Abstract
The leukotrienes are inflammatory mediators derived from arachidonic acid. It was demonstrated that the priming of leukocytes with phorbol-12-myristate-13-acetate (PMA) leads to the increased formation of 5-lipoxygenase (5-LO) products in parallel with the increased association of 5-LO with the nucleus and the activation of kinases that can phosphorylate 5-LO in vitro. Stimulation of the monocytic cell line Mono Mac 6 with calcium ionophore gave low 5-LO product formation and no detectable redistribution of 5-LO. However, after priming of Mono Mac 6 cells with phorbol esters, ionophore led to the association of 45% to 75% of cellular 5-LO with the nuclear membrane, to 5-LO kinase activation, to enhanced release of arachidonate, and to substantial leukotriene synthesis. Similar results were obtained for human polymorphonuclear leukocytes stimulated with low-dose ionophore. In addition, for each cell type, PMA priming up-regulated leukotriene biosynthesis in the presence of exogenous arachidonic acid. A protein kinase inhibitor, calphostin C, reduced the association of 5-LO with the nucleus and 5-LO kinase activity, and the formation of 5-LO products was inhibited. These results suggest that PMA up-regulates leukotriene biosynthesis not only by increasing the release of endogenous arachidonate, but also by increasing the capacity for 5-LO phosphorylation and for the translocation of 5-LO to the nucleus in leukocytes.
Introduction
Leukotrienes (LTs) are mediators of inflammatory responses formed primarily in different types of leukocytes, and, in leukotriene biosynthesis, 5-lipoxygenase (5-LO) catalyzes the conversion of arachidonic acid (AA) to leukotriene A4.1 The cellular distribution of 5-LO in unstimulated cells differs among different cell types. In peripheral blood neutrophils, differentiated HL-60 cells, and peritoneal macrophages, 5-LO is localized mainly in the cytosol, whereas in resting alveolar macrophages, rat basophilic leukemia cells, mouse bone marrow–derived mast cells, and Langerhans cells of human skin, the enzyme is partly or predominantly present in the soluble compartment of the nucleus. On cell activation, both cytosolic and nuclear soluble 5-LO can translocate to the nuclear envelope, leading to colocalization with cytosolic phospholipase A2 (cPLA2) and 5-lipoxygenase activating protein (FLAP) (for review, see2). An N-terminal β-barrel domain of 5-LO is important for this calcium-stimulated membrane association.3,4Treatment of rat neutrophils with inflammatory agents in vivo, or adherence of neutrophils to glass in vitro, led to nuclear import of cytosolic 5-LO and a concomitant increased capacity for subsequent LT biosynthesis.5 Such import into a soluble compartment within the nucleus depends on nuclear localization sequences, which appear to be present both in the N-terminal part of 5-LO and close to the C-terminus.6-9 Proteins interacting with 5-LO may also influence its subcellular distribution and nuclear import. 5-LO contains a Src homology 3-binding motif that may enable its interaction with, for example, growth factor receptor–bound protein 2 and with cytoskeletal proteins.10 In addition, it was recently found that 5-LO is able to interact with coactosin-like protein,11 implying a relation to the actin network of the cell. Finally, phosphorylation of 5-LO can be a factor influencing its activity.12-14
Differentiation of Mono Mac 6 (MM6) cells with transforming growth factor β (TGF-β) and calcitriol (VD3) leads to substantial 5-LO protein expression and strong 5-LO activity on stimulation with ionophore in the presence of exogenous AA.15 However, stimulation with ionophore alone gave only marginal leukotriene formation.16 Several reports have documented the up-regulation of leukotriene synthesis by phorbol esters in various cell types. Here we demonstrate that the priming of MM6 cells and of human polymorphonuclear leukocytes (PMNLs) with phorbol-12-myristate-13-acetate (PMA) results in substantially increased 5-LO product formation, in parallel with an increased association of 5-LO with the nucleus and with 5-LO kinase activation.
Materials and methods
Materials
RPMI 1640 medium was from Gibco BRL (Life Technologies, Rockville, MD), and fetal calf serum and bovine insulin were obtained from Sigma (St Louis, MO). Human TGF-β1 was purified from outdated platelets as described,17 and VD3 was obtained from Biomol (Plymouth Meeting, PA). Human recombinant 5-LO was expressed and purified as described.18[γ-32P]Adenosine triphosphate (ATP; 110 TBq/mmol) and [3H]arachidonic acid were purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). PMA and mezerein were from Sigma, calphostin C was from RBI (Natick, MA), MAPKAP kinase 2 (MK2) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), and high-performance liquid chromatography (HPLC) solvents were from Rathburn Chemicals (Walkerburn, Scotland).
Cells
MM6 cells19 were maintained in RPMI 1640 medium with glutamine supplemented with 10% fetal calf serum, 100 μg/mL streptomycin, 100 U/mL penicillin, 1 mM sodium pyruvate, 1× nonessential amino acids, 1 mM oxalacetic acid, and 10 μg/mL bovine insulin. All cultures were seeded at a density of 2 × 105 cells/mL. MM6 cells were treated with 2 ng/mL TGF-β and 50 nM VD3 for 4 days. Cells were harvested by centrifugation (200g for 10 minutes at room temperature) and washed once in phosphate-buffered saline (PBS), pH 7.4. Human PMNLs were isolated from leukocyte concentrates obtained from healthy donors at Karolinska Hospital (Stockholm, Sweden) as described.20
Determination of 5-lipoxygenase products
For assays of intact cells, PMNLs (5 × 106) or MM6 cells (3 × 106) were finally resuspended in 1 mL PGC buffer (PBS containing 1 mg/mL glucose and 1 mM CaCl2) and preincubated as indicated. The reaction was started by the addition of ionophore A23187 with or without exogenous AA at the indicated concentrations. After 10 minutes at 37°C, the reaction was stopped with 1 mL methanol and 30 μL 1 N HCl, and 500 μL PBS was added. After centrifugation (800g,10 minutes, room temperature) the samples were applied to C-18 solid-phase extraction columns (100 mg; IST, Mid Glamorgan, United Kingdom), preconditioned with 1 mL methanol and 1 mL water. The columns were washed with 1 mL water and 1 mL water–methanol (75/25, vol/vol). 5-LO metabolites were eluted with 300 μL methanol. The extract was diluted with 120 μL water, and 100 μL diluted extract was analyzed by HPLC as described21 using a C-18 Radial-Pak column (Waters) eluted with methanol–water–acetic acid 75:25:0.1 (vol/vol/vol) at a flow rate of 1.2 mL/min. Amounts of different metabolites were determined by peak area integration. 5-LO product formation is expressed as pmol 5-LO metabolites per 106 cells, which includes LTB4 and its all-trans isomers 5(S),12(S)-dihydroxy-6,10-trans-8,14-cis-eicosatetraenoic acid (5(S),12(S)-DiHETE), and 5(S)-hydro(peroxy)-6-trans-8,11,14-cis-eicosatetraenoic acid (5-H(p)ETE).
For assays of cell homogenates, cells (3-5 × 106) were finally resuspended in 1 mL PBS (with 1 mg/L glucose, without Ca2+ or Mg2+) and cooled on ice. EDTA (1 mM) was added, and the cells were sonicated (3 × 5 seconds on ice). Then ATP (1 mM) and other agents (as indicated) were added, the sample was preincubated for 30 seconds at 37°C, and the incubation was started by the addition of CaCl2 and AA (1 mM and 40 μM final concentrations, respectively). After 10 minutes at 37°C, the incubation was stopped with 1 mL methanol and 30 μL 1 N HCl and was further processed as above.
Determination of [3H]arachidonic acid release from phospholipids
Cells were harvested and washed as described above, resuspended at 2 × 106/mL in RPMI 1640 medium containing 4.8 nM [3H]AA (corresponding to 0.25 μCi/mL; specific activity, 200 Ci/mmol), and incubated for 90 minutes at 37°C in 5% CO2 atm. Thereafter, cells were collected by centrifugation and were washed twice with PBS and once with PBS containing 0.5 mg/mL fatty acid-free albumin to remove unincorporated [3H]AA. Labeled cells (5 × 106) were resuspended in 1 mL PGC buffer containing 2 mg/mL fatty acid-free albumin. The samples were preincubated at 37°C with or without additives before the addition of ionophore A23187 at the indicated concentrations. After 10 minutes, the reaction was stopped with 1 mL methanol and 30 μL 1 N HCl, and 500 μL PBS was added. After centrifugation (800g, 10 minutes), samples were applied to C-18 solid-phase extraction columns (100 mg) preconditioned with 1 mL methanol and 1 mL water. The columns were washed with 1 mL water and 1 mL water–methanol (75/25, vol/vol). [3H]AA was extracted with 500 μL methanol. Extracts were analyzed by reverse-phase HPLC. A C-18 Radial-Pak column (Waters) was eluted with methanol–water–trifluoro-acetic acid 85:15:0.007 (vol/vol/vol) at a flow rate of 1.2 mL/min. Radioactivity was detected with a β-RAM HPLC flow-through monitor system (Inus System) using Monoflow 2 scintillation liquid coupled on-line to UV spectrophotometers. Quantitative determination was performed by peak area integration.
Subcellular fractionation by detergent lysis
Isolated MM6 cells (1 × 107) or human PMNLs (3 × 107) were resuspended in 1 mL PGC buffer. The cells were preincubated in the presence or absence of additives at 37°C before the addition of ionophore A23187 at the indicated concentrations. After another 5-minute incubation period at 37°C, the samples were chilled on ice for 3 to 5 minutes and centrifuged (200g, 5 minutes, 4°C). Pellets were then suspended in 500 μL ice-cold NP-40-lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 1 mM EDTA, 0.1% NP-40, 1 mM phenylmethylsulfonyl fluoride [PMSF], 60 μg/mL soybean trypsin inhibitor, and 10 μg/mL leupeptin), vortexed (3 × 5 seconds), kept on ice for 10 minutes, and centrifuged (800g, 10 minutes, 4°C). Resultant supernatants (nonnuclear fractions) were transferred to a new tube, and the pellets (nuclear fractions) were resuspended in 500 μL ice-cold relaxation buffer (50 mM Tris-HCl, pH 7.4, 250 mM sucrose, 25 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM PMSF, 60 μg/mL soybean trypsin inhibitor, 10 μg/mL leupeptin). Both nuclear and nonnuclear fractions were centrifuged again (800g, 10 minutes, 4°C) for further purification. Lysis of cells and integrity of nuclei were confirmed by light microscopy with trypan blue exclusion. Nuclei in relaxation buffer were disrupted by sonication (3 × 5 seconds). Aliquots of nuclear and nonnuclear fractions were immediately mixed with the same volume of SDS-b, heated for 6 minutes at 95°C, and analyzed for 5-LO protein by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. Lamin B, a ubiquitous protein exclusively present in the nuclear membrane, was used as a marker to indicate the correct fractionation.
Immunoblot analysis of subcellular fractions
Aliquots (25 μL) of pair-wise subcellular fractions (cytosol and nucleus), corresponding to equal amounts of cells, were mixed with 4 μL glycerol/0.1% bromphenolblue (1:1, vol/vol) and analyzed by SDS-PAGE using a Mini Protean system (Bio-Rad) on a 4% to 15% linear gradient gel. After electroblot to nitrocellulose membrane (Amersham Pharmacia), membranes were blocked with 5% nonfat dry milk in 50 mM Tris-HCl, pH 7.4 and 100 mM NaCl (Tris-buffered saline [TBS]) for 1 hour at room temperature. Membranes were washed and incubated with anti–5-LO antiserum (1551, affinity purified on a 5-LO column) for 2 to 3 hours at room temperature. Then membranes were washed with TBS and incubated with 1:1000 dilution of alkaline phosphatase–conjugated antirabbit IgG (Sigma) for 2 hours at room temperature. After washing with TBS and TBS plus 0.1% NP40, 5-LO protein was visualized with the alkaline phosphatase substrates nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Sigma) in detection buffer (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 5 mM MgCl2). Densitometry was performed with a Gel Doc 1000 instrument and the Molecular Analyst software (Bio-Rad); relative intensities (arbitrary units) given under the blot bands are comparable within each figure.
In vitro kinase assay
For preparation of immunoprecipitates (IPs), MM6 cell incubations were stopped by the addition of 2 vol ice-cold stop-buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, and 2 mM Na3VO4) and cooled on ice. After approximately 2 minutes on ice, cells were pelleted (500g, 3 minutes, 4°C), and lysed by the addition of ice-cold lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.5% NP-40, 50 mM NaF, 2 mM Na3VO4, 25 mM β-glycerophosphate, 10 mM sodium pyrophosphate, 10 mM 4-nitrophenyl phosphate, 1 mM PMSF, 5 μM ZnCl2, 10 μg/mL leupeptin, and 60 μg/mL soybean trypsin inhibitor). During 10 minutes in this buffer, the suspension was vortexed repeatedly (5-second bursts) to assure complete lysis. Supernatants were obtained by centrifugation of the lysates (16 000g, 10 minutes, 4°C) and kept on ice. To immunoprecipitate MK2, supernatants corresponding to 2 × 107 MM6 cells were incubated with 5 μL MK2-antibody for 2 hours at 4°C. The immune complexes were precipitated (2 hours at 4°C) with 20 μL Protein A/G Plus-Agarose (Santa Cruz Biotechnology) and washed twice with lysis buffer and twice with kinase buffer. MK2-IPs were used immediately for in vitro kinase assays. Purified recombinant 5-LO (3 μg) was incubated with MK2-IPs from MM6 cells in kinase buffer (25 mM HEPES, pH 7.5, 25 mM MgCl2, 25 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4) containing ATP (100 μM) and [γ-32P]ATP (2 μCi/mL). Final volume was 20 μL, and incubation time was 30 minutes at 30°C. The reaction was terminated by the addition of SDS-b and heating at 95°C for 6 minutes. Samples were separated by SDS-PAGE, and phosphorylated proteins were visualized by autoradiography of the dried gel.
In-gel kinase assay
For the preparation of PMNL total cell lysates, incubations were stopped by the addition of the same volume of SDS-b, vortexed, and heated at 95°C for 6 minutes. Total cell lysates of PMNL, corresponding to 0.5 × 106 cells, were loaded on 10% SDS-PAGE gels containing 0.15 mg/mL purified recombinant human 5-LO. After electrophoresis, kinase activity in the gels was analyzed as described.14
Results
Priming with PMA up-regulates 5-lipoxygenase translocation and formation of products in MM6 cells
Because differentiated MM6 cells possess a high capacity for leukotriene production, it was of interest to study the subcellular distribution of 5-LO. Localization of the enzyme was assessed by means of subcellular fractionation using detergent lysis (0.1% NP-40) and 5-LO immunoblotting. This technique, which was used before for studies of 5-LO translocation in leukocytes,22 23 yields a nuclear fraction containing intact nuclei and a nonnuclear fraction containing cytosol, plasma membrane, endoplasmic reticulum, Golgi apparatus, and cytoskeletal proteins.
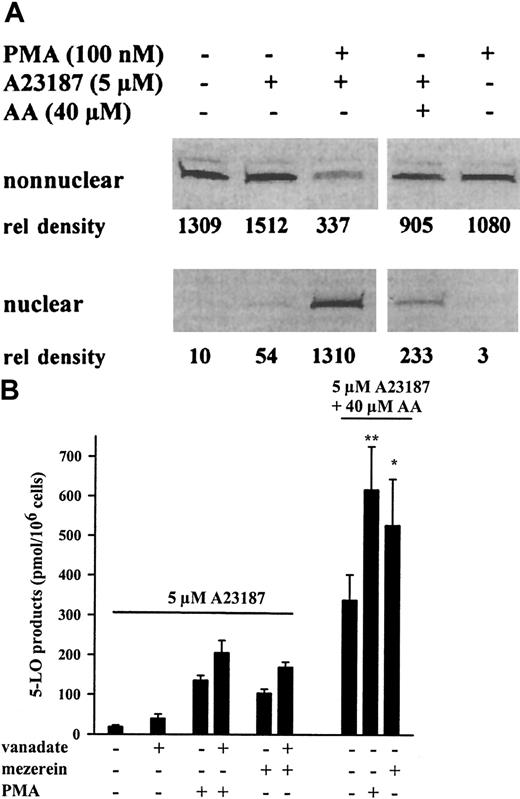
As illustrated in Figure 1A, 5-LO protein was found exclusively in the nonnuclear fraction, both in resting differentiated MM6 cells and after stimulation with ionophore (5 μM). However, priming with the protein kinase C (PKC) activator PMA (100 nM) for 10 minutes and subsequent stimulation with 5 μM ionophore led to a redistribution of approximately 45% to 75% of the cytosolic 5-LO to the nuclear envelope, as determined by densitometric analysis of 5-LO immunoblots. After PMA and ionophore stimulation, nuclear 5-LO was always found in the 100 000g pellet (membrane associated) and not in the soluble fraction of the nucleus. Treatment of MM6 cells with PMA only (without ionophore) gave no 5-LO translocation. Interestingly, ionophore stimulation in the presence of exogenously added AA (no PMA) resulted in a slight increase in the amount of 5-LO associated with the nucleus (approximately 20%). It was recently reported that exogenous AA (no ionophore) added to adenosine-depleted PMNLs induced the translocation of 5-LO to nuclear structures, and it was suggested that AA acts through a mechanism that requires an autocrine stimulatory loop by LTB4.24
Translocation of 5-LO and up-regulation of 5-LO product formation in MM6 cells.
(A) Western blot analysis of 5-LO in subcellular fractions from MM6 cells. Cells (1 × 107 in 1 mL PGC buffer) were first incubated (primed) for 10 minutes at 37°C with or without PMA (100 nM). Then A23187 (5 μM) or A23187 and AA (5 and 40 μM, respectively) were added as indicated, and the incubations were continued for another 5 minutes. Cell fractionation and immunoblotting were performed as described in “Materials and methods.” Pair-wise samples (nonnuclear, nuclear) correspond to the identical cell numbers. The relative intensities of blot bands were determined by densitometry (arbitrary units). Similar results were obtained in 2 additional experiments. (B) 5-LO product formation in intact MM6 cells (3 × 106 in 1 mL PGC buffer) was determined by HPLC as described in “Materials and methods.” Cells were primed for 10 minutes with PMA (100 nM), mezerein (100 nM), and sodium orthovanadate (1 mM) before stimulation for 10 minutes with A23187 (5 μM) or A23187and AA (5 and 40 μM, respectively), as indicated. Results are given as mean + SE of 3 independent experiments. Studentt test; **P < .01; *P < .05.
Translocation of 5-LO and up-regulation of 5-LO product formation in MM6 cells.
(A) Western blot analysis of 5-LO in subcellular fractions from MM6 cells. Cells (1 × 107 in 1 mL PGC buffer) were first incubated (primed) for 10 minutes at 37°C with or without PMA (100 nM). Then A23187 (5 μM) or A23187 and AA (5 and 40 μM, respectively) were added as indicated, and the incubations were continued for another 5 minutes. Cell fractionation and immunoblotting were performed as described in “Materials and methods.” Pair-wise samples (nonnuclear, nuclear) correspond to the identical cell numbers. The relative intensities of blot bands were determined by densitometry (arbitrary units). Similar results were obtained in 2 additional experiments. (B) 5-LO product formation in intact MM6 cells (3 × 106 in 1 mL PGC buffer) was determined by HPLC as described in “Materials and methods.” Cells were primed for 10 minutes with PMA (100 nM), mezerein (100 nM), and sodium orthovanadate (1 mM) before stimulation for 10 minutes with A23187 (5 μM) or A23187and AA (5 and 40 μM, respectively), as indicated. Results are given as mean + SE of 3 independent experiments. Studentt test; **P < .01; *P < .05.
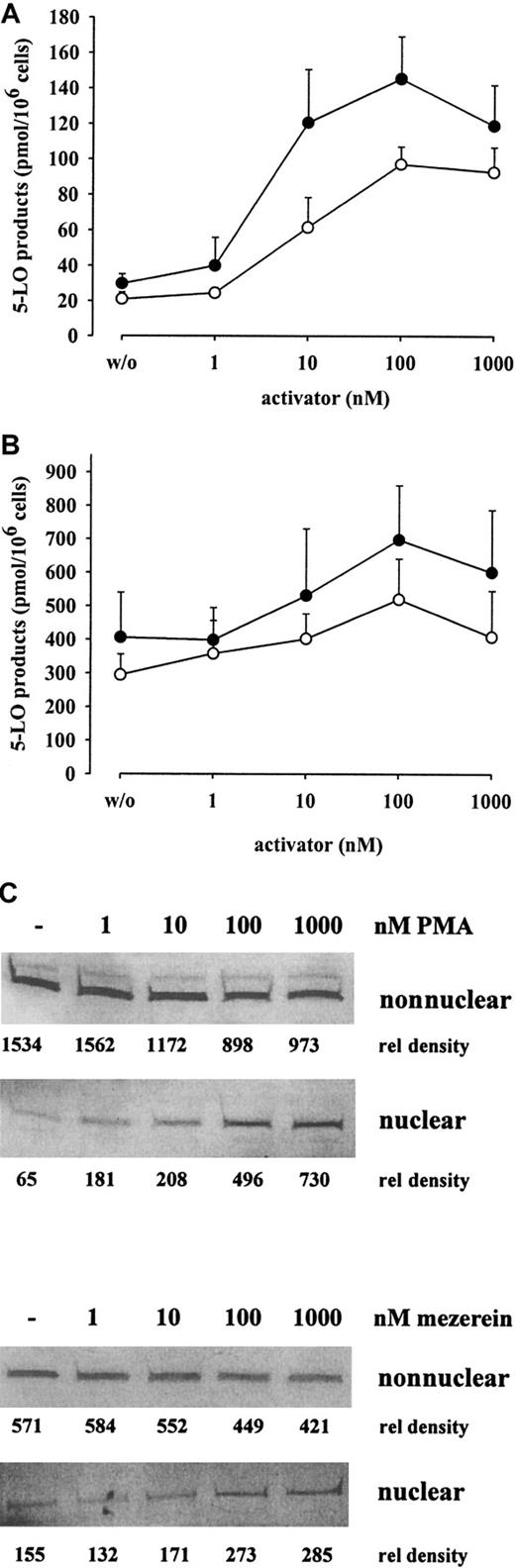
In Figure 1B the 5-LO product formation in MM6 cells is shown. Activity was high after stimulation with 5 μM ionophore in the presence of exogenous AA (40 μM) (338 ± 64 pmol/106 cells), but only marginal formation of 5-LO products was detectable (18.8 ± 4 pmol/106 cells) after stimulation with ionophore alone. However, the priming of MM6 cells with PMA or the PMA-analogue mezerein (100 nM each) for 10 minutes and subsequent stimulation with 5 μM ionophore led to a 5- to 7-fold increase in 5-LO products (136 ± 12 and 104 ± 10 pmol/106 cells). When exogenous AA was present in the incubation mixture, PMA or mezerein led to a 1.5- to 2-fold increase in 5-LO products (Figure 1B), which was statistically significant. The priming effects of PMA and mezerein on product formation were dose dependent, both in the absence and the presence of exogenous AA; a correlation with 5-LO products and the appearance of 5-LO protein in the nucleus were found (Figure2).
Dose response of PMA and mezerein for 5-LO product formation and 5-LO translocation in MM6 cells.
Intact MM6 cells (3 × 106 in 1 mL PGC buffer) were primed for 10 minutes at 37°C with the indicated concentrations of PMA (●) or mezerein (○). Then cells were stimulated with 5 μM A23187 (A) or A23187 and AA (5 μM and 40 μM, respectively) (B) for 10 minutes, and 5-LO product formation was determined by HPLC. Results are given as mean + SE; n = 3-5. (C) MM6 cells (1 × 107 in 1 mL PGC buffer) were primed with the indicated concentrations of PMA or mezerein for 10 minutes at 37°C before stimulation with 5 μM A23187 for 5 minutes. Cells were fractionated, and nonnuclear and nuclear fractions were analyzed for 5-LO by immunoblotting as described. Similar results were obtained in one additional experiment.
Dose response of PMA and mezerein for 5-LO product formation and 5-LO translocation in MM6 cells.
Intact MM6 cells (3 × 106 in 1 mL PGC buffer) were primed for 10 minutes at 37°C with the indicated concentrations of PMA (●) or mezerein (○). Then cells were stimulated with 5 μM A23187 (A) or A23187 and AA (5 μM and 40 μM, respectively) (B) for 10 minutes, and 5-LO product formation was determined by HPLC. Results are given as mean + SE; n = 3-5. (C) MM6 cells (1 × 107 in 1 mL PGC buffer) were primed with the indicated concentrations of PMA or mezerein for 10 minutes at 37°C before stimulation with 5 μM A23187 for 5 minutes. Cells were fractionated, and nonnuclear and nuclear fractions were analyzed for 5-LO by immunoblotting as described. Similar results were obtained in one additional experiment.
PMA-primed redistribution of 5-LO to the nucleus was a rapid process and correlated with the time course of leukotriene production. Product formation and translocation of 5-LO were detectable 30 seconds after ionophore addition, maximal leukotriene formation was determined after 3 minutes, and maximal association of 5-LO with the nuclear membrane was obtained after 2.5 to 5 minutes (data not shown). 4α-Phorbol, used as a negative control in phorbol ester receptor studies, had no effect (data not shown). For 5-LO translocation and product formation, it was necessary to preincubate with PMA or mezerein before ionophore was added; simultaneous addition had no effect. Preincubation for longer than 10 to 15 minutes resulted in impaired priming. Treatment of MM6 cells with PMA or mezerein in the absence of ionophore failed to induce product formation. Addition of vanadate (a protein tyrosine phosphatase inhibitor) to the incubations potentiated the effects of PMA and mezerein on 5-LO product formation and translocation but yielded only marginal effect by itself. Co-addition of cycloheximide (100 μM) had no effect, indicating that protein synthesis was not required for the up-regulation of 5-LO product formation and translocation by either PMA or mezerein.
Priming with PMA up-regulates 5-lipoxygenase translocation and formation of products in PMNLs
Previously, Liles et al25 demonstrated that human neutrophils stimulated with low-dose ionophore (0.4 μM) required the co-addition of PMA for the release of arachidonate and 5-LO metabolites. Thus, we examined the effects of PMA and mezerein on 5-LO translocation and product formation in human PMNLs. 5-LO distribution in PMNLs was assessed as described for MM6 cells using detergent lysis (0.1% NP-40) and Western blot analysis of subcellular fractions.
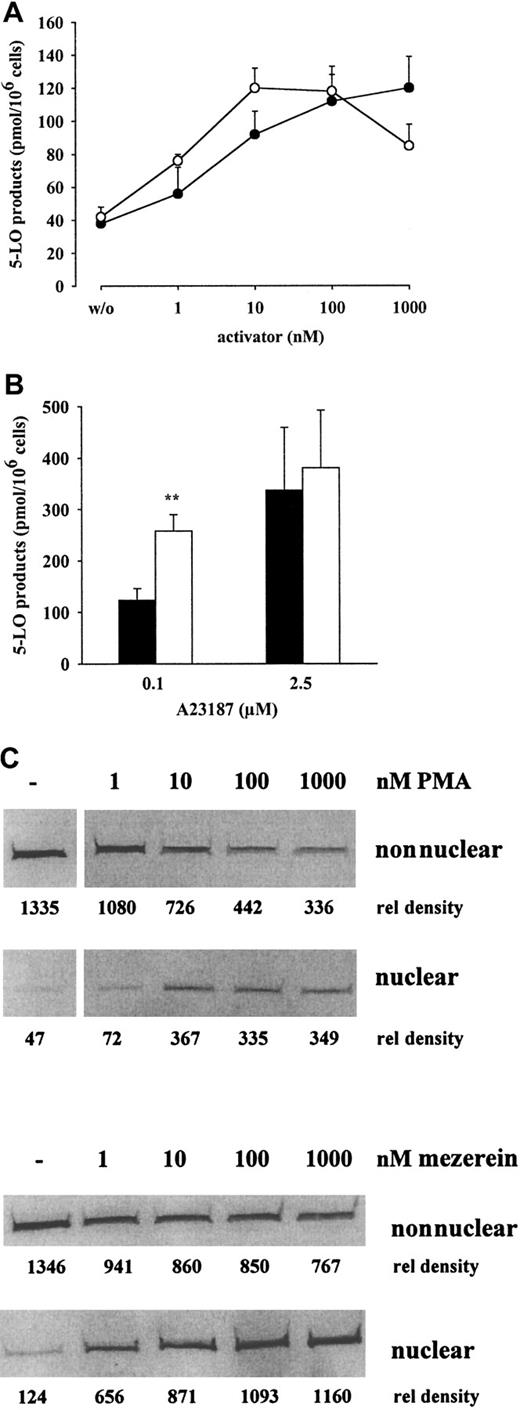
5-LO product formation in PMNLs stimulated with ionophore (0.1-10 μM) was enhanced after 5 minutes of priming with PMA (100 nM). The PMA effect ranged from 3-fold (at 0.1 μM ionophore) to 1.4-fold (at 10 μM ionophore) (Figure 3A). In resting PMNLs, 5-LO was detected only in the nonnuclear fraction, and, after stimulation with ionophore (up to 10 μM), there was a dose-dependent enrichment of 5-LO in the nuclear fraction (Figure 3B). After stimulation with low-dose ionophore (with or without exogenous AA), 5-LO product formation was relatively low, but priming with PMA or mezerein increased the response (Figure4A-B). The effect of PMA-priming on product formation in cells stimulated with 0.1 μM A23187 in the presence of exogenous AA (Figure 4B, left) was statistically significant. After stimulation with low-dose ionophore, only a small amount of 5-LO was associated with the nucleus, but priming with PMA or mezerein increased translocation in a dose-dependent manner (Figure4C), and there was a correlation between 5-LO product formation and the amount of 5-LO protein bound to the nucleus (compare Figures 4A and4C). The negative control 4α-phorbol had no effect, and the treatment of PMNLs with PMA or mezerein in the absence of ionophore failed to induce 5-LO product formation or translocation, as found for MM6 cells (data not shown).
Dose response of A23187 regarding 5-LO product formation and 5-LO translocation in PMNLs.
For determination of 5-LO product formation in intact cells (A), human PMNLs (5 × 106 in 1 mL PGC buffer) were preincubated in the absence (●) or presence of 100 nM PMA (○) for 5 minutes at 37°C before stimulation with indicated concentrations ofA23187 for an additional 10 minutes. 5-LO product formation was determined by HPLC. Results are given as mean + SE; n = 3. For determination of 5-LO distribution (B), PMNLs (3 × 107in 1 mL PGC buffer) were activated with the indicated concentrations ofA23187 for 5 minutes. Cell fractionation and immunoblotting were performed as described.
Dose response of A23187 regarding 5-LO product formation and 5-LO translocation in PMNLs.
For determination of 5-LO product formation in intact cells (A), human PMNLs (5 × 106 in 1 mL PGC buffer) were preincubated in the absence (●) or presence of 100 nM PMA (○) for 5 minutes at 37°C before stimulation with indicated concentrations ofA23187 for an additional 10 minutes. 5-LO product formation was determined by HPLC. Results are given as mean + SE; n = 3. For determination of 5-LO distribution (B), PMNLs (3 × 107in 1 mL PGC buffer) were activated with the indicated concentrations ofA23187 for 5 minutes. Cell fractionation and immunoblotting were performed as described.
Dose response of PMA and mezerein for priming of 5-LO product formation and 5-LO translocation in PMNLs.
(A) To determine the effect on 5-LO product formation, PMNLs (5 × 106 cells in 1 mL PGC buffer) were primed with the indicated concentrations of PMA (●) or mezerein (○) for 5 minutes at 37°C before stimulation with 0.1 μM A23187 for an additional 10 minutes. 5-LO product formation was determined by HPLC. Results are given as mean ± SE; n = 3. (B) To determine the effect on 5-LO product formation in the presence of exogenous AA, PMNLs were primed for 5 minutes at 37°C with (■) or without PMA (▪) (100 nM) before stimulation with 0.1 or 2.5 μM A23187 in the presence of AA (40 μM) for 10 more minutes. Results are given as mean + SE; n = 3. (C) To determine the effect on 5-LO translocation, human PMNLs (3 × 107 in 1 mL PGC buffer) were primed with the indicated concentrations of PMA or mezerein for 5 minutes at 37°C and were subsequently stimulated with 0.1 μM ionophore for 10 minutes. Cells were fractionated, and soluble and nuclear fractions were analyzed for 5-LO by immunoblotting as described. Similar results were obtained in one additional experiment. Student t test; **P < .01.
Dose response of PMA and mezerein for priming of 5-LO product formation and 5-LO translocation in PMNLs.
(A) To determine the effect on 5-LO product formation, PMNLs (5 × 106 cells in 1 mL PGC buffer) were primed with the indicated concentrations of PMA (●) or mezerein (○) for 5 minutes at 37°C before stimulation with 0.1 μM A23187 for an additional 10 minutes. 5-LO product formation was determined by HPLC. Results are given as mean ± SE; n = 3. (B) To determine the effect on 5-LO product formation in the presence of exogenous AA, PMNLs were primed for 5 minutes at 37°C with (■) or without PMA (▪) (100 nM) before stimulation with 0.1 or 2.5 μM A23187 in the presence of AA (40 μM) for 10 more minutes. Results are given as mean + SE; n = 3. (C) To determine the effect on 5-LO translocation, human PMNLs (3 × 107 in 1 mL PGC buffer) were primed with the indicated concentrations of PMA or mezerein for 5 minutes at 37°C and were subsequently stimulated with 0.1 μM ionophore for 10 minutes. Cells were fractionated, and soluble and nuclear fractions were analyzed for 5-LO by immunoblotting as described. Similar results were obtained in one additional experiment. Student t test; **P < .01.
Calphostin C inhibits 5-lipoxygenase translocation and formation of products
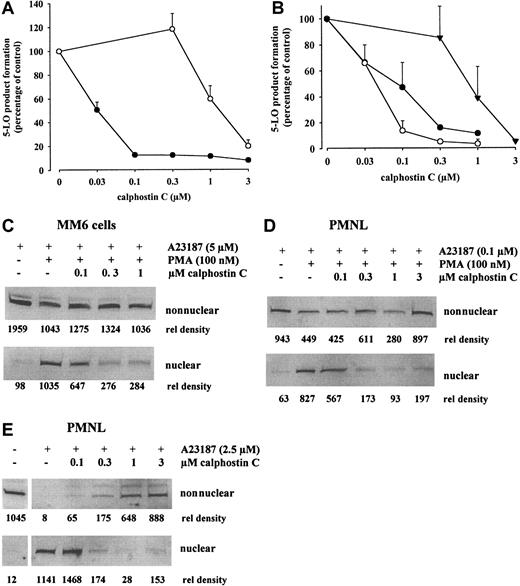
Calphostin C interacts with the regulatory domain of protein kinase C by competing for the binding site of diacylglycerols and phorbol esters. Thus, calphostin C binds to other proteins with a phorbol ester binding site.26 Calphostin C blocked PMA-primed 5-LO product formation and association with nuclei in MM6 cells and PMNLs (Figure 5A-E). The effects were dose dependent, and a correlation between inhibition of product formation and translocation of 5-LO was found. At 0.1 μM calphostin C, the inhibitory effects regarding both 5-LO product formation and translocation were comparable for PMA-primed MM6 cells (stimulated with 5 μM ionophore) and for PMA-primed PMNLs (stimulated with 0.1 μM ionophore). In addition, for nonprimed PMNLs challenged with 2.5 μM ionophore, calphostin C blocked 5-LO product formation and translocation. When exogenous AA was present in the incubations, concentrations of calphostin C approximately 10 times higher were required to inhibit product formation (Figure 5A-B), similar to the doses needed to inhibit 5-LO in crude cell homogenates. Thus, for cell homogenates the IC50 for calphostin C was approximately 2 μM, and the direct effect on 5-LO at 0.1 μM calphostin C was negligible.
Calphostin C inhibits 5-LO product formation and 5-LO redistribution in MM6 cells and PMNLs.
To determine the effects on 5-LO product formation or translocation, the indicated cell numbers (in 1 mL PGC buffer) were incubated with the indicated concentrations of calphostin C for 30 minutes at 37°C under ordinary fluorescent light. Then PMA (100 nM) was added for the indicated times before stimulation with ionophore A23187. 5-LO product formation was determined by HPLC. Results are given as mean + SE; n = 3. Alternatively, cells were fractionated, and soluble and nuclear fractions were analyzed for 5-LO by immunoblotting as described. (A) Effect on 5-LO product formation in MM6 cells (3 × 106 in 1 mL). Cells were primed with PMA for 10 minutes, before stimulation with 5 μM ionophore (●) or 5 μM ionophore plus 40 μM AA (○) for 10 minutes. (B) Effect on 5-LO product formation in PMNLs (5 × 106 in 1 mL). Cells were primed with PMA for 5 minutes, before stimulation with 0.1 μM ionophore (○) for 10 minutes. Nonprimed cells were stimulated with 2.5 μM ionophore (●) or 2.5 μM ionophore plus 40 μM AA (▾) for 10 minutes. (C) Effect on translocation in MM6 cells (1 × 107 in 1 mL). Cells were primed with PMA for 10 minutes, before stimulation with 5 μM ionophore for 5 minutes. Similar results were obtained in 2 additional experiments. (D) Effect on translocation in PMA-primed PMNLs (3 × 107 in 1 mL). Cells were primed with PMA for 5 minutes, before stimulation with 0.1 μM ionophore for 5 minutes. Similar results were obtained in 2 additional experiments. (E) Effect on translocation in PMNLs stimulated only with ionophore (3 × 107 cells in 1 mL). Nonprimed cells were stimulated with 2.5 μM ionophore for 5 minutes. Similar results were obtained in one additional experiment.
Calphostin C inhibits 5-LO product formation and 5-LO redistribution in MM6 cells and PMNLs.
To determine the effects on 5-LO product formation or translocation, the indicated cell numbers (in 1 mL PGC buffer) were incubated with the indicated concentrations of calphostin C for 30 minutes at 37°C under ordinary fluorescent light. Then PMA (100 nM) was added for the indicated times before stimulation with ionophore A23187. 5-LO product formation was determined by HPLC. Results are given as mean + SE; n = 3. Alternatively, cells were fractionated, and soluble and nuclear fractions were analyzed for 5-LO by immunoblotting as described. (A) Effect on 5-LO product formation in MM6 cells (3 × 106 in 1 mL). Cells were primed with PMA for 10 minutes, before stimulation with 5 μM ionophore (●) or 5 μM ionophore plus 40 μM AA (○) for 10 minutes. (B) Effect on 5-LO product formation in PMNLs (5 × 106 in 1 mL). Cells were primed with PMA for 5 minutes, before stimulation with 0.1 μM ionophore (○) for 10 minutes. Nonprimed cells were stimulated with 2.5 μM ionophore (●) or 2.5 μM ionophore plus 40 μM AA (▾) for 10 minutes. (C) Effect on translocation in MM6 cells (1 × 107 in 1 mL). Cells were primed with PMA for 10 minutes, before stimulation with 5 μM ionophore for 5 minutes. Similar results were obtained in 2 additional experiments. (D) Effect on translocation in PMA-primed PMNLs (3 × 107 in 1 mL). Cells were primed with PMA for 5 minutes, before stimulation with 0.1 μM ionophore for 5 minutes. Similar results were obtained in 2 additional experiments. (E) Effect on translocation in PMNLs stimulated only with ionophore (3 × 107 cells in 1 mL). Nonprimed cells were stimulated with 2.5 μM ionophore for 5 minutes. Similar results were obtained in one additional experiment.
Priming with PMA up-regulates 5-LO kinase activation in MM6 cells and PMNL: inhibition by calphostin C
In the course of these studies, we found that the activation of MM6 cells or PMNLs, for example with ionophore, led to the stimulation of MAPKAP kinases that can phosphorylate 5-LO in vitro.14Thus, the effect of PMA-priming on the activation of 5-LO kinases was determined. For MM6 cells, in vitro kinase assays were performed using MK2-IPs from nonprimed or primed cells. PMA priming gave a clear up-regulation of the ionophore-induced MK2 activity with 5-LO as substrate, and this was almost entirely prevented by calphostin C (1 μM) (Figure 6). For PMNLs, in-gel kinase assays were performed with total cell lysates. For cells stimulated with low-dose ionophore, PMA priming gave a clear up-regulation of the 40-kd 5-LO kinase (possibly MK3; compare14), whereas the MK2 band at 47 kd increased only slightly (Figure 7A). When calphostin C (1 μM) was present during PMA priming, the up-regulation of the 40-kd kinase activity was practically abolished. For cells stimulated with high-dose ionophore (no priming), calphostin C counteracted the activation of 5-LO kinases both at 40 and 47 kd (Figure 7B).
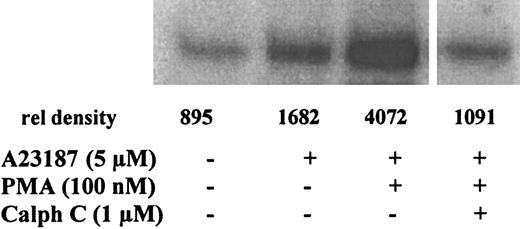
PMA up-regulates 5-LO phosphorylation by MK2 in MM6 cells: inhibition by calphostin C.
Phosphorylation of 5-LO by immunoprecipitates of MK2 from MM6 cells was determined by in vitro kinase assays. Differentiated MM6 cells (2.5 × 107 in 1 mL PGC buffer) were incubated with the indicated concentrations of calphostin C for 30 minutes at 37°C under ordinary fluorescent light. Then PMA (100 nM) was added for 10 more minutes before stimulation with 5 μM A23187 for 3 minutes at 37°C. Incubations were stopped by the addition of 2 vol ice-cold stop-buffer. MK2 was immunoprecipitated, and aliquots of the MK2-IPs corresponding to the same cell numbers were subjected to in vitro kinase assay with 5-LO (3 μg) as substrate. Results are representative of at least 3 separate experiments.
PMA up-regulates 5-LO phosphorylation by MK2 in MM6 cells: inhibition by calphostin C.
Phosphorylation of 5-LO by immunoprecipitates of MK2 from MM6 cells was determined by in vitro kinase assays. Differentiated MM6 cells (2.5 × 107 in 1 mL PGC buffer) were incubated with the indicated concentrations of calphostin C for 30 minutes at 37°C under ordinary fluorescent light. Then PMA (100 nM) was added for 10 more minutes before stimulation with 5 μM A23187 for 3 minutes at 37°C. Incubations were stopped by the addition of 2 vol ice-cold stop-buffer. MK2 was immunoprecipitated, and aliquots of the MK2-IPs corresponding to the same cell numbers were subjected to in vitro kinase assay with 5-LO (3 μg) as substrate. Results are representative of at least 3 separate experiments.
PMA up-regulates 5-LO kinase activation in PMNLs: inhibition by calphostin C.
PMNLs (5 × 107 in 1 mL PGC buffer) were preincubated at 37°C in the presence or absence of calphostin C, as indicated, for 30 minutes under ordinary fluorescent light. (A) Cells were primed with PMA (100 nM) for 10 minutes before stimulation with 0.1 μM A23187 for 3 minutes at 37°C. Incubations were terminated by the addition of the same volume of SDS-b, vortexed and heated at 95°C for 6 minutes. Aliquots corresponding to 0.5 × 106 PMNLs were electrophoresed on a 10% SDS-polyacrylamide gel polymerized with 0.15 mg/mL purified recombinant 5-LO. In-gel kinase assay was performed as described in “Materials and methods.” Positions of standard proteins are indicated. (B) Nonprimed cells were stimulated with 2.5 μM A23187 for 3 minutes at 37°C and analyzed for 5-LO phosphorylation by in-gel kinase assay. Results are representative of 3 separate experiments.
PMA up-regulates 5-LO kinase activation in PMNLs: inhibition by calphostin C.
PMNLs (5 × 107 in 1 mL PGC buffer) were preincubated at 37°C in the presence or absence of calphostin C, as indicated, for 30 minutes under ordinary fluorescent light. (A) Cells were primed with PMA (100 nM) for 10 minutes before stimulation with 0.1 μM A23187 for 3 minutes at 37°C. Incubations were terminated by the addition of the same volume of SDS-b, vortexed and heated at 95°C for 6 minutes. Aliquots corresponding to 0.5 × 106 PMNLs were electrophoresed on a 10% SDS-polyacrylamide gel polymerized with 0.15 mg/mL purified recombinant 5-LO. In-gel kinase assay was performed as described in “Materials and methods.” Positions of standard proteins are indicated. (B) Nonprimed cells were stimulated with 2.5 μM A23187 for 3 minutes at 37°C and analyzed for 5-LO phosphorylation by in-gel kinase assay. Results are representative of 3 separate experiments.
Priming with PMA up-regulates the release of [3H]arachidonic acid in MM6 cells and PMNL: inhibition by calphostin C
Activation of PKC by PMA was previously shown to increase AA release in various leukocytes.25 27-29 We analyzed the PMA effect on the release of [3H]AA from phospolipids in MM6 cells and human PMNLs. As shown in Figure8, differentiated MM6 cells released [3H]AA after ionophore (5 μM) treatment, and comparable amounts of [3H]AA were released in ionophore (2.5 μM)-stimulated PMNLs. Priming of MM6 cells and PMNLs with 100 nM PMA or mezerein resulted in 5- to 7-fold and 2- to 3-fold enhancements of AA release, respectively. It should be observed that in contrast to PMNLs, MM6 cells showed no or little 5-LO product formation on stimulation with high-dose ionophore alone, though comparable amounts of AA were released in both cell types. Finally, when PMNLs were stimulated with low-dose ionophore (0.1 μM), the effect of PMA-priming on AA release was more prominent than when PMNLs were stimulated with high-dose ionophore (2.5 μM).
[3H]arachidonic acid release in MM6 cells and human PMNLs.
MM6 cells or freshly isolated human PMNLs (2 × 106/mL in RPMI 1640 medium) were prelabeled with 0.25 μCi/mL [3H]AA for 90 minutes at 37°C and 5% CO2. After the removal of unincorporated [3H]AA, cells (5 × 106 in 1 mL PGC buffer, containing 2 mg/mL fatty acid-free albumin) were primed in the absence or presence of PMA or mezerein for 10 minutes at 37°C, A23187 was added at the indicated concentrations, and the incubations continued for another 10 minutes. Free (nonesterified) [3H]AA was determined by HPLC as described in “Materials and methods.” Results are given as mean ± SE; n = 3.
[3H]arachidonic acid release in MM6 cells and human PMNLs.
MM6 cells or freshly isolated human PMNLs (2 × 106/mL in RPMI 1640 medium) were prelabeled with 0.25 μCi/mL [3H]AA for 90 minutes at 37°C and 5% CO2. After the removal of unincorporated [3H]AA, cells (5 × 106 in 1 mL PGC buffer, containing 2 mg/mL fatty acid-free albumin) were primed in the absence or presence of PMA or mezerein for 10 minutes at 37°C, A23187 was added at the indicated concentrations, and the incubations continued for another 10 minutes. Free (nonesterified) [3H]AA was determined by HPLC as described in “Materials and methods.” Results are given as mean ± SE; n = 3.
The effects of calphostin C (0.1 μM) on [3H]AA release were determined (data not shown). Preincubation time with the inhibitor was 30 minutes and was followed by 10-minute priming and 10-minute incubation with ionophore. For PMA-primed MM6 cells stimulated with ionophore A23187 (5 μM), [3H]AA release was 42% ± 7% of control (n = 3). For PMA-primed PMNLs stimulated with low-dose ionophore A23187 (0.1 μM), [3H]AA release was 26% and 17% of control (2 experiments). For nonprimed PMNLs stimulated with high-dose ionophore A23187 (2.5 μM), [3H]AA release was 55% and 43% of control (2 experiments).
Discussion
In this article, we report that the pre-incubation of phagocytic cells with PMA led to the up-regulation of 5-LO product formation on subsequent stimulation with calcium ionophore A23187, which coincided with increased 5-LO association with the nucleus and 5-LO kinase activation. These effects were observed for the monocytic human cell line MM6 and for human PMNLs prepared from peripheral blood. For MM6 cells stimulated with ionophore, there was practically no association with the nucleus unless the cells had been primed with PMA or with the PMA-analogue mezerein (Figures 1A, 2C). For PMNLs, ionophore alone at high doses resulted in translocation (Figure 3B), but PMA (or mezerein) showed a clear up-regulation of the effect of low-dose ionophore (Figure 4C). Thus, it appears that in PMNLs (but not in MM6 cells) treated with high-dose ionophore, endogenous signal molecules such as diacylglycerol, whose action can be mimicked by PMA priming, are generated or activated. Because cycloheximide had no effect, PMA priming should not depend on protein synthesis. In addition, for MM6 cells (but not for murine RAW 264.7 macrophages), it was found that PMA was required (in addition to lipopolysaccharide [LPS]), for secretion of tumor necrosis factor α.30
Up-regulation of cellular leukotriene biosynthesis by PMA has been observed for neutrophils and macrophages,12,25,27,29,31and in some of these studies25,27 it was demonstrated that PMA augmented the ionophore induced release of [3H]AA from prelabeled cells. However, it was suggested that PMA could also up-regulate 5-LO activity by unknown mechanisms.12 We found that PMA priming up-regulated AA release, 5-LO kinase activity, and 5-LO translocation, all of which may contribute to increased 5-LO product formation from endogenous substrate. For PMA-primed MM6 cells stimulated with ionophore A23187, LT biosynthesis was maximal after 3 minutes, maximal association of 5-LO with the nucleus occurred within 2.5 to 5 minutes, and 5-LO kinase activity was prominent 2 to 3 minutes after the addition of ionophore. 5-LO translocation can be divided in the transport process (from cytosol to the vicinity of the nucleus or into the nucleus) and the association with the nuclear membrane.2 Our data indicate that PMA-priming augments the association of 5-LO with the nuclear membrane. Phosphorylation could also influence the nuclear import of 5-LO, as suggested by the isolation of small amounts of phosphorylated 5-LO from a genomic DNA fraction of ionophore-stimulated HL-60 cells.13 In vitro adherence of neutrophils caused the rapid import of 5-LO into the nucleus, resulting in an increased capacity for subsequent ionophore-induced LTB4synthesis.5 This appears to be different from the PMA-priming effect because the transfer to the nucleus occurred first, after stimulation of the PMA-primed cells with ionophore (Figure 1). After this treatment, the nuclear 5-LO was associated with the nuclear 100 000g pellet (membrane fraction). Another difference was that PMA priming of PMNLs did not lead to a requirement for higher concentrations of ionophore (Figure 3A), as was found after the elicitation by adhesion.5 The priming of human PMNLs with LPS up-regulated fMLP-induced 5-LO translocation to the nucleus and 5-LO activity.32 Because neither LPS nor PMA alone induced nuclear association, it appears that the priming effects of LPS and PMA could be similar. FLAP resides permanently at the nuclear membrane,23,33 and this protein is believed to present substrate to 5-LO.34 FLAP mRNA is expressed in MM6 cells,35 and FLAP protein has been detected in MM6 cell nuclear fractions (O.W., unpublished observation, April 1998). cPLA2 also migrates to this locale after the stimulation of PMNLs with ionophore.23 36 Our results agree with the concept that endogenous arachidonate is released at the nuclear membrane and that 5-LO has to translocate to this compartment for efficient LTA4 production.
Interestingly, when MM6 cells were incubated with exogenous 40 μM AA (together with ionophore, no PMA priming), 5-LO translocation was slightly stimulated (Figure 1A), as noted earlier for PMNLs.24 The slight association with the nuclear membrane might contribute to the high 5-LO product formation observed in such incubations (Figure 1B). However, a more plausible explanation is that the large amount of 5-LO remaining in the cytosol converted exogenous substrate efficiently regardless of translocation. Thus, PMA-priming effects related to 5-LO translocation may not be obvious when exogenous substrate is provided. It should not be concluded that simply because PMA-priming of MM6 cells is not required when exogenous substrate is present, PMA-priming primarily consists in the up-regulation of endogenous AA release. The importance of translocation for the conversion of endogenous substrate is emphasized by comparison of the results after stimulation of MM6 cells and PMNLs with high-dose ionophore only (no PMA). Under these incubation conditions, the release of AA was similar in both cell types (Figure 8). However, in MM6 cells, no translocation was detectable (Figure 1A) and product formation was low (Figure 1B), whereas in PMNLs, translocation occurred and 5-LO product formation was substantial (Figure 3). Nevertheless, PMA priming also resulted in a slight (approximately 2-fold) increase in 5-LO activity in the presence of exogenous AA, both in MM6 cells (Figures1B, 2B) and in PMNLs stimulated with low-dose ionophore (Figure 4B). This may indicate that the association of 5-LO with the nuclear membrane is favorable when exogenous substrate is used. Indeed published reports indicate that FLAP stimulated the conversion of exogenous substrate.34 Alternatively, the effect of PMA on cellular 5-LO product formation in the presence of exogenous AA could reflect effects of phosphorylation unrelated to 5-LO translocation to the nucleus.
Recently, we reported that 5-LO can be phosphorylated by p38 kinase–regulated MAPKAP kinases and that sodium arsenite leads to the strong activation of 5-LO kinases in parallel with the up-regulation of 5-LO activity.14 Apparently, PMA priming of ionophore-induced 5-LO activity, as described here for MM6 cells and PMNLs, is another experiment in which the up-regulation of kinases active on 5-LO can be observed with the up-regulation of 5-LO product formation. In fact, in PMA-priming experiments, 5-LO kinase activity was always up-regulated when 5-LO translocated to the nucleus and high 5-LO product formation was observed. For PMNLs particularly, the activity of the 5-LO kinase migrating at 40 kd varied with translocation and 5-LO activity (Figure 7). It was suggested that this is MAPKAP kinase 3 (MK3).14 A possible pathway for the stimulation of MK3 by PKC can be visualized because PKC activates ERK1/2, which can activate MK3.37 In these studies we attempted to immunoprecipitate 5-LO from cells pre-incubated with inorganic 32Pi. However, we were unable to convincingly demonstrate radiolabeling of 5-LO by this method, which may indicate that only a small fraction of the cell supply of 5-LO is subject to phosphorylation.
Calphostin C competes with diacylglycerols and phorbol esters for the binding site on PKC and other PMA-binding proteins.26 We found that calphostin C inhibited PMA-primed 5-LO product formation in MM6 cells and in PMNLs (Figure 5). In both cell types AA release, 5-LO kinase activation, and 5-LO association with the nucleus were reduced (0.1-1 μM calphostin C). Interestingly, also in nonprimed PMNLs stimulated with only high-dose ionophore, 5-LO product formation, release of AA, activation of 5-LO kinases, and 5-LO translocation were all inhibited by calphostin C (Figures 5, 7). A23187 alone did not induce 5-LO translocation to the nucleus in MM6 cells. A plausible explanation is that ionophore could not activate certain kinase cascades in MM6 cells, whereas this occurred in PMNLs. This is supported by the observation that calphostin C inhibited 5-LO product formation similarly in PMA-primed MM6 cells or PMNLs stimulated with ionophore and in nonprimed PMNLs incubated only with high-dose ionophore. However, when exogenous AA was present, the effect of calphostin C on product formation was much reduced, probably because the conversion of exogenous substrate now occurred in the cytosol. Although calphostin C blocked PMA-primed activation of 5-LO kinases and 5-LO translocation, a direct connection between these events remains to be determined. The possibility of phosphorylation of the other proteins important for 5-LO activity should not be excluded.
The roles of calcium and phosphorylation for cPLA2 activity have been studied extensively. Calcium is a primary determinant, whereas phosphorylation at Ser505, outside the calcium-binding C2 domain, is of varying importance for cPLA2 activity in different cell types and for different stimuli (for review, see38). For example, in macrophages stimulated with high-dose ionophore, the sustained increase in calcium caused AA release without an absolute requirement of cPLA2 phosphorylation by MAP kinases, but phosphorylation was essential when cells were stimulated with zymozan (this causes a transient increase in calcium).39 In Sf9 cells expressing recombinant cPLA2, the C2 domain was necessary and sufficient for calcium-induced translocation to the nuclear envelope, and the C2 domain was required but not sufficient when cells were treated with okadaic acid (okadaic acid causes phosphorylation of cPLA2).40 However, relations between cPLA2 phosphorylation and activity are not entirely clear,41,42 and other mediators (phosphatidylinositol 4,5-bisphosphate)43 and more complex roles for MAPK pathways44 have also been suggested to regulate cPLA2 activation and membrane binding.
The mode of regulation by calcium seems to be similar for 5-LO and cPLA2. Thus, calcium leads to membrane association and to the stimulation of enzyme activities. It was recently found that an N-terminal β-sandwich in 5-LO mediates both calcium stimulation of activity and association with the nuclear membrane as the C2 domain in cPLA2.3,4 Possibly, similar regulation principles apply to both cPLA2 and 5-LO, and one may speculate that the phosphorylation of 5-LO could differ significantly in different cell types and for different stimuli. Interactions of 5-LO with other proteins10 11 that could possibly be subject to phosphorylation may also govern 5-LO activation and translocation. In summary, our observations strengthen the concept that signal transduction pathway(s) involving phorbol ester receptors, resulting in kinase activation, is/are important not only for cPLA2 but also for 5-LO activity in the cell.
We thank Drs Dieter Steinhilber and Pontus Larsson Forsell for helpful discussions and Hélène Ax:son Johnson for expert technical assistance. O.W. received a Karolinska Institute Guest Scientist fellowship.
Supported by grants from the Swedish Medical Research Council (03X-217), from the European Union, and from the Verum Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Olof Rådmark, Department of Medical Biochemistry and Biophysics, Division of Physiological Chemistry II, Karolinska Institutet, S-171 77 Stockholm, Sweden; e-mail:olof.radmark@mbb.ki.se.



![Fig. 8. [3H]arachidonic acid release in MM6 cells and human PMNLs. / MM6 cells or freshly isolated human PMNLs (2 × 106/mL in RPMI 1640 medium) were prelabeled with 0.25 μCi/mL [3H]AA for 90 minutes at 37°C and 5% CO2. After the removal of unincorporated [3H]AA, cells (5 × 106 in 1 mL PGC buffer, containing 2 mg/mL fatty acid-free albumin) were primed in the absence or presence of PMA or mezerein for 10 minutes at 37°C, A23187 was added at the indicated concentrations, and the incubations continued for another 10 minutes. Free (nonesterified) [3H]AA was determined by HPLC as described in “Materials and methods.” Results are given as mean ± SE; n = 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/8/10.1182_blood.v97.8.2487/5/m_h80810918008.jpeg?Expires=1770222459&Signature=P99Ao~5KnVgBipISBK2txvkR6J592c-szvoruykLfLQJxpSFu~mp0tky3CphFDmE6iey9mZMF4WKcZoFKkIAGVhP2ruubUYEHh0p9dHhZjWojVgYvqpA4J91saDjZexaKl6URnAyDB43vstyFlO~0BYQbDRxBOSt3FNJXJa1r0DThbggVZE9xhVvNE4OxVntnrGnkkaVe-sZb3ziuaFvKwOsFwVIrtwyJ27xQCmK11yQ~t3oo53TeATDu8GnpYNNcHqnssWn8Wbt88I9ut-~U61yBwbgNOGDoNLote3TOYc~nZXhQ-fqWVxHwBdJIMQpecnf8UPmdIZi34zyN2Kmcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal