We read with interest the article by Kunzmann et al, which states cytoreductive effects of aminobisphosphonates mediated by gamma delta T cells (γδ cells).1 All tested aminobisphosphonates induced significant expansion of γδ cells. This proliferative response was interleukin-2 (IL-2) dependent, whereas activation of γδ T cells (up-regulation of CD25 and CD69) occured in the absence of exogenous cytokines. Pamidronate-treated bone marrow (BM) cultures of patients with multiple myeloma showed significant reduced plasma cell survival compared with untreated cultures. When γδ T cells were depleted in BM before treatment, cytoreductive effects were completely abrogated. These results are supported by findings that we gained from pure γδ T cell cultures.2 We found that γδ T cells isolated from peripheral blood can kill human neuroblastoma cells efficiently (100% specific lysis at an effector-to-target cell [E/T] ratio of 20:1). The cytotoxic effects of γδ T cells were not restricted to neuroblastoma cells. γδ cells were also cytotoxic against human Burkitt lymphomas Daudi (32.7% specific lysis at an E/T ratio of 20:1; identical to the data from Kunzman et al1) and Raji (10.3%), human colon carcinoma cell line Colo 205 (23.1%), erytholeukemia cell line K562 (100%), and human neuroepithelioma cell line SK-N-MC (100%).2 These data raise the possibility of using this in a clinical setting. We enriched TCR γδ–expressing T cells out of peripheral blood mononuclear cells (PBMNCs) by positive selection. γδ T cells were magnetically and fluorescence labeled using a hapten-modified anti–TCR γδ antibody and fluorescein isothiocyanate–conjugated antihapten microbeads (both from Miltenyi Biotech, Bergisch Gladbach, Germany). Subsequent positive selection was carried out with LS+/VS+ columns according to the protocol supplied by Miltenyi Biotech. The efficiency of positive selection was evaluated by flow cytometric analysis. Fifty milliliters of peripheral blood from a healthy adult donor normally yielded approximately 2 to 14 × 106 γδ T cells. The γδ cells were then tested in stimulation assays to determine optimum propagation and optimum proliferation for ex vivo generation in order to get reasonable amounts for clinical applications. The proliferation-inducing capacities of clodronate (Disodium(dichloromethylene)-diphosphonatetetrahydrate, a bisphosphonate like Isopentenylpyrophosphate, but nontoxic), IL-2, PHA, IL-15, OKT3 (antiCD3; bound), or combinations of these were tested in pure γδ T cell cultures. Our choice to use clodronate as a proliferation-inducing substance was also based on the observation made by Berenson et al,3 who had reported that survival rates were significantly raised in a group of patients who had recieved bisphosphonate pamidronate as infusions additionally to salvage chemotherapy. We were interested in whether these findings were in context with γδ T cell cytotoxicity and based on a possible selective (specific) induction of γδ T cell proliferation.
We chose clodronate for our study instead of pamidronate since it is a classic bisphosphonate, and substantial differences in the hematologic response to initial treatments with aminobisphosphonates have been reported.4-6 The optimized concentration of clodronate for in vitro assays was predetermined using different concentrations (75, 60, 45, 30, and 15 μg/mL) in proliferation assays. Best results were obtained with a single dose of 37.5 μg of clodronate per milliliter of growth medium. Higher concentrations of clodronate resulted in a strong inhibitory effect on cell proliferation.2 (Optimized doses are obtainable in vivo: recommended medication in vivo is 300 mg clodronate intravenously within 2 hours. This equals an initial concentration of 75 μg clodronate per milliliter of blood in a patient with 4 liters of blood.)
6-3[H]-thymidine incorporation was measured on day 14 of the culture period. Clodronate, PHA, or OKT3 alone showed effects that were comparable to untreated control cells (proliferation 1.7, 1.6, and 1 times control, respectively). IL-15 and IL-2 increased proliferation 7 and 20 times, respectively. Maximum proliferation was induced by the combination of IL-2/clodronate or IL-2/OKT3 (increase of proliferation by a factor of 35.8 and 57.8 times that of control, respectively). Both combinations clearly exhibited more than just an additive effect. When IL-2–treated γδ T cells were set as control, increases in proliferation by additional treatment with clodronate, PHA, and OKT3 were 74.4%, 38.5%, and 182%, respectively. A comparison between proliferative effects on γδ T cells of the most effective dual combination (OKT3 and IL-2) with triple combination (OKT3, IL-2, and clodronate) after 14 days in culture revealed a 15% enhancement of proliferation with the latter (in 3 individual assays) (Figure1). This might be due to an additional, different stimulation mechanism induced by clodronate. Isopentenylpyrophosphate or BCG (BCG Vaccine Behring; Chiron-Behring, Marburg, Germany) failed to increase proliferation of OKT3 and IL-2–treated cells.
Proliferation of γδ T lymphocytes.
Fifty thousand γδ T cells were seeded per well in 96-well microtiter plates. Growth medium was supplemented with indicated drugs and proliferation measured by 6-3[H]-thymidine incorporation assay. Replicates were set up 10-fold. Columns represent the number of counts per minute (mean ± SD) of 6-3[H]-thymidine incorporation.
Proliferation of γδ T lymphocytes.
Fifty thousand γδ T cells were seeded per well in 96-well microtiter plates. Growth medium was supplemented with indicated drugs and proliferation measured by 6-3[H]-thymidine incorporation assay. Replicates were set up 10-fold. Columns represent the number of counts per minute (mean ± SD) of 6-3[H]-thymidine incorporation.
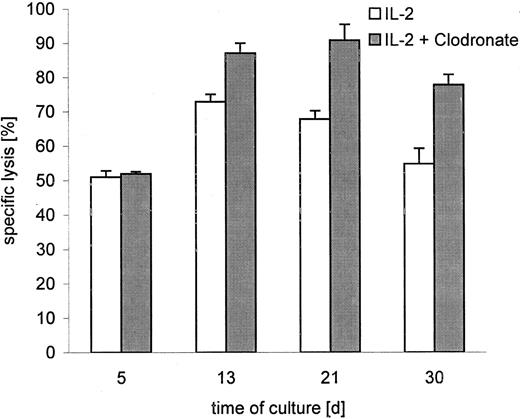
Since we had tested clodronate for induction of proliferation, it was an interesting proposition to determine whether clodronate modifies the cytotoxic activities of γδ T cells, in addition to its proliferative properties. γδ T cells were grown with and without clodronate in the presence of IL-2. Cell cytotoxicity was determined at E/T ratios of 20:1 to 1.2:1 in BATDA release (a nonradioactive cell cytotoxicity–determining assay with a high sensitivity comparable to chromium release).7 No difference in cytolytic activity compared with an IL-2-only–treated control group was seen after 5 days, but clear effects were detectable on days 13, 21, and 30 (Figure 2). Clodronate-treated γδ T cells clearly exhibited higher cytotoxic activities toward neuroblastoma cells than untreated control cells (at an E/T ratio of 20:1, killing was 31.3% ± 9.9% SD). This was also observed at the lowest tested E/T ratio of 1.2:1, where cytotoxicity was even 48.6% ± 17% SD higher than cytotoxicity of the control group. Our data agree with Kunzmann et al1 that proliferation response of γδ T cells to (amino)bisphosphonates are IL-2–dependent. Kunzmann et al1 found only a slight effect on γδ T cell proliferation by bisphosphonate clodronate, compared with the dramatic increase of γδ cells in PBMNC cultures induced by aminobisphosphonates. This seems reasonable since PBMNC cultures offer the possibility of cross-reactions with cytokine producing/releasing immune competent cells and/or interactions with a variety of other effector cells and γδ T cells. Thus, effects mediated by aminobisphosphonates might be primary or the end of a cascade of reactions in PBMNC cultures. Secondary side effects cannot be excluded. But nevertheless, in a γδ T cell–restricted system, bisphosphonates induce measurable effects on proliferation and cytotoxicity of γδ T cells. These data and the capacity of efficient killing of human malignant cells by γδ T cells suggest an important role for them in immunological strategies of cancer therapy, where aminobisphosphonates and bisphosphonate clodronate mediate γδ T–cell proliferation and activation.
Cytotoxicity of γδ T cells against neuroblastoma cells.
γδ T lymphocytes were cultured for a period of 30 days with IL-2 or with IL-2 and clodronate. Columns represent the percentage of specific lysis in a cytotoxicity-determining assay (BATDA release)4compared with control (maximum release) at different time points. Results are shown for E/T ratio 20:1.
Cytotoxicity of γδ T cells against neuroblastoma cells.
γδ T lymphocytes were cultured for a period of 30 days with IL-2 or with IL-2 and clodronate. Columns represent the percentage of specific lysis in a cytotoxicity-determining assay (BATDA release)4compared with control (maximum release) at different time points. Results are shown for E/T ratio 20:1.

![Fig. 1. Proliferation of γδ T lymphocytes. / Fifty thousand γδ T cells were seeded per well in 96-well microtiter plates. Growth medium was supplemented with indicated drugs and proliferation measured by 6-3[H]-thymidine incorporation assay. Replicates were set up 10-fold. Columns represent the number of counts per minute (mean ± SD) of 6-3[H]-thymidine incorporation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2917/6/m_h80911086001.jpeg?Expires=1769091716&Signature=eojTZPDaEKCbTWYFnxmA0ds1GdhbzSu1~6S0F8~iBsauUd8i-uXclZ-pgc-yifWujnNPANi2MZKWgljSVrqfnrAdNMhz0a43ox4fWTN7WOcKCuV1o6K1Y5f5NjP5h7C4QLGIpqpfs9o0nyJPry06w2pNwkmZ28CCYfAFENdJZJsdOtvKn3yxD3fvUs97yXL6BNv4X~dSrhQ5sOg9BvezKEW-JRBlY8bdpBYzMqvSJbLHB-m5FK53wIYbwKwBZ0-KA4pM1xt5m4tJ913w3Of~FLYImwnQSmMnLmm5R1Ehk-ERqIpbIC-so25aAlpOkpXBG8bKhk1h8C3EG699~HTYvg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal