Abstract
Antigen-presenting cells (APCs) from subcutaneous mouse MOPC315 plasmacytoma phagocytosed immunoglobulin G–coated magnetic beads, enabling efficient isolation within 2 hours by magnetic separation (APC-MB). Cell morphology was heterogeneous, with some of the cells having dendrites. The surface phenotype of purified tumor APCs-MB was CD11b+, CD11c+, CD40+, CD80+, CD86+, and MHC class II+. Tumor APCs-MB expressed messenger RNA for fractalkine and ABCD-1 chemokines, and for CC-type chemokine receptors CCR5 and CCR7, indicating the presence of mature dendritic cells (DCs). Visualized at a single cell level within 4 hours after disruption of the tumor, APCs-MB induced rapid Ca++ mobilization in MHC class II–restricted tumor idiotype (Id)–specific cloned CD4+ T cells. In long-term assays, tumor APCs-MB induced proliferation of naive T cells from Id-specific T-cell receptor transgenic mice. The results suggest that tumor APCs-MB represent a heterogeneous cell population that includes myeloid-derived DCs of various stages of maturation. A considerable fraction (≥ 15%) of DCs is spontaneously primed with tumor-specific antigen.
Introduction
T cells are thought to be important in immune responses to tumors.1 To become activated, naive T cells need to interact with antigen-presenting cells (APCs) for recognition of MHC-presented tumor-specific peptides and costimulatory molecules. This poses a problem for initiation of antitumor T-cell responses because tumor cells often only express (reduced amounts of) class I molecules and neither class II nor CD80/86 molecules.
The problem would be solved if tumor-specific antigen (TSA) is transferred to highly stimulatory dendritic cells (DCs) that possess all features required for activation of naive T cells. Indirect genetic and functional evidence indicates that TSA is transferred to professional APCs.2-4 Direct evidence exists from 2 manipulated model systems. In one report, transfection of tumor cells with interleukin 3 (IL-3) was required to detect macrophage-like cells in the tumor spontaneously presenting a class I–restricted pseudotumor antigen (ovalbumin) to CD8+ cells.5 In another study, when tumor cells were transduced with both granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40L, shared macrophage and DC-like cells were able to present endogenous viral tumor-associated antigen in a class I–restricted fashion.6 Our own recent work with unmanipulated myeloma cells shows that TSA (myeloma protein variable region idiotype [Id]) is transferred to tumor APCs with DC-like features for class II presentation to CD4+ cells.7 This might explain the previously reported resistance of Id-specific T-cell receptor (TCR)–transgenic mice to the class II–negative MOPC315 myeloma, mediated by CD4+ T cells.8 9
The maturation stage of DCs influences capture and presentation of antigen.10,11 Recently, it was described that myeloid-derived immature DCs phagocytosed latex microbeads injected intracutaneously in normal mice. A fraction of these cells migrated to draining lymph nodes (LNs). Phagocytic DCs were able to stimulate alloreactive T cells in vitro.12 It is important to establish whether similar phagocytic APCs exist in tumors and whether they can present TSA to T cells. By use of a magnetic bead (MB) purification method, we rapidly (2 hours) segregated phagocytic APCs-MB from subcutaneous MOPC315 (immunoglobulin [Ig]Aλ2315) plasmacytomas. This allowed us to analyze their microscopic appearance and expression of various cell surface markers as well as chemokines and chemokine receptors. To test rapidly whether freshly isolated APCs-MB could stimulate myeloma protein L-chain [91-107 Id(λ2315)]–specific, I-Ed–restricted CD4+ cells, we employed a Ca++ mobilization assay, which takes only a few hours to complete. Moreover, this digital imaging assay allowed analysis of encounters of single tumor DCs and T cells.
Materials and methods
Model system
MOPC315 is a class II–negative mouse plasmacytoma that secretes IgA myeloma protein M315 with a unique λ2 chain, λ2315. A CDR3 Id determinant in Vλ2315 (aa 91-107) is presented on I-Ed MHC class II molecules to CD4+ T cells.13-15 Residues 94, 95, and 96 are crucial for Id-specific T-cell recognition16; these 3 residues are nongermline due to somatic mutations in the Vλ2 gene segment of MOPC315.17
Mice
The λ2315-specific, TCR (Vα1, Jα19; Vβ8.2, Jβ1.2)-transgenic mice, backcrossed to BALB/c for more than 15 generations, have been described earlier.18 19 The hemizygous mice were of specific pathogen free (SPF) standard and bred behind a barrier. The transgenic and nontransgenic animals used in this study were offspring from (BALB/c × hemizygous TCR-transgenic) F1 crosses and were typed by staining of peripheral blood lymphocytes with clonotype-specific GB113 monoclonal antibody (mAb). The study was approved by the Committee for Experiments on Animals, The National Hospital, Oslo, Norway.
Cell lines and injection of tumor cells
MOPC31520 and J558 (IgAλ1) are transplantable BALB/c plasmacytomas obtained from American Type Culture Collection (ATCC, Manassas, VA). Retrovirally enhanced green fluorescent protein (EGFP)-neo transduced MOPC315 cells were kindly obtained from Dr Brad Howard (University of Kiel, Germany). Mice (6-12 weeks old) were injected subcutaneously in the interscapular region with high loads of MOPC315 (2 × 106) cells or J558 (5 × 105) cells.8,9 The tumors grew locally, but infrequent metastatic cells were found with progressive tumor growth (unpublished data, October 2000). M315 and J558 myeloma protein concentrations in serum were assayed by enzyme-linked immunosorbent assay (ELISA).8 D2SC/1 (D2SC) is a DC line derived from the mouse spleen, showing characteristics of immature DCs.21
Preparation of APCs
MB-purified APCs.
MBs (φ 4.5 μm, M450; Dynal, Oslo, Norway) were coated with fluorescein isothiocyanate (FITC)–labeled mAb TIB95 (mouse IgG2a, with specificity for H-2Kk,g,r,p; ATCC). Tumors and spleens were minced with scissors. Tissue pieces were placed in a tube containing fetal calf serum (FCS)–supplemented (10%) RPMI 1640/HEPES medium with collagenase (400 U/mL; Sigma, St Louis, MO) and DNase (0.33 mg/mL; Sigma) and dispersed by intermittent pipetting with a wide-bored pipette for 45 minutes at 22°C. Residual fragments were gently crushed against a stainless steel mesh with a glass rod. Released cells were pooled and washed twice. The cell suspension was incubated with coated MBs for 30 minutes at 37°C. For most experiments, an estimated (based on flow cytometry) bead/APC ratio of 3:1 was used. Cells that had phagocytosed MBs were extracted by exposure for 1 to 3 minutes at 22°C to a Dynal magnetic extractor. The magnetic extraction procedure was repeated twice. For T-cell stimulation assays, APCs-MB were irradiated with 2000 rads, a dose that inhibited growth of MOPC315 and J558 cell lines by 98% and 94%, respectively.
Spleen DCs.
Dendritic cells from spleens of BALB/c mice without tumors were isolated by density gradient centrifugation and transient adherence to plastic according to previously described methods.7
Th1- and Th2-polarized cells and T-cell clone
Th1- and Th2-polarized cells were obtained from LN cells of TCR-transgenic severe combined immunodeficiency (SCID) mice by incubation of 5 × 105/mL LN cells with 2000 rad-irradiated BALB/c spleen cells (2.5 × 106/mL) in the presence of 4 μg/mL of synthetic peptide. For Th1, we added 2 μg/mL of anti–IL-411B11 and 1 ng/mL of recombinant mouse IL-12 (R&D Systems, Minneapolis, MN), whereas for Th2, IL-4 (20 U/mL) was added to the medium. Th1 or Th2 cells were used in T-cell stimulation assays on day 10 after their last stimulation. Th1 and Th2 phenotypes were verified by testing interferon γ (IFNγ) and IL-4 in supernatants of polarized T cells restimulated on day 10. The Id(λ2315)-specific 7A10B2 Th1 clone has been described.13 Complete tissue culture medium was RPMI 1640 with HEPES (Gibco, Paisley, Scotland) with 10% FCS (Gibco) and supplements.22
T-cell assays
Stimulators.
Irradiated (2000 rad) APCs were cultured with T cells in 280 μL volumes in triplicate in 96-well microtiter plates.
Responders.
Sources and numbers/well of cells used were the following: 5 × 104 LN cells from TCR-transgenic SCID mice; 105 LN cells from TCR transgenic or nontransgenic mice; 1.6 × 105 TCR-transgenic SCID spleen cells; 3 × 105 TCR-transgenic or nontransgenic spleen cells; or 2 × 104 Th1- or Th2-polarized T cells or cloned 7A10B2 cells. These numbers were chosen, based on flow cytometry, so that there should be approximately 2 × 104 Id-responsive CD4+ cells/well.
Positive control.
To test the ability of the APCs to stimulate Id-specific CD4+ cells, a parallel set of cultures received an optimal concentration of 91 to 101 (λ2315) synthetic peptide (4 μg/mL).
Assays.
After 60 hours, 80 μL supernatants were withdrawn for determination of IL-2 and IFNγ.8 Immediately afterward, cultures were pulsed with 10 μCi 3HTdR in 20 μL complete medium, harvested 16 hours later, and counted by use of a Betamax scintillation counter (Hewlett-Packard, CA).
Reverse transcriptase–polymerase chain reaction
Total RNA from approximately 5 × 105 cells was extracted by using TRIzol reagent (Life Technologies, Täby, Sweden). Complementary DNA (cDNA) was synthesized by incubation for 1 hour at 37°C in a 20 μL reaction buffer containing MMLV reverse transcriptase (RT), enzyme buffer, oligo pd(T)12-18, dNTP, and RNase inhibitor (RNAguard) all from Amersham Pharmacia Biotech (Uppsala, Sweden). Amplification of cDNA (0.5 μL in a 25 μL reaction volume) was done by polymerase chain reaction (PCR) with PfuTurbo polymerase (Stratagene, La Jolla, CA), using the following conditions: 5 minutes at 95°C, followed by 30 (glutaraldehyde phospho-dehydrogenase [GAPDH]), 32 (CCR5, CCR7, and fractalkine), 33 (CD19), or 35 (ABCD-1, CD3ε) cycles of denaturation at 95°C for 45 seconds, annealing at 58°C (54°C for CD19; 62°C for CD3ε) for 45 seconds, and elongation at 72°C for 45 seconds. GAPDH served as a control for quantity and integrity of cDNA input. CCR5 and CCR7 were amplified in the same reaction tube. Sequences of the following primers were published elsewhere: GAPDH,23 CCR5,24CCR7,25 fractalkine,26 ABCD-1,27CD3ε,28 and CD19.28
Antibodies and flow cytometry
The following commercially available mAbs were used, conjugated with either FITC, phycoerythrin (PE), allophycocyanin, or biotin: CD1d (1B1), CD4 (RM4.5), CD8 (53-6.7), CD11b (M1/70), CD11c (HL3), CD13 (R3-242), CD19 (1D3), CD40 (3/23), CD80 (16-10A1), CD86 (GL-1), CD138 (281-2), and Ly-6G (Gr-1; RB6-8C5) (Pharmingen, San Diego, CA). The various fluorochrome-conjugated, isotype-matched control mAbs were from Pharmingen. Some mAbs were protein A– or protein G–Sepharose purified and conjugated with biotin by the following in-house procedures: CD11c (HB224, ATCC), pan-MHC class II (TIB120, ATCC), I-Ed class II (14.4.4S, ATCC; 13/4,29). Biotinylated antibodies were detected with streptavidin-Cy-Chrome (Pharmingen). Combinations of the above listed mAbs were used for double, triple, or quadruple stainings of cells. Stainings were essentially done as described earlier.18 19 Samples were acquired on a FACScan or FACScalibur (Becton Dickinson, Mountain View, CA) and analyzed by the CellQuest program.
Immunofluorescence
Tumor tissue was embedded in OCT compound (Tissue-Tek, Sakura FineTek, Torrance, CA) and snap-frozen in isopentane that had been precooled on dry ice. Frozen sections with a thickness of 4 μm were cut on a cryotome and mounted on L-polylysine–coated glass slides (Polysine Microslides, Menzel-Gläser, Germany). The preparations were air dried overnight, fixed in acetone for 10 minutes, and stored at −20°C. Sections were rehydrated in phosphate-buffered saline (PBS) before staining. All Abs were diluted in PBS containing 30% normal mouse serum. Washings with 2 changes of PBS were carried out between each of the incubation steps described below. Mounting was done with ImmunoMount (Shandon, Pittsburgh, PA).
Cells binding mouse IgG were detected by sequential incubation of sections with normal mouse IgG (10 mg/mL; Sigma) and goat antimouse IgG conjugated with Cy2 (Jackson Immunoresearch Laboratories, West Grove, PA). For CD11b detection, Cy2-stained or unstained sections were first blocked with avidin/biotin and then sequentially incubated with rat anti-CD11b mAb (M1-70; Pharmingen), biotinylated rabbit antirat IgG antibodies (Vector Laboratories, Burlingame, CA), and, finally, streptavidin-Cy3 (Molecular Probes, Eugene, OR). Nuclei were detected with 4, 6-diamino-2-phenylindoldihydrochloride. Images of stained sections were taken with a CCD camera (Hamamatsu, Japan) and layered with Adobe Photoshop software.
Measurement of cytosolic free Ca++ in single T cells
Measurement of Ca++ in single cells was done essentially as previously described.30 MB-purified APCs (0.5-1 × 105) were seeded in 6-mm wells (plastic cylinders attached to glass cover slips with a silicone rubber [RTV 118; GE Silicones, Bergen op Zoom, Holland]) 1 to 5 hours before the experiment and incubated at 37°C. The cells were washed with PBS twice after 1 hour. Cloned Th1 7A10B2 cells were kept on ice prior to loading with 10 μM of the fluorescent Ca++ indicator fura-2 (Molecular Probes, Eugene, OR). Labeling was done for 60 minutes at room temperature followed by centrifugation and resuspension. Labeled T cells were used as quickly as possible, but they could be kept on ice for up to 5 hours before use. The wells with the APCs were placed in a 37°C chamber on the table of an inverted microscope, and the registration was started when the fura-2 loaded 2 × 105 T cells were added to the well. The Ca++ imaging and registration software has been developed and described previously.31 The cytosolic Ca++concentration was calculated as described previously.32The threshold is defined as the difference between the maximal Ca++ level and the basal level before the response.
Results
Tumor dendritic CD11b+ cells bind IgGs and phagocytose IgG-coated MBs
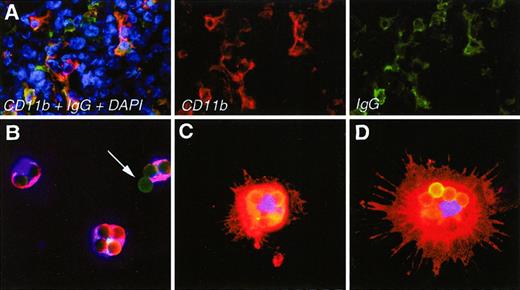
To assess molecular and cellular markers in freshly isolated tumor APCs (DCs and macrophages), we established an expeditious method of isolation (approximately 2 hours) by use of MBs. The basis for this method was the observation that in sections of MOPC315 tumors, the CD11b+ cells bound mouse IgG, suggesting that they had Fc receptors (Figure 1A). Thus, we coated 4.5 μm MBs with FITC-conjugated mouse IgG2a (TIB95) and incubated them with collagenase/Dnase-dispersed tumor cells. Cells with beads were easily extracted by use of a magnet, and the recovered fraction was denoted APC-MB. Figure 1B-D suggests that most MBs were phagocytosed, but some were also attached to the surface (arrow, Figure1B). The number of such beads/cell varied from 1 to 40, with an average of 3 to 10 (see also Figure 5E). To verify the phagocytic capacity of tumor APCs-MB, we incubated them with human red blood cells (RBCs) coated with human immunoglobulin and then lysed the RBCs with a hypotonic solution, preserving the APCs-MB. Some APCs-MB had RBCs within their cellular perimeter, whereas no extracellular RBCs were present (data not shown). This implied that isolated APCs-MB were indeed phagocytic. Immunostaining after extraction of APCs-MB showed that the cells were CD11b+(Figure 1B-D). Some cells had a DC appearance; these were generally larger and contained many phagocytosed beads per cell (Figure 1C,D). Other cells were smaller with no observable dendrites and contained fewer beads per cell (Figure 1B). APCs-MB could be isolated from MOPC315 tumors established in TCR-transgenic as well as BALB/c mice. (TCR-transgenic mice were injected with high loads of MOPC315 cells to partly overcome their resistance to Id+tumors.9) Mainly intermediate to large size tumors (0.5-3 g) were used throughout these studies.
Microscopic appearance of APCs in sections of tumors and after isolation as APCs-MB.
(A) Immunohistochemical staining of APCs with CD11b mAb (red) and mouse IgG plus antimouse IgG (green) in sections of small subcutaneous MOPC315 tumors (0.2-0.5 g) in TCR-transgenic mice. Cell nuclei are in blue. (B-D) Examples of stained APCs-MB, purified, attached to slides, and fixed within 2 hours after dissection of MOPC315 tumors. Anti-CD11b in red, MBs conjugated with mouse IgG2a-FITC in green, nuclei in blue. The arrow indicates a bead that has not been internalized. Beads are 4.5 μm in diameter.
Microscopic appearance of APCs in sections of tumors and after isolation as APCs-MB.
(A) Immunohistochemical staining of APCs with CD11b mAb (red) and mouse IgG plus antimouse IgG (green) in sections of small subcutaneous MOPC315 tumors (0.2-0.5 g) in TCR-transgenic mice. Cell nuclei are in blue. (B-D) Examples of stained APCs-MB, purified, attached to slides, and fixed within 2 hours after dissection of MOPC315 tumors. Anti-CD11b in red, MBs conjugated with mouse IgG2a-FITC in green, nuclei in blue. The arrow indicates a bead that has not been internalized. Beads are 4.5 μm in diameter.
The purity of the extracted APCs-MB was determined by flow cytometry. For this purpose, we used EGFP–neo transduced MOPC315 cells that had been selected twice by cloning by limiting dilution. Subclone 7.1, expressing more than 95% of highly positive green fluorescent protein (GFP) cells (data not shown), was used for tumor injections. APCs-MB from MOPC315-7.1 tumors contained less than 1% of GFP-positive cells (data not shown).
APCs-MB derived from the tumor resemble DCs by cell-surface markers
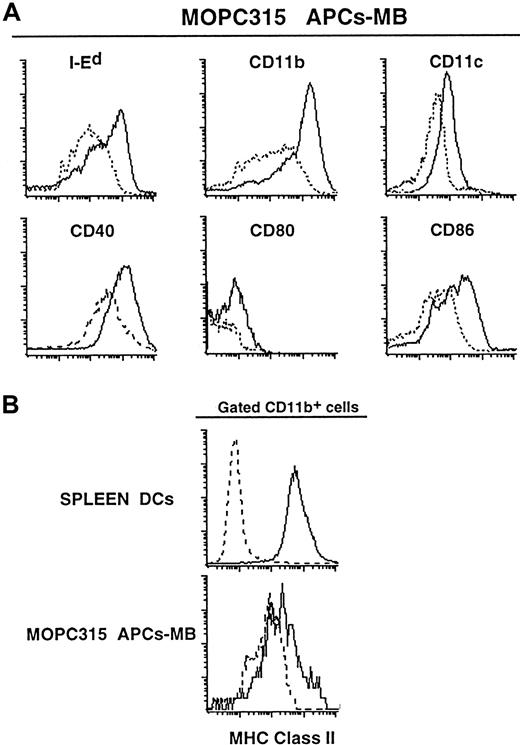
APCs-MB were heterogeneous with respect to expression of MHC class II and CD80/86 molecules as assessed by flow cytometry. However, a large majority of APCs-MB were I-Ed+, CD11b+, CD11c+, CD40+, CD80+, and CD86+ (Figure 2A,B). The APCs-MB were CD4−, CD8α−, CD95L−, and Ly-6G− (data not shown). Hence, tumor APCs-MB had a phenotype similar to that of APC I and APC II previously isolated from MOPC315 tumor by density gradient centrifugation and adherence (compare Figure 2A with stainings in Dembic et al7). Tumor CD11b+ APCs-MB appeared to have a lower expression of class II molecules than conventionally purified spleen DCs (Figure 2B) and spleen CD11b+ APCs-MB (data not shown). If MOPC315 tumor would produce vascular endothelial growth factor (VEGF) as it has been reported for many other cancers, this might have affected the level of class II expression perhaps by interfering with DC maturation.33-35 However, the large unspecific signal of tumor APCs-MB makes the comparison somewhat difficult (Figure 2B). The heterogeneity of class II expression is consistent with the APCs-MB being a mixture of various types of DCs and/or macrophages. The yield of APCs-MB was 0.5% to 2%, which is similar to the previously observed level of CD11b+, class II+ cells purified by adherence from MOPC315 tumors of intermediate and large sizes.7
Phenotypic profiles of APCs-MB, isolated within 2 hours from MOPC315 tumors (1-3 g) in BALB/c mice.
(A) APCs-MB (containing phagocytosed mouse IgG FITC-coated MBs giving a signal in the FL-1 channel) were double-stained with anti-CD11b–allophycocyanin and either biotinylated I-Ed-, CD11c-, or CD40-specific mAbs; or triple-stained with anti-CD11b–allophycocyanin, biotinylated anti-CD11c, and either PE-conjugated anti-CD11b, -CD80, or -CD86 mAbs. FITC+APCs-MB were gated, and stainings for indicated mAbs are shown as histograms (full lines) overlaid with corresponding isotype-matched control Abs (dashed lines). Events (1 × 105) were acquired for each sample. (B) Tumor APCs-MB and conventionally purified DCs (APC-I) from spleen60 were triple-stained with PE–anti-CD11b, anti-CD11c–allophycocyanin, and biotinylated anti–class II mAbs. Gated CD11b + cells were analyzed for the expression of class II molecules (full lines) overlaid with isotype-matched control Abs (dashed lines). Approximately 1 × 105 or 5 × 104 events were acquired for the spleen or tumor APCs, respectively.
Phenotypic profiles of APCs-MB, isolated within 2 hours from MOPC315 tumors (1-3 g) in BALB/c mice.
(A) APCs-MB (containing phagocytosed mouse IgG FITC-coated MBs giving a signal in the FL-1 channel) were double-stained with anti-CD11b–allophycocyanin and either biotinylated I-Ed-, CD11c-, or CD40-specific mAbs; or triple-stained with anti-CD11b–allophycocyanin, biotinylated anti-CD11c, and either PE-conjugated anti-CD11b, -CD80, or -CD86 mAbs. FITC+APCs-MB were gated, and stainings for indicated mAbs are shown as histograms (full lines) overlaid with corresponding isotype-matched control Abs (dashed lines). Events (1 × 105) were acquired for each sample. (B) Tumor APCs-MB and conventionally purified DCs (APC-I) from spleen60 were triple-stained with PE–anti-CD11b, anti-CD11c–allophycocyanin, and biotinylated anti–class II mAbs. Gated CD11b + cells were analyzed for the expression of class II molecules (full lines) overlaid with isotype-matched control Abs (dashed lines). Approximately 1 × 105 or 5 × 104 events were acquired for the spleen or tumor APCs, respectively.
APCs-MB from tumor have chemokine and chemokine receptor messenger RNA profile consistent with DCs
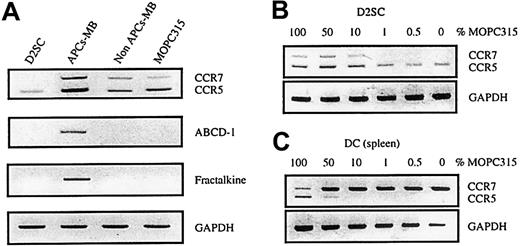
Tumor APCs-MB expressed chemokine messenger RNA (mRNA) transcripts of MIP1α and regulated on activation normal T cell expressed and secreted (data not shown). However, both of these chemokines have been described to be expressed in macrophages as well as on induction in mature DCs. We thus examined APCs-MB for the presence of DC markers like ABCD-1 and fractalkine (ABCD-3) transcripts.25,26,36-38 These chemokines are not expressed by mouse macrophages derived from lymphocyte-deficient Rag2−/− bone marrow.37 RT-PCRs for both chemokines gave positive results with tumor APCs-MB but not with any other sample (Figure 3A), indicating the presence of DCs in tumor APCs-MB. APC-MB was not contaminated with activated B cells, a possible source of ABCD-1,27 because no CD19+ cells were found by flow cytometry. Moreover, CD19 mRNA was undetectable in cultured MOPC315 cells and tumor APC-MB samples (data not shown). Therefore, tumor APCs-MB include cells (DCs-MB) that have a chemokine-transcript profile consistent with DCs. Importantly, ABCD-1 and fractalkine are up-regulated in mature mouse DCs.37 Thus their presence in tumor DCs-MB, but not in the immature DC line D2SC21 (Figure 3A), suggest that some tumor DCs-MB have a mature phenotype.
Chemokine and chemokine receptor expression in APCs-MB purified from MOPC315 tumors.
APCs-MB were purified from MOPC315 tumors (1-2 g) in BALB/c mice, the remaining cells (non–APC-MB) consisted mainly of tumor cells (A). In addition, D2SC, an immature spleen-derived DC line, and in vitro-cultured MOPC315 tumor cells, were analyzed (B,C). Expression of ABCD-1, fractalkine (ABCD-3), CCR5, and CCR7 was analyzed by RT-PCR. GAPDH served as a control for the cDNA input in RT-PCR.
Chemokine and chemokine receptor expression in APCs-MB purified from MOPC315 tumors.
APCs-MB were purified from MOPC315 tumors (1-2 g) in BALB/c mice, the remaining cells (non–APC-MB) consisted mainly of tumor cells (A). In addition, D2SC, an immature spleen-derived DC line, and in vitro-cultured MOPC315 tumor cells, were analyzed (B,C). Expression of ABCD-1, fractalkine (ABCD-3), CCR5, and CCR7 was analyzed by RT-PCR. GAPDH served as a control for the cDNA input in RT-PCR.
Immature DCs express CCR5,39 whereas mature DCs down-regulate CCR539 and up-regulate CCR7 mRNA.25,40 Therefore, we tested tumor DCs-MB for the expression of CCR5 and CCR7 mRNA by dual RT-PCR assay. Purified APCs-MB from MOPC315 tumors had both CCR5 and CCR7 transcripts (Figure 3A). However, because MOPC315 cells also expressed CCR5 and to a lesser extent CCR7 mRNAs, the CCR5 and CCR7 transcripts could originate from tumor cells that were either contaminating the APC-MB preparations or that had been phagocytosed by APCs-MB. These possibilities appear unlikely, because both the CCR5 and CCR7 signals were stronger in APC-MB than in the MOPC315 tumor sample itself, whereas the GAPDH signal was the same (Figure 3A). To investigate this issue in a more conclusive manner, we mixed cDNA from MOPC315 cells with cDNAs from CCR5+ (D2SC) or CCR7+ (spleen DCs, isolated by the transient adherence method from mice without tumors) cells. CCR5 (Figure 3C) and CCR7 (Figure 3B) signals were undetectable in mixes that contained less than 10% of cDNA from tumor cells. As contamination of APCs-MB with GFP-transduced MOPC315 cells was below 1%, it is improbable that CCR5 and CCR7 signals were generated by copurified (or engulfed) MOPC315 cells. Other sources of CCR5 and CCR7 signals could be CCR5+ Th1 cells41 and CCR7+-activated T and B cells (for a review see Jung and Littman42 and Baggiolini43). However, APCs-MB did not contain CD3ε or CD19 mRNA signals (data not shown), excluding copurified T and B cells as possible sources. Concerning the CCR5 signal, it could be caused by macrophages that are known to be CCR5+.44 Therefore, we tested the expression of the transcription factor TFEC, thought to be macrophage specific.45 Tumor APCs-MB gave positive RT-PCR signals, but the immature DC line D2SC/1 also tested positive (not shown). These results suggest that macrophages and/or immature DCs could be sources of CCR5 in APCs-MB. The CCR7 signal, however, strongly suggests that mature DCs were present in the APCs-MB, as they are the only remaining known source for the CCR7 transcripts.
APCs-MB from tumors stimulate T cells in vitro
APCs-MB isolated from Id+ MOPC315 tumors induced, in the absence of added λ2315 synthetic peptide, in vitro proliferation of Id-specific naive CD4+ cells, polarized Th1 and Th2 cells tested on day 10, and cloned Th1 7A10B2 cells (Figure4). Measurements of lymphokines (IL-2 for naive cells, IFNγ for Th1, IL-4 for Th2) in supernatant gave concordant results (data not shown). We used J558 APCs-MB as controls, because J558 is a BALB/c myeloma that produces IgAλ1, which is close to MOPC315 IgAλ2 isotype, but yet idiotypically dissimilar. Id specificity was evident because nontransgenic T cells did not respond, and because APCs-MB isolated from Id-negative J558 tumors were not stimulatory (Figure 4). The Id-specific responses were not due to contaminating MOPC315 cells because contamination was less than 1%, and by virtue of MOPC315 cells being class II negative and thus unable to stimulate directly class II–restricted Id-specific T cells.7 Consistent with previous findings with DCs purified on the basis of density and adherence,7 Id priming was much more pronounced for tumor APCs-MB than spleen APCs-MB isolated from the same mice, which were not stimulatory at all (Figure 4).
Tumor APCs-MB are TSA (Id) primed and stimulate TSA (Id)-specific T cells.
Large (1.5-3 g) Id+ tumors (MOPC315) and Id−tumors (J558) were established in BALB/c mice. APCs-MB were isolated from tumors or spleens and tested for their ability to induce proliferation of the indicated types of responder T cells. APC-MB (stimulators): ■, J558; ▪, MOPC315;  , spleen (MOPC315–tumor-bearing mice).
, spleen (MOPC315–tumor-bearing mice).
Tumor APCs-MB are TSA (Id) primed and stimulate TSA (Id)-specific T cells.
Large (1.5-3 g) Id+ tumors (MOPC315) and Id−tumors (J558) were established in BALB/c mice. APCs-MB were isolated from tumors or spleens and tested for their ability to induce proliferation of the indicated types of responder T cells. APC-MB (stimulators): ■, J558; ▪, MOPC315;  , spleen (MOPC315–tumor-bearing mice).
, spleen (MOPC315–tumor-bearing mice).
Ca++ mobilization in T cells occurs within minutes after the contact with APCs-MB
To avoid DC maturation in vitro, we wished to investigate the ability of freshly isolated APCs-MB to stimulate T cells, in a rapid assay for T-cell activation. To this end, we measured Ca++mobilization in T cells within minutes after exposure to APCs by digital imaging.30 The technique has the additional advantage that productive APC to T-cell interaction can be observed at a single cell level.30
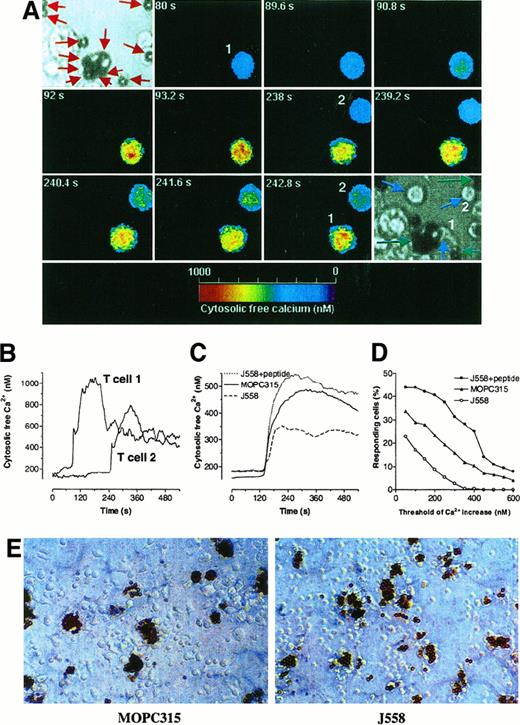
An example of 2 fura-2 loaded cloned Th1 cells mobilizing Ca++ in response to DC-MB from Id+ tumors is shown in Figure 5A, and traces are shown in Figure 5B. A stimulatory DC-MB is visible in the upper-left corner of Figure 5A; this cell is evidently phagocytic, as it has engulfed MBs (red arrows). The Ca++ responses in 7A10B2 Th1 cells were Id specific, because they were larger on interaction with APC-MB from Id+ tumors than with APC-MB from Id− tumors (Figure 5C,D). The high background responses with APCs-MB from J558 tumors may be due to a large number of class II, costimulatory, and adhesion molecules on such cells, which all together can induce a weak Ca++ signal in unspecific T cells, as described by others.46 APCs-MB from J558 tumors were potent APCs because, when they were deliberately pulsed with synthetic Id peptide, the 7A10B2 cells responded with a strong Ca++ signal (Figure 5C,D). The slopes of response rate curves for MOPC315 and J558, in the absence of added Id peptide (Figure 5D), have an average 15% overall difference. The actual response rates could be higher than that, because the layers of APCs were not confluent, and some observed T cells might actually never have met an APC. This suggests that MOPC315 tumor APCs-MB contain at least 15% cells that are spontaneously primed with tumor-specific Id peptide. T cells became blasts within 2 days after the incubation with APCs-MB from Id+ MOPC315, but not with APCs-MB that were isolated from Id− tumor (J558) (Figure 5E). The blasts were clustered close to APCs-MB (marked by brown beads) from MOPC315 tumors (Figure5E).
Functional analyses of tumor DC-T cell interactions.
DCs-MB were purified from large MOPC315 or J558 tumors established in BALB/c mice. (A) Single-cell analysis of Ca++ mobilization in T cells that interact with DC-MB. Two fura-2 loaded T cells that interact with DCs-MB from MOPC315 are shown. Image 1: Light microscopic image of DCs before adding T cells. MBs are indicated by red arrows. Image 2-11: Pseudo-colored images of the cytosolic-free Ca++ concentration in T cells taken at different time points after addition of T cells to the wells. Image 12: Light microscopic image taken after the Ca++ registration, demonstrating the interaction between the 2 T cells (numbered and indicated by blue arrows) and DCs-MB (indicated by green arrows). T cell No. 1 evidently contacts 2 DCs-MB. An additional T cell (not numbered) came into the field after 242 seconds, has no contact with DC-MB, and is indicated by a blue arrow. Note that the T cells are smaller in the light microscopic images than in the pseudo-color images due to optical artifacts. (B) Ca++ traces of 2 different single T cells depicted in A. (C) Average Ca++ responses in cloned Id-specific Th1 7A10B2 cells stimulated by DC-MB from Id+ and Id− tumors in the presence or absence of synthetic Id peptide. Only cells with a response above 100 nM (ie, the threshold) are included. The responses were synchronized with a start of the signal set at 120 seconds. Number of analyzed-responding T cells: MOPC315 DC-MB, n = 81; J558 DC-MB, n = 39; and J558 DC-MB pulsed with λ2315 peptide, n = 28. (D) Frequency of responding T cells as a function of the magnitude of Ca++mobilization. Number of T cells analyzed: MOPC315 DC-MB, n = 280; J558 DC-MB, n = 229; and J558 DC-MB pulsed with λ2315peptide, n = 64. (E) Light microscopic images of cloned Id-specific T-cells 7A10B2, Th1 cultured for 2 days with DCs-MB from MOPC315 or J558 tumors.
Functional analyses of tumor DC-T cell interactions.
DCs-MB were purified from large MOPC315 or J558 tumors established in BALB/c mice. (A) Single-cell analysis of Ca++ mobilization in T cells that interact with DC-MB. Two fura-2 loaded T cells that interact with DCs-MB from MOPC315 are shown. Image 1: Light microscopic image of DCs before adding T cells. MBs are indicated by red arrows. Image 2-11: Pseudo-colored images of the cytosolic-free Ca++ concentration in T cells taken at different time points after addition of T cells to the wells. Image 12: Light microscopic image taken after the Ca++ registration, demonstrating the interaction between the 2 T cells (numbered and indicated by blue arrows) and DCs-MB (indicated by green arrows). T cell No. 1 evidently contacts 2 DCs-MB. An additional T cell (not numbered) came into the field after 242 seconds, has no contact with DC-MB, and is indicated by a blue arrow. Note that the T cells are smaller in the light microscopic images than in the pseudo-color images due to optical artifacts. (B) Ca++ traces of 2 different single T cells depicted in A. (C) Average Ca++ responses in cloned Id-specific Th1 7A10B2 cells stimulated by DC-MB from Id+ and Id− tumors in the presence or absence of synthetic Id peptide. Only cells with a response above 100 nM (ie, the threshold) are included. The responses were synchronized with a start of the signal set at 120 seconds. Number of analyzed-responding T cells: MOPC315 DC-MB, n = 81; J558 DC-MB, n = 39; and J558 DC-MB pulsed with λ2315 peptide, n = 28. (D) Frequency of responding T cells as a function of the magnitude of Ca++mobilization. Number of T cells analyzed: MOPC315 DC-MB, n = 280; J558 DC-MB, n = 229; and J558 DC-MB pulsed with λ2315peptide, n = 64. (E) Light microscopic images of cloned Id-specific T-cells 7A10B2, Th1 cultured for 2 days with DCs-MB from MOPC315 or J558 tumors.
Collectively, the findings show that phagocytic APCs, freshly isolated from Id+ tumors, can induce Ca++ responses in cloned Id-specific 7A10B2 T cells, visualized at the single cell level, and that this response can lead to blastogenesis of T cells.
Discussion
Indirect functional evidence for transfer of TSA to professional APCs3,4,47 needs to be supplanted by direct demonstration and characterization of such cells in the tumor. Recently, TSA-primed tumor APCs of a macrophage type5 or shared macrophage and DC type6 have been reported. In these cases, IL-35 and CD40L/GM-CSF6 transfection of tumor cells were employed to detect the TSA-primed APC. Perhaps these types of APCs were artificially induced by such manipulations of the tumor cells.5,6 In the present model, we used unmanipulated myeloma cells injected subcutaneously, but we could, nevertheless, detect tumor DCs spontaneously primed with TSA.7 We have now further characterized these TSA-primed DCs obtained by a novel, fast isolation procedure, allowing lineage determination and testing of their ability to stimulate T cells at the single cell level.
The TSA (Id)-primed APCs, isolated within 2 hours by their ability to phagocytose IgG2a-coated MBs, were heterogeneous, with a large fraction that was class II+, CD11b+, CD11c+, CD40+, CD80+, and CD86+. This phenotype is compatible with that of myeloid-derived DCs. The phagocytic APCs-MB expressed mRNAs for ABCD-1 and fractalkine, which are specifically up-regulated with maturation of mouse DCs.25,26,36,37 In addition, tumor APCs-MB have increased expression of transcripts for CCR7, another marker of mature DCs. Therefore, at least a fraction of isolated phagocytic APCs-MB have mature DC markers. Phagocytosis has been ascribed to immature DCs,12 and this capability is down-regulated on maturation (for a review, see Banchereau and Steinman10). Therefore, our results raise the question of whether mature DC-like cells in tumors are unusual in that they have retained the capacity to phagocytose. Alternatively, the DCs could represent a transitional stage in which cells still have the capacity to phagocytose but have up-regulated ABCD-1, fractalkine, and CCR7 expression. Also possible, maturation of phagocytic DCs might have been triggered by mere extraction of DCs out of their environment and subsequent manipulation,48,49 like adherence to plastic and/or digestion of surface molecules by collagenase during the 2-hour isolation procedure. Furthermore, ex vivo maturation could have also been induced by the binding and phagocytosis of IgG-coated magnetic beads, because Fc-receptor–mediated signals have been described to do so.50 However, the latter is unlikely to have a major impact, because Fc-receptor signals required 24 hours to induce maturation of DCs.50 Apart from mature CCR7+, ABCD-1+, and fractalkine+ DCs, isolated tumor APCs-MB probably include CCR5+ immature DCs and/or macrophages.
It has recently been described that intracutaneously injected latex microbeads are phagocytosed in situ by dendritic cells. On migration to draining LNs, such DCs were capable of stimulating alloreactive T cells in vitro.12 The IgG2a MB phagocytic DCs in subcutaneous tumors may correspond to the phagocytic DCs found in normal skin.12 In addition, we show that the phagocytic DCs are spontaneously primed with tumor-specific (Id) antigen and are able to stimulate Id-specific T cells.
How do the DCs become primed with Id? First, because they are phagocytic, they could engulf and process apoptotic tumor cells through a pathway that has been described for cross-priming.51-53Because MOPC315 plasmacytoma cells have large amounts of myeloma protein in their secretory organelles, this could be an important mechanism for class II–restricted Id presentation on tumor-derived DCs. Second, the tumor DCs could endocytose, possibly via Fc receptors, myeloma protein in the extracellular fluid. In this respect, the local concentration of M315 in tumor tissue could be extremely high, facilitating Id priming of tumor DCs. Furthermore, tumor DCs could have particularly high numbers of Fc receptors of particular specificities, eg, for IgA (M315). It should be emphasized that myeloma protein, like M315, is produced and secreted at high levels compared to other TSA. Therefore, it will be important to investigate whether tumor DCs also become primed with other types of TSAs, which are produced at lower levels or remain cell bound. The occurrence of DCs-MB spontaneously primed with Id can be confirmed in a different tumor model: A20 lymphoma cells transfected with Id-containing vector (K. Lundin and B.B., unpublished results, October 2000).
An important question is whether DCs in tumor have reached a maturation stage in which they are functionally competent to stimulate T cells. The known stimuli for DC maturation include viral and microbial constituents, inflammatory cytokines, and CD40 ligand.11Evidently, ex vivo tumor DCs-MB were able to induce proliferation of and IL-2 production in Id-specific CD4+ T cells of a naive phenotype in vitro. Because proliferation and lymphokine assays take several days to complete, DCs could have matured and become functionally competent during the assays. Therefore, we here employed a rapid Ca++ mobilization assay for T-cell activation. The results show that, within a total of 4 hours from disruption of the tumor, APCs-MB were able to stimulate cloned Id-specific T cells. Whether APCs-MB can also stimulate naive Id-specific CD4+ T cells in this assay is difficult to determine, because naive cells are far less efficient at mobilizing Ca++ (data not shown).
In proliferation and lymphokine assays, in which thousands of APCs and T cells interact in a well, it is difficult to accurately define the frequency and the phenotype of stimulatory APCs. On the basis of the single T-cell Ca++ mobilization assay, the lower estimate for frequency of APCs-MB with the ability to stimulate T cells is 15%. Because the digital imaging Ca++ mobilization assay allows visualization of single APC-MB and T cells, it might in the future be possible to exactly pinpoint the phenotype of stimulatory Id-primed APCs by staining the interacting cells with mAbs, immediately after conclusion of the Ca++ assay. The data presented and discussed elsewhere in this study would suggest that the APCs-MB that induced Ca++ mobilization belong to the DC lineage.
Id-specific CD4+ cells protect transgenic mice against a challenge with class II–negative MOPC315 cells,8,9 but the mechanism remains to be fully elucidated. How do tumor DCs and Id-specific CD4+ T cells contribute to the protection? It has been suggested that immature DCs in tissues down-regulate CCR5 and up-regulate CCR7 during maturation.54 CCR7 binds secondary lymphoid tissue chemokine (CCL21) that is expressed on endothelial cells of lymphatic vessels and forms a gradient in tissues surrounding them. According to this scheme, Id-primed CCR7+tumor DCs could migrate via lymphatics to draining LNs and induce activation and proliferation of naive Id-specific CD4+cells (Z.D. and B.B., unpublished data, October 2000). The activated Id-specific CD4+ T cells could differentiate into effectors (or memory cells), extravasate in the tumor, and become reactivated by Id-primed DCs residing in the tumor. This sequence of events is consistent with the presence of activated, proliferating T cells juxtaposed to class II+ CD11b+ subendothelial cells (corresponding to the current DC-MB?) in sections of MOPC315 tumors in TCR-transgenic mice.7 Reactivated CD4+ T cells in the tumor could then kill cancer cells by a bystander mechanism, eg, via CD95L or IFNγ expression55(B. Bogen, unpublished results, October 2000). This might represent a mechanism that can diminish tumor take and in vivo progression of the tumor (K. Lundin and B.B., unpublished data, October 2000).
Protection of TCR-transgenic mice can be overcome by injection of high loads of MOPC315 tumor cells, as done in the present experiments. In such tumor-bearing TCR-transgenic mice, Id-primed DCs prevail, and peripheral T-cell tolerance develops by deletion.56 Could the Id-primed tumor DCs have Janus-like features, initially eliciting tumor-toxic CD4+ responses, and later inducing T-cell tolerance? Conceivably, Id-primed DCs might directly tolerize Id-specific T cells in several ways, for example, by excessive and prolonged stimulation, leading to T-cell deletion by clonal exhaustion. Alternatively, tumor DCs could contribute to tolerance by migration to LNs and transfer of Id (TSA) to tolerogenic resident DCs, as previously suggested for self antigen-primed DCs.11 57-59
We thank Peter Hofgaard, Hilde Omholt, and Olav Schreurs for technical assistance.
Supported by grants from the Norwegian Research Council, Medinnova (The National Hospital, Oslo), and the Norwegian Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Zlatko Dembic, Institute of Immunology, The National Hospital, University of Oslo, 0027 Oslo, Norway; e-mail:zlatko.dembic@labmed.uio.no.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal