Abstract
Human herpesvirus 8 (HHV-8) is a recently discovered gammaherpesvirus that is the etiologic agent of Kaposi sarcoma (KS). The natural history of primary HHV-8 infection, including clinical outcome and host immune responses that may be important in preventing disease related to HHV-8, has not been elucidated. The present study characterized the clinical, immunologic, and virologic parameters of primary HHV-8 infection in 5 cases detected during a 15-year longitudinal study of 108 human immunodeficiency virus type 1 seronegative men in the Multicenter AIDS Cohort Study. Primary HHV-8 infection was associated with mild, nonspecific signs and symptoms of diarrhea, fatigue, localized rash, and lymphadenopathy. There were no alterations in numbers of CD4+ or CD8+ T cells or CD8+ T-cell interferon γ (IFN-γ) production to mitogen or nominal antigen. CD8+ cytotoxic T-lymphocyte precursor (CTLp) and IFN-γ reactivity were detected during primary HHV-8 infection, with broad specificity to 5 lytic cycle proteins of HHV-8 encoded by open reading frame 8 (ORF 8; glycoprotein B homolog of Epstein-Barr virus), ORF 22 (gH homolog), ORF 25 (major capsid protein homolog), ORF 26 (a minor capsid protein homolog), or ORF 57 (an early protein homolog), in association with increases in serum antibody titers and appearance of HHV-8 DNA in blood mononuclear cells. CD8+ T-cell responses to HHV-8 decreased by 2 to 3 years after primary infection. This antiviral T-cell response may control initial HHV-8 infection and prevent development of disease.
Introduction
Human herpesvirus 8 (HHV-8, also called Kaposi sarcoma–associated herpesvirus) is a recently discovered gammaherpesvirus that is the etiologic agent of Kaposi sarcoma (KS).1 There is currently little knowledge of the clinical syndromes associated with primary HHV-8 infection or immune responses to the virus. Primary infection of adults with Epstein-Barr virus (EBV), the other human gammaherpesvirus, can commonly result in a prolonged, although self-limiting, symptomatic mononucleosis illness with long-term expansion of activated CD8+ T cells and inhibition of T-cell reactivity to mitogens and viral antigens.2-8 Recently, primary infection with HHV-8 was reported in one adult patient infected with human immunodeficiency virus-1 (HIV-1), which was associated with fever, arthralgia, and cervical lymphadenopathy.9 It is not clear, however, whether these clinical signs and symptoms were related to the underlying HIV-1 infection in this patient.
Virus-specific CD8+ T cells are likely to be central in host control of primary HHV-8 infection. CD8+ T cells specific for lytic cycle proteins of EBV, including structural, immediate-early (IE), and early proteins,10-14 appear prominent in EBV mononucleosis. Thus, they may play a major role in limiting the primary phase of EBV infection.3,11,15Indeed, 0.5% to 6%, and as much as 44%, of circulating CD8+ cells are specific for EBV lytic peptides by HLA tetramer staining during infectious mononucleosis.16,17 It has also been shown that IE proteins are frequent targets for EBV-specific, CD8+ T cells during persistent infection.14,18 Similarly, infection of mice with murine herpesvirus 68 (MHV-68), another gammaherpesvirus, results in expansion of CD8+ cytotoxic T-lymphocyte precursors (CTLp's) specific for MHV-68 lytic proteins.19,20 These are detectable in both lymph nodes and at the site of local infection after primary infection in mice,20 and could be involved in control of MHV-68 infection.21,22 In contrast, only 2 studies to date have been able to detect CD8+ T-cell reactivity specific for HHV-8.23 24 Moreover, the levels of anti-HHV-8 CD8+ T-cell responses measured by bulk CTL assays used in these cross-sectional studies were relatively low, with narrow specificity for different HHV-8 proteins.
In this report, we conducted the first prospective study of HIV-1–negative adults with documented seroconversion to HHV-8 infection to identify clinical syndromes, potential immunosuppressive states and immune responses, and virologic markers related to this primary gammaherpesvirus infection. We used CTLp and single-cell interferon γ (IFN-γ) enumeration methods for more sensitive and quantitative assessment of anti-HHV-8 CD8+ T-cell responses, and compared these for 5 lytic cycle proteins of HHV-8. The results show that primary HHV-8 infection is associated with nonspecific, mild symptoms and generates CD8+ T-cell responses that are broadly specific for the different HHV-8 lytic proteins in association with HHV-8 seroconversion and the appearance of HHV-8 DNA in peripheral blood mononuclear cells (PBMCs). These CD8+ T-cell responses may be important in immune control of HHV-8 infection.
Patients, materials, and methods
Patients
The study participants were 119 HIV-1 uninfected, white men (median age, 47 years; range, 39-61 years) from the Pittsburgh portion of the Multicenter AIDS Cohort Study (MACS), an ongoing, longitudinal study of the natural history of HIV-1 infection in homosexual men that began recruitment of subjects in 1984.25 Written, informed consent was obtained from all volunteers in the study. The MACS subjects were assessed for clinical signs and symptoms, and blood samples were obtained for cryopreservation of PBMCs, plasma, and serum, at approximately 6-month intervals from 1984 through 1999. Serologic testing for HHV-8–specific antibodies (Abs) was done on the first and last serum samples available from the Pittsburgh MACS specimen repository from the 119 HIV-1 seronegative men who were still active in the study and had sufficient numbers of cryopreserved PBMC samples collected over at least 8 of the 6-month interval visits. Those men who tested negative for HHV-8 Abs in the first serum and positive in the last serum were chosen for further analysis. Sera from all of the interval clinic visits of these men were then assayed for HHV-8 Abs. The date of seroconversion was estimated as the midpoint between the last HHV-8 seronegative visit and first HHV-8 seropositive visit. The men seronegative for HHV-8 and HIV-1 (ie, 102 of the 119 men) served as controls for the clinical aspects of the study. For this analysis, we used any self-reported symptoms and any abnormal lymph nodes found on physical examination at the visits surrounding HHV-8 seroconversion. We compared the frequency of signs and symptoms noted at the first seropositive visit in the HHV-8 seroconverters (ie, rash, diarrhea, fatigue, or abnormal lymph nodes) to that observed among the 102 seronegative men. For the latter, we randomly selected their visit to correspond to the same visits analyzed for the HHV-8 seroconverters.
Viral serology
Antibodies against HHV-8 lytic antigens were determined by an indirect immunofluorescence assay using the body cavity B-cell lymphoma (BCBL)-1 cell line that contains the HHV-8 genome.26BCBL-1 cells were treated with 20 ng/mL tetradecanoyl phorbol acetate for 3 to 5 days to induce HHV-8, collected by centrifugation, rinsed with phosphate-buffered saline (PBS), and resuspended in a small volume of PBS. Aliquots of cells were placed in individual wells on a 12-well Teflon-coated glass slide, air dried, and fixed with ice-cold acetone for 20 minutes. Following fixation, the slides were air dried and stored at −20°C. For each assay, the fixed cells were first incubated in PBS containing 10% goat serum for 1 hour at 37°C to block nonspecific binding. The blocking buffer was removed and primary Ab (diluted in PBS containing 10% goat serum) was added. The slides were incubated for 1 hour at 37°C. Two dilutions (1:50 and 1:100) of primary Ab (participant's sera) were tested for each serum sample. The cells were next washed extensively in PBS, treated with the secondary Ab (fluorescein isothiocyanate [FITC]-goat antihuman IgG diluted 1:200 in PBS) and incubated at 37°C for 0.5 to 1 hour. The cells were again washed extensively in PBS and glass coverslips added. For each assay, we confirmed the specificity of the fluorescence using cells incubated with primary but no secondary Ab, and cells incubated with secondary but no primary Ab. For each batch of serum samples tested, known HHV-8 positive and negative sera were included. All serum samples were tested twice in a blinded fashion and assessed microscopically by the same reader. Assays using the induced BCBL-1 cells were examined for the presence of whole cell immunofluorescence. The serum samples were serially diluted to determine the Ab titers (reciprocal of the last positive dilution). The cutoff titer for positive reactivity was 50.
An enzyme immunoassay (rLAV EIA, Genetic Systems, Redmond, WA) and an immunoblot assay (Biorad Laboratories, Hercules, CA) were used for detection of antibodies to HIV-1 as per the manufacturer's instructions. The 5 immunology study subjects were seropositive for cytomegalovirus (CMV) Abs by passive latex agglutination (Becton Dickinson, Mountain View, CA).
Polymerase chain reaction assay for HHV-8 DNA
DNA isolated from PBMCs was analyzed by polymerase chain reaction (PCR) for the presence of HHV-8 DNA. PCR analyses were performed using primers that amplified a 233-bp fragment of the minor capsid gene encoded by open reading frame (ORF) 26.27 28For nested PCR experiments, 5 μL of each PCR reaction was amplified with a primer set located internal to the ORF 26 primers. The nested primers were 5′-ATGGTCGTGCCGCAGCAACTGG-3′ and 5′-CGCCCCATAAATGACACATTGG-3′. Cellular DNA was isolated using the genomic DNA isolation kit from Gentra Systems (Minneapolis, MN). PCR was performed on samples equivalent to the amount of DNA from 5 × 104 or 2.5 × 105 cells. Amplification was done for 5 minutes at 94°C for 35 cycles with a single cycle consisting of 1 minute at 94°C, 1 minute at 58°C, 1 minute at 72°C, and 7 minutes at 72°C. PCR products were separated on 2% agarose gels, transferred to nytran membranes, and hybridized with an internal oligonucleotide probe labeled with 32P. The sequence of oligonucleotide probe was TGCAGCAGCTGTTGGTGTACCACAT. PCR products that hybridized to the probe were detected by image analysis with an Imagequant PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Construction of recombinant vaccinia viruses containing HHV-8 lytic cycle proteins
The ORF for glycoprotein B (gB; ORF 8) was obtained as the plasmid gB-Blunt-pCR. The ORFs for glycoprotein H (gH; ORF 22), major capsid protein (MCP; ORF 25), a minor capsid protein (MiCP; ORF 26), and an IE protein (ORF 57) were amplified by PCR using the HHV-8 genome contained in the BCBL-1 cell line as the template. The primer sets used for PCR amplification were: (gH) 5′-CACCTAGAGGATCCGACATGCAGGGTC, 3′-TAAAAAATCTAAAGCTTTATTGACCG; (MCP) 5′-CTCGAGCGCTGGATCCATGGAGGCG, 3′-CACGATGAAAGCTTTCGAGCC; (MiCP) 5′-GGCTAGTCATATGGCACTCGAC, 3′-CACGATGAAAGCTTTCGAGCC; and (IE) 5′-CTCCCTGCGAATTCGCATGATAATTG, 3′-GTTACATGGAATTCACGGGAGACAC. The amplified ORFs were cloned into the vaccinia virus shuttle vector, pSC11, and recombinant vaccinia viruses constructed by transfection of cells infected with wild-type vaccinia virus (WR) as described previously.29,30 Each virus was plaque purified 3 times. Rabbit polyclonal Abs against MiCP and mouse polyclonal Abs against MCP were generated by immunization of animals with purified bacterial fusion proteins. Mouse polyclonal Abs against IE protein were generated by immunization of animals with a peptide corresponding to amino acids EYYRPGDVMGLLNVLV (single-letter amino acid codes). Rabbit antiserum against gB and gH was generated against recombinant proteins. Identification of the HHV-8 proteins expressed by the recombinant vaccinia viruses was determined by immunoblot analysis of infected cell lysates as described previously.29 30Protein bands corresponding to the predicted sizes of the individual proteins were noted in vaccinia virus recombinant-infected cell lysates as 120 kd (gB), 74 kd (gH), 150 kd (MCP), 32 kd (MiCP), and 27 kd (IE).
Limiting dilution and bulk CTL assays
The CTLp frequencies were assessed using a modification of our limiting dilution assay for HIV-1.31 On day 0, autologous, EBV-transformed B-lymphocyte cell lines (B-LCL) were infected overnight with recombinant vaccinia viruses in RPMI medium (Life Technologies, Gaithersburg, MD) supplemented with 15% fetal calf serum (FCS), at 37°C in 5% CO2. On day 1, these cells (stimulators) were inactivated with psoralen (10 μg/mL, Sigma, St Louis, MO) and long-wave UV light (40 mW/cm2) for 30 minutes, then resuspended at the concentration of 5 × 104 cells/mL in stimulation medium (RPMI 1640 medium with 15% FCS plus human recombinant interleukin 2 [IL-2; 100 U/mL; Chiron, Emeryville, CA]). Allogeneic PBMCs (feeder cells) from normal donors were γ-irradiated (4000 Rad) and resuspended at 1 × 106 cells/mL. Frozen PBMCs (effectors) were thawed and resuspended in stimulation medium at different concentrations (160 000, 120 000, 60 000, 30 000, 10 000, 5000, 2500, and 0 [control] cells/mL). The mean ± SE viability of the thawed PBMCs was 92% ± 1% (n = 37). Stimulators, feeders, and effectors were plated into round-bottom 96-well plates (Becton Dickinson) in 50 μL, 50 μL, and 100 μL, respectively. Twenty-four wells were used for each different concentration. The wells without effectors were used as the negative controls. The plates were incubated at 37°C in 5% CO2for 14 days, and the medium was changed every 3 to 4 days. On day 13, the targets were prepared by radiolabeling infected autologous B-LCL with 51Cr (100 μCi/mL, Dupont NEN, Boston, MA) for 16 hours at 37°C in 5% CO2. On day 14, target cells were washed and added to the effectors. The effector cells were divided into 2 sets, with one set added to 24 replicate wells containing VSC11 infected target cells as controls for 4 hours at 37°C in 5% CO2, and the other set incubated under the same conditions with targets cells infected with vaccinia virus vectors expressing individual recombinant HHV-8 proteins. The plates were then harvested and counted for radioactivity in a gamma counter. Mean spontaneous release and mean maximum lysis were calculated for wells with targets alone and with detergent, respectively. Wells were scored as positive for CTL recognition only if the level of specific lysis was more than 10%. Frequency values were determined with 95% confidence limits from the cell input number at which 37% of the wells were negative for recognition of the targets using the method of maximum likelihood, fulfilling single hit kinetics by χ2 analysis.
For bulk CTL lysis, stimulators, feeders, and effectors were treated as described for the limiting dilution assay, except that the concentrations for stimulators and effectors were 5 × 104 and 2.5 × 105, respectively. On day 14, the target cells were washed, and the effectors were harvested and plated at E (effector):T (target) ratio 20:1, 10:1, and 5:1 in triplicate. The plates were then incubated for 4 hours, harvested, and counted in the gamma counter. The percentage of specific cytotoxicity was calculated as (experimental release minus spontaneous release) / (maximum release minus spontaneous release) times 100.
The mean ± SE levels of CTLp's for B-LCL infected with the control VSC11 were 17 ± 3/106 PBMCs and 7.6% ± 0.7% (E:T, 20:1), 5.8% ± 0.5% (E:T, 10:1), and 3.7% ± 0.3% (E:T, 5:1) for bulk CTL lysis (n = 185). Results for both CTLp's and the bulk CTL assays are presented as percentage virus-specific lysis, which equals percent specific lysis against the HHV-8 antigen-expressing targets minus percent specific lysis against the mock antigen-expressing targets.
Single-cell IFN-γ production assay
For assessing the production of IFN-γ, nitrocellulose-bottomed 96-well plates (Millipore, Bedford, MA) were precoated with 10 μg/mL of an anti-IFN-γ monoclonal antibody (mAb), 1-DIK (Mabtech, Decatur, GA) overnight at 4°C. The coated plates were washed 4 times with PBS to remove unbound Ab and then blocked for 2 hours with RPMI 1640 supplemented with 10% human serum. Frozen PBMCs were thawed and added to the wells with the autologous, infected B-LCL at effectors/stimulators ratio of 2:1 and incubated overnight at 37°C and 5% CO2. The cells were discarded the following day by washing the plates 6 times with PBS containing 0.05% Tween 20, and the second biotinylated anti-IFN-γ mAb, 7-B6-1 biotin (Mabtech), was added at 2 μg/mL and incubated for 2 hours at 37°C in 5% CO2. The plates were further washed 6 times with PBS/0.05% Tween 20, followed by addition of avidin horseradish peroxidase-conjugated streptavidin (Vectastain Elite kit, Vector Laboratories, Burlingame, CA) to the wells and incubation for 1 hour at room temperature. The plates were washed 3 times with PBS/0.05% Tween 20 and 3 times with PBS. The spots were developed by addition of 3-amino-9-ethyl-carbazole (Sigma) diluted in 0.1 M acetate buffer, pH 5, containing H2O2. Reactions were allowed for about 5 minutes and were stopped by washing under running tap water. After drying, spots were counted using a dissection microscope. The results were expressed as the number of spots per 106 PBMCs or CD8+ T cells. Stimulation with phorbol myristate acetate (PMA) and ionomycin (1 ng/mL and 1 μM, respectively) was used as a positive control, and stimulation with medium and mock vaccinia virus-infected B-LCL was used as a negative control.
The mean ± SE level of IFN-γ–producing cells induced by B-LCL infected with the control VSC11 was 67 ± 15/106 PBMCs (n = 37). Results for the IFN-γ assays are presented as the number of virus-specific spot-forming cells per 106 PBMCs, which is the number of spot-forming cells induced by the HHV-8 antigen-expressing targets per 106 PBMCs minus the number of spot-forming cells induced by the mock antigen-expressing targets per 106 PBMCs.
Blocking assay using HLA class I and class II antibodies
Targets and stimulators were washed and suspended at a concentration of 1 × 106 cells/mL. The mAbs against HLA class I (W6/32, Accurate Chemical, Westbury, NY) or HLA class II (I3, Beckman-Coulter, Fullerton, CA) were added at a dilution to the targets on day 14 of the CTL assay or to the stimulators on day 1 of the single-cell IFN-γ production assay, and incubated at 37°C in 5% CO2 for 45 minutes. The remainder of the experiments were performed as described above.
CD4+ and CD8+ T-cell enrichment
Immunomagnetic beads specific for CD4 and CD8 (M-450, Dynal, Lake Success, NY) were used for negative selection. Frozen PBMCs (10 × 106) were thawed and mixed with either CD4 or CD8 beads for 1 hour at 4°C in a 2-mL plastic tube (Nalgene, Rochester, NY). The tube was then placed on a magnet for 3 to 5 minutes, the supernatants containing either CD8+-enriched or CD4+-enriched T cells were removed. The T cells enriched with CD4+ or CD8+ were used as effectors in both the bulk CTL and the single-cell IFN-γ production assays.
T-cell phenotyping
For immunophenotyping, frozen PBMCs were thawed and washed in Hanks balanced salt solution supplemented with 2% FCS and 0.1% NaN3 and incubated (30 minutes at 4°C) with one of the following mAbs: FITC- or phycoerythrin (PE)-conjugated mAbs specific for CD3, CD4, CD14, CD38, CD28, HLA-DR, or CD45 (Becton Dickinson); CD45RA and CD45RO (Immunotech, Westbrook, ME); and PE-Cy*5 conjugated anti-CD4 and anti-CD8 mAb (Immunotech). After incubation, the PBMCs were washed with PBS and fixed with 1% paraformaldehyde. Surface expression was analyzed using an EPICS Elite flow cytometer (Beckman-Coulter).
Statistical analysis
The Fisher exact test was used for comparison of clinical signs and symptoms. The 2-tailed, paired Student t test was used for comparing levels of IFN-γ production and CTLp's. Nonparametric correlations of CTLp's and IFN-γ–producing cells were assessed using the 2-tailed Spearman rank correlation test. The 3-way repeated measures ANOVA was used for pattern analysis.
Results
Incidence and clinical signs and symptoms of primary HHV-8 infection
We chose to assess the clinical and laboratory components of primary HHV-8 infection in HIV-1–negative persons in the MACS to avoid potential complications of underlying HIV-1 infection. In this group of homosexual men persistently seronegative for HIV-1, the HHV-8 seroprevalence rate was 9.2% (11 of 119) at study entry in 1984-1985, which is similar to the seroprevalence reported in the general population.1 From April 1984 through March 1999, 6 of the 108 HHV-8 seronegative men seroconverted to HHV-8. One of the 6 HHV-8 seroconverters was excluded from this investigation because of insufficient numbers of interim clinic visits to determine the time of seroconversion.
As of March 1999, the overall incidence rate for HHV-8 infection was 3.7/1000 person-years (5 of 1361). However, the incidence was not constant over the years. These HHV-8 seroconversions occurred earlier in the study with the incidence rates being 10.1, 19.9, 9.8, and 9.8/1000 person-years in 1985, 1986, 1990, and 1991, respectively, with no subsequent HHV-8 seroconversions after 1991.
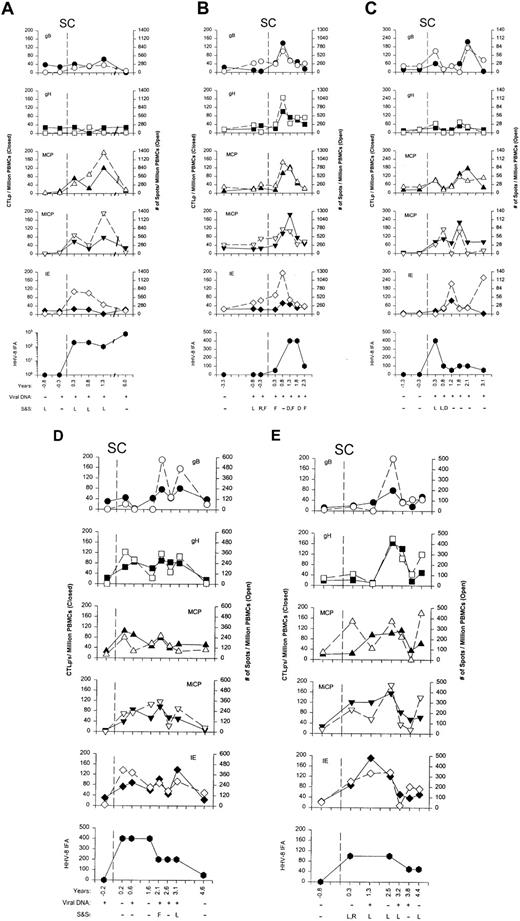
No specific, severe illness was associated with primary HHV-8 infection as noted in the extensive clinical assessment taken at the time of the study subjects' clinic visits. However, several nonspecific, mild clinical signs and symptoms were documented near the time of seroconversion in 4 of the 5 men: mild, transient cervical and submental lymphadenopathy and diarrhea in subjects 20 and 22 (Figure1A,C), fatigue and localized rash on the right ankle in subject 21 (Figure 1B), and cervical and submental lymphadenopathy and localized facial rash in subject 24 (Figure 1E). No signs and symptoms were associated with seroconversion in subject 23 (Figure 1D). To determine whether these signs and symptoms were significantly associated with HHV-8 seroconversion, we compared these findings from the first seropositive visit for the HHV-8 seroconverters with the same clinic visits in the 102 men who remained HHV-8 seronegative; 80% of the HHV-8 seroconverters reported at least one symptom compared to 31.4% of the seronegative men (P = .043).
Antigen reactivity.
(A-E) HHV-8–specific CTLp and IFN-γ production to 5 lytic cycle antigens, HHV-8 Ab titers, HHV-8 DNAemia, and signs and symptoms in subjects 20 to 24. CTLp's are shown as the closed symbols, whereas the numbers of IFN-γ–producing cells are the open symbols. SC is the estimated time of seroconversion; years are the times of the clinic visits before and after HHV-8 seroconversion; viral DNA is PCR detection of HHV-8 DNA in PBMCs; S&S represent symptoms of rash (R), fatigue (F), and diarrhea (D) reported by the study subject for the 6 month time interval before the specific visits, and signs of lymphadenopathy (L) detected on physical examination at the particular study visit.
Antigen reactivity.
(A-E) HHV-8–specific CTLp and IFN-γ production to 5 lytic cycle antigens, HHV-8 Ab titers, HHV-8 DNAemia, and signs and symptoms in subjects 20 to 24. CTLp's are shown as the closed symbols, whereas the numbers of IFN-γ–producing cells are the open symbols. SC is the estimated time of seroconversion; years are the times of the clinic visits before and after HHV-8 seroconversion; viral DNA is PCR detection of HHV-8 DNA in PBMCs; S&S represent symptoms of rash (R), fatigue (F), and diarrhea (D) reported by the study subject for the 6 month time interval before the specific visits, and signs of lymphadenopathy (L) detected on physical examination at the particular study visit.
Longitudinal analysis of T-cell phenotypes in primary HHV-8 infection
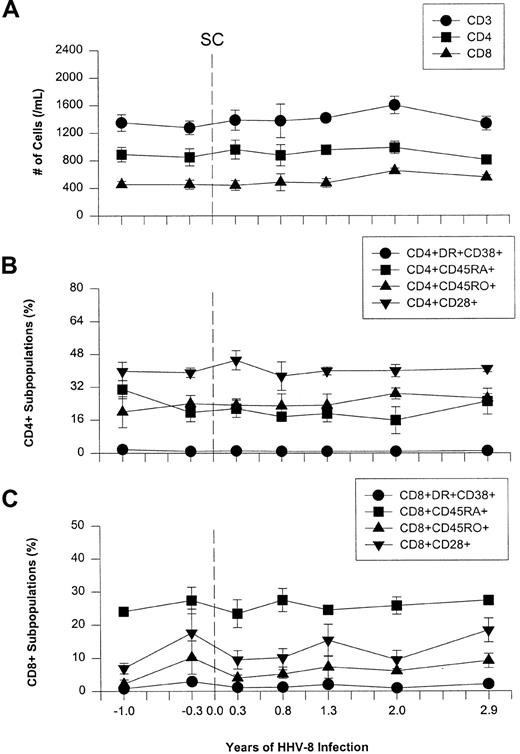
We assessed T-cell phenotypes, including memory and activation cell markers, to determine whether there were any changes during primary HHV-8 infection. This is because expansion of activated CD8+ T cells is a classic finding in EBV mononucleosis of adults.2-4 No changes were seen in T-cell numbers expressing lineage CD3, CD4, and CD8 molecules, naı̈ve/memory cell markers CD45RA and CD45RO, or activation and costimulatory markers CD38, CD28, and HLA-DR, after primary infection with HHV-8 (Figure2).
Longitudinal flow analysis of T-cell phenotypes in the 5 HHV-8 seroconverters.
(A) CD3+, CD4+, and CD8+ T-cell numbers. (B) Naı̈ve/memory and activation cell markers CD45RA, CD45RO, HLA-DR, CD38, and CD28 in the CD4+ T- cell subpopulation. (C) Naı̈ve/memory and activation cell markers in the CD8+ cell subpopulation. The data are mean ± SE for at least 3 individuals at each timepoint. Time points −1.0, 2.0, and 2.9 years represent the average of 2 actual data points for −1.3 and −0.8, 1.8, and 2.1, and 2.6 and 3.1 years, respectively.
Longitudinal flow analysis of T-cell phenotypes in the 5 HHV-8 seroconverters.
(A) CD3+, CD4+, and CD8+ T-cell numbers. (B) Naı̈ve/memory and activation cell markers CD45RA, CD45RO, HLA-DR, CD38, and CD28 in the CD4+ T- cell subpopulation. (C) Naı̈ve/memory and activation cell markers in the CD8+ cell subpopulation. The data are mean ± SE for at least 3 individuals at each timepoint. Time points −1.0, 2.0, and 2.9 years represent the average of 2 actual data points for −1.3 and −0.8, 1.8, and 2.1, and 2.6 and 3.1 years, respectively.
Effects of HHV-8 infection on CD8+ T-cell reactivity to CMV and PMA/ionomycin
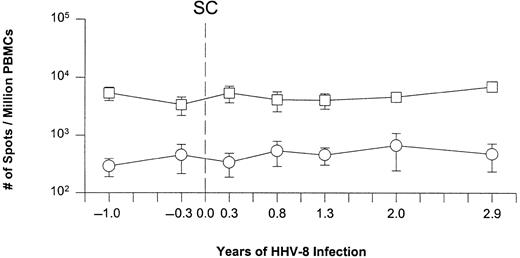
The immunosuppressive effects of primary HHV-8 infection were analyzed in the HHV-8 seroconverters by assessing the numbers of CD8+ T cells responding to a specific recall antigen and a mitogen. There were no significant changes in the numbers of IFN-γ–producing cells either to the CMV pp65 matrix protein, which is a major CTL target,32,33 or to the mitogen PMA/ionomycin, after seroconversion to HHV-8 (Figure3). Thus, primary HHV-8 infection differs from primary symptomatic EBV infection that is classically associated with transient immunosuppression.5-7
Single-cell IFN-γ production induced by CMV pp65 antigen and PMA/ionomycin in the 5 subjects during primary HHV-8 infection.
Response to CMV, ○; to PMA/ionomycin, ■. The data are the mean ± SE for at least 3 individuals at each time point. SC represents the estimated time of seroconversion to HHV-8 in these individuals.
Single-cell IFN-γ production induced by CMV pp65 antigen and PMA/ionomycin in the 5 subjects during primary HHV-8 infection.
Response to CMV, ○; to PMA/ionomycin, ■. The data are the mean ± SE for at least 3 individuals at each time point. SC represents the estimated time of seroconversion to HHV-8 in these individuals.
Anti–HHV-8 CTLp's and IFN-γ–producing CD8+ T cells during primary HHV-8 infection
All of the 5 study subjects developed broad antigen reactivity by CD8+ CTLp's and IFN-γ–producing T cells to at least 3 of the 5 HHV-8 lytic cycle proteins concurrent with the appearance of serum antibodies to the virus and viral DNA in the PBMCs (Figure 1A-E). Bulk CTL responses specific for the HHV-8 lytic cycle proteins also developed coincident with seroconversion and DNAemia, similar to the CTLp responses (data not shown). In 3 of the 5 cases (Figure 1A,B,D), there was evidence of HHV-8 DNA in the blood approximately 2 to 9 months before the estimated time of seroconversion and increase in titers of HHV-8–specific Ab. Notably, in subject 21, anti-HHV-8 T-cell IFN-γ responses were detected concurrently with HHV-8 DNA at 3 and 9 months before seroconversion (eg, 280 spots and 340/106PBMCs for gB, and 350 and 420/106 PBMCs for IE protein, respectively; Figure 1B). This indicates that in some primary HHV-8 infections, DNAemia precedes production of detectable anti-HHV-8 serum antibodies but is usually coincident with appearance of CD8+ T-cell immunity to HHV-8.
The frequencies of CTLp's and IFN-γ–producing cells specific for the 5 lytic cycle proteins in the 5 individuals increased to peak levels by 1 to 2 years after seroconversion before decreasing to lower levels, together with declines in Ab titers (Figure 1A-E). Viral DNA persisted in the blood of most of the subjects for several years after seroconversion. HLA blocking and T-cell enrichment studies showed that the HHV-8–specific CTL reactivity and IFN-γ production were mediated by HLA class I associated, CD8+ T cells (Figure4A-D).
Association of HLA class I and CD8+ T cells with HHV-8–specific CTL reactivity and IFN-γ production.
(A) Blockage of HHV-8–specific CTL responses to gH (▨) and MiCP (▩) by anti-HLA class I but not class II in representative subject 14. (B) Blockage of HHV-8–specific IFN-γ production induced by gB (■), gH (▨), and IE protein (▧) by anti-HLA class I but not class II Abs in representative subject 21. (C) HHV-8 specific CTL responses to gH (▨) and MiCP (▩) mediated by enriched CD8+ T cells, and not by enriched CD4+ T cells, in representative subject 14 at E:T ratios of 20:1, 10:1, and 5:1. (D) HHV-8–specific IFN-γ production induced by gB (■), gH (▨), and IE protein (▧) in enriched CD8+ T cells, and not in enriched CD4+ T cells, in representative subject 21.
Association of HLA class I and CD8+ T cells with HHV-8–specific CTL reactivity and IFN-γ production.
(A) Blockage of HHV-8–specific CTL responses to gH (▨) and MiCP (▩) by anti-HLA class I but not class II in representative subject 14. (B) Blockage of HHV-8–specific IFN-γ production induced by gB (■), gH (▨), and IE protein (▧) by anti-HLA class I but not class II Abs in representative subject 21. (C) HHV-8 specific CTL responses to gH (▨) and MiCP (▩) mediated by enriched CD8+ T cells, and not by enriched CD4+ T cells, in representative subject 14 at E:T ratios of 20:1, 10:1, and 5:1. (D) HHV-8–specific IFN-γ production induced by gB (■), gH (▨), and IE protein (▧) in enriched CD8+ T cells, and not in enriched CD4+ T cells, in representative subject 21.
Analysis of these data by a 3-way repeated measures ANOVA for the limiting dilution and single-cell IFN-γ production assays confirmed that there was a significant increase in these 2 HHV-8 specific immune parameters in relation to time after seroconversion (Figure5). Furthermore, there were no differences in these CD8+ T-cell responses to the 5 separate HHV-8 antigens by parametric (r = 0.52,P = .29) and nonparametric (r = 0.31,P = .61) analysis. Results from the bulk lysis CTL assay were similar to those from the limiting dilution and single-cell IFN-γ production assays for all 5 proteins (data not shown).
Pattern analysis of CTLp and IFN-γ T-cell reactivity to the 5 HHV-8 lytic cycle antigens during HHV-8 primary infection (mean ± SE).
Time is grouped into 5 separate intervals, that is, 0, 1 to 12 months, 13 to 30 months, 31 to 40 months, and 41+ months. These periods were chosen in a manner designed to maximize the number of patients contributing observations to each time interval (ie, 5, 5, 5, 4, and 3 patients). When a patient contributed more than a single observation to an interval, the values were averaged. Time and HHV-8 antigen were crossed with study subject, which was treated as a random factor. This analysis demonstrated that time was the most significant predictor of the level of T-cell response (P < .001) for both the limiting dilution CTLp assay and the single-cell IFN-γ production assay. ●, gB; ■, gH; ▴, MCP; ▵, MiCP; ♦, IE.
Pattern analysis of CTLp and IFN-γ T-cell reactivity to the 5 HHV-8 lytic cycle antigens during HHV-8 primary infection (mean ± SE).
Time is grouped into 5 separate intervals, that is, 0, 1 to 12 months, 13 to 30 months, 31 to 40 months, and 41+ months. These periods were chosen in a manner designed to maximize the number of patients contributing observations to each time interval (ie, 5, 5, 5, 4, and 3 patients). When a patient contributed more than a single observation to an interval, the values were averaged. Time and HHV-8 antigen were crossed with study subject, which was treated as a random factor. This analysis demonstrated that time was the most significant predictor of the level of T-cell response (P < .001) for both the limiting dilution CTLp assay and the single-cell IFN-γ production assay. ●, gB; ■, gH; ▴, MCP; ▵, MiCP; ♦, IE.
The frequencies of HHV-8 specific, IFN-γ–producing cells were significantly higher than the CTLp frequencies (Table1). The anti-HHV-8 CD8+T-cell responses during the years after primary HHV-8 infection were less overall than the recall T-cell responses to CMVpp65 matrix protein; that is, the mean ± SE of the IFN-γ responses to CMV was 427 ± 61 (P < .01) compared to each of the HHV-8 proteins listed in Table 1.
Comparison between cytotoxic T-lymphocyte precursors and single-cell interferon γ production
| HHV-8 protein . | Assay . | Mean (± SE) . | P value* . |
|---|---|---|---|
| gB | CTLp | 50 (± 8) | .0008 |
| IFN-γ | 172 (± 35) | ||
| gH | CTLp | 50 (± 7) | .0008 |
| IFN-γ | 203 (± 44) | ||
| MCP | CTLp | 69 (± 8) | .0016 |
| IFN-γ | 273 (± 59) | ||
| MiCP | CTLp | 82 (± 8) | .0014 |
| IFN-γ | 282 (± 56) | ||
| IE | CTLp | 55 (± 8) | .000093 |
| IFN-γ | 292 (± 51) |
| HHV-8 protein . | Assay . | Mean (± SE) . | P value* . |
|---|---|---|---|
| gB | CTLp | 50 (± 8) | .0008 |
| IFN-γ | 172 (± 35) | ||
| gH | CTLp | 50 (± 7) | .0008 |
| IFN-γ | 203 (± 44) | ||
| MCP | CTLp | 69 (± 8) | .0016 |
| IFN-γ | 273 (± 59) | ||
| MiCP | CTLp | 82 (± 8) | .0014 |
| IFN-γ | 282 (± 56) | ||
| IE | CTLp | 55 (± 8) | .000093 |
| IFN-γ | 292 (± 51) |
HHV-8 indicates human herpesvirus 8; gB, glycoprotein B; CTLp, cytotoxic T-lymphocyte precursors; IFN-γ, interferon γ; gH, glycoprotein H; MCP, major capsid protein; MiCP, minor capsid protein IE, intermediate-early (proteins).
2-tailed, paired Student t test.
Discussion
This study provides the first description of the natural history of primary HHV-8 infection in healthy adults negative for HIV-1. We identified a relatively low prevalence of HHV-8 infection (9.2%) at entry into the MACS in 1984-1985, and an overall incidence rate for HHV-8 infection of 3.7/1000 person-years between 1984 and 1999, which is similar to the recent report of primary HHV-8 seroconversions among HIV-1 uninfected homosexual men in an Amsterdam study.34These rates, along with a decreasing trend in incident HHV-8 infection by calendar year, were not unexpected in our cohort of men persistently negative for HIV-1. HHV-8 seroprevalence in these men was similar to that in the general adult population (0%-8%),1 and lower than the 30% to 37% seroprevalence noted at study entry in HIV-1–infected homosexual men in the MACS.35,36 In this regard, we have observed a dramatic decrease in HIV-1 seroconversion in the MACS.37 Thus, under the hypothesis that among homosexual men, the same sexual behaviors are risk factors for HIV-1 and HHV-8 infection, a similar temporal decline in HHV-8 infection should be observed.
There was no specific, severe illness associated with primary HHV-8 infection. However, transient, mild signs and symptoms of diarrhea, fatigue, localized rash, and cervical and submental lymphadenopathy were noted near the time of seroconversion to HHV-8 in 4 of the 5 seroconverters. This significantly differed from clinical findings in the 102 men who remained HHV-8 seronegative. Notably, the ratio of symptomatic to asymptomatic primary infections for the other human gammaherpesvirus, EBV, ranges from 3:1 to 1:10 in different adult populations.8 Thus, although HHV-8 does not appear to be a common cause of a severe, primary clinical syndrome in normal adults, it may be related to a mild syndrome that will require confirmation in larger studies of primary HHV-8 infection.
Our finding that there were no significant alterations in numbers of T-cell phenotypes or activated T cells during primary HHV-8 infection also differs from primary, symptomatic EBV infection in adults, which has a prolonged increase in the numbers of CD8+CD45RO+ HLA-DR+ T cells.2-4 Moreover, we did not find a suppressive effect of HHV-8 primary infection on either CMV-specific or mitogen-stimulated CD8+ T-cell IFN-γ responses in these normal adults. This again differs from the temporal, immunosuppressive effects of symptomatic primary EBV infection on T-cell function.5-7These differences between the 2 human gammaherpesvirus infections must be tempered, however, in that there are no comparable studies on the effects of asymptomatic primary EBV infections on T-cell populations and function. Indeed, a recent study showed that there is no depression of CD4+ T-cell immunity to nominal antigen or mitogen in primary asymptomatic CMV infection in adolescents.38 This is in contrast to the significant suppression in such T-cell functions found during symptomatic CMV infection of adults.39
Of importance is that we documented a distinct, persistent HLA class I restricted, CD8+ cell cytolytic and IFN-γ response to HHV-8 lytic cycle proteins associated with primary HHV-8 infection. This supports a role for these cell-mediated immune responses in the control of initial HHV-8 infection. It also concurs with reports that CD8+ T-cell responses to lytic cycle proteins of EBV develop during primary, symptomatic EBV infection.11,17Similar to primary EBV infection, there was broad antigenic reactivity of CD8+ T cells to this group of HHV-8 lytic cycle proteins after primary HHV-8 infection. That is, the 5 persons infected with HHV-8 had CD8+ T-cell reactivity to at least 3 of the 5 HHV-8 proteins, using 2 different measures of CD8+ T-cell immunity, that is, CTL lysis and single-cell IFN-γ production. Moreover, there was no dominant T-cell response in these men for a specific HHV-8 protein. Because these 5 HHV-8 proteins have some homology with EBV,40 there was a potential for cross-reactivity by anti-EBV CD8+ T cells. However, the temporal relationship between the CD8+ T-cell responses, HHV-8–specific Ab, and HHV-8 DNAemia in our study indicate that these T-cell responses were specific for the HHV-8 proteins.
Lytic cycle proteins were also immunogenic for the humoral Ab system, because Ab titers to HHV-8 lytic cycle proteins paralleled the T-cell responses. A high prevalence of antibodies for other HHV-8 capsid proteins has been detected in HHV-8 seropositive individuals,41,42 supporting the immunogenicity of lytic cycle proteins of the virus. A viremic (DNAemia) phase appeared 2, 3, and 9 months prior to the appearance of detectable humoral Ab responses to HHV-8 in 3 subjects and occurred coincident or after seroconversion in the other 2 subjects. This may be related in part to variation in the actual time of initial HHV-8 infection, which was at some point within the approximately 6-month interval between the last HHV-8 Ab-negative and first HHV-8 Ab-positive results. Of interest is that the earliest DNAemia, found at both 9 and 3 months before seroconversion in one subject, was coincident with the appearance of anti-HHV-8 IFN-γ production by the study subject's CD8+T cells. Thus, both HHV-8 DNA and T-cell immunity to HHV-8 may in some cases precede appearance of antiviral antibodies in the serum as has been found in other viral infections.43
The early presence of DNAemia in some of our individuals is similar to the recent Amsterdam study, which found HHV-8 DNA in the serum of 2 of their 7 cases of primary HHV-8 infections in HIV-1 negative persons 3 months prior to the determined date of seroconversion.34Interestingly, 6 of their 11 HIV-1–infected participants also had HHV-8 DNA-positive sera up to 2 years before seroconversion, suggesting that there may be higher levels of HHV-8 DNAemia in HIV-1–infected persons before the presence of detectable Ab. However, our results contrast with the Amsterdam study in that we detected viral DNA in PBMCs for several years after seroconversion, whereas they detected viremia in the serum for only a few months after seroconversion. This discrepancy may be due to a lower viral DNA copy number in serum compared to PBMCs as the infection evolves into a persistent, latent state. The sporadic, HHV-8 DNA-negative samples in 2 of our 5 subjects after seroconversion may be related to relatively low levels of this DNA in their peripheral blood, as has been reported for some healthy, HHV-8–seropositive, HIV-1–negative individuals.44
The levels of CD8+ T-cell reactivity to most of the HHV-8 lytic cycle proteins declined several years after primary infection. This may be related to a decrease in lytic replication of the virus and resultant lower antigenic load. This could also explain why relatively low CTL reactivity to HHV-8 lytic cycle proteins has been found in cross-sectional studies of persons with unknown duration of HHV-8 infection.23 24 Indeed, viral DNA persisted in the PBMCs for at least 2 years after seroconversion in the 5 subjects in this study, suggesting an establishment of chronic, latent HHV-8 infection. CD8+ T-cell responses to HHV-8 could be operative in controlling reactivation of virus in this latent phase of HHV-8 infection.
As recently reported for other viral infections,45 we found that the number of cells producing IFN-γ was a more sensitive parameter of anti-HHV-8 T-cell reactivity than CTLp's. That is, the mean number of IFN-γ–producing CD8+ T cells was significantly higher than the number of CTLp's for each of the 5 HHV-8 proteins. Of further interest is that the number of IFN-γ–producing T cells induced by CMV pp65 antigen was significantly higher than that induced by each of the 5 HHV-8 proteins. This implies that the CD8+ memory T-cell response to this CMV lytic protein is more robust than the response to HHV-8. We are currently using these approaches as well as HLA class I tetramer analysis46 to delineate the fine specificity CD8+ T-cell responses to peptides derived from these HHV-8 proteins.
Our results support the role of CD8+ T cells specific for HHV-8 lytic proteins in control of primary HHV-8 infection. Recent cross-sectional studies found lower anti-HHV-8 CTL23,24and CD4+ T-cell responses in HHV-8–seropositive, HIV-1–infected persons.47 Longitudinal studies are needed to delineate the function of HHV-8–specific T-cell reactivity in the development of HHV-8–associated diseases such as KS during HIV-1 infection and immunosuppressive drug treatment in organ and tissue transplant recipients.
This study was part of the requirements for MS and PhD degrees by Q.J.W. in the Department of Infectious Diseases and Microbiology of the University of Pittsburgh Graduate School of Public Health. We thank Dr Xiao-Li Huang and Dr Zheng Fan for research suggestions; Laurie Johnson, Christine Kalinyak, Xiao Mao, Luann Borowski, Susan McQuiston, Gayle Springer, and Bill Buchanan for technical and clinical assistance; and the Pitt Men's Study MACS staff and volunteers for their dedication and support.
Supported in part by grants from the US Public Health Service (P30 CA47904, R01 CA82053, R01 CA75957, R03 CA81600 and U01 AI35041).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Charles R. Rinaldo Jr, Department of Infectious Diseases and Microbiology, Graduate School of Public Health, University of Pittsburgh, A427 Crabtree Hall, 130 De Soto St, Pittsburgh, PA 15261; e-mail: rinaldo+@pitt.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal