Abstract
Arachidonic acid (AA) generated by phospholipase A2(PLA2) is thought to be an essential cofactor for phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. Both enzymes are simultaneously primed by cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor–α (TNF-α). The possibility that either unprimed or cytokine-primed responses of PLA2 or NADPH oxidase to the chemotactic agents formyl-methionyl-leucyl-phenylalanine (FMLP) and complement factor 5a (C5a) could be differentially inhibited by inhibitors of the mitogen-activated protein (MAP) kinase family members p42ERK2 (PD98059) and p38SAPK(SB203580) was investigated. PD98059 inhibited the activation of p42ERK2 by GM-CSF, TNF-α, and FMLP, but it did not inhibit FMLP-stimulated superoxide production in either unprimed or primed neutrophils. There was no significant arachidonate release from unprimed neutrophils stimulated by FMLP, and arachidonate release stimulated by calcium ionophore A23187 was not inhibited by PD98059. In contrast, PD98059 inhibited both TNF-α– and GM-CSF–primed PLA2 responses stimulated by FMLP. On the other hand, SB203580 inhibited FMLP-superoxide responses in unprimed as well as TNF-α– and GM-CSF–primed neutrophils, but failed to inhibit TNF-α– and GM-CSF–primed PLA2 responses stimulated by FMLP, and additionally enhanced A23187-stimulated arachidonate release, showing that priming and activation of PLA2 and NADPH oxidase are differentially dependent on both the p38SAPK and p42ERK2 pathways. Studies using C5a as an agonist gave similar results and confirmed the findings with FMLP. In addition, methyl arachidonyl fluorophosphonate (MAFP), the dual inhibitor of c and iPLA2 enzymes, failed to inhibit superoxide production in primed cells at concentrations that inhibited arachidonate release. These data demonstrate that NADPH oxidase activity can be dissociated from AA generation and indicate a more complex role for arachidonate in neutrophil superoxide production.
Introduction
The production of the oxygen radical superoxide and the unsaturated fatty acid arachidonic acid (AA) are both essential for phagocyte function, the former mediating microbicidal activity,1,2 and the latter being the rate-limiting step for eicosanoid synthesis.3,4 The activity of the enzymes that produce superoxide and arachidonate, namely nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and phospholipase A2(PLA2), respectively, are tightly regulated, and the activity of both enzymes can be enhanced rapidly by many-fold if phagocytes are exposed to cytokines, growth factors, and inflammatory mediators such as granulocyte-macrophage colony-stimulating factor (GM-CSF),5-7 tumor necrosis factor–α (TNF-α),8,9 interleukin-8 (IL-8),10 and LPS11,12 during the process known as cell priming.13
There is evidence that the activation of the NADPH oxidase may require AA. Early work showed that exogenous arachidonate added to neutrophils was a potent activator of superoxide production.14,15Arachidonate may act by directly modifying the molecular components of the NADPH oxidase and is reported to increase the affinity of NADPH for its binding site on the oxidase16 and regulate a proton channel17 situated on the gp91phoxprotein.18 Arachidonate may also facilitate the formation of the NADPH oxidase complex on the membrane by enabling the translocation of cytosolic proteins,19 increasing the affinity of guanosine 5′-triphosphate (GTP) binding sites in the plasma membrane,20 and enhancing the dissociation of the rac G protein from its regulatory molecule, GDI.21 Additionally, arachidonate may act as a signal transduction molecule by activating protein kinase cascades, such as protein kinase C,22,23 mitogen-activated protein kinase (MAPK), p42ERK2,23,24 and stress-activated protein kinase, p38SAPK,23 or by modulating intracellular calcium levels25,26 upstream from the NADPH oxidase. AA that is required for NADPH oxidase activity may derive from several lipid sources through the activity of PLA2. Several classes of PLA2, namely Group II,9 Group V sPLA2,27 Group IV complementary (c)PLA2,9,28 and Group VI iPLA2,29 can release arachidonate from human neutrophils. cPLA2 was recently demonstrated to be essential for NADPH oxidase activity in human myeloid PLB-985 cells via activation of a proton channel.30 31
Arachidonate may also mediate the priming of the NADPH oxidase.6 Although not directly activating superoxide production, 1 μM arachidonate caused enhancement of NADPH oxidase activity when the cells were subsequently stimulated by the chemotactic peptide FMLP.7 In addition, inhibition of arachidonate release by mepacrine inhibited the priming of NADPH oxidase by GM-CSF without inhibiting superoxide production by unprimed cells.7 At present it is unclear which PLA2enzymes are activated during cytokine-mediated priming of arachidonate release, although it was recently reported that both Group IV cPLA2 and Group II sPLA2 are activated by TNF priming of neutrophils.9 Furthermore, different PLA2 may be involved in arachidonate release for eicosanoid production rather than for superoxide generation.32
The mechanisms by which cytokines regulate the different PLA2 have not been fully elucidated. Serine phosphorylation is important for regulating cPLA2.33,34Several kinases are reported to phosphorylate cPLA2including protein kinase C,35 and 12-O-tetradecanoylphorbol 13-acetate (TPA) (the potent agonist of protein kinase C) in combination with calcium ionophore induces massive arachidonate release.36 The kinases p42ERK2,35,37 and p38SAPK,34 can also induce phosphorylation of cPLA2. In platelets stimulated with collagen or thrombin, both serine (S)505 and S727 residues of cPLA2 are phosphorylated, and inhibition of p38SAPK was shown to partially inhibit the phosphorylation of PLA2 on both S505 and S727.34 As only the S505 residue lies within a MAPK consensus sequence, the involvement of another kinase downstream of p38SAPK is suggested. Both GM-CSF6,28 and TNF-α38 induce phosphorylation of cPLA2 as well as activating p42ERK2,39,40 and p38SAPK, respectively.40 Therefore, these kinases may play a role in cytokine-driven, arachidonate-mediated priming of NADPH oxidase. However, GM-CSF also activates PI-3 kinase as well as members of the src kinase family, lyn and yes,41which could also be involved in priming events.
The aim of this study was to investigate the role of p42ERK2 and p38SAPK as well as AA production in the activation and priming of the neutrophil respiratory burst. We show that NADPH oxidase and PLA2 have a differential dependence on p42ERK2 and p38SAPK activity and that arachidonate release is not obligatory for eliciting the priming of FMLP- or C5a-stimulated superoxide production.
Materials and methods
Materials
Cytokines.
Stock solutions of recombinant human (rh)GM-CSF (expressed inEscherichia coli) (Hoechst, Hounslow, England) and rhTNF-α (R&D Systems, Europe Abingdon, England) were prepared in sterile phosphate-buffered saline (PBS, pH 7.4) (Gibco-BRL Life Technologies, Paisley, Scotland) containing 1% (vol/vol) fetal calf serum (FCS) (Gibco) and stored at −20°C.
Agonists.
Inhibitors.
Inhibitors included the 5-lipoxygenase activating protein inhibitor MK88642 (gift from Merck-Frosst, Kirkland, Quebec, Canada). A 100 μM stock solution in DMSO was prepared immediately prior to use. A stock solution of N-ethyl-maleimide (NEM) (Sigma) in 100 mM PBS was prepared daily. Stock solutions of 30 mM PD98059 (Calbiochem-Novabiochem, La Jolla, CA) and 30 mM SB203580 (Alexis, Nottingham, England) in DMSO were stored at −20°C and diluted 1000-fold into reaction mixtures. Methyl arachidonyl fluorophosphonate (MAFP) was supplied in solution in methyl acetate (Cayman Chemical, Ann Arbor, MI). The solvent was evaporated under nitrogen, and MAFP was reconstituted with DMSO at 50 mM and stored at −80°C.
Purification of neutrophils
Peripheral venous blood from healthy adult donors was anticoagulated with 2 mM ethylenediamine tetraacetic acid (EDTA) (pH 7.4), and the neutrophils were purified by dextran sedimentation of erythrocytes, centrifugation through Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden), and hypotonic lysis of the remaining erythrocytes as described previously.43 Sterile preparations and procedures were used throughout to minimize contact of cells with endotoxin and to reduce inadvertant priming. Cells were resuspended in PBS supplemented with 0.9 mM calcium, 0.5 mM magnesium, and 5 mM glucose (PBSG).
Measurement of p38 MAP kinase activity
Purified human neutrophils (2 × 107 cells per mL) were preincubated for 30 minutes at 37°C with either 1, 5, 10, 20, 30, or 40 μM SB203580 or with DMSO diluent and then stimulated for 1 minute with either 1 μM FMLP or with PBS diluent. The cells were pelleted by a brief centrifugation step at 12 000g for 30 seconds and lysed by incubation for 30 minutes in 0.5 mL ice-cold lysis buffer—20 mM Tris-HCl (tris[hydroxymethyl] aminomethane–hydrochloride) (pH 8.0), 137 mM sodium chloride (NaCl), 1 mM magnesium dichloride (MgCl2), 1 mM calcium dichloride (CaCl2), 10% glycerol (vol/vol), 1% Nonidet P40 (Sigma) (vol/vol), 2 mM EDTA, 1 mM sodium orthovanadate, 5 mM sodium pyrophosphate, 1 mM sodium fluorine (NaF), and 1 mM β glycerophosphate—containing the following protease inhibitors: 1 mM phenylmethanesulphonyl fluoride (PMSF), 10 μg/mL leupeptin, 10 μg/mL aprotinin, 10 μg/mL pepstatin A, 1 mM diisopropylfluorophosphate (DIFP), and 2 mM Pefabloc SC (4-(2-aminoethyl)-benzensulfonyl fluoride, hydrochloride) (Roche Diagnostics, Lewes, England).
The lysates were centrifuged at 12 000g for 10 minutes at 4°C, and the supernatants were incubated on ice for 30 minutes with 2 μg/mL of a polyclonal anti-p38 MAPK antibody (C-20; Santa Cruz Biotechnology, Santa Cruz, CA) and then with an equal volume of protein G Sepharose beads (Pharmacia Biotech) for 30 minutes at 4°C with rotation. The immune complexes were washed twice with wash buffer—0.5% Triton X-100, 1 mM EDTA, 50 mM Tris-HCl (pH 8.0), and 100 mM NaCl—and once with kinase buffer—25 mM Tris-HCl (pH 8.0), 10 mM MgCl2, and 2 mM MnCl2—and finally resuspended in 10 μL assay dilution buffer containing 200 ng GST-MAPKAPK-2 (residues 46-400 of rhMAPKAPK-2 with an N-terminal glutathione S-transferase tag and a C-terminal myc epitope) (Upstate Biotechnology, Lake Placid, NY).
We added 2 μL magnesium/ATP (adenosine 5′-triphosphate) cocktail (prepared according to the manufacturer's instructions) and 0.185 MBq (5 μCi) γ–phosphorous-32 (γ-32P)–ATP (specific activity, 11 × 1016Bq/mM [3000 Ci/mM]) (Amersham Pharmacia Biotech, Little Chalfont, England) to give a final volume of 36 μL, and the reaction mixtures were incubated for 15 minutes at 30°C. Next, 9 μL of 4 times Laemmli sample buffer was added, and the samples were boiled for 10 minutes. Proteins were separated by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), the gel was dried, and MAPKAP kinase-2 phosphorylation was detected by autoradiography. Aliquots of the samples equivalent to 1-2 × 105 cells were analyzed for total p38 content by immunoblotting as described below.
Detection of p38 phosphorylation by immunoblotting
Neutrophils (1 × 106 cells per mL) were stimulated with either diluent (0.01% FCS), 1 μM FMLP, 500 U/mL TNF-α, or 10 ng/mL GM-CSF at 37°C. At timed intervals 100-μL samples were taken, and the reaction was terminated by addition of 2 mM NEM followed by rapid centrifugation at 12 000g for 30 seconds. The pellet was resuspended in 50 μL ice-cold lysis buffer (composition as given above, but without Pefabloc). We added 50 μL of 2 times Laemmli sample buffer, and the samples were heated at 95°C for 10 minutes. Proteins equivalent to 1-2 × 105 cells per lane were analyzed by 15% SDS-PAGE (acrylamide:bis percentage, 30:0.8) at 150 V for 3.5 hours. The resolved proteins were transferred to PVDF membranes (Immobilon; Millipore, Watford, England). The nonspecific binding sites were blocked in Tris-buffered saline/Tween-20 (TBS-T)—10 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.05% Tween 20—supplemented with 5% (wt/vol) nonfat dried milk for 2 hours at room temperature. The blots were incubated overnight at 4°C with either a goat polyclonal primary p38 MAPK or phosphospecific p38 MAPK rabbit antibody (New England Biolabs, Beverly, MA) (1:1000 dilution, specifically against tyrosine 182 and threonine 180). The membranes were washed 3 times with TBS-T and then incubated for 1 hour at room temperature with a 1:2000 dilution of either secondary horseradish peroxidase–conjugated antirabbit or antigoat immunoglobulin G (IgG) antibodies (Dako, High Wycombe, England). After 2 washes with TBS-T and one wash with TBS, the phosphorylated p38 and total p38 MAP kinase bands were detected by enhanced chemiluminescence (ECL) (Amersham Pharmacia).
Measurement of superoxide production
Superoxide generation was measured at 37°C by the superoxide dismutase–inhibitable reduction of ferri-cytochrome c in a dual-beam spectrophotometer as previously described.43Purified human neutrophils (1 × 106 cells per mL) were incubated in 1-mL cuvettes with or without inhibitors (PD98059 for 10 minutes or SB203580 for 30 minutes) prior to addition of either 500 U/mL TNF-α, 10 ng/mL GM-CSF, or 0.01% FCS diluent for 30 minutes. The samples were stimulated with 1 μM FMLP or 100 ng/mL C5a for 10 minutes, and the reactions were stopped with NEM at a final concentration of 2 mM.
Release of hydrogen-3–AA
Release of hydrogen-3 (3H)–AA from purified human neutrophils was measured as previously described and confirmed using thin-layer chromatography.7 We incubated 5 × 106 cells per mL in PBS, 5 mM glucose, and 0.01% vol/vol FCS with 0.38 MBq (0.5 μCi) 5,6,8,9,11,12,14,15-3H-AA (specific activity, 7.33 TBq/mM (202 Ci/mM) (Amersham Pharmacia) for 2 hours at room temperature. The radio-labeled cells were then centrifuged at 300g for 7 minutes to remove unincorporated radioactivity and then washed 3 times in PBS. The pellet was resuspended to 2 × 106 cells per mL in PBSG. Neutrophil samples (0.5 mL in duplicate) were incubated with 200 nM MK886 (to inhibit metabolism of AA by the 5-lipoxygenase pathway) for 5 minutes at 37°C. Then cells were incubated with either 0.01% FCS as diluent control, 500 U/mL TNF-α, or 10 ng/mL GM-CSF for 20 minutes followed by stimulation with 1 μM calcium ionophore A23187, 1 μM FMLP, or 100 ng/mL C5a for 20 minutes at 37°C. The reaction was stopped by placing the samples on ice and then centrifuged at 12 000g for 4 minutes; 0.4-mL aliquots of the supernatants were assayed for radioactivity by scintillation spectroscopy.
Phosphorylation of p42ERK2 measured by gel retardation assay
Samples of stimulated neutrophils were prepared for Western blot analysis as described above. Proteins equivalent to 1-2 × 105 cells per lane were separated by 15% SDS-PAGE (acrylamide:bis percentage, 15:0.075 [200:1]) for 15 hours at 120 V. After electrophoresis, proteins were electrophoretically transferred onto PVDF membranes (Millipore). Nonspecific binding sites on the membrane were blocked in TBS-T/5% (wt/vol) nonfat dried milk for 2 hours at room temperature. Membranes were incubated with 0.2 μg/mL of a rabbit primary anti-p42ERK2 antibody at a 1:1000 dilution in TBS-T/5% milk (C-14) for 1 hour and then with a secondary horseradish peroxidase–conjugated goat antirabbit IgG antibody at a 1:2000 dilution for 1 hour at room temperature. After 2 washes with TBS-T and one wash with TBS, phosphorylated p42ERK2 was detected by ECL as a band with retarded mobility.
Immunoprecipitation of cPLA2 and detection of phosphorylation by gel retardation
Purified neutrophils (2 × 107 cells per mL) were incubated at 37°C for 30 minutes with either PD98059 or DMSO as diluent control. Cells were then stimulated with either GM-CSF or FCS diluent for 5 minutes at 37°C. Immunodetection of phosphorylated cPLA2 was performed according to the method of Kramer et al.44 The reaction was terminated by addition of 1 mL ice-cold lysis buffer (final concentrations, 1% Triton X-100, 0.5% SDS, 0.75% deoxycholate, 10 mM EDTA, 1 mM PMSF, 10 μg/mL leupeptin, 10 μM pepstatin A, 100 μg/mL aprotinin, 50 mM NaF, 200 μM Na3VO4, 10 mM Na4P2O7, and 1 μM microcystin) for 15 minutes. The cell lysates were subjected to centrifugation at 12 000g for 15 minutes at 4°C. The supernatants were incubated with a rabbit antihuman cPLA2 antibody (N-216, Santa Cruz Biotechnology) at a 1:500 dilution for 2 hours followed by 25 μL protein A Sepharose beads for 30 minutes at 4°C. The immunoprecipitates were washed 4 times with 1 mL wash buffer (0.5% Triton X-100 and 150 mM NaCl [pH 7.4]), then twice with wash buffer containing 750 mM NaCl, and finally twice with the initial wash buffer. The samples were resuspended in 2 times Laemmli sample buffer, incubated for 10 minutes at 60°C, and subjected to 10% SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Hybond C extra, Amersham Pharmacia). The membranes were blocked overnight in 3% bovine serum albumin and then incubated with rabbit antihuman cPLA2 antibody at 1:500 dilution for 2 hours at room temperature. The membranes were washed 3 times with wash buffer and incubated with a secondary horseradish peroxidase–conjugated goat antirabbit IgG antibody at a dilution of 1:2000 for 1 hour. The membrane was washed 3 times with wash buffer, and immunoreactive cPLA2 bands were detected using ECL.
Statistical analysis
The data presented are the mean ± SE of the number of experiments given in the text. Analyses to determine the statistical significance employed the Student paired t test.
Results
Activation of neutrophil p38SAPK by agonists and cytokines
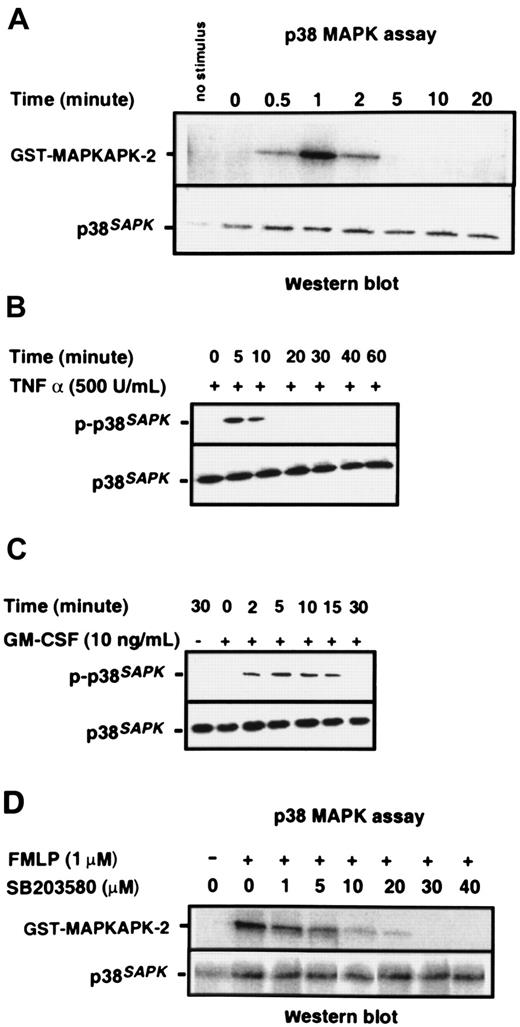
Purified neutrophils were stimulated with 1 μM chemotactic peptide FMLP; p38SAPK immunoprecipitates were prepared, and their kinase activity was measured by the phosphorylation of a MAPKAPK-2 substrate, as described in “Materials and methods.” Figure 1A shows that p38SAPKwas rapidly and transiently stimulated by FMLP. Activity was detected within 30 seconds, was maximal at one minute, but was no longer detectable at 5 minutes. Figure 1B-C shows that the cytokines TNF-α and GM-CSF also stimulate the activation of p38SAPK in purified neutrophils, and phosphorylation of this molecule is determined by immunoblotting of whole cell lysates with a p38SAPK phosphospecific antibody. GM-CSF–induced phosphorylation of p38SAPK was detectable within 2 minutes and was sustained for 15 minutes (Figure 1C), whereas TNF-α–induced phosphorylation was slightly slower in onset and more transient (Figure1B). The kinetics of p38SAPK phosphorylation stimulated by FMLP, when determined by immunoblotting with the phosphospecific antibody, was similar to the activation of p38SAPK activity (data not shown). The optimum inhibitory concentration of the p38SAPK inhibitor SB20358045 46 was determined using the p38 kinase assay. The data in Figure 1D show that SB203580 inhibited the phosphorylation of MAPKAPK-2 in a dose-dependent manner with approximately 1 μM IC50 and complete inhibition at 30 μM.
Activation of p38SAPK in purified neutrophils.
(A) PMN were stimulated with 1 μM FMLP as indicated in the figure, and p38SAPK was immunoprecipitated and subjected to a kinase assay using glutathione S-transferase–tagged MAPKAP kinase-2 as substrate in the presence of γ–32P-ATP. Samples were then subjected to Western blotting and autoradiography as described in “Materials and methods.” The top panel shows the autoradiograph of the region of the blot corresponding to MAPKAP kinase-2. The bottom panel shows the membrane reprobed with a p38SAPK-specific antibody to check for equal loading of p38 kinase in each lane. PMN were also stimulated with (B) TNF-α and (C) GM-CSF followed by preparation of cell lysates, separation of proteins by SDS-PAGE, and immunoblotting with antibodies specific for phosphorylated p38SAPK (top panel) and total cell p38SAPK(bottom panel). (D) Neutrophils (2 × 107 cells per mL) were incubated with the p38 kinase inhibitor SB203580 at various doses or DMSO diluent prior to stimulation with FMLP for 1 minute. Preparation of p38SAPK immunoprecipitates and p38 kinase assay was performed as described in panel A. The top panel shows the autoradiograph of the region of the membrane corresponding to MAPKAP kinase-2, and the bottom panel shows the membrane reprobed with a p38SAPK-specific antibody.
Activation of p38SAPK in purified neutrophils.
(A) PMN were stimulated with 1 μM FMLP as indicated in the figure, and p38SAPK was immunoprecipitated and subjected to a kinase assay using glutathione S-transferase–tagged MAPKAP kinase-2 as substrate in the presence of γ–32P-ATP. Samples were then subjected to Western blotting and autoradiography as described in “Materials and methods.” The top panel shows the autoradiograph of the region of the blot corresponding to MAPKAP kinase-2. The bottom panel shows the membrane reprobed with a p38SAPK-specific antibody to check for equal loading of p38 kinase in each lane. PMN were also stimulated with (B) TNF-α and (C) GM-CSF followed by preparation of cell lysates, separation of proteins by SDS-PAGE, and immunoblotting with antibodies specific for phosphorylated p38SAPK (top panel) and total cell p38SAPK(bottom panel). (D) Neutrophils (2 × 107 cells per mL) were incubated with the p38 kinase inhibitor SB203580 at various doses or DMSO diluent prior to stimulation with FMLP for 1 minute. Preparation of p38SAPK immunoprecipitates and p38 kinase assay was performed as described in panel A. The top panel shows the autoradiograph of the region of the membrane corresponding to MAPKAP kinase-2, and the bottom panel shows the membrane reprobed with a p38SAPK-specific antibody.
Effect of inhibition of p38SAPK on neutrophil superoxide production and AA release
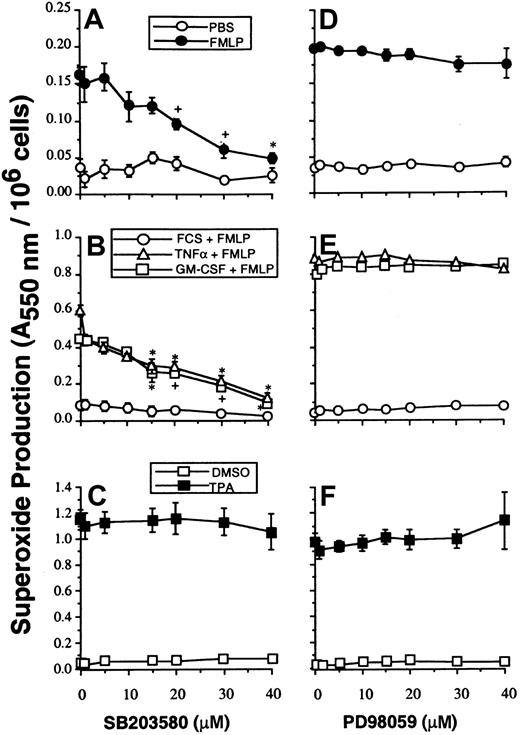
Studies with SB203580 were performed to determine whether p38SAPK has a role in mediating the priming effects of GM-CSF and TNF-α on either PLA2 or NADPH oxidase activity stimulated with FMLP. Neutrophils were preincubated with SB203580, and NADPH oxidase activity was measured by the superoxide dismutase–inhibitable reduction of cytochrome c as described in “Materials and methods.” Figure2A-B shows that SB203580 inhibited unprimed as well as GM-CSF– and TNF–primed superoxide production stimulated by FMLP. SB203580, at doses that inhibited p38SAPK, did not inhibit NADPH oxidase activity stimulated by the receptor-independent agonist TPA and thus did not inhibit the assembly and activation of the oxidase stimulated via PKC (Figure 2C).
The effect of SB203580 and PD98059 on neutrophil superoxide production.
(A, D) Neutrophils were preincubated with inhibitors at the range of concentrations indicated, for optimal times as described in “Materials and methods,” before stimulation for 10 minutes with 1 μM FMLP and measurement of superoxide production. (B, E) Parallel samples were primed by incubation with either FCS diluent, GM-CSF, or TNF-α for 30 minutes before stimulation with FMLP as in panels A and D. (C, F) Cells were stimulated with 100 ng/mL TPA for 5 minutes.
The effect of SB203580 and PD98059 on neutrophil superoxide production.
(A, D) Neutrophils were preincubated with inhibitors at the range of concentrations indicated, for optimal times as described in “Materials and methods,” before stimulation for 10 minutes with 1 μM FMLP and measurement of superoxide production. (B, E) Parallel samples were primed by incubation with either FCS diluent, GM-CSF, or TNF-α for 30 minutes before stimulation with FMLP as in panels A and D. (C, F) Cells were stimulated with 100 ng/mL TPA for 5 minutes.
PLA2 activity was determined as the extracellular release of 3H-AA from prelabeled phospholipid stores in neutrophils whose 5-lipoxygenase activity had been fully inhibited by the highly specific inhibitor MK886.42 This allowed maximal detection of PLA2 rather than 5-lipoxygenase activity and allowed the effect of the kinase inhibitors on PLA2 to be determined without interference from any possible effect on the downstream metabolism of AA. In 14 experiments the amount of AA released from non-primed neutrophils stimulated with FMLP was not significantly greater than background activity (2340 ± 239 cpm/106 unstimulated cells and 2474 ± 230 cpm/106 FMLP-stimulated cells), as we previously reported,7 but AA release was significantly greater than background in FMLP-stimulated cells primed with either TNF-α (4294 ± 667 cpm/106 cells) (n = 7,P = .02) or GM-CSF (4660 ± 539 cpm/106cells) (n = 7, P = .001).
The data presented in Table 1 show that when neutrophils were preincubated with SB203580 under the conditions that gave complete inhibition of p38SAPK and significant inhibition of NADPH oxidase activity, there was no significant inhibition of either GM-CSF– or TNF-primed FMLP-stimulated AA release. To confirm these data, neutrophils treated with SB203580 was also stimulated with 1 μM calcium ionophore A23187, and AA release was measured. Table 1 shows that no inhibition of AA release from either unprimed or primed cells was apparent; in fact, SB203580 significantly enhanced AA release from ionophore-stimulated cells that had been primed with TNF-α.
Effect of the p38 MAP kinase inhibitor SB203580 on cytokine-mediated priming of arachidonate release in neutrophils
| Stimulus, 1 μM . | SB203580, μM . | Priming agent, % control . | ||
|---|---|---|---|---|
| FCS . | TNF-α . | GM-CSF . | ||
| FMLP | 0 | — | 100+ | 100 |
| 1 | — | 136 ± 36 | 125 ± 11 | |
| 5 | — | 113 ± 27 | 159 ± 24 | |
| 10 | — | 107 ± 26 | 145 ± 18 | |
| 15 | — | 107 ± 23 | 149 ± 19 | |
| 20 | — | 109 ± 33 | 130 ± 19 | |
| 30 | — | 91 ± 20 | 78 ± 30 | |
| A23187 | 0 | 100 | 100 | 100 |
| 1 | 74 ± 13 | 187 ± 12* | 130 ± 12 | |
| 5 | 91 ± 12 | 199 ± 19† | 138 ± 16 | |
| 10 | 76 ± 23 | 211 ± 14* | ND | |
| 15 | 80 ± 28 | 216 ± 27† | 115 ± 8 | |
| 20 | 97 ± 21 | 206 ± 27† | 115 ± 9 | |
| 30 | 112 ± 31 | 182 ± 22† | 127 ± 11 | |
| Stimulus, 1 μM . | SB203580, μM . | Priming agent, % control . | ||
|---|---|---|---|---|
| FCS . | TNF-α . | GM-CSF . | ||
| FMLP | 0 | — | 100+ | 100 |
| 1 | — | 136 ± 36 | 125 ± 11 | |
| 5 | — | 113 ± 27 | 159 ± 24 | |
| 10 | — | 107 ± 26 | 145 ± 18 | |
| 15 | — | 107 ± 23 | 149 ± 19 | |
| 20 | — | 109 ± 33 | 130 ± 19 | |
| 30 | — | 91 ± 20 | 78 ± 30 | |
| A23187 | 0 | 100 | 100 | 100 |
| 1 | 74 ± 13 | 187 ± 12* | 130 ± 12 | |
| 5 | 91 ± 12 | 199 ± 19† | 138 ± 16 | |
| 10 | 76 ± 23 | 211 ± 14* | ND | |
| 15 | 80 ± 28 | 216 ± 27† | 115 ± 8 | |
| 20 | 97 ± 21 | 206 ± 27† | 115 ± 9 | |
| 30 | 112 ± 31 | 182 ± 22† | 127 ± 11 | |
Purified neutrophils were incubated with SB203580 for 30 minutes at 37°C before priming with either 500 U/mL TNF-α, 10 ng/mL GM-CSF, or 0.01% FCS diluent for 20 minutes followed by stimulation with agonist as indicated for 15 minutes. Arachidonate release was measured as described in “Materials and methods.” Basal release of arachidonate in unstimulated samples was subtracted from the stimulated value, and the data were expressed as a percentage of the DMSO diluent control. Absolute values expressed as cpm/106 cells for (1) FMLP stimulation: FCS, 118 ± 100; TNF, 1493 ± 461; and GM-CSF, 1317 ± 321 and (2) A23187 stimulation: FCS, 2584 ± 786; TNF, 4856 ± 1094; and GM-CSF, 11 306 ± 3332. In all cases the release of arachidonate from unprimed FMLP-stimulated cells was not significantly different from background. The data shown are the mean ± SE of 4 experiments.
ND indicates not determined; FCS, fetal calf serum; TNF-α, tumor necrosis factor–α; GM-CSF, granulocyte-macrophage colony-stimulating factor; FMLP, formyl-methionyl-leucyl-phenylalanine.
.01 > P > .001 indicates significant difference between samples with inhibitor.
.05 > P > .01 indicates significant difference between samples without inhibitor.
Activation of neutrophil p42ERK2 by agonists and cytokines
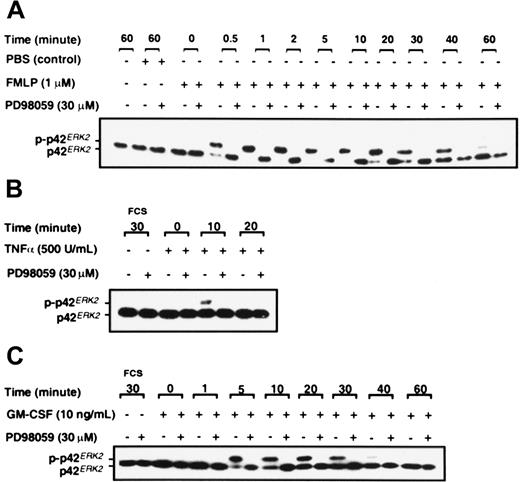
Neutrophils were stimulated with FMLP, GM-CSF, and TNF-α, and analysis of p42ERK2 phosphorylation was by gel retardation assay as described in “Materials and methods.” Figure3 shows that FMLP, GM-CSF, and TNF-α activate p42ERK2 in neutrophils in addition to activating p38SAPK. FMLP stimulated the phosphorylation of p42ERK2 within 30 seconds, and the activation was sustained for at least 40 minutes (Figure 3A). TNF-α stimulation of p42ERK2 was only transient, with a weak band being detected at 10 minutes after stimulation (Figure 3B), whereas GM-CSF induced more sustained activation (Figure 3C). Activation of p42ERK2 was inhibited by preincubation of neutrophils with the noncompetitive MEK1 inhibitor PD98059,47 as shown in Figure 3. Data from dose-response studies showed that complete inhibition of p42ERK2 activation in neutrophils stimulated by GM-CSF was achieved at 10 μM PD98059 (data not shown). Inhibition of p42ERK2 kinase by PD98059 was achieved rapidly, a preincubation of 5 minutes was sufficient to fully inhibit the enzyme, and inhibition was sustained for at least 60 minutes after GM-CSF stimulation (data not shown).
Effect of PD98059 on the phosphorylation of p42ERK2.
Purified neutrophils were incubated with either DMSO or 30 μM PD98059 for 30 minutes before stimulation with either (A) 1 μM FMLP, (B) 500 U/mL TNF-α, or (C) 10 ng/mL GM-CSF or FCS diluent for the times indicated. Phosphorylation of p42ERK2 was measured by gel retardation as described in “Materials and methods.” The data shown are from a single experiment that was performed twice with similar results.
Effect of PD98059 on the phosphorylation of p42ERK2.
Purified neutrophils were incubated with either DMSO or 30 μM PD98059 for 30 minutes before stimulation with either (A) 1 μM FMLP, (B) 500 U/mL TNF-α, or (C) 10 ng/mL GM-CSF or FCS diluent for the times indicated. Phosphorylation of p42ERK2 was measured by gel retardation as described in “Materials and methods.” The data shown are from a single experiment that was performed twice with similar results.
Effect of inhibition of p42ERK2 MAP kinase on neutrophil superoxide production and AA release
Figure 2D-E shows that under the conditions where p42ERK2 activation was completely blocked, there was no observable concomitant inhibition of either unprimed (n = 4), GM-CSF–primed (n = 3), or TNF-α–primed (n = 3) NADPH oxidase activity stimulated by FMLP. Neither did PD98059 at any dose inhibit TPA-stimulated NADPH oxidase activity (n = 3) (Figure 2F). However, the data given in Table 2 show that PD98059 did partially inhibit both GM-CSF– and TNF-α–primed FMLP-stimulated PLA2 responses. To confirm the inhibitory effect of PD98059 on arachidonate release, studies were performed using 1 μM calcium ionophore A23187 as stimulant. In 4 experiments, AA release from unprimed neutrophils stimulated by A23187 was not significantly inhibited by PD98059 at any concentration, whereas GM-CSF and TNF-α priming of AA release stimulated by A23187 was inhibited in a dose-dependent fashion (Table 2).
Effect of the MEK kinase inhibitor PD98059 on cytokine-mediated priming of arachidonate release in neutrophils
| Stimulus, 1 μM . | PD98059, μM . | Priming agent, % control . | ||
|---|---|---|---|---|
| FCS . | TNF-α . | GM-CSF . | ||
| FMLP | 0 | — | 100+ | 100 |
| 1 | — | 80 ± 3* | 88 ± 10 | |
| 5 | — | 61 ± 3* | 49 ± 25 | |
| 10 | — | 52 ± 8† | 50 ± 3* | |
| 15 | — | 53 ± 24 | 46 ± 8* | |
| 20 | — | 28 ± 6* | 40 ± 8† | |
| 30 | — | 30 ± 4* | 57 ± 4* | |
| A23187 | 0 | 100 | 100 | 100 |
| 1 | 79 ± 19 | 82 ± 8 | 79 ± 11 | |
| 5 | 71 ± 22 | 66 ± 17 | 67 ± 14 | |
| 10 | 80 ± 25 | 48 ± 5† | 59 ± 8† | |
| 15 | 73 ± 24 | 42 ± 8† | 46 ± 15† | |
| 20 | 86 ± 18 | 44 ± 8† | 54 ± 8† | |
| 30 | 80 ± 21 | 38 ± 4* | 40 ± 7* | |
| Stimulus, 1 μM . | PD98059, μM . | Priming agent, % control . | ||
|---|---|---|---|---|
| FCS . | TNF-α . | GM-CSF . | ||
| FMLP | 0 | — | 100+ | 100 |
| 1 | — | 80 ± 3* | 88 ± 10 | |
| 5 | — | 61 ± 3* | 49 ± 25 | |
| 10 | — | 52 ± 8† | 50 ± 3* | |
| 15 | — | 53 ± 24 | 46 ± 8* | |
| 20 | — | 28 ± 6* | 40 ± 8† | |
| 30 | — | 30 ± 4* | 57 ± 4* | |
| A23187 | 0 | 100 | 100 | 100 |
| 1 | 79 ± 19 | 82 ± 8 | 79 ± 11 | |
| 5 | 71 ± 22 | 66 ± 17 | 67 ± 14 | |
| 10 | 80 ± 25 | 48 ± 5† | 59 ± 8† | |
| 15 | 73 ± 24 | 42 ± 8† | 46 ± 15† | |
| 20 | 86 ± 18 | 44 ± 8† | 54 ± 8† | |
| 30 | 80 ± 21 | 38 ± 4* | 40 ± 7* | |
Purified neutrophils were incubated with PD98059 for 10 minutes at 37°C before priming and stimulation with agonist and measurement of arachidonate release as described in the legend to Table 1. Basal release of arachidonate in unstimulated samples was subtracted from the stimulated value, and the data were expressed as a percentage of DMSO diluent control. Absolute values were expressed as cpm/106cells for (1) FMLP stimulation: FCS, 123 ± 142; TNF, 2563 ± 950; and GM-CSF, 1130 ± 30; and (2) A23187 stimulation; FCS, 2472 ± 779; TNF, 5266 ± 1251; and GM-CSF, 10 050 ± 524. Release of arachidonate from unprimed FMLP-stimulated cells was not significantly different from background, therefore the percent of control values was not determined. The data shown are the mean ± SE of 4 experiments.
See Table 1 for abbreviations.
.01 > P > .001 indicates significant difference between samples with inhibitor.
.05 > P > .01 indicates significant difference between samples without inhibitor.
Effect of the MAP kinase inhibitors on the phosphorylation of cPLA2
To investigate whether the target class of PLA2 that was inhibited by PD98059 was cPLA2, we investigated the effects of PD98059 on cPLA2 phosphorylation as determined by gel retardation. The data in Figure4 show that phosphorylation of cPLA2 was stimulated by GM-CSF and that this was not inhibited by either 20 or 30 μM PD98059, doses that completely inhibit p42ERK2 activity (Figure 3A-C).
Effect of PD98059 on the phosphorylation of cytosolic PLA2.
Purified neutrophils were preincubated with PD98059 or DMSO vehicle at the doses indicated in the figure for 30 minutes before stimulation with 10 ng/mL GM-CSF for 5 minutes. Migration of cPLA2immunoprecipitates was determined by SDS-PAGE followed by Western blotting with an anti-cPLA2 antibody.
Effect of PD98059 on the phosphorylation of cytosolic PLA2.
Purified neutrophils were preincubated with PD98059 or DMSO vehicle at the doses indicated in the figure for 30 minutes before stimulation with 10 ng/mL GM-CSF for 5 minutes. Migration of cPLA2immunoprecipitates was determined by SDS-PAGE followed by Western blotting with an anti-cPLA2 antibody.
The effect of the PLA2 inhibitor MAFP on superoxide production and arachidonate release
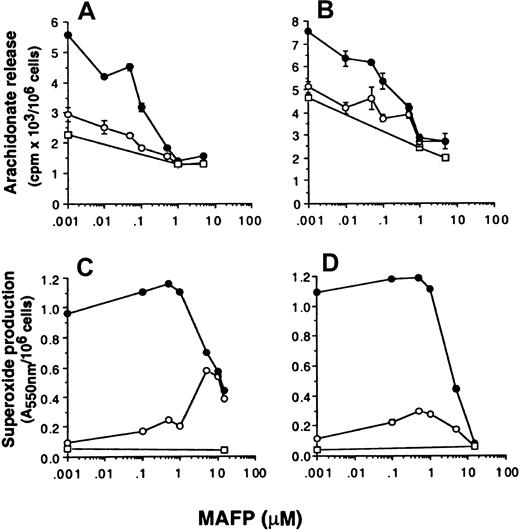
The data so far presented suggest that superoxide production and arachidonate release can be dissociated by selective inhibition of either p42ERK2 or p38SAPK. To further investigate whether superoxide production can occur independently from arachidonate production, the effect of PLA2 inhibitors on the respiratory burst and AA release was measured. Neutrophils were preincubated with the dual c and iPLA2 inhibitor MAFP48 49 before priming and stimulation with FMLP, and the effect on NADPH oxidase and arachidonate release was measured. Figure 5 shows that MAFP inhibited FMLP-stimulated AA release primed by GM-CSF or TNF-α with approximately 0.1 μM IC50, whereas this compound did not inhibit either unprimed or primed FMLP-stimulated NADPH oxidase activity unless used at a much higher concentration of 5 μM. In control experiments measuring the activation of a different signal transduction pathway, we showed that these high doses of MAFP did not inhibit signal transducer and activator of transcription (STAT)5b activation in neutrophils stimulated with GM-CSF (data not shown), thus the inhibitory effects of MAFP were not due to generalized cellular toxicity.
Effect of MAFP on neutrophil arachidonate release and superoxide production.
Neutrophils were preincubated with MAFP at the range of concentrations indicated in the text, followed by priming with either (A, C) 10 ng/mL GM-CSF (●) or (B, D) 500 U/mL TNF-α (●) or FCS diluent (○) and stimulated with either 1 μM FMLP (○) or PBS (■). (A, B) Arachidonate release was measured as described in “Materials and methods.” Stimulation with FMLP was for 30 minutes. (C, D) Superoxide production was measured by the reduction of cytochrome c in a dual-beam spectrophotometer. Stimulation with FMLP was for 10 minutes.
Effect of MAFP on neutrophil arachidonate release and superoxide production.
Neutrophils were preincubated with MAFP at the range of concentrations indicated in the text, followed by priming with either (A, C) 10 ng/mL GM-CSF (●) or (B, D) 500 U/mL TNF-α (●) or FCS diluent (○) and stimulated with either 1 μM FMLP (○) or PBS (■). (A, B) Arachidonate release was measured as described in “Materials and methods.” Stimulation with FMLP was for 30 minutes. (C, D) Superoxide production was measured by the reduction of cytochrome c in a dual-beam spectrophotometer. Stimulation with FMLP was for 10 minutes.
Studies with C5a
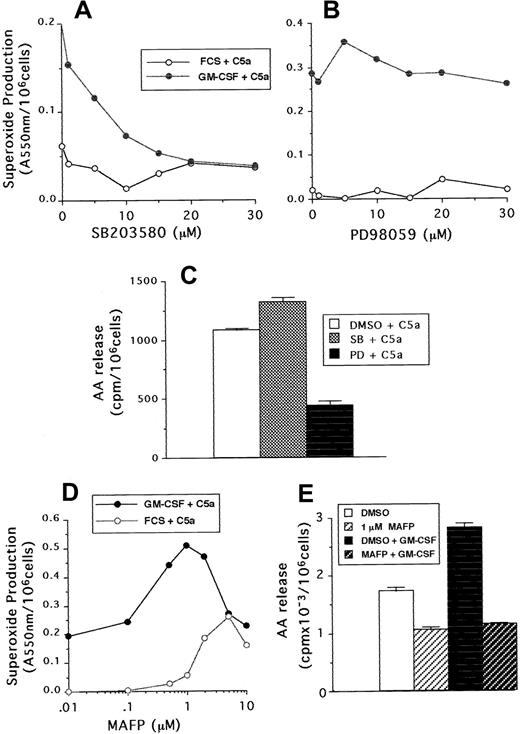
Neutrophil superoxide production and AA release stimulated by C5a were also measured in samples that had been preincubated with either PD98059, SB203580, or MAFP. Although C5a was a weaker agonist than FMLP, similar results were found with regard to the sensitivity of neutrophils to the MAP kinase and phospholipase inhibitors. Figure6A-B shows that superoxide production was inhibited by SB203580 in a dose-dependent manner, but not by PD98059, whereas these compounds had the reverse effect on arachidonate release (Figure 6C). In addition, 1 μM MAFP enhanced both unprimed and GM-CSF–primed C5a-stimulated superoxide production (Figure 6D), but inhibited to basal levels C5a-stimulated AA release (Figure6E).
The effect of SB203580, PD98059, and MAFP on neutrophil responses stimulated by C5a.
(A,B) Superoxide production following incubation with either SB203580 or PD98059 at the doses indicated in the figure, followed by priming with GM-CSF or cytokine diluent and stimulation with 100 ng/mL C5a. (C) AA release from GM-CSF–primed cells preincubated with either 30 μM SB203580, PD98059, or DMSO diluent and stimulated with 100 ng/mL C5a. (D) Superoxide production from GM-CSF– or diluent-primed cells, preincubated with either MAFP at the doses indicated or DMSO diluent, followed by stimulation with 100 ng/mL C5a. (E) AA release from neutrophils incubated with or without 1 μM MAFP and then primed by incubation with 10 ng/mL GM-CSF or cytokine diluent, followed by stimulation with 100 ng/mL C5a.
The effect of SB203580, PD98059, and MAFP on neutrophil responses stimulated by C5a.
(A,B) Superoxide production following incubation with either SB203580 or PD98059 at the doses indicated in the figure, followed by priming with GM-CSF or cytokine diluent and stimulation with 100 ng/mL C5a. (C) AA release from GM-CSF–primed cells preincubated with either 30 μM SB203580, PD98059, or DMSO diluent and stimulated with 100 ng/mL C5a. (D) Superoxide production from GM-CSF– or diluent-primed cells, preincubated with either MAFP at the doses indicated or DMSO diluent, followed by stimulation with 100 ng/mL C5a. (E) AA release from neutrophils incubated with or without 1 μM MAFP and then primed by incubation with 10 ng/mL GM-CSF or cytokine diluent, followed by stimulation with 100 ng/mL C5a.
Discussion
Our data extend previous findings that FMLP,50-52 GM-CSF,53-55 and TNF-α54-56 can activate p38SAPK in human neutrophils. The kinetics of activation of p38SAPK by GM-CSF and TNF-α were broadly similar to, but more prolonged than, that of FMLP. The p38 kinase inhibitor SB203580 had approximately 1 μM IC50 in the p38SAPK kinase assay and inhibited FMLP- and C5a-stimulated superoxide production both in unprimed and GM-CSF– or TNF-α–primed cells. This suggests that p38SAPK has a role in activating the neutrophil respiratory burst stimulated by FMLP (in confirmation of previous reports54,55,57) and by C5a. However, there was no detected effect of p38SAPK inhibition on superoxide production stimulated by TPA, which is in contrast to the recent findings of Lal et al.58
Parallel experiments measuring arachidonate release stimulated by FMLP and C5a showed little inhibition attributable to blocking p38SAPK, and in fact, arachidonate release was enhanced by 1-20 μM SB203580. The enhancing effect of SB203580 on arachidonate release was further confirmed in cells stimulated with calcium ionophore. These results show that (1) SB203580 was not a global inhibitor of either FMLP or C5a signaling upstream of the NADPH oxidase, and thus the site of action of p38SAPK is likely to be the oxidase itself; (2) priming and activation of neutrophil AA release is not dependent on p38SAPK; and (3) the signal transduction pathways important for activating the NADPH oxidase via FMLP or C5a receptors in either primed or unprimed cells are not the same as those for activating PLA2.
Our observations are in agreement with those of Syrbu et al,38 who showed that SB203580 does not inhibit the phosphorylation of cPLA2 in FMLP-stimulated neutrophils, as determined by a gel-shift assay. However, Syrbu et al38 report that SB203580 did partially inhibit FMLP-stimulated AA release from intact cells. The differences between these findings and ours may be due to differences in techniques used, as Syrbu et al did not use an inhibitor of 5-lipoxygenase in these assays and therefore measured the additive effects of SB203580 on both PLA2 and 5-lipoxygenase. Indeed, we have shown that SB203580 does partially inhibit 5-lipoxygenase,59 but it fails to inhibit AA release in cells where 5-lipoxygenase is blocked with MK886. SB203580 similarly inhibits other enzymes, such as cyclo-oxygenase 2, which are downstream of PLA2.60
In contrast, inhibition of p42ERK2 had no effect on superoxide production in unprimed or primed cells. Unprimed cells stimulated with calcium ionophore, but not FMLP or C5a, released arachidonate, but this release was not inhibited significantly by the p42ERK2 pathway inhibitor. A dose-dependent, but partial, inhibition of arachidonate release was only seen in cytokine-primed cells. The data support other reports that activation of the FMLP-stimulated respiratory burst is not dependent on p42ERK2,61-63 but indicate that primed PLA2 responses are dependent on this kinase. Our data show that activation of NADPH oxidase and PLA2 in GM-CSF– or TNF-α–primed PMN can be dissociated by either blocking p38SAPK or p42ERK2 kinase pathways and suggest that arachidonate release is not required for the activation of the respiratory burst by FMLP or C5a. It should be noted that inhibitors, such as the MAP kinase inhibitors, may have other effects when used in complex biological systems than when used in single enzyme assays. Thus, differential effects may also reflect different affinities and concentration dependence of the end points that are studied.
To examine further the dissociation of the activation pathways from the FMLP receptor to NADPH oxidase and arachidonate release, the effect of the PLA2 inhibitor MAFP48,49 on these enzyme systems was studied. We previously showed that both GM-CSF–primed oxidase and PLA2 activity were inhibited by mepacrine, a relatively nonspecific inhibitor of sPLA2, which suggests a possible role for this enzyme in activating the primed oxidase.7 Superoxide production by unprimed neutrophils was not inhibited by mepacrine, suggesting that only the enhanced superoxide production in response to priming was dependent on concomitant arachidonate production. However, concentrations of MAFP that inhibited FMLP- and C5a-stimulated arachidonate release primed by GM-CSF and TNF-α did not inhibit the NADPH oxidase. This supports the hypothesis that arachidonate production in neutrophils is not exclusively required for respiratory burst activity. Our experiments with MAFP do not exclude the possibility that a small amount of AA is produced by other noncytosolic PLA2, which is sufficient to allow superoxide production to occur. Of relevance, bromoenol lactone, which is reported to be a more potent inhibitor of iPLA2than MAFP,64,65 did inhibit both PLA2 and NADPH oxidase activities (data not shown). However, it was recently reported that BEL has the property of directly binding to the p67phox protein66 and thus may inhibit NADPH oxidase by a mechanism which is independent from that inhibiting PLA2 activity.
The ability to dissociate FMLP- and C5a-stimulated NADPH oxidase activity from arachidonate release has important implications for the role of arachidonate in respiratory burst activity. Arachidonate is thought to interact with the NADPH oxidase in several ways,15-21and specific binding sites for arachidonate on the p67phox protein have been recently elucidated.66 However, recent work with leukemic cells lines induced to differentiate to mature cells have revealed conflicting results. Lowenthal and Levy31 showed that human myeloid PLB-985 cells transfected with antisense cPLA2 lack respiratory burst activity following differentiation with either retinoic acid or vitamin D3, but that activity was restored by addition of exogenous arachidonate. In contrast we previously showed that U937 cells differentiated with interferon-γ develop a robust superoxide response to the agonists TPA, FMLP, and cross-linking FcγRII, all in the absence of detectable AA release or leukotriene production.67 More recently others confirmed the lack of AA release in U937 cells, unless they are differentiated with vitamin D3, and showed that the development of oxidase activity with cell maturation depended on the cosynthesis of PLD.68 The data support the hypothesis that there are alternative pathways for activating the NADPH oxidase other than via AA generated by PLA2.
Our data reveal a greater sensitivity of AA release stimulated by calcium ionophore to PD98059 in primed versus unprimed neutrophils; thus it is possible that a different PLA2 enzyme may be responsible for AA release in primed cells compared to unprimed cells. The PD98059-sensitive PLA2 in primed cells may not be cPLA2, as Syrbu et al38 have shown that PD98059 inhibits neither FMLP- nor TNF-α–stimulated phosphorylation of this enzyme. Similarly, we find that PD98059 does not inhibit GM-CSF–mediated phosphorylation of cPLA2.
The aim of this work was to investigate the signaling pathway responsible for priming PLA2 activity and to compare and contrast it with those pathways responsible for the priming and activation of the respiratory burst. Our results indicate that superoxide production is dependent on p38SAPK, which is in agreement with previous work.54,55,57 58 By contrast, when we looked at PLA2, there was no dependence on the p38SAPK pathway, but there was a dependence on p42ERK2 MAP kinase activity only in cytokine-primed cells. Blocking arachidonate release with either a p42ERK2 pathway inhibitor or PLA2 inhibitor did not concomitantly reduce NADPH oxidase activity. Thus the signaling pathways from the GM-CSF and TNF-α receptor for priming PLA2 activity differ from those responsible for priming the NADPH oxidase, thereby emphasizing the heterogeneity and complexity of both growth factor receptor signaling and the requirement of arachidonate for NADPH oxidase activation.
The authors are grateful to Dr Asim Khwaja for help with determining p38 kinase activity and Dr Helen Wheadon for performing the STAT5b assay.
Supported by a grant from the Kay Kendall Leukaemia Fund, London, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David C. Linch, Department of Haematology, The Royal Free and University College London Medical School, 98, Chenies Mews, London WC1E 6HX, England; e-mail: d.linch@ucl.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal