + IL-7 . | PMA + ionomycin . |
|---|---|---|---|---|---|---|---|---|
| 1 | γc+ | 1 | 53 ± 10 | 1052 ± 212 | 13 962 ± 1812 | 10 575 ± 682 | 4088 ± 234 | 27 312 ± 2057 |
| mγc-BMT | 1 | 53 ± 14 | 1349 ± 231 | 2864 ± 74 | 4291 ± 503 | 2309 ± 212 | 35 899 ± 182 | |
| XSCID | 1 | 64 ± 10 | 271 ± 69 | 244 ± 64 | 228 ± 16 | 279 ± 61 | ND | |
| 2 | γc+ | 1 | 67 ± 4 | 1587 ± 147 | 26 434 ± 1249 | 15 363 ± 3493 | 4823 ± 61 | ND |
| mγc-BMT | 1 | 70 ± 7 | 1218 ± 48 | 1756 ± 254 | 1685 ± 86 | 1740 ± 229 | ND | |
| XSCID | 4 | 58 ± 7 | 85 ± 9 | 71 ± 4 | 81 ± 11 | 77 ± 12 | 2788 ± 518 | |
| 3 | γc+ | 1 | 42 ± 7 | 1484 ± 579 | 16 516 ± 94 | 7842 ± 1037 | 5683 ± 739 | 9183 ± 1417 |
| mγc-BMT | 2 | 48 ± 11 | 765 ± 32 | 1181 ± 615 | 1134 ± 550 | 994 ± 600 | 8086 ± 2102 |
| Experiment . | . | n . | Medium . | ConA . | ConA + IL-2 . | ConA + IL-4 . | ConA + IL-7 . | PMA + ionomycin . |
|---|---|---|---|---|---|---|---|---|
| 1 | γc+ | 1 | 53 ± 10 | 1052 ± 212 | 13 962 ± 1812 | 10 575 ± 682 | 4088 ± 234 | 27 312 ± 2057 |
| mγc-BMT | 1 | 53 ± 14 | 1349 ± 231 | 2864 ± 74 | 4291 ± 503 | 2309 ± 212 | 35 899 ± 182 | |
| XSCID | 1 | 64 ± 10 | 271 ± 69 | 244 ± 64 | 228 ± 16 | 279 ± 61 | ND | |
| 2 | γc+ | 1 | 67 ± 4 | 1587 ± 147 | 26 434 ± 1249 | 15 363 ± 3493 | 4823 ± 61 | ND |
| mγc-BMT | 1 | 70 ± 7 | 1218 ± 48 | 1756 ± 254 | 1685 ± 86 | 1740 ± 229 | ND | |
| XSCID | 4 | 58 ± 7 | 85 ± 9 | 71 ± 4 | 81 ± 11 | 77 ± 12 | 2788 ± 518 | |
| 3 | γc+ | 1 | 42 ± 7 | 1484 ± 579 | 16 516 ± 94 | 7842 ± 1037 | 5683 ± 739 | 9183 ± 1417 |
| mγc-BMT | 2 | 48 ± 11 | 765 ± 32 | 1181 ± 615 | 1134 ± 550 | 994 ± 600 | 8086 ± 2102 |
Shown are the mean values ± SEM of incorporated [3H]-thymidine (cpm) obtained from triplicate cultures.
ConA indicates concanavalin A; IL, interleukin; ND, not determined; for other abbreviations, see Table 1.
Immunophenotypic analysis of thymocytes
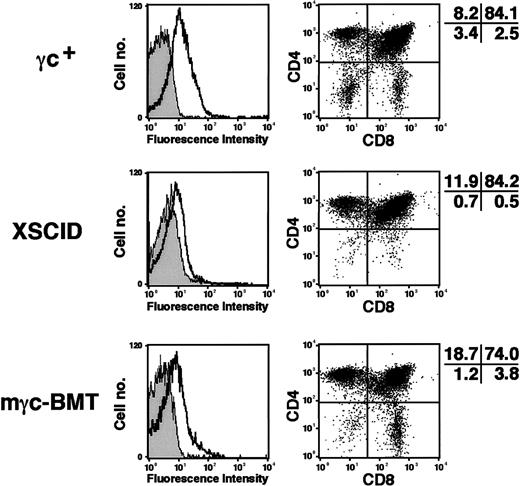
Although the repopulation of thymus in XSCID mice after retroviral-mediated gene therapy was suggested in a previous report by the increased thymocyte numbers,18 detailed immunophenotypic analysis was not reported. We deemed it important to determine the extent of normalization of thymic subpopulations induced in Δγc+-XSCID mice by corrective gene transfer. We assessed thymic cellularity and found a dramatic increase of thymocyte counts (average, 3.7 × 107) in 3 of 6 treated animals, whereas untreated Δγc+-XSCID or mock-BMT mice showed much lower counts ranging between 3 × 105 and 4 × 106. As expected, the expression of mγc was detected on thymocytes from all animals by flow cytometry (Figure4, left panels). When stained for CD4 and CD8, untreated Δγc+-XSCID animals showed minimal numbers of CD4−/CD8− and CD8+thymocytes, consistent with previous observations.28 29 In contrast, marked increases of CD4−/CD8− and CD8+ cells were observed in thymocytes obtained from treated animals (Figure 4, right panels). Taking into account the significant increase in thymocyte numbers observed in treated mice, the improvement of CD4−/CD8− and CD8+cellularity appears even more significant.
Immunophenotyping of thymocytes.
Thymocytes obtained from wild-type γc+, untreated Δγc+-XSCID (XSCID), and gene-corrected Δγc+-XSCID animals (mγc-BMT) were analyzed. Left panels show mγc expression on unfractionated thymocytes. Shown are the staining profiles of isotypic control mAbs (gray histograms) and mγc-specific mAbs (open histograms). Right panels show CD4 versus CD8 staining of thymocytes. Percentages of cells in each quadrant are shown. Results are representative of 2 independent experiments.
Immunophenotyping of thymocytes.
Thymocytes obtained from wild-type γc+, untreated Δγc+-XSCID (XSCID), and gene-corrected Δγc+-XSCID animals (mγc-BMT) were analyzed. Left panels show mγc expression on unfractionated thymocytes. Shown are the staining profiles of isotypic control mAbs (gray histograms) and mγc-specific mAbs (open histograms). Right panels show CD4 versus CD8 staining of thymocytes. Percentages of cells in each quadrant are shown. Results are representative of 2 independent experiments.
Bcl-2 expression and cell survival in thymocytes
Recent studies have suggested that diminished Bcl-2 expression and reduced cell survival may explain the poor thymic cellularity in XSCID mice.28 29 To test whether restored γc-mediated signaling had positive effects on these parameters, we examined Bcl-2 levels and cell viability of thymocytes by flow cytometry. We confirmed the marked reduction of Bcl-2 expression, especially in CD4−/CD8− and CD8+ fractions of thymocytes obtained from untreated Δγc+-XSCID animals (Figure 5A). In contrast, thymocytes obtained from mγc-BMT mice showed clear improvement of Bcl-2 levels in all thymocyte fractions, particularly in CD4−/CD8− and CD8+ cells (Figure5A). When cell viability was assessed in thymocytes kept in culture for 24 hours, extensive cell death was observed in CD4−/CD8− and CD8+ cells derived from untreated Δγc+-XSCID mice (Figure 5B, white bars) compared to normal thymocytes (black bars). Consistent with the enhanced Bcl-2 levels, thymocytes of mγc-BMT animals showed clear improvement of cell survival in these 2 fractions (Figure 5B, gray bars). These results demonstrated that the retroviral-mediated gene correction resulted in the reversion of the defects observed in Δγc+-XSCID thymocytes, most likely because of the restoration of γc-mediated signaling pathways (eg, IL-7) required for normal thymocyte homeostasis in vivo.
Enhanced Bcl-2 expression and cell survival after stem cell gene correction.
(A) Bcl-2 expression levels in thymocyte fractions. Thymocytes were stained for surface CD4 and CD8 expression, then stained intracellularly with either isotypic control mAb (gray histograms) or antimouse Bcl-2 mAb (open histograms). Shown are the results analyzed by gating CD4−/CD8− (DN) cells, CD4+/CD8+ (DP), or either CD4+ or CD8+ cells. Representative results of 2 independent experiments are presented. (B) In vitro assessment of thymocyte cell death. After 24 hours of culture, cells were stained for CD4 and CD8, then incubated with 7-AAD. The percentage of 7-AAD+ cells was determined in each thymocyte fraction by gating on CD4−/CD8−, CD4+/CD8+, CD4+, and CD8+ cells. Shown are the mean values of 2 independent experiments. ▪, γc+, untreated normal mice; ■, XSCID, untreated Δγc+-XSCID mice; ░, mγc-BMT, Δγc+-XSCID mice transplanted with mγc-transduced BM cells.
Enhanced Bcl-2 expression and cell survival after stem cell gene correction.
(A) Bcl-2 expression levels in thymocyte fractions. Thymocytes were stained for surface CD4 and CD8 expression, then stained intracellularly with either isotypic control mAb (gray histograms) or antimouse Bcl-2 mAb (open histograms). Shown are the results analyzed by gating CD4−/CD8− (DN) cells, CD4+/CD8+ (DP), or either CD4+ or CD8+ cells. Representative results of 2 independent experiments are presented. (B) In vitro assessment of thymocyte cell death. After 24 hours of culture, cells were stained for CD4 and CD8, then incubated with 7-AAD. The percentage of 7-AAD+ cells was determined in each thymocyte fraction by gating on CD4−/CD8−, CD4+/CD8+, CD4+, and CD8+ cells. Shown are the mean values of 2 independent experiments. ▪, γc+, untreated normal mice; ■, XSCID, untreated Δγc+-XSCID mice; ░, mγc-BMT, Δγc+-XSCID mice transplanted with mγc-transduced BM cells.
Discussion
An extensive series of in vitro13-17 and in vivo18-20 preclinical experiments performed by several laboratories since the early 1990s has culminated in the recent exciting results of the first clinical trial of gene therapy for XSCID that has shown good immune reconstitution in treated patients for more than 1 year.21 Important information on the safety and efficacy of γc gene transfer into HSCs was obtained using different mouse strains of XSCID, all carrying null-mutations of the γc.18-20 However, whether the residual expression of γc could result in dominant-negative effects, thus potentially reducing the therapeutic effects of gene transfer, still need to be formally investigated.
We found that retroviral-mediated expression of the normal γc molecule into hematopoietic stem cells of mice carrying a truncated γc resulted in the restoration of cellular and humoral immune functions in transplanted mice. In particular, thymic development and mature T lymphocyte numbers and functions were reconstituted to levels close to those observed in control animals. At partial contrast, total numbers of peripheral B lymphocytes and NK cells detected in treated animals were lower than normal. These findings are similar to those of previously published reports18-20,30 and may indicate that different levels of transgene expression may be needed for the complete normalization of B- and NK-cell development. Regardless of the low total numbers of B lymphocytes that developed in treated mice in these previous studies, humoral immunity was clearly improved.18-20,30 The reconstitution of cytokine responses in the B cells of these animals, however, had not been studied in detail. IL-4 is known to induce IgG1 and IgE isotype switching in LPS-activated mouse B cells,31 and γc is thought to be indispensable for this response.6 32 To examine IL-4 signaling in XSCID B cells after genetic correction, we opted for an in vitro immunoglobulin isotype switching assay that enables the functional assessment on a single-cell basis. We showed that B cells newly developed in treated animals switched to IgG1+ or IgE+ cells in response to IL-4, whereas untreated Δγc+-XSCID counterparts failed to do so. These results indicate that the γc expressed in B cells by gene transfer is functionally competent in vivo and further support the notion that humoral responses can be corrected in XSCID mice by gene transfer. However, it is important to note that, because B lymphopoiesis differs significantly in mice and humans and because of the differences existing between the B-cell phenotype of the murine model and of humans with XSCID, these results may not directly translate to human trials.
We have paid particular attention to the analysis of thymocyte populations in treated mice in terms of immunophenotype and function because studies of these critical cells are obviously precluded in humans treated by gene therapy. By assessing proliferative responses to various stimulants, we could demonstrate that thymocytes developed in treated animals responded to the addition of IL-2, IL-4, or IL-7, indicating the functional reconstitution of the respective γc-containing receptors. The proliferative response, however, was not as strong as that observed in normal thymocytes (Table 2). Soudais et al20 have also reported that the enhancement of ConA mitogenic responses by the addition of IL-2 or IL-7 in splenocytes derived from gene therapy–treated XSCID animals was reduced compared to that in normal counterparts. These partial responses may be attributed to lower expression levels of transduced γc compared to normal or to the lack of physiological regulation of γc expression, which is under the constitutive transcriptional control of the retroviral long terminal repeat. At present, it is unclear whether higher levels of γc expression would result in normal performances of developing lymphocytes in XSCID recipients treated by gene therapy or whether activation-mediated induction of γc expression is necessary to achieve full immune reconstitution. With the available technology, achievement of high levels of transgene expression above a hypothetical threshold may be more feasible than attempting the reconstitution of the physiological mechanisms of γc regulation.
By immunophenotyping thymocytes developed in mγc-BMT mice, we observed a dramatic increase of both CD4−/CD8− and CD8+ cells, the thymic subsets markedly affected in γc-deficiency mice.28,29 The higher Bcl-2 expression levels and the concomitant improvement of cell survival observed in these fractions are most likely attributable to the reconstitution of functional γc-containing receptors because γc-dependent cytokines have been shown to enhance lymphocyte cell survival by maintaining the levels of antiapoptotic molecules including Bcl-2 and Bcl-xL.33,34 Specifically, because reduced Bcl-2 levels are observed in CD4−/CD8−thymocytes of IL-7–deficient mice,35 signaling through the reconstituted IL-7 receptors leading to the expansion of thymic precursors is primarily responsible for thymic reconstitution in our treated animals, though new lines of evidence have recently challenged the role of γc-dependent cytokines in the up-regulation of Bcl-2 during positive selection.36
Analysis of our mice also showed that the copy numbers of integrated MNDmγc provirus clearly varied among the different tissues. Whereas BM cells contained 0.06 to 0.1 copies/cell of the mγc transgene, splenocytes and thymocytes showed much higher proviral copy numbers, indicating preferential repopulation of these peripheral lymphoid tissues by the gene-corrected cells. In gene therapy experiments using Jak3-deficient SCID mice, Bunting et al30 also observed higher proviral copy numbers in lymphoid cells than in myeloid lineages. These observations are in line with the results of our competition experiments in murine XSCID BMT models37 and with the overall clinical experience of allogeneic BMT in patients with XSCID, and they further support the notion that after stem cell gene therapy, gene-corrected cells have a selective growth advantage over defective cells in the reconstitution of lymphoid compartments in conditions affecting the γc/Jak3-signaling pathway. It was especially striking that the thymocytes in this study showed nearly one copy of the mγc transgene per cell, suggesting that most cells that repopulated each recipient's thymus were gene-corrected. Several explanations are possible for these findings, including the enhanced ability of thymic precursors expressing γc to home and engraft in thymic tissues and the preferential survival of γc-positive cells in the thymic environment.
In summary, we have demonstrated that retroviral-mediated gene correction could reconstitute the immune system in a murine XSCID model expressing truncated γc and that the transduced γc enabled cytokine-signaling in lymphoid cells by γc-dependent receptors. These findings have important implications because they suggest that corrective gene transfer could be successful in XSCID patients who carry mutations of the γc gene, leading to the expression of truncated γc proteins. Of note, in the current series of experiments, we have used the identical vector and transduction conditions we had previously used in γc-null XSCID mice with similar overall efficacy,19 thus indicating that the presence of the truncated mγc did not hinder the therapeutic effects of gene transfer. One possible explanation for these findings is that the levels of wild-type γc expression obtained in gene-corrected cells were high enough to form sufficient numbers of functional receptors that could successfully compete with mutant γc-containing receptors for cytokine binding.
Several patients with XSCID have been found to carry mutations leading to truncated γc protein expressed on the cell surface24; one patient treated with gene therapy expressed one of these mutant forms, which, however, did not seem to interfere with the successful outcome of the procedure. Although it is unclear whether these results can be translated to all mutations compatible with residual surface expression of γc, the clinical experience with this particular patient complements our results in the Δγc+-XSCID animals and suggests that residual expression of γc may not pose a significant problem for gene therapy of XSCID. Additional studies are needed to determine whether the same conclusions are applicable to all γc mutations (deletions or missense mutations) that are compatible with residual surface expression of the γc protein but that interfere with normal cytokine signaling.
We thank Mrs Stacie M. Anderson for excellent technical assistance and Dr David M. Bodine for insightful discussions and advice.
Supported in part by Japanese Society for the Promotion of Science Research Fellowships for Japanese Biomedical and Behavioral Researchers at the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Fabio Candotti, Clinical Gene Therapy Branch, National Human Genome Research Institute, National Institutes of Health, 10 Center Dr, Bldg 10, Rm 10C103, MSC 1851, Bethesda, MD 20892-1851; e-mail: fabio@nhgri.nih.gov.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal