Abstract
The experiences of 69 (38 marrow and 31 peripheral blood stem cell [PBSC]) donors participating in a randomized trial comparing allogeneic bone marrow with PBSC transplantation were studied. Marrow was collected by means of standard harvest techniques and general or regional anesthesia. PBSC donors were treated with 5 to 7 days of filgrastim at a dose of 16 μg/kg/d and underwent 1 to 3 days of apheresis to obtain 5 × 106 CD34+ cells per kilogram recipient weight. Donors completed questionnaires describing their health experiences before, during, and then weekly after donation until return to baseline status. Both marrow and PBSC donors reported minimal fluctuation in symptoms measuring emotional status. In contrast, both groups of donors reported deterioration in physical status starting with administration of filgrastim (PBSC donors) or after the marrow collection procedure. The symptom burden reported was similar, with pain a prominent symptom for both groups. Equivalent mean levels of maximal pain, average pain, and pain duration through the day were reported, although toxicity peaks occurred at different time points during the harvest procedures. All PBSC donors but only 79% of marrow donors reported good physical status by 14 days after the harvest procedures. These data demonstrate similar levels of physical discomfort for hematopoietic stem cell donors regardless of the collection procedure used, but a quicker resolution of symptoms for PBSC donors.
Introduction
The ability to collect large quantities of hematopoietic stem cells (HSCs) from the peripheral blood provides an alternative to bone marrow as a cell source for allogeneic or autologous HSC transplantation. The potential advantages to the patient of transplantation of peripheral blood stem cells (PBSCs) include more rapid hematological recovery and reduced transplantation costs.1-5 Although PBSC recipients may experience increased risks of chronic graft-versus-host disease,6 a recently completed randomized study comparing these 2 sources of cells for allogeneic transplantation demonstrated reduced peri-transplantation mortality and improved overall survival for patients who received PBSCs from HLA-matched sibling donors.7
The HSC donor may also benefit from the selection of PBSCs.8 Advantages for the donor include the avoidance of a surgical procedure conducted under general or regional anesthesia that may result in 1 or more days of hospitalization. Although administration of hematopoietic cytokines over several days is necessary to mobilize hematopoietic stem cells into the peripheral circulation, no long-term sequelae of cytokine administration have yet been reported.9 The alterations in blood counts and hepatic enzyme levels that occur are transient.10 11
Although many publications retrospectively review the incidence of donor complaints after either bone marrow harvesting or PBSC mobilization and collection,10-15 few reports have focused prospectively on the donation experience,15 and none on the relative experiences that can be determined in the setting of a randomized trial. In this study, we report a prospective comparison of donor experiences for marrow and PBSC donors participating in a randomized study comparing transplantation for recipients of HSCs from either of these 2 sources. The focus of this report is on the common medical complications documented by the medical staff and the range and severity of physical complaints reported by the donors.
Patients and methods
Patient and donor selection
Donors eligible for this study of the donation experience were also participants in a randomized, nonblinded phase III study comparing bone marrow and PBSCs as the source of hematopoietic stem cells for allogeneic transplantation in the treatment of malignant diseases. Patient eligibility criteria included a diagnosis of a hematological malignancy, age between 12 and 55 years, and the availability of an HLA-matched family member donor. Donor eligibility criteria included age older than 12 years and the absence of preceding illness that would otherwise preclude HSC donation. All donors underwent predonation health screening with a medical history and physical examination followed by appropriate health- and age-related laboratory and radiological testing. Informed consent for enrollment in this phase III study was obtained on consent forms approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (Seattle, WA).
Collection of bone marrow
Bone marrow was collected on the day of infusion from the posterior (and anterior, if deemed necessary by the harvesting physician) iliac crests of the donor in the hospital operating room by means of procedures similar to those previously described.16 Donors received either general or regional anesthesia. The goal of the harvest procedure was to collect 10 to 15 mL bone marrow per kilogram recipient weight. One unit of autologous whole blood was collected at least 7 days before the harvest procedure if the anticipated volume of marrow to be collected exceeded either 500 mL or 10 mL/kg donor weight. Anesthesia and immediately postoperative pain and antinausea medications were not prescribed by protocol, but left to the judgment of the anesthesiologist and the bone marrow collection team. Similarly, the transfusion of homologous blood was left to the discretion of the physician caring for the donor.
After surgery, donors were cared for in the Ambulatory Surgery Center (ASC) until the discharge criteria defined for blood pressure, pulse, pain control, and dietary intake were met. Donors who did not meet these criteria within 6 to 8 hours after the harvest procedure were admitted to the hospital for further care. All donors were provided with combination pain medication (325 mg acetaminophen with 5 mg oxycodone) on discharge from the hospital.
All donors were examined by nursing staff in the outpatient clinic on the day after the harvest procedure and thereafter as needed. Routine blood tests were not obtained after the bone marrow harvest procedure.
Mobilization and collection of PBSCs
PBSC donors were treated with filgrastim (Neupogen) (Amgen, Thousand Oaks, CA) at a daily morning dose of 16 μg/kg administered by subcutaneous injection. The donors were assessed daily by staff nurses during the mobilization period, and peripheral blood counts were obtained, but no donor required adjustment of the filgrastim dose because of markedly elevated white blood cell count or somatic toxicity. On the fifth day of treatment with filgrastim (the day prior to transplantation), the donors underwent collection of PBSCs by apheresis commencing at least 4 hours after the filgrastim injection. The first PBSC collection was stored overnight at 4°C. The criterion for adequate PBSC collection was a target number of at least 5 × 106 CD34+ cells per kilogram patient weight. Donors who did not achieve this goal on the first day's collection received an additional dose of filgrastim and underwent a second apheresis collection on the following day. Oral pain medication (acetaminophen plus oxycodone or the equivalent) was provided to PBSC donors if requested, but not as a routine component of the treatment regimen.
Leukapheresis was performed with the use of a continuous-flow blood cell separator (Spectra, version 4.7) (Cobe BCT, Englewood, CO).17 Venous access was established by either peripheral vein or temporary central venous catheter inserted into the subclavian or femoral vein. Anticoagulant, consisting of 10 U heparin per milliliter acid citrate dextrose A (ACD-A), was infused at a ratio of 1 mL anticoagulant to 30 mL whole blood. An additional 40 mL of this solution was placed in the collection bag at the start of the procedure. Inlet flow rate was maintained at 50 to 100 mL/min as tolerated by the donor with a maximum ACD-A infusion rate of 0.8 mL/min/kg donor weight. The collection rate was 1.5 mL/min. A set volume of 12 L blood processed per apheresis procedure was prescribed.
The donors were evaluated and blood counts were measured on the day after the completion of the apheresis procedures. Blood counts and chemistry profiles, including hepatic enzyme measurements, were obtained at intervals up to 3 weeks after the discontinuation of filgrastim.
Self-report questionnaires measuring health status
The donors were requested to complete questionnaires describing their health experiences during the previous 24 hours at the time points indicated in Table 1. For purposes of timing filgrastim administration, HSC harvest procedures, and completion of questionnaires, day 0 is defined as the day of HSC infusion. PBSC and marrow donors underwent different procedures, and the timing of the evaluations was designed to assess the experience of the mobilization therapy for PBSC donors (questionnaires administered on the third and fifth days of filgrastim administration) and the recovery from the donation process for both groups of donors (questionnaires administered on the day after cell collection and weekly, thereafter). The same questionnaires, measuring both physical and emotional experiences, were used for both groups of donors. In this paper, which primarily describes physical responses during the recovery period, we report data obtained with the following measures: (1) Physical status and emotional status numerical rating scales. These 11-point response scales range from 0 (very poor) to 10 (excellent). (2) The Brief Pain Inventory, which is widely used to assess clinical pain.18 It provides measures of average pain, worst pain, pain duration, and pain interference during sleep on 11-point numerical rating scales, with 0 denoting no pain or dysfunction and 10 denoting maximal pain or dysfunction. (3) Words Describing Pain, from the McGill Pain Questionnaire.19 This lists a series of adjectives commonly used to describe pain or discomfort. The score on this measure was the number of adjectives checked. (4) A Clinical Symptom Checklist. This presented a list of 23 symptoms or adverse experiences, such as fatigue, nausea, and trouble concentrating, that sometimes occur during the donor recovery period. Each of these symptoms was rated on Likert-style response scales with categories describing the frequency during the past 24 hours: “not at all,” “a little,” “quite a bit,” and “very much.” The symptom burden score was the mean response obtained across the 23 scales with the categories coded numerically as 1, 2, 3, and 4, respectively.
Questionnaires and timing of assessments
| Timing* . | Marrow donors . | PBSC donors . | P value1-153 . | ||
|---|---|---|---|---|---|
| Questionnaire† . | No.‡ . | Questionnaire† . | No.‡ . | ||
| Baseline | A, B | 38 | A, B | 30 | — |
| Day −3 | — | — | A | — | |
| Day −1 | — | — | A | — | |
| Day +1 | A, B | 34 | A, B | 25 | .49 |
| Day +7 | A | 30 | A | 23 | 1.00 |
| Day +14 | A | 29 | A | 21 | .59 |
| Day +21 | A | 26 | A | 19 | .80 |
| Day +281-155 | A | 24 | A | 18 | .81 |
| 6 months | A, B | A, B | |||
| Timing* . | Marrow donors . | PBSC donors . | P value1-153 . | ||
|---|---|---|---|---|---|
| Questionnaire† . | No.‡ . | Questionnaire† . | No.‡ . | ||
| Baseline | A, B | 38 | A, B | 30 | — |
| Day −3 | — | — | A | — | |
| Day −1 | — | — | A | — | |
| Day +1 | A, B | 34 | A, B | 25 | .49 |
| Day +7 | A | 30 | A | 23 | 1.00 |
| Day +14 | A | 29 | A | 21 | .59 |
| Day +21 | A | 26 | A | 19 | .80 |
| Day +281-155 | A | 24 | A | 18 | .81 |
| 6 months | A, B | A, B | |||
PBSC indicates peripheral blood stem cell.
Day −3 is the third day of filgrastim administration; day −1 is the fifth day of filgrastim administration and the first day of apheresis. Day +1 is the day after marrow harvesting and the day after the completion of the last apheresis procedure if more than one was performed.
Questionnaire A indicates questionnaires on physical status, emotional status, symptom checklist, brief pain inventory, and McGill Pain Questionnaire; questionnaire B indicates profile of mood states and Better Person Inventory.
Number of donors who returned questionnaires at the indicated time point.
Exact P value (permutation test) for the null hypothesis that the proportions lost to follow-up are equal in the 2 treatment populations at each assessment point.
Donors were requested to complete questionnaire A on a weekly basis until return to baseline status as determined by the donor.
Primary outcomes for this analysis were physical status, emotional status, worst pain, average pain, pain duration, pain interference during sleep, words describing pain, and symptom burden. Other outcomes, such as the individual responses to the Clinical Symptom Checklist, were treated descriptively as secondary end points. Although repeated measures were obtained according to the schedule in Table 1, we defined primary clinical trial end points for each donor as the worst score measured on each primary outcome during the period from day −3 to day +14. These summary end points provided a basis for comparing overall group experiences despite the unique schedules for marrow and PBSC donors, which tended to produce toxicity peaks at different time points.
Statistical analysis
This is a prospective study of donor experiences for PBSC and marrow donors enrolled in a randomized study of these 2 sources of HSCs. Donor characteristics, as well as characteristics of the marrow and PBSC harvest procedures are described by means of summary statistics, such as median values and ranges. Examinations of the differences between donor groups were performed by means of Student t test for independent samples for continuous variables, or by chi-square analysis for dichotomous variables. Comparisons of response frequencies were conducted by means of exact small-sample permutation tests.
Because there were several correlated primary end points, the cumulative risk of type I error was substantial. We therefore first conducted a simultaneous test, using multivariate analysis of variance, of the hypothesis that the population end points had equal means; the P value for this test controls for multiple correlated outcomes. The significance of this test indicated that some combination of primary end points discriminated between donor groups. The significance of the multivariate test permitted additional univariate comparisons among the end points. We compared donor group mean differences in individual primary end points using ttests for independent samples with no additional adjustment to theP values for multiple comparisons. We consideredP values less than .05 to be evidence of nonzero population differences.
As Table 1 indicates, donors were lost to follow-up at various times before day 28. However, Table 6 suggests that in most of these cases, peak toxicities had probably already occurred before the donor was lost to follow-up; if this assumption is true, the summary end points remain valid and should not bias treatment comparisons. To check this assumption, we corroborated our primary findings with analogous analyses based on multiple imputation of missing data.20These analyses, which impute missing scores conditional on prior observations, are less sensitive to missing data bias than analyses based on observed cases only.21 Since the imputations are taken multiply, the original uncertainties in the data are preserved. Agreement between the 2 kinds of analyses suggests the primary analysis is not sensitive to missing data bias.
Results
A total of 69 donors were enrolled on this randomized study. The number of CD34+ cells collected from one 54-year-old female donor after 2 apheresis procedures totaled only 74 × 106cells (1.01 × 106/kg recipient weight). PBSC collection was deemed a failure for this donor, and she underwent bone marrow harvesting 3 days after the second apheresis procedure. Data obtained for this donor were not used in this analysis. Donor demographics and baseline comparability between the 2 groups of evaluable donors are shown in Table 2. Both groups were of similar age and gender, and both groups had similar numbers of donors who reported chronic medical problems in the preharvest evaluation. The 2 groups did not significantly differ on any baseline characteristic or in their baseline scores on the primary outcome measures (P = .43, multivariate test).
Donor demographics and baseline comparability
| . | Marrow donors . | PBSC donors . | Pvalue . |
|---|---|---|---|
| Evaluable donors | 38 | 30* | .45 |
| Age, y (range) | 42.5 (28-63) | 41 (22-61) | .37 |
| Male gender (%) | 22 (42) | 16 (53) | .81 |
| Weight, kg (range) | 84 (51.4-151.8) | 75 (47.6-124.0) | .07 |
| Chronic illness (%) | 7 (18) | 8 (27) | .42 |
| Chronic medication (%) | 7 (18) | 6 (20) | .87 |
| Multivariate psychometric profile | — | — | .43 |
| . | Marrow donors . | PBSC donors . | Pvalue . |
|---|---|---|---|
| Evaluable donors | 38 | 30* | .45 |
| Age, y (range) | 42.5 (28-63) | 41 (22-61) | .37 |
| Male gender (%) | 22 (42) | 16 (53) | .81 |
| Weight, kg (range) | 84 (51.4-151.8) | 75 (47.6-124.0) | .07 |
| Chronic illness (%) | 7 (18) | 8 (27) | .42 |
| Chronic medication (%) | 7 (18) | 6 (20) | .87 |
| Multivariate psychometric profile | — | — | .43 |
The number of donors is shown for each characteristic, except for age and weight, for which mean (range) is shown.
PBSC indicates peripheral blood stem cell.
Of 31 PBSC donors enrolled, 1 was unable to complete the donation satisfactorily.
Marrow harvesting
A total of 38 donors underwent harvesting of bone marrow. The specifics of the marrow harvesting procedure are shown in Table3. Almost all the donors complained of pain and received opioid analgesics during the immediate postoperative period, despite receiving opioids (fentanyl) as a component of the anesthesia. Complaints of dizziness and nausea documented by the ASC staff were also common despite the blood component support and aggressive hydration with crystalloid solutions (average, 3.4 L) in the operating and postoperative recovery rooms. One donor received homologous blood in addition to the unit of autologous blood.
Description of bone marrow collection procedures
| . | No. . |
|---|---|
| ASA rank | |
| 1 | 26 |
| 2 | 7 |
| 3 | 5 |
| Anesthesia | |
| General | 36 |
| Spinal | 2 |
| Harvest sites | |
| Posterior | 37 |
| Anterior and posterior | 1 |
| Duration3-150 | |
| Anesthesia, min, median (range)3-151 | 87.5 (48-159) |
| Harvest procedure, min, median (range)3-152 | 62.5 (27-120) |
| Day-surgery stay, min, median (range)3-153 | 376.0 (201-700) |
| Blood replacement | |
| 0 U | 3 |
| 1 U | 34 |
| > 1 U | 1 |
| Postoperative complications3-155 | |
| Pain | 32 |
| Nausea | 10 |
| Hypotension | 13 |
| Hemorrhage | 5 |
| . | No. . |
|---|---|
| ASA rank | |
| 1 | 26 |
| 2 | 7 |
| 3 | 5 |
| Anesthesia | |
| General | 36 |
| Spinal | 2 |
| Harvest sites | |
| Posterior | 37 |
| Anterior and posterior | 1 |
| Duration3-150 | |
| Anesthesia, min, median (range)3-151 | 87.5 (48-159) |
| Harvest procedure, min, median (range)3-152 | 62.5 (27-120) |
| Day-surgery stay, min, median (range)3-153 | 376.0 (201-700) |
| Blood replacement | |
| 0 U | 3 |
| 1 U | 34 |
| > 1 U | 1 |
| Postoperative complications3-155 | |
| Pain | 32 |
| Nausea | 10 |
| Hypotension | 13 |
| Hemorrhage | 5 |
The number of donors is shown, except for duration.
ASA indicates American Society of Anesthesiologists.
The duration of stay in the Ambulatory Surgery Center (ASC) excludes the 3 donors who were hospitalized overnight and a fourth donor who was subsequently hospitalized for complications of marrow harvesting.
Duration of anesthesia.
Time from start to completion of harvesting.
Length of stay in the ASC from start of anesthesia to discharge.
The number of donors with the indicated complications, as documented by the written notes of the ASC nursing staff. Of the donors reporting pain, 26 received additional opioid medications during their stay in the ASC unit. All donors were provided with oral opioid medications on discharge from the hospital. Donors described as experiencing hemorrhage required change of dressing before discharge from ASC.
Two donors were hospitalized overnight and 1 donor was hospitalized for 2 nights because of postoperative hypotension, nausea, and/or pain. A fourth donor was hospitalized 6 days after the procedure after he developed deep-vein thrombosis (DVT), presenting to the clinic with complaints of worsening leg and buttock pain.
Collection of PBSCs
A total of 30 evaluable donors underwent harvesting of PBSCs; the specifics of the apheresis procedures are shown in Table4. Only one collection procedure was required to reach the target dose of at least 5 × 106CD34+ cells per kilogram recipient weight for 19 donors, whereas 3 procedures were required for 1 donor. Two donors required placement of apheresis catheters because of inadequate arm veins; for one donor, the catheter was placed in the femoral vein whereas in the other, the catheter was placed in the subclavian vein. The complications of the apheresis procedures documented by the staff of the apheresis unit were minor and included needle infiltration and citrate reaction, although no donor required premature discontinuation of the procedure. One donor subsequently developed superficial phlebitis of an arm vein that resolved with symptomatic therapy. A second donor developed a large, painful hematoma at the site of the femoral vein apheresis catheter, which also resolved in several days with symptomatic treatment.
Description of apheresis collection procedures
| . | No. . |
|---|---|
| No. procedures | |
| 1 | 18 |
| 2 | 11 |
| 3 | 1 |
| Venous access | |
| Vein/vein | 28 |
| Catheter4-150 | 2 |
| Blood volume processed, L, median (range) | 12 (10.8-20.0) |
| Duration of apheresis procedure, min, median (range) | 152 (121-241) |
| Apheresis complications | |
| Needle infiltration | 2 |
| Citrate reaction | 6 |
| Phlebitis | 1 |
| Hematoma | 1 |
| Report of pain4-151 | |
| Day 1 | 1/27 |
| Day 2 | 11/24 |
| Day 3 | 22/24 |
| Day 4 | 24/25 |
| Day 5 | 23/24 |
| . | No. . |
|---|---|
| No. procedures | |
| 1 | 18 |
| 2 | 11 |
| 3 | 1 |
| Venous access | |
| Vein/vein | 28 |
| Catheter4-150 | 2 |
| Blood volume processed, L, median (range) | 12 (10.8-20.0) |
| Duration of apheresis procedure, min, median (range) | 152 (121-241) |
| Apheresis complications | |
| Needle infiltration | 2 |
| Citrate reaction | 6 |
| Phlebitis | 1 |
| Hematoma | 1 |
| Report of pain4-151 | |
| Day 1 | 1/27 |
| Day 2 | 11/24 |
| Day 3 | 22/24 |
| Day 4 | 24/25 |
| Day 5 | 23/24 |
Data show the number of donors, except for blood volume processed and duration.
The donor treated with a femoral vein catheter underwent processing of 20 L blood in order to reach the target dose of CD34+ cells from one collection procedure.
Data show the number of donors with reports of pain documented by the nursing staff each morning before the administration of filgrastim. Donor complaints were not documented for all visits. The denominator indicates the total number of donors with nursing notes for each day of filgrastim administration. Day 1 is the first day of filgrastim administration; day 5 is the first day of apheresis (day −1).
The PBSC donors were evaluated daily by a member of the nursing staff before administration of filgrastim. Only one donor never reported bone pain or other symptoms to the nursing staff during this period of time (Table 4). Opioid medications were prescribed for 17 of the 30 donors during the period of filgrastim administration.
Health status self-report measures
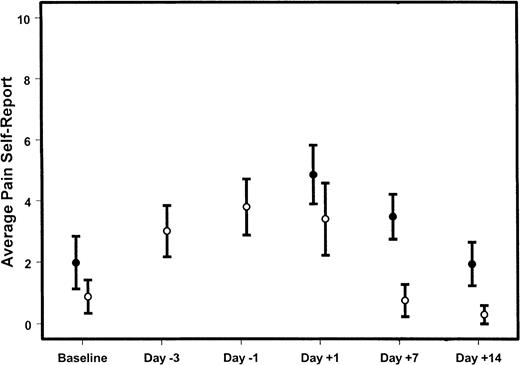
The proportions of patients returning questionnaires did not differ by group (Table 1). Emotional status and physical status numerical rating scale responses were summarized for each group of donors at intervals up to 14 days after donation (Figure1A-B). On average, neither the bone marrow nor the PBSC donors reported much fluctuation in their emotional status during the donation process. However, both groups reported a worsening of physical status as a result of the harvest procedures. Physical status and emotional status were measured only once during the predonation period (baseline) for bone marrow donors, and we assume that these were stable during this time. Bone marrow donors reported a considerable decrease in physical status on the day after donation, with gradual improvement during the 2 weeks after donation. In contrast, PBSC donors reported a considerable decrease in physical status during the several days filgrastim was administered. Initial resolution of symptoms was evident 1 day after discontinuation of filgrastim, and these donors had, on average, reported a return to baseline physical status by 7 days after donation.
Self-reports from donors on emotional and physical status.
Shown are the mean (± 95% confidence intervals) emotional status (panel A) and physical status (panel B) self-reports for marrow (●) or PBSC (○) donors at varying times during the donation procedures. Day 0 is the day of HSC infusion. Day −3 is the third day of filgrastim administration for PBSC donors; day −1 is the fifth day of filgrastim administration and the first day of apheresis. If the confidence intervals do not overlap, the group means differ significantly (P < .05) at that time point.
Self-reports from donors on emotional and physical status.
Shown are the mean (± 95% confidence intervals) emotional status (panel A) and physical status (panel B) self-reports for marrow (●) or PBSC (○) donors at varying times during the donation procedures. Day 0 is the day of HSC infusion. Day −3 is the third day of filgrastim administration for PBSC donors; day −1 is the fifth day of filgrastim administration and the first day of apheresis. If the confidence intervals do not overlap, the group means differ significantly (P < .05) at that time point.
The 2 groups differed significantly (P = .009) on the multivariate test of equal means for the primary end points, indicating that some combination of the primary end points discriminated between marrow and PBSC donors. For example, the average emotional status exceeded the average physical status in both groups, but the magnitude of this difference was significantly greater (P = .008) in the marrow group (mean difference of 2.65 between emotional and physical status scores) than in the PBSC group (mean difference, 1.43). Pain reports also interacted across groups, with the gap between worst pain and average pain significantly greater (P = .01) for the PBSC group (mean difference, 1.77) than for the marrow group (mean difference, 0.97). The significance of the multivariate test rejects the null hypothesis that all end points are equal across both groups and supports subsequent examination of multiple univariate comparisons. Comparisons of these primary end points are shown in Table 5. These summary comparisons are based on a donor's maximum rating of each self-report measure observed during the period of filgrastim administration (PBSC donors) and the first 2 weeks after donation (all donors). In the univariate comparisons, the 2 groups of donors did not differ for any of these measurements of the donation experience. When data were controlled statistically for baseline differences, the conclusions remained unchanged.
Summary comparisons
| . | Marrow donors . | PBSC donors . | P value . |
|---|---|---|---|
| Physical status5-150 | 3.74 | 4.40 | .23 |
| Emotional status5-150 | 6.38 | 5.83 | .40 |
| Worst pain5-150 | 6.06 | 6.30 | .72 |
| Average pain5-150 | 5.09 | 4.53 | .37 |
| Pain duration5-150 | 7.09 | 6.53 | .46 |
| Pain interference with sleep5-150 | 5.06 | 5.33 | .74 |
| Words describing pain5-151 | 4.50 | 4.90 | .52 |
| Symptom burden5-152 | 1.90 | 1.76 | .18 |
| . | Marrow donors . | PBSC donors . | P value . |
|---|---|---|---|
| Physical status5-150 | 3.74 | 4.40 | .23 |
| Emotional status5-150 | 6.38 | 5.83 | .40 |
| Worst pain5-150 | 6.06 | 6.30 | .72 |
| Average pain5-150 | 5.09 | 4.53 | .37 |
| Pain duration5-150 | 7.09 | 6.53 | .46 |
| Pain interference with sleep5-150 | 5.06 | 5.33 | .74 |
| Words describing pain5-151 | 4.50 | 4.90 | .52 |
| Symptom burden5-152 | 1.90 | 1.76 | .18 |
Data show the average maximum for each item through the first 14 days after donation but also including the period of filgrastim administration for PBSC donors. The differences between the groups were tested separately for each end point by means of t tests for independent samples.
PBSC indicates peripheral blood stem cell.
This was rated on an 11-point scale of 0 (minimum) to 10 (maximum).
Data show the average number of words used.
The mean score for the 23 symptoms or adverse experiences measured on a 4-point scale.
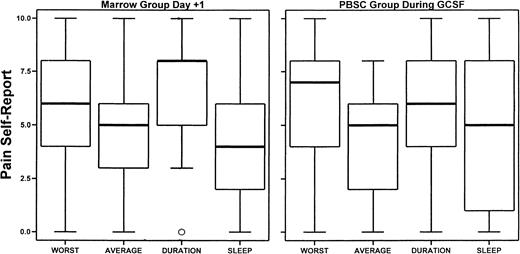
PBSC donors reported a considerable amount of pain during the days filgrastim was administered, with a quick return to baseline after completion of the harvest procedures (Figure2). Marrow donors reported greater average pain, maximal pain, and duration of pain 7 days after donation; this difference was almost resolved by 14 days after donation (Figure2, Table 6). It is notable that some PBSC donors reported a high and persistent level of pain on both the third and fifth day of administration of filgrastim (Figure3). Figure4 compares the distributions of pain measures at the times of peak intensity for each group: the third and fifth days of filgrastim administration and the day after marrow collection. The distributions of average and maximum intensity of pain experienced were very similar for marrow and PBSC donors. Although typical levels of pain duration and interference with sleep were roughly similar for these 2 groups of donors at their respective peaks, PBSC donors reported greater ranges, particularly for sleep disturbance.
Average pain reported by donors.
Shown is the mean (± 95% confidence intervals) for the average pain reported by marrow (●) or PBSC (○) donors at varying times during the donation procedures. Day 0 is the day of HSC infusion. Day −3 is the third day of filgrastim administration for PBSC donors; day −1 is the fifth day of filgrastim administration and the first day of apheresis. If the confidence intervals do not overlap, the group means differ significantly (P < .05) at that time point.
Average pain reported by donors.
Shown is the mean (± 95% confidence intervals) for the average pain reported by marrow (●) or PBSC (○) donors at varying times during the donation procedures. Day 0 is the day of HSC infusion. Day −3 is the third day of filgrastim administration for PBSC donors; day −1 is the fifth day of filgrastim administration and the first day of apheresis. If the confidence intervals do not overlap, the group means differ significantly (P < .05) at that time point.
Recovery of donor health status for 3 key outcomes
| . | Physical status6-150,6-151 . | Average pain6-150,6-152 . | Symptom burden6-153,6-155 . | Lost to follow-up . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Good . | Moderate . | Poor . | Mild . | Moderate . | Severe . | Mild . | Moderate . | Severe . | New . | Cumulative . | |
| Day 1 | |||||||||||
| BMT (no.) | 6 | 8(1) | 18(3) | 15(1) | 10 | 8(3) | 9(1) | 13(1) | 11(2) | 4 | 4 |
| % | 18.8 | 25.0 | 56.3 | 45.5 | 30.3 | 24.2 | 27.3 | 39.4 | 33.3 | ||
| PBSC (no.) | 12 | 4(2) | 7 | 15(1) | 3(2) | 5 | 12(1) | 8(1) | 3(1) | 3 | 3 |
| % | 57.2 | 17.4 | 30.4 | 65.2 | 13.0 | 21.7 | 52.2 | 34.8 | 13.0 | ||
| Day 7 | |||||||||||
| BMT (no.) | 14 | 10 | 6(1) | 19 | 11(1) | 0 | 24 | 4 | 2(1) | 1 | 5 |
| % | 46.7 | 3.3 | 20.0 | 63.3 | 36.7 | 0 | 80.0 | 13.3 | 6.7 | ||
| PBSC (no.) | 21(3) | 1 | 1 | 22(3) | 1 | 0 | 22(3) | 1 | 0 | 3 | 6 |
| % | 91.3 | 4.3 | 4.3 | 95.7 | 4.3 | 0 | 95.7 | 4.3 | 0 | ||
| Day 14 | |||||||||||
| BMT (no.) | 23(1) | 4(1) | 2 | 26(2) | 3 | 0 | 25(2) | 4 | 0 | 2 | 7 |
| % | 79.3 | 13.8 | 6.9 | 89.7 | 10.3 | 0 | 86.2 | 13.8 | 0 | ||
| PBSC (no.) | 21(2) | 0 | 0 | 21(2) | 0 | 0 | 21(2) | 0 | 0 | 2 | 8 |
| % | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | ||
| Day 21 | |||||||||||
| BMT (no.) | 25(3) | 1 | 0 | 23(3) | 2 | 1 | 25(3) | 1 | 0 | 3 | 10 |
| % | 96.2 | 3.8 | 0 | 88.5 | 7.7 | 3.8 | 96.2 | 3.8 | 0 | ||
| PBSC (no.) | 18(1) | 1 | 0 | 19(1) | 0 | 0 | 16 | 3(1) | 0 | 1 | 9 |
| % | 94.7 | 5.3 | 0 | 100 | 0 | 0 | 84.2 | 15.8 | 0 | ||
| Day 28 | |||||||||||
| BMT (no.) | 22 | 1 | 1 | 22 | 2 | 0 | 23 | 1 | 0 | 106-154 | |
| % | 91.7 | 4.2 | 4.2 | 91.7 | 8.3 | 0 | 95.8 | 4.2 | 0 | ||
| PBSC (no.) | 18 | 0 | 0 | 16 | 1 | 1 | 16 | 1 | 1 | 96-154 | |
| % | 100 | 0 | 0 | 88.9 | 5.6 | 5.6 | 88.9 | 5.6 | 5.6 | ||
| . | Physical status6-150,6-151 . | Average pain6-150,6-152 . | Symptom burden6-153,6-155 . | Lost to follow-up . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Good . | Moderate . | Poor . | Mild . | Moderate . | Severe . | Mild . | Moderate . | Severe . | New . | Cumulative . | |
| Day 1 | |||||||||||
| BMT (no.) | 6 | 8(1) | 18(3) | 15(1) | 10 | 8(3) | 9(1) | 13(1) | 11(2) | 4 | 4 |
| % | 18.8 | 25.0 | 56.3 | 45.5 | 30.3 | 24.2 | 27.3 | 39.4 | 33.3 | ||
| PBSC (no.) | 12 | 4(2) | 7 | 15(1) | 3(2) | 5 | 12(1) | 8(1) | 3(1) | 3 | 3 |
| % | 57.2 | 17.4 | 30.4 | 65.2 | 13.0 | 21.7 | 52.2 | 34.8 | 13.0 | ||
| Day 7 | |||||||||||
| BMT (no.) | 14 | 10 | 6(1) | 19 | 11(1) | 0 | 24 | 4 | 2(1) | 1 | 5 |
| % | 46.7 | 3.3 | 20.0 | 63.3 | 36.7 | 0 | 80.0 | 13.3 | 6.7 | ||
| PBSC (no.) | 21(3) | 1 | 1 | 22(3) | 1 | 0 | 22(3) | 1 | 0 | 3 | 6 |
| % | 91.3 | 4.3 | 4.3 | 95.7 | 4.3 | 0 | 95.7 | 4.3 | 0 | ||
| Day 14 | |||||||||||
| BMT (no.) | 23(1) | 4(1) | 2 | 26(2) | 3 | 0 | 25(2) | 4 | 0 | 2 | 7 |
| % | 79.3 | 13.8 | 6.9 | 89.7 | 10.3 | 0 | 86.2 | 13.8 | 0 | ||
| PBSC (no.) | 21(2) | 0 | 0 | 21(2) | 0 | 0 | 21(2) | 0 | 0 | 2 | 8 |
| % | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | ||
| Day 21 | |||||||||||
| BMT (no.) | 25(3) | 1 | 0 | 23(3) | 2 | 1 | 25(3) | 1 | 0 | 3 | 10 |
| % | 96.2 | 3.8 | 0 | 88.5 | 7.7 | 3.8 | 96.2 | 3.8 | 0 | ||
| PBSC (no.) | 18(1) | 1 | 0 | 19(1) | 0 | 0 | 16 | 3(1) | 0 | 1 | 9 |
| % | 94.7 | 5.3 | 0 | 100 | 0 | 0 | 84.2 | 15.8 | 0 | ||
| Day 28 | |||||||||||
| BMT (no.) | 22 | 1 | 1 | 22 | 2 | 0 | 23 | 1 | 0 | 106-154 | |
| % | 91.7 | 4.2 | 4.2 | 91.7 | 8.3 | 0 | 95.8 | 4.2 | 0 | ||
| PBSC (no.) | 18 | 0 | 0 | 16 | 1 | 1 | 16 | 1 | 1 | 96-154 | |
| % | 100 | 0 | 0 | 88.9 | 5.6 | 5.6 | 88.9 | 5.6 | 5.6 | ||
Numbers in parentheses indicate donors who were lost to follow-up following that assessment. P values correspond to exact permutation tests of equal distributions across donor groups.
BMT indicates bone marrow transplant; PBSC, peripheral blood stem cell.
The 3 categories for physical status and average pain correspond to 0-4, 5-6, and 7-10 divisions on an 11-point scale.
P = .042 for day 1; P = .002 for day 7; P = .102 for day 14; P = .672 for day 21; P = 1.000 for day 28.
P = .284 for day 1; P = .005 for day 7; P = .186 for day 14; P = .501 for day 21; P = .756 for day 28.
The 2 categories for symptom burden were defined by breakpoints at 1.5 and 2.0 on the mean score, which ranged from 1.0 to 4.0 across 23 symptoms.
P = .111 for day 1; P = .307 for day 7; P = .103 for day 14; P = .195 for day 21; P = .712 for day 28.
P = .79, exact test of equality of proportions of cases lost to follow-up.
Severity and duration of average pain reported by PBSC donors.
This shows the severity (x-axis) and duration through the day (y-axis) of average pain reported by PBSC donors on day −1 (day 5 of filgrastim administration). Points in the upper-right quadrant correspond to donors' exceeding the pain scale midpoints for both severity and duration.
Severity and duration of average pain reported by PBSC donors.
This shows the severity (x-axis) and duration through the day (y-axis) of average pain reported by PBSC donors on day −1 (day 5 of filgrastim administration). Points in the upper-right quadrant correspond to donors' exceeding the pain scale midpoints for both severity and duration.
Pain as reported by marrow and PBSC donors.
Shown is the median of pain (worst, average, duration, and during sleep) on the day after marrow donation or during the period of filgrastim administration (day −3 or day −1) as reported by marrow or PBSC donors, respectively. The box ends and median are the 25th, 50th (median), and 75th percentiles. The whiskers extend to the extremes unless there are outliers by an objective criterion (one marrow donor reported “0” for duration of pain).
Pain as reported by marrow and PBSC donors.
Shown is the median of pain (worst, average, duration, and during sleep) on the day after marrow donation or during the period of filgrastim administration (day −3 or day −1) as reported by marrow or PBSC donors, respectively. The box ends and median are the 25th, 50th (median), and 75th percentiles. The whiskers extend to the extremes unless there are outliers by an objective criterion (one marrow donor reported “0” for duration of pain).
Since the measurement scales for the outcomes listed in Table 5 lack intrinsic clinical meaning, we also present the key findings for 3 primary outcomes using a more interpretable classification system. In Table 6, we display the numbers and proportions of donors with mild (or good), moderate, or severe (or poor) pain, physical status, and symptom burden at each time point, on the basis of the classification scheme suggested by Serlin et al.22 Health status is in the good range for 80% to 100% of the donors by day 14, and 90% to 100% of donors by day 28. Table 6 also denotes (in the entries in parentheses) the numbers of donors in each category who failed to return questionnaires subsequent to any particular assessment. Although the overall loss to follow-up did not differ significantly (P = .79) across groups, there was a tendency for early marrow dropouts to have more symptomatology than early PBSC dropouts. Later dropouts in both groups had low levels of symptomatology. All statistical conclusions remained unchanged under multiple imputation of missing data, so the pattern of missing data did not affect the interpretation (P = .004 for the multivariate test of primary end points under multiple imputation).
Pain was the primary complaint of both groups of donors, but the frequency of other symptoms commonly reported by both PBSC and marrow donors is listed in Table 7. Both PBSC and marrow donors reported the presence of a wide variety of somatic complaints associated with the donation process. Table 7 shows that these symptoms had nearly resolved by 7 days after HSC donation in both groups, but that the severity of the discomfort and the number of different complaints were greater for the marrow donors than for PBSC donors 7 days after the completion of the donation.
Symptom checklist
| Marrow donors7-150 . | Day +1 . | Day +7 . | ||||||
|---|---|---|---|---|---|---|---|---|
| Not at all . | A little . | Quite a bit . | Very much . | Not at all . | A little . | Quite a bit . | Very much . | |
| Need rest | 1 | 7 | 13 | 12 | 6 | 17 | 4 | 3 |
| Shortness of breath | 27 | 4 | 1 | 1 | 25 | 4 | 1 | 0 |
| Felt weak | 5 | 14 | 7 | 7 | 12 | 14 | 3 | 1 |
| Difficulty walking | 1 | 11 | 9 | 12 | 8 | 16 | 4 | 2 |
| Difficulty sleeping | 9 | 17 | 4 | 2 | 11 | 16 | 0 | 2 |
| Loss of appetite | 12 | 11 | 7 | 3 | 25 | 3 | 2 | 0 |
| Tired | 1 | 14 | 9 | 9 | 5 | 19 | 3 | 3 |
| Sore throat | 12 | 16 | 3 | 2 | 28 | 5 | 0 | 0 |
| Nausea | 11 | 14 | 4 | 3 | 26 | 3 | 1 | 0 |
| Vomited | 23 | 5 | 4 | 1 | 30 | 0 | 0 | 0 |
| Marrow donors7-150 . | Day +1 . | Day +7 . | ||||||
|---|---|---|---|---|---|---|---|---|
| Not at all . | A little . | Quite a bit . | Very much . | Not at all . | A little . | Quite a bit . | Very much . | |
| Need rest | 1 | 7 | 13 | 12 | 6 | 17 | 4 | 3 |
| Shortness of breath | 27 | 4 | 1 | 1 | 25 | 4 | 1 | 0 |
| Felt weak | 5 | 14 | 7 | 7 | 12 | 14 | 3 | 1 |
| Difficulty walking | 1 | 11 | 9 | 12 | 8 | 16 | 4 | 2 |
| Difficulty sleeping | 9 | 17 | 4 | 2 | 11 | 16 | 0 | 2 |
| Loss of appetite | 12 | 11 | 7 | 3 | 25 | 3 | 2 | 0 |
| Tired | 1 | 14 | 9 | 9 | 5 | 19 | 3 | 3 |
| Sore throat | 12 | 16 | 3 | 2 | 28 | 5 | 0 | 0 |
| Nausea | 11 | 14 | 4 | 3 | 26 | 3 | 1 | 0 |
| Vomited | 23 | 5 | 4 | 1 | 30 | 0 | 0 | 0 |
| PBSC donors7-150 . | Day −1 . | Day +7 . | ||||||
|---|---|---|---|---|---|---|---|---|
| Not at all . | A little . | Quite a bit . | Very much . | Not at all . | A little . | Quite a bit . | Very much . | |
| Need rest | 6 | 9 | 7 | 5 | 12 | 9 | 2 | 0 |
| Shortness of breath | 23 | 4 | 0 | 0 | 22 | 1 | 0 | 0 |
| Felt weak | 9 | 11 | 7 | 0 | 20 | 3 | 0 | 0 |
| Difficulty walking | 9 | 14 | 4 | 0 | 21 | 2 | 0 | 0 |
| Difficulty sleeping | 8 | 12 | 5 | 2 | 13 | 8 | 1 | 1 |
| Loss of appetite | 17 | 7 | 2 | 1 | 21 | 2 | 0 | 0 |
| Tired | 3 | 12 | 7 | 4 | 9 | 13 | 1 | 0 |
| Sore throat | 22 | 5 | 0 | 0 | 22 | 1 | 0 | 0 |
| Nausea | 22 | 4 | 0 | 0 | 23 | 0 | 0 | 0 |
| Vomited | 27 | 0 | 0 | 0 | 23 | 0 | 0 | 0 |
| PBSC donors7-150 . | Day −1 . | Day +7 . | ||||||
|---|---|---|---|---|---|---|---|---|
| Not at all . | A little . | Quite a bit . | Very much . | Not at all . | A little . | Quite a bit . | Very much . | |
| Need rest | 6 | 9 | 7 | 5 | 12 | 9 | 2 | 0 |
| Shortness of breath | 23 | 4 | 0 | 0 | 22 | 1 | 0 | 0 |
| Felt weak | 9 | 11 | 7 | 0 | 20 | 3 | 0 | 0 |
| Difficulty walking | 9 | 14 | 4 | 0 | 21 | 2 | 0 | 0 |
| Difficulty sleeping | 8 | 12 | 5 | 2 | 13 | 8 | 1 | 1 |
| Loss of appetite | 17 | 7 | 2 | 1 | 21 | 2 | 0 | 0 |
| Tired | 3 | 12 | 7 | 4 | 9 | 13 | 1 | 0 |
| Sore throat | 22 | 5 | 0 | 0 | 22 | 1 | 0 | 0 |
| Nausea | 22 | 4 | 0 | 0 | 23 | 0 | 0 | 0 |
| Vomited | 27 | 0 | 0 | 0 | 23 | 0 | 0 | 0 |
Data show the number of donors with each complaint at the specified times before or after donation. The day is in reference to the day of hematopoietic stem cell infusion (day 0).
Discussion
In this randomized study, we found that the peak symptom burden and level of pain reported by the PBSC donors did not differ significantly from those reported by the bone marrow donors. Both groups of donors reported considerable somatic toxicity, as has been described in other retrospective studies of marrow or PBSC donation. However, the temporal patterns of discomfort and recovery differed for these 2 groups. In general, the apheresis procedures used to collect PBSCs were well tolerated. The administration of filgrastim to mobilize PBSCs into the peripheral circulation was associated with considerable somatic toxicity, and a proportion of donors reported pain that was both severe and persistent during the days of drug administration. In contrast, the symptoms experienced by marrow donors were a direct result of the anesthesia and multiple needle punctures. The filgrastim-induced symptoms quickly resolved after discontinuation of this medication. PBSC donors reported high and persistently stable levels of pain for several days before transplantation, but recovered quickly after the discontinuation of filgrastim administration. Virtually all PBSC donors reported a return to baseline health status by 14 days after donation. Bone marrow donors, in contrast, experienced more prolonged pain, and only 80% reported return to baseline status by 14 days after donation, a finding consistent with what has been reported by marrow donors for unrelated donor transplantation.15
Although no single end point differed between these 2 groups in univariate comparisons, the significance (P = .009) of the multivariate test implies that a combination of end points, such as the difference between emotional and physical status and between average and worst pain during the day, did differ. Since emotional status can reflect physical status, expectations of the donors may play a role in these interactions. It is possible that PBSC donors expected fairly minimal side effects, perhaps comparable to those accompanying routine blood donations, and their emotional status may have suffered when symptoms were worse than expected. Marrow donors, by contrast, may have had more realistic expectations for their side effects and may have felt relatively better for meeting the challenges of donation. In addition, marrow donors experienced physical discomfort after the completion of the donation process so that the peak physical discomfort would fall at a time of less anxiety. It is possible that the greater difference between worst pain and average pain during a 24-hour period reported by the PBSC donors reflected a temporal effect of filgrastim administration that would not be detected in this current study and that has not been reported by others.
Reports by others suggest that the level of discomfort experienced by patients and donors undergoing filgrastim treatment for mobilization of PBSCs may be related to the dose of filgrastim administered.11 Stroncek et al11 found that 10% to 15% of healthy research subjects treated with filgrastim at doses up to 10 μg/kg/d required a dose reduction or discontinuation of filgrastim administration because of somatic toxicity. We used a higher dose than was administered in that research study in order to achieve higher levels of CD34+ cells in the peripheral circulation. None of the donors in this study required dose reduction, possibly because the sibling donors of cells for transplantation may be more highly motivated than research subjects. However, in this study, opioid-containing medications were prescribed to more than half of the donors to reduce pain, a situation unlike that in Stroncek's study. Had the current study used lower doses of filgrastim, it could have diminished the intensity of pain perceived in the PBSC group, but could have also prolonged the pain if additional apheresis procedures were required. We did not attempt to quantify actual use of analgesic or other medications by either group of donors, nor did we attempt to influence the outcome of this study by prospectively modifying the collection procedures described in this report by using nonstandard anesthesia or apheresis regimens.
This study was not intended to detect long-term health consequences of the donation procedure, and the numbers of donors enrolled were too small to detect differences in the incidence of serious adverse events for bone marrow or PBSC collection procedures. The experiences of the donors described in this study are consistent with those described in previous publications on the experiences of bone marrow or PBSC donors. Complications can arise from various aspects of the collection procedure. Bone marrow donors are exposed to an anesthetic procedure, blood loss, and multiple needle punctures that can damage the ilium or contiguous structures.12,23 The incidence of hospitalization in this study was similar to that of other retrospective reports of outpatient marrow harvesting.24In contrast, PBSC donors are exposed to cytokine administration, and a small proportion of donors will require placement of an apheresis catheter because of inadequate venous access for the collection procedure. The safety of administering filgrastim to donors with chronic medical illnesses is uncertain,8,25 and the donor with unstable health may best be cared for in the intensive setting of the operating room rather than the outpatient apheresis unit. However, administration of filgrastim or other agents to mobilize HSCs into the peripheral circulation has no recognized long-term sequelae and appears safe for most donors, including the elderly.26Life-threatening complications associated, at least temporarily, with the donation procedure have been reported for both collection procedures, but serious toxicity from either procedure is rare.8,12 27 In this study, 2 bone marrow donors and 1 PBSC donor experienced complications that could be considered serious adverse events (for marrow, DVT and homologous blood transfusions; for PBSC, hematoma).
The two groups of donors underwent markedly different procedures, but the validated scales used for quantifying pain and other symptoms allow comparison between these groups. Although PBSC donors returned to baseline more quickly after donation, these donors experienced several days of filgrastim-associated symptoms, and these data do not demonstrate that donation of PBSCs is much easier for the donor than donation of marrow. Such factors as donor health and the treatment plan of the recipient must be considered in choosing the source of HSCs for transplantation. It is unlikely that modifications of the marrow harvesting technique will affect the postoperative discomfort experienced by the marrow cell donor. Symptoms such as nausea are most likely related to the anesthesia and postoperative pain medications and may be partially alleviated by the use of other agents, but the pain reported is a direct consequence of marrow harvesting. In contrast, most of the discomfort experienced by the PBSC donor is a consequence of cytokine administration and not from the apheresis procedure. Peak levels of CD34+ cells in the peripheral blood reliably occur after 5 days of cytokine administration,28 and the somatic discomfort experienced by the PBSC donor appears early in this course. Alternative mobilization schemes, possibly with cytokine or chemokine regimens yet to be developed, have the potential to further facilitate the donation process and, by reducing the need for multiple days of cytokine administration, decrease the toxicity experienced by the PBSC donor. In the meantime, counseling of donors and better pain management strategies may help in the management of the somatic complaints experienced by the PBSC donor.
We thank the many people involved with the collection of hematopoietic stem cells for transplantation at the Fred Hutchinson Cancer Research Center, including the families and friends of the donors and patients who supported them during the transplantation procedures.
Supported in part by an unrestricted gift from Amgen; National Cancer Institute grants CA18029 and CA15704; and a grant from the Jose Carreras Foundation Against Leukemia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Scott D. Rowley, Hackensack University Medical Center, 20 Prospect Ave, Suite 400, Hackensack, NJ 07601; e-mail:srowley@humed.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal