Abstract
Adhesion of polymorphonuclear leukocytes (PMNLs) to activated platelets requires a P-selectin–triggered, tyrosine kinase–dependent adhesiveness of Mac-1 and is accompanied by tyrosine phosphorylation of a 110-kd protein (P-110) in PMNLs. Inhibitors of SRC tyrosine kinases were found to inhibit PMNL adhesion to activated platelets or to P-selectin expressing Chinese hamster ovary (CHO-P) cells and the tyrosine phosphorylation of P-110. Adhesion of PMNLs to activated platelets or to CHO-P cells stimulated activity of LYN and HCK. Monoclonal antibody blockade of P-selectin or β2-integrins reduced the activation of both kinases. In PMNLs either adherent to platelets or aggregated by P-selectin–IgG chimera, Mac-1 was rapidly redistributed to the Triton X-100–insoluble cytoskeletal fraction, and large clusters of Mac-1 colocalized with patches of F-actin at the sites of cell-cell contact. In PMNLs stimulated by P-selectin–IgG chimera, SRC kinase inhibition impaired Mac-1 clustering, F-actin accumulation, and CD18 redistribution to the cytoskeleton. Disruption of the actin filament network by cytochalasin D prevented PMNL-platelet adhesion and P-selectin–induced PMNL aggregation and impaired the clustering of Mac-1. In agreement with the requirement for the β2-integrin in the functional up-regulation of LYN and HCK, integrin blockade by monoclonal antibodies resulted in a complete inhibition of P-selectin–induced Mac-1 clustering and F-actin accumulation. Taken together, the results indicate that, after an initial P-selectin–triggered β2-integrin interaction with the ligand, SRC kinases are activated and allow the remodeling of cytoskeleton-integrin linkages and integrin clustering that finally strengthen cell-cell adhesion. This model highlights a new role for SRC kinases in a regulatory loop by which the Mac-1 promotes its own adhesive function.
Introduction
Polymorphonuclear leukocyte (PMNL)-platelet interactions occur at sites of vascular injury, and inflammatory and thrombotic states are associated with circulating PMNL-platelet aggregates. Recent studies in patients and in experimental animal models suggest that PMNL-platelet aggregates may play a role in the vascular response to injury that occurs after erosion or rupture of an atherosclerotic plaque or during coronary angioplasty or stent placement.1 The interaction of PMNLs with activated platelets is coordinated by an adhesion cascade in which P-selectin binds to P-selectin glycoprotein ligand-1 (PSGL-1)2,4 on leukocytes to promote the initial tethering of the cells. Subsequent firm adhesion is mediated by integrin αMβ2 (CD11b/CD18 or Mac-1)5-8 binding to either platelet GPIb9 or to fibrinogen bound to platelet GPIIb/IIIa or αVβ3.10
The ability of the β2-integrin to bind the ligands, allowing the shear-resistant cell-cell adhesion, is functionally regulated by the cell through an active process involving intracellular signaling.11 It is generally accepted that these processes are triggered by G-protein–coupled receptors for chemokines or by lipid chemoattractant coexpressed with the selectins on the cell surface.12 For example, PMNL-platelet interactions are strengthened by platelet-associated PAF and CXC chemokines.10 Moreover P-selectin binding to its receptor(s) on PMNLs may promote β2-integrin adhesiveness in human2,13 and in mouse14 cells. We recently reported that in cell suspensions subjected to high-speed rotatory motion, P-selectin expressed either on activated platelets or on P-selectin–transfected Chinese hamster ovary (CHO-P) cells was able to promote a Mac-1–dependent PMNL-platelet adhesion or PMNL–CHO-P cell adhesion; similarly, soluble recombinant P-selectin–IgG chimera induced Mac-1–mediated PMNL homologous aggregation.2 The formation of β2-integrin–mediated PMNL aggregates required tyrosine kinase activity and was accompanied by tyrosine phosphorylation of a major protein of approximately 110 kd (P-110). Blockade of Mac-1 by specific monoclonal antibodies (mAbs) against the α or β chain of Mac-1 abolished tyrosine phosphorylation of P-110 in PMNLs interacting with either activated platelets or CHO-P cells and in PMNLs challenged with soluble P-selectin–IgG chimera, suggesting that the engagement of Mac-1 with its ligand on the neighboring cell was necessary for tyrosine kinase(s) activation. These observations raised the question of the nature of protein tyrosine kinases and of the possible mechanism(s) by which they regulate Mac-1 adhesiveness triggered by P-selectin.
Tyrosine kinases belonging to the SRC family have been implicated in the outside-in signaling that follows β2-integrin ligand binding and leads to a number of functional responses, such as degranulation and respiratory burst in leukocytes.15 Moreover, in different cell types, SRC and other members of this family play a pivotal role in actin cytoskeleton reorganization and in integrin-cytoskeleton interactions that are necessary for rapid cell spreading on extracellular matrix.16-18 Previous studies indicated that the interaction of the cytoplasmic tail of the β2 chain with cytoskeletal components and cytoskeletal reorganization represent important postligand binding events that regulate the adhesive function of the β2-integrin CD11a/CD18.19,20 β2-integrins are constitutively linked to the cytoskeletal protein talin21; as a consequence of cell activation, calpain-dependent proteolysis of talin causes a transient dissociation of the β2 tail from the cytoskeleton22 and facilitates integrin diffusion into clusters. Re-attachment of the β2 tail to another cytoskeletal protein, α-actinin, finally stabilizes the integrin-cytoskeleton interaction and promotes firm adhesion.21
In the present study, we demonstrate a crucial role of the SRC family, most probably LYN and HCK, in the regulation of Mac-1 adhesiveness triggered by P-selectin. Moreover, our results support the concept that the SRC kinase-dependent signaling initiates from the integrin itself and regulates Mac-1–cytoskeleton interaction and Mac-1 clustering at the site of cell-cell contact. This study highlights a new role of SRC kinases in a regulatory loop by which Mac-1 promotes its own function.
Materials and methods
Chemicals
Hydroethidine (HE) and rhodamine phalloidin were from Molecular Probes (Eugene, OR). 2′,7′-Bis-(2-carboxyethyl)-5(6)carboxy–fluorescein triacetoxy methyl ester (BCECF-am), n-formyl-methyl-leucyl-phenylalanine (fMLP), prostaglandin E1 (PGE1), HEPES, EGTA, thrombin from human plasma (2000 U/mg NIH protein), and cytochalasin D were purchased from Sigma Chemical (St Louis, MO). Kinase inhibitors 3,4,3′,5′-tetrahydroxy-trans-stilbene (piceatannol), 4-amino-5-(-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP1), and tyrphostin AG 490 were purchased from Alexis (San Diego, CA). The MEK inhibitor PD98059, SRC tyrosine kinase inhibitor 4-amino-5-(clorophenyl)-7-(t-butyl)pyrazolo[3,4-d] pyrimidine (PP2) and its negative control 4-amino-7-phenilpyrazol[3,4-d] pyrimidine (PP3) were from Calbiochem (La Jolla, CA). Paraformaldehyde (PFA) was from Fluka (Milan, Italy). Immobilized Protein A on Trysacryl was from Pierce (Rockford, IL). [γ-32P]ATP and enhanced chemiluminescence Western blotting system (ECL kit) and the ECL protein biotinylation module were from Amersham Pharmacia Biotech (Little Chalfont, Buckinghamshire, United Kingdom). fMLP was dissolved in dimethyl sulfoxide (DMSO) at concentrations of 100 μM, stored at −20°C, and diluted in isotonic saline just before use. Thrombin was dissolved in saline at concentrations of 50 U/mL and was stored at −20°C until use. BCECF-am and HE were dissolved in DMSO at concentrations of 1 mg/mL and 8 mg/mL, respectively, stored at −20°C, and used within 4 weeks. Reagents for electrophoresis and Western blot analysis were pure grade.
Antibodies
The anti-CD18 IB423 and the anti–P-selectin WAPS 12.224 mAbs (American Tissue Type Culture Collection, Manassas, VA) were purified from mouse ascites or hybridoma cell supernatant using Protein G–Sepharose affinity column. The following mAbs were kindly provided: the anti-CD18 KIM 12725 by Dr M. K. Robinson (Exploratory Research CellTech Therapeutics Limited, Slough, England), the anti-CD11b OKM10 by Ortho Diagnostic System (Raritan, NJ), and the anti–PSGL-1 PL226 by Dr R. P. McEver and Dr K. L. Moore (WK Warren Medical Research Institute, University of Oklahoma, Oklahoma City). The rabbit anti-FGR antibody27 was kindly provided by Dr G. Berton (Institute of General Pathology, University of Verona, Italy). Rabbit anti-LYN and HCK antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-LYN, anti-FGR, and anti-HCK antibodies were biotinylated using the ECL-protein biotinylation module. The recombinant horseradish peroxidase–conjugated antiphosphotyrosine mAb RC20 was from Transduction Laboratories (Exeter, United Kingdom). Alexa Fluor 488 goat anti–mouse IgG conjugate was from Molecular Probes. Goat anti–mouse IgG peroxidase conjugate was from Calbiochem. Fluorescein isothiocyanate (FITC) conjugate antihuman CD11b mAb (clone 44) was from Sigma. Human P-selectin–IgG fusion proteins were kindly provided by Genetics Institute (Cambridge, MA).28
Preparation of polymorphonuclear leukocytes, platelets, and culture of CHO-P cells
Blood was collected from healthy volunteers who had not received any medication for at least 2 weeks. PMNLs and platelets were isolated as previously reported.8 After they were washed in HEPES-Tyrode buffer containing 2 μM PGE1 and 5 mM EGTA, platelets were stimulated with 0.5 U/mL thrombin for 2 minutes at room temperature and fixed with 1% PFA at room temperature for 1 hour. CHO cells stably transfected with the cDNA encoding for human P-selectin were kindly provided by Genetics Institute (Cambridge, MA) and cultured as previously reported.29 Immediately before the experiments, CHO-P cells were detached by incubating the monolayer with 5 mM EGTA and 5 mM EDTA for 10 minutes, washed twice in HEPES-Tyrode, and resuspended in the same buffer at a concentration of 107/mL.
Experimental conditions
All the experiments were performed under the following standard conditions: cells were incubated in a final volume of 500 μL in siliconized glass tubes (internal diameter, 6 mm; ChronoLog; Mascia Brunelli, Milan, Italy), and the tubes were placed in an aggregometer (Platelet Ionized Calcium Aggregometer; Mascia Brunelli) at 37°C with stirring (1000 rpm) obtained by an iron bar (4 mm long) rotated under a magnetic field. Although the shear rate produced by this stirring speed cannot be precisely quantified, it should approximate 250 seconds−1.30 PMNLs were incubated in standard conditions alone or with platelets (1:5 or 1:10 ratio) or CHO-P cells (5:1 ratio) or 10 μg/mL P-selectin–IgG chimera. Given that P-selectin–IgG chimera contains the Fc portion of the human IgG, PMNLs were preincubated for 10 minutes with 50 μg/mL nonimmune human IgG, before stimulation with P-selectin–IgG chimera, to exclude a possible effect of this portion mediated by the Fc receptor on PMNLs. For PSGL-1 cross-linking experiments, PMNLs were preincubated at 4°C for 15 minutes with 20 μg/mL anti–PSGL-1 antibody PL2, rapidly centrifuged, and incubated in standard conditions with 10 μg/mL rabbit anti–mouse IgG F(ab′)2 fragment.
For blocking studies, antibodies were preincubated at saturating concentration (20 μg/mL) with the desired cell fraction for 15 minutes at 4°C. PMNLs were preincubated with tyrosine kinase inhibitors for 1 minute at room temperature or with cytochalasin D for 2 minutes at room temperature.
For adhesion experiments, PMNLs were stained with the vital red fluorescent dye HE (20 μg/mL per 5 × 107 PMNL/mL) for 30 minutes at 4°C. Platelets and CHO-P cells were loaded with the green fluorescent dye BCECF by incubating PRP or CHO-P cell suspensions with 2 μg/mL acetoxymethyl ester (BCECF-am) for 30 minutes at 37°C. The formation of PMNLs platelets and PMNLs–CHO-P cells mixed conjugates was evaluated by double-color flow cytometry, as previously described.8 Percentages of PMNLs binding platelets (PMNL (+) %) and of CHO-P cells binding PMNLs (CHO-P (+) %) were reported.
In vitro kinase assay
PMNLs were pretreated with 2 mM di-isopropyl fluorophosphate for 30 minutes at 4°C and washed with HEPES-Tyrode buffer before incubation with platelets (1:5 ratio) or CHO-P cells (5:1 ratio) in standard conditions. Samples were immediately centrifuged at 200g for 10 minutes at 4°C. Cell lysis was performed by adding to the cell pellet RIPA (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 1% sodium deoxycholate) or Triton buffer (1% Triton X-100, 25 mM Tris-HCl, pH 7.4, 37.5 mM NaCl) containing protease and phosphatase inhibitors (1 mM EDTA, 5 μg/mL aprotinin, leupeptin and pepstatin A, 1 mM phenylmethylsulfonyl fluoride, 10 μM PAO, 200 μM sodium orthovanadate, and 1 mM dithiothreitol). After 1 hour at 4°C, lysates were clarified by centrifugation, adjusted to the same protein concentration (300 μg for FGR and LYN and 600 μg for HCK) with lysis buffer, and precleared with anti–mouse rabbit IgG bound to immobilized Protein A on Trysacryl (Pierce).
Immunoprecipitations were performed by incubating the samples for 3 hours at 4°C with 10 μL protein A, previously preadsorbed with specific anti-LYN, anti-HCK, or anti-FGR antibodies. Immune complexes were washed and divided in half. One part was resuspended in kinase buffer (20 mM HEPES, pH 7.5, 10 mM MnCl2, 1 mM DTT) containing 10 μCi [γ-32P]ATP (specific activity, 5000 Ci/mmol). The reaction was stopped after 10 minutes by the addition of 2× reducing Laemmli boiling buffer, and samples were subjected to 10% SDS–polyacrylamide gel electrophoresis (PAGE). After drying, gels were exposed at −70°C to X-Omat AR films. To control that equal immunoprecipitation occurred in the samples, the remaining part of immunoprecipitates were subjected to 10% SDS-PAGE, transferred to nitrocellulose membranes, and analyzed by Western blotting with biotinylated anti-LYN, anti-HCK, or anti-FGR antibodies. In preliminary experiments, the amount of HCK and LYN was controlled in immunoprecipitates from the same number of PFA-fixed, thrombin-activated platelets and CHO-P cells that were used to stimulate PMNLs. In platelet immunoprecipitates, only negligible amounts of LYN were found, compared to those present in PMNLs, but neither HCK nor LYN could be detected in CHO-P cell immunoprecipitates. In agreement, the activity of both kinases was undetectable in platelet and CHO-P cell lysates.
Isolation of polymorphonuclear leukocyte cytoskeleton
Samples were lysed for 10 minutes at 4°C with 500μL of 2× cytoskeleton stabilization buffer (CSK)31 as follows: 50 mM PIPES pH 6.9, 8 M glycerol, 2 mM MgCl2, 0.4% Triton X-100, 4 mM EGTA, 4 mM EDTA, 4 mM sodium orthovanadate, 2 mM PMSF, 20 μg/mL aprotinin, 20 μg/mL leupeptin, 10 μg/mL pepstatin A, and 20 μM PAO. Then cell lysates were centrifuged for 10 minutes at 14 000 rpm in Eppendorf microfuge, and pellets (corresponding to Triton X-100–insoluble fractions) were washed once with 1× CSK, resuspended with RIPA buffer (30 minutes at 4°C) and added with the same volumes of 2× Laemmli boiling buffer containing protease and phosphatase inhibitors. Amounts of Triton X-100–insoluble fraction corresponding to 0.5 × 106 PMNLs were subjected to 6% SDS-PAGE and transferred to nitrocellulose sheets, and CD18 was analyzed by the anti-CD18 monoclonal antibody KIM 127 (0.1 μg/mL; 1 hour at room temperature) followed by goat anti–mouse IgG peroxidase conjugate.
Tyrosine phosphorylation experiments
PMNLs were incubated alone or with platelets or CHO-P cells, as described for adhesion experiments. The reaction was stopped by adding 1 volume of 2× reducing Laemmli buffer. Samples were boiled for 10 minutes and centrifuged for 10 minutes at 7000g. Then 100 μL aliquots, corresponding to 1.25 × 106 total PMNL lysate, were loaded into 7.5% to 12.5% gradient SDS polyacrylamide gels and transferred onto nitrocellulose sheets, and the tyrosine-phosphorylated proteins were revealed with the recombinant horseradish peroxidase-conjugated antiphosphotyrosine antibody RC20 (0.1 μg/mL, 30 minutes at 37°C). Detection was performed by chemiluminescence using the ECL kit, and bands were visualized by autoradiography. Western blot analysis of lysates of platelets and CHO-P cells alone indicated that tyrosine-phosphorylated proteins, including P-110, found in mixed PMNLs-platelets and PMNLs–CHO-P cells lysates were derived from PMNLs. This was further supported by the evidence that the pattern of protein tyrosine phosphorylation in PMNLs-platelets, PMNLs–CHO-P cells, and PMNLs challenged with soluble P-selectin was overimposable (not shown).
Confocal microscopy
Samples were incubated with 5 μg/mL anti-CD11b antibody OKM10 for 30 minutes at 4°C and fixed with 2% PFA. After they were washed, samples were treated for 15 minutes at 4°C with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and 50 mM NH4Cl, to quench autofluorescence and to block nonspecific interactions, and they were incubated for 30 minutes at 4°C with 20 μg/mL goat anti–mouse Alexa Fluor 488 IgG antibody. In samples in which PMNL aggregation was induced by antibody-mediated PSGL-1 engagement, Mac-1 was stained by FITC-conjugated mAb 44 before fixation. After Mac-1 staining, PMNLs were then permeabilized with PBS containing 0.05% saponin and 0.5% BSA for 30 minutes at 4°C, and filamentous actin (F-actin) was stained with 1 μg/mL rhodamine-phalloidin in PBS containing 0.01% saponin and 0.5% BSA for 30 minutes at 4°C. Samples were resuspended in Mowiol, plated on a glass coverslip, and observed with a LSM 510 laser scanning microscope equipped with Axiovert 100 M-BP (Zeiss, Milano, Italy). Optical Z-sections from each sample were taken with 0.3 μm Z-step from the top to the bottom of the cell.
Results
Activity of tyrosine kinases belonging to the SRC family is required for polymorphonuclear leukocyte adhesion to P-selectin–expressing cells and for P-110 phosphorylation
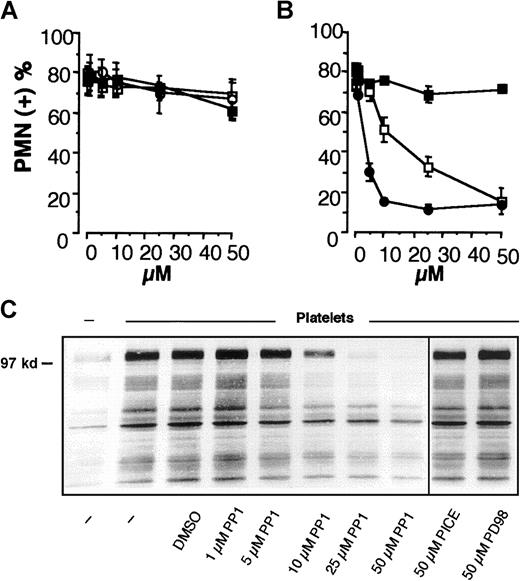
We previously reported that the adhesion of PMNLs to activated platelets requires a P-selectin–triggered, tyrosine kinase-dependent adhesiveness of Mac-1 and is accompanied by tyrosine phosphorylation of P-110 in PMNL.2 To identify the tyrosine kinases involved in the regulation of Mac-1 adhesiveness stimulated by P-selectin, specific inhibitors of different classes of tyrosine kinases, which have already been implicated in the regulation of integrin function in leukocytes,31-34 were tested for their ability to prevent PMNL adhesion to activated platelets. Inhibitors of SRC tyrosine kinases PP1 and PP235 inhibited the formation of mixed PMNL-platelet conjugates in a concentration-dependent manner with the IC50 of 17.2 and 3.8 μM, respectively, whereas the inactive analog PP3 was ineffective (Figure1A). In contrast, the Jak-2 tyrosine kinase inhibitor tyrphostin AG 490,36 the Syk inhibitor piceatannol,37 and the MEK inhibitor PD9805938 did not significantly modify PMNL-platelet adhesion (Figure 1B). Likewise, only SRC kinase inhibitors prevented the tyrosine phosphorylation of the P-110 protein (Figure 1C). This indicates that SRC tyrosine kinase activity may be required for Mac-1 adhesiveness triggered by P-selectin.
Activity of SRC tyrosine kinases is required for PMNL adhesion to activated platelets and for platelet-induced PMNL P-110 tyrosine phosphorylation.
HE-loaded PMNLs were preincubated for 1 minute at 37°C with different concentrations of (A) PP1 (■), PP2 (●), PP3 (▪); (B) tyrphostin AG 490 (■), piceatannol (○), PD98059 (▪) before the addition to PFA-fixed, BCECF-loaded thrombin-activated platelets. Mixed-cell suspensions were coincubated at 37°C and 1000 rpm stirring (standard conditions). The interaction was stopped at 2 minutes by the addition of 1 vol of 2% PFA, and samples were processed for FACS analysis. Data report the percentage of PMNLs displaying the platelet green fluorescent marker. Values are means ± SEM (n = 5). (C) PMNLs were preincubated for 1 minute at 37°C with different concentrations of PP1 or with 50 μM piceatannol or PD98059 before coincubation with PFA-fixed, thrombin-activated platelets. Coincubation in standard conditions was stopped at 2 minutes, and samples were processed for analysis of protein tyrosine phosphorylation. The figure shows the Western blot of samples from a representative of 3 experiments.
Activity of SRC tyrosine kinases is required for PMNL adhesion to activated platelets and for platelet-induced PMNL P-110 tyrosine phosphorylation.
HE-loaded PMNLs were preincubated for 1 minute at 37°C with different concentrations of (A) PP1 (■), PP2 (●), PP3 (▪); (B) tyrphostin AG 490 (■), piceatannol (○), PD98059 (▪) before the addition to PFA-fixed, BCECF-loaded thrombin-activated platelets. Mixed-cell suspensions were coincubated at 37°C and 1000 rpm stirring (standard conditions). The interaction was stopped at 2 minutes by the addition of 1 vol of 2% PFA, and samples were processed for FACS analysis. Data report the percentage of PMNLs displaying the platelet green fluorescent marker. Values are means ± SEM (n = 5). (C) PMNLs were preincubated for 1 minute at 37°C with different concentrations of PP1 or with 50 μM piceatannol or PD98059 before coincubation with PFA-fixed, thrombin-activated platelets. Coincubation in standard conditions was stopped at 2 minutes, and samples were processed for analysis of protein tyrosine phosphorylation. The figure shows the Western blot of samples from a representative of 3 experiments.
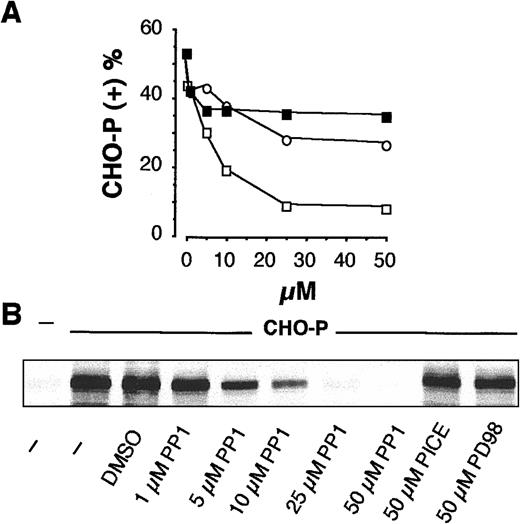
To confirm this hypothesis, CHO-P cells or a soluble recombinant P-selectin–IgG chimera was used to stimulate β2-integrin–mediated interactions on PMNLs. In mixed-cell suspensions, CHO-P cells formed P-selectin– and β2-integrin–mediated mixed cell conjugates with PMNLs, indicating that CHO-P cells express P-selectin and a functional β2-integrin ligand (not shown and reference 2). As reported in Figure2, SRC kinase inhibition prevented PMNL adhesion to CHO-P cells and tyrosine phosphorylation of the P-110, whereas piceatannol and PD98059 were ineffective. Similar results were obtained when Mac-1–mediated homologous PMNL aggregation and P-110 phosphorylation were induced by soluble recombinant P-selectin–IgG chimera: PP1 inhibited both phenomena in a concentration-dependent manner with IC50 of approximately 3 μM (data not shown). Taken together, these results strongly support the hypothesis that tyrosine kinases of the SRC family play a pivotal role in the functional up-regulation of Mac-1 induced by P-selectin.
Activity of SRC tyrosine kinases is required for PMNL adhesion to CHO-P cells and for CHO-P cell–induced P-110 tyrosine phosphorylation.
(A) HE-loaded PMNLs were preincubated for 1 minute at 37°C with different concentrations of PP1 (■), PD98059 (▪), or piceatannol (○), before addition to BCECF-loaded CHO-P cells. Mixed-cell suspensions were coincubated in standard conditions, the interaction was stopped at 2 minutes, and samples were processed for FACS analysis. The percentage of CHO-P cells binding PMNLs from representative of 2 different experiments was reported. (B) PMNLs were preincubated for 1 minute at 37°C with different concentrations of PP1 or with 50 μM piceatannol or PD98059 before coincubation with CHO-P cells in standard conditions. The interaction was stopped at 2 minutes, and samples were processed for analysis of protein tyrosine phosphorylation. The figure shows the tyrosine-phosphorylated 110-kd protein from a representative of 2 different experiments.
Activity of SRC tyrosine kinases is required for PMNL adhesion to CHO-P cells and for CHO-P cell–induced P-110 tyrosine phosphorylation.
(A) HE-loaded PMNLs were preincubated for 1 minute at 37°C with different concentrations of PP1 (■), PD98059 (▪), or piceatannol (○), before addition to BCECF-loaded CHO-P cells. Mixed-cell suspensions were coincubated in standard conditions, the interaction was stopped at 2 minutes, and samples were processed for FACS analysis. The percentage of CHO-P cells binding PMNLs from representative of 2 different experiments was reported. (B) PMNLs were preincubated for 1 minute at 37°C with different concentrations of PP1 or with 50 μM piceatannol or PD98059 before coincubation with CHO-P cells in standard conditions. The interaction was stopped at 2 minutes, and samples were processed for analysis of protein tyrosine phosphorylation. The figure shows the tyrosine-phosphorylated 110-kd protein from a representative of 2 different experiments.
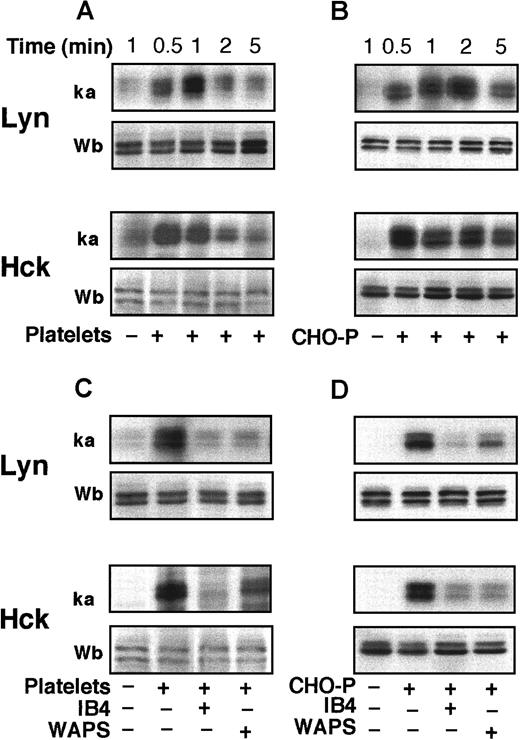
Polymorphonuclear leukocyte adhesion to platelets or CHO-P cells induces a CD18-dependent activation of LYN and HCK
PMNLs express the SRC tyrosine kinases LYN, HCK, and FGR.15 To determine the activity of these enzymes, we immunoprecipitated the kinases and measured their autophosphorylation using an in vitro kinase assay. A rapid stimulation of the activity of LYN and HCK occurred in PMNLs adherent to activated platelets or CHO-P cells (Figure 3A,B). Western blot analysis with biotinylated anti-LYN or anti-HCK antibodies showed that equal amounts of both kinases were immunoprecipitated in the samples. Lysates of fixed platelets or CHO-P cells alone did not show any detectable LYN and HCK kinase activity (data not shown). The activity of FGR was not consistently stimulated after PMNL coincubation with platelets or CHO-P cells (data not shown). mAbs to either P-selectin or β2-integrins reduced the activation of both LYN and HCK in PMNLs mixed with activated platelets or CHO-P cells (Figure 3C,D), indicating that P-selectin and β2-integrin–mediated events are necessary for the activation of SRC tyrosine kinases.
Platelet and CHO-P cell adhesion to PMNL induces CD18-dependent activation of LYN and HCK.
PMNLs were coincubated with PFA-fixed and -activated platelets (A, C) or CHO-P cells (B, D) for different times (A, B) or 2 minutes (C, D) in standard conditions. Where indicated (C, D), PMNLs were preincubated with the anti-CD18 antibody IB4 (20 μg/mL) for 10 minutes at 4°C, and platelets or CHO-P cells were preincubated with the anti–P-selectin antibody, WAPS 12.2 (20 μg/mL), for 10 minutes at room temperature. Samples were lysed by the addition of RIPA or Triton X-100–containing buffer. LYN and HCK were immunoprecipitated, and immune complexes were in part subjected to in vitro kinase assay and in part analyzed by Western blotting with biotinylated anti-LYN and anti-HCK antibodies. The figure shows autoradiograms of phosphorylated kinases (ka) and Western blots (Wb) from a representative of 3 different experiments.
Platelet and CHO-P cell adhesion to PMNL induces CD18-dependent activation of LYN and HCK.
PMNLs were coincubated with PFA-fixed and -activated platelets (A, C) or CHO-P cells (B, D) for different times (A, B) or 2 minutes (C, D) in standard conditions. Where indicated (C, D), PMNLs were preincubated with the anti-CD18 antibody IB4 (20 μg/mL) for 10 minutes at 4°C, and platelets or CHO-P cells were preincubated with the anti–P-selectin antibody, WAPS 12.2 (20 μg/mL), for 10 minutes at room temperature. Samples were lysed by the addition of RIPA or Triton X-100–containing buffer. LYN and HCK were immunoprecipitated, and immune complexes were in part subjected to in vitro kinase assay and in part analyzed by Western blotting with biotinylated anti-LYN and anti-HCK antibodies. The figure shows autoradiograms of phosphorylated kinases (ka) and Western blots (Wb) from a representative of 3 different experiments.
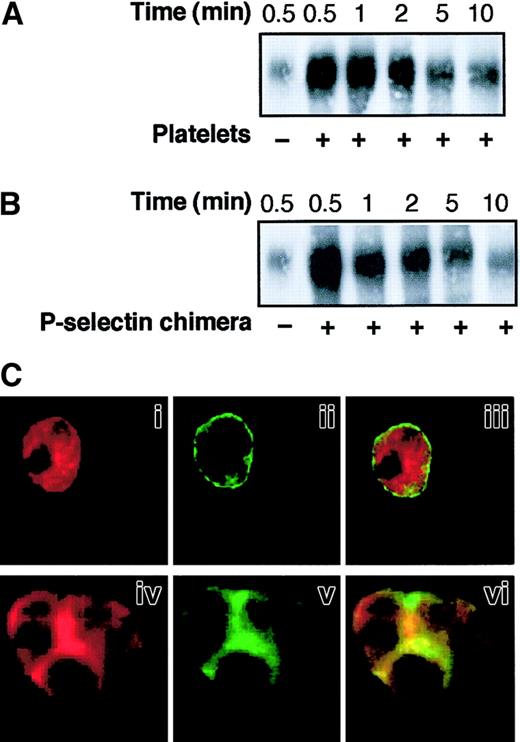
P-selectin triggers CD18 redistribution to Triton X-100–insoluble cytoskeletal fraction and colocalization of Mac-1 clusters with F-actin patches at the site of cell-cell contact: role of SRC tyrosine kinases
SRC tyrosine kinases are essential for actin cytoskeletal re-organization39 and may modulate integrin-cytoskeleton interactions,18 which, in turn, regulate β2-integrin adhesiveness.20 40 We investigated whether SRC tyrosine kinases promote the adhesive function of Mac-1 triggered by P-selectin through the regulation of integrin-cytoskeleton interaction and integrin distribution on the PMNL membrane. The association of β2-integrins with the Triton X-100–insoluble cytoskeletal fraction was examined in PMNLs incubated alone, in mixed PMNL-platelet aggregates, and in PMNL aggregates induced by soluble P-selectin–IgG chimera. A rapid increase in the amount of CD18 in the Triton X-100–insoluble fraction occurred when platelets were added to PMNLs (Figure 4A). The redistribution of CD18 to the Triton X-100–insoluble fraction was maximal at 30 seconds and declined thereafter, reflecting the kinetics of PMNL-platelet conjugate formation (data not shown and reference 2). Exposure of PMNLs to P-selectin–IgG chimera also resulted in a rapid association of CD18 with the cytoskeleton (Figure 4B). Western blot analysis using the specific anti-CD11b mAb OKM10 confirmed that Mac-1 was redistributed to the Triton X-100–insoluble cytoskeleton in PMNLs aggregated by P-selectin (data not shown).
P-selectin triggers CD18 redistribution to Triton X-100–insoluble cytoskeletal fraction and colocalization of Mac-1 clusters with F-actin patches at the site of cell-cell contact.
PMNLs were incubated with platelets (A) or 10 μg/mL P-selectin–IgG chimera (B) for different times in standard conditions. Samples were lysed with CSK buffer, and proteins associated with the Triton X-100–insoluble cytoskeletal fraction were subjected to 6% SDS-PAGE and transferred to Immobilon membrane. The presence of CD18 was analyzed by Western blotting using the anti-CD18 antibody KIM127. The figure shows the results representative of 5 different experiments. (C) PMNLs were incubated for 2 minutes in standard conditions in the absence (i-iii) or in the presence of P-selectin–IgG chimera (iv-vi). After incubation, CD11b and F-actin were stained using the anti-CD11b antibody OKM10, followed by goat anti–mouse Alexa Fluor 488 antibody and rhodamine-phalloidin, respectively, and processed for confocal laser scanning microscopy. Images represent confocal micrographs from the middle third of a single unstimulated cell (i-iii) and of an aggregate of 3 cells (iv-vi). Panels i and iv show F-actin staining, and ii and v show CD11b staining. In the overlay (iii, vi), yellow represents colocalization of the 2 stainings. The figure reports the results of a representative of 5 different experiments.
P-selectin triggers CD18 redistribution to Triton X-100–insoluble cytoskeletal fraction and colocalization of Mac-1 clusters with F-actin patches at the site of cell-cell contact.
PMNLs were incubated with platelets (A) or 10 μg/mL P-selectin–IgG chimera (B) for different times in standard conditions. Samples were lysed with CSK buffer, and proteins associated with the Triton X-100–insoluble cytoskeletal fraction were subjected to 6% SDS-PAGE and transferred to Immobilon membrane. The presence of CD18 was analyzed by Western blotting using the anti-CD18 antibody KIM127. The figure shows the results representative of 5 different experiments. (C) PMNLs were incubated for 2 minutes in standard conditions in the absence (i-iii) or in the presence of P-selectin–IgG chimera (iv-vi). After incubation, CD11b and F-actin were stained using the anti-CD11b antibody OKM10, followed by goat anti–mouse Alexa Fluor 488 antibody and rhodamine-phalloidin, respectively, and processed for confocal laser scanning microscopy. Images represent confocal micrographs from the middle third of a single unstimulated cell (i-iii) and of an aggregate of 3 cells (iv-vi). Panels i and iv show F-actin staining, and ii and v show CD11b staining. In the overlay (iii, vi), yellow represents colocalization of the 2 stainings. The figure reports the results of a representative of 5 different experiments.
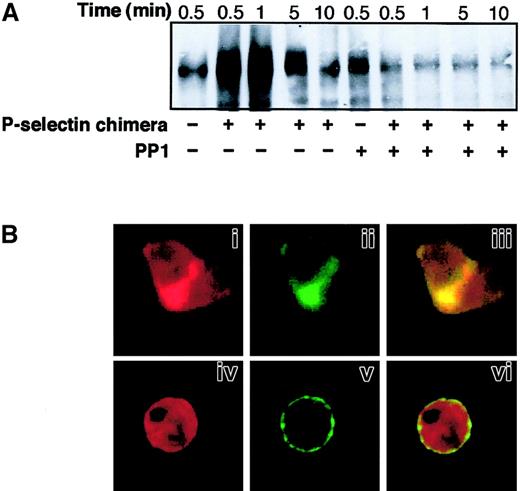
To determine whether association with the Triton X-100–insoluble cytoskeleton correlated with surface redistribution, PMNLs were dual labeled with mAb OKM10 to CD11b and rhodamine phalloidin to detect F-actin and were examined by confocal fluorescence microscopy. In resting PMNLs (Figure 4C), CD11b was almost evenly distributed over the PMNL surface (i), and F-actin was homogeneously distributed inside the cell (ii). In PMNL aggregates induced by P-selectin–IgG chimera, large clusters of CD11b were localized at the site of cell-cell contact (v) and were superimposed on or surrounded by F-actin patches (iv and vi).
The role of SRC tyrosine kinases in Mac-1 association with the cytoskeleton and clustering triggered by P-selectin was investigated using inhibitors of their activity. At concentrations needed to inhibit P-selectin–induced PMNL adhesion, PP1 impaired CD18 redistribution to the Triton X-100–insoluble fraction in PMNLs stimulated by P-selectin–IgG chimera (Figure 5A) and in PMNL-platelet mixed aggregates (data not shown). Similar results were obtained with the SRC tyrosine kinase inhibitor PP2 (data not shown). Confocal microscopy (Figure 5B) showed that in PMNLs pretreated with PP1 before stimulation with P-selectin, CD11b was uniformly distributed as a peripheral ring (v) and F-actin was homogeneously distributed (iv). These data demonstrate that SRC tyrosine kinase activity is required for Mac-1 association to the cytoskeleton and clustering at the sites of cell-cell contact in PMNL aggregates induced by P-selectin.
SRC tyrosine kinase activity is required for P-selectin–triggered CD18 redistribution to Triton X-100–insoluble cytoskeletal fraction and colocalization of Mac-1 clusters with F-actin patches at the site of cell-cell contact.
PMNLs were preincubated for 1 minute at 37°C with DMSO or 25 μM PP1 before the addition of 10 μg/mL P-selectin–IgG chimera. (A) Coincubation in standard conditions was stopped at different times. Samples were processed, and the presence of CD18 in Triton X-100–insoluble cytoskeletal fraction was analyzed as in Figure 4. (B) PMNLs, pretreated with DMSO (i-iii) or with 25 μM PP1 (iv-vi), were stimulated by 10 μg/mL P-selectin–IgG chimera for 2 minutes. After stimulation, CD11b and F-actin were stained and processed for confocal laser scanning microscopy as in Figure 4. Panels i, ii, and iii show an aggregate of 2 cells. Panels i and iv show F-actin staining, ii and v show CD11b staining. In the overlay (iii, vi), yellow represents colocalization of the 2 stainings. The figure is representative of results obtained in 5 different experiments.
SRC tyrosine kinase activity is required for P-selectin–triggered CD18 redistribution to Triton X-100–insoluble cytoskeletal fraction and colocalization of Mac-1 clusters with F-actin patches at the site of cell-cell contact.
PMNLs were preincubated for 1 minute at 37°C with DMSO or 25 μM PP1 before the addition of 10 μg/mL P-selectin–IgG chimera. (A) Coincubation in standard conditions was stopped at different times. Samples were processed, and the presence of CD18 in Triton X-100–insoluble cytoskeletal fraction was analyzed as in Figure 4. (B) PMNLs, pretreated with DMSO (i-iii) or with 25 μM PP1 (iv-vi), were stimulated by 10 μg/mL P-selectin–IgG chimera for 2 minutes. After stimulation, CD11b and F-actin were stained and processed for confocal laser scanning microscopy as in Figure 4. Panels i, ii, and iii show an aggregate of 2 cells. Panels i and iv show F-actin staining, ii and v show CD11b staining. In the overlay (iii, vi), yellow represents colocalization of the 2 stainings. The figure is representative of results obtained in 5 different experiments.
Engagement of PSGL-1 by a monoclonal antibody triggers formation of Mac-1 clusters and F-actin patches in polymorphonuclear leukocytes
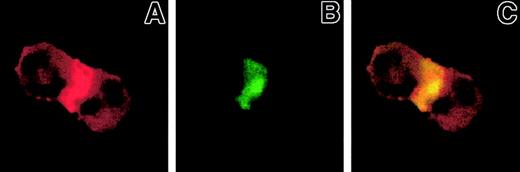
Because PSGL-1 is the major leukocyte ligand for P-selectin, we examined the effect of PSGL-1 cross-linking on the cellular localization of Mac-1. PSGL-1 was engaged by mAb PL2 and was cross-linked by anti–mouse IgG F(ab′)2 fragment. Cross-linking of PSGL-1 induced PMNL aggregation, organization of F-actin (Figure 6A), clustering of CD11b (Figure 6B), and colocalization of CD11b and F-actin at the sites of PMNL-PMNL adhesion (Figure 6C). These results support the conclusion that P-selectin induces Mac-1 activation through the binding to PSGL-1.
Engagement of PSGL-1 by a monoclonal antibody triggers the formation of Mac-1 clusters and F-actin patches in PMNL.
PMNLs were preincubated with the anti–PSGL-1 antibody PL2 (20 μg/mL) and then were washed and incubated for 3 minutes in the presence of rabbit anti–mouse IgG F(ab′)2 fragments (10 μg/mL) in standard conditions. Samples were stained with FITC-conjugated anti-CD11b antibody and rhodamine-phalloidin and processed for confocal laser scanning microscopy; A and B show F-actin and CD11b staining, respectively. In the overlay (C), yellow represents colocalization of the 2 stainings. The figure shows an aggregate of 2 cells. Results were reported from a representative of 3 experiments.
Engagement of PSGL-1 by a monoclonal antibody triggers the formation of Mac-1 clusters and F-actin patches in PMNL.
PMNLs were preincubated with the anti–PSGL-1 antibody PL2 (20 μg/mL) and then were washed and incubated for 3 minutes in the presence of rabbit anti–mouse IgG F(ab′)2 fragments (10 μg/mL) in standard conditions. Samples were stained with FITC-conjugated anti-CD11b antibody and rhodamine-phalloidin and processed for confocal laser scanning microscopy; A and B show F-actin and CD11b staining, respectively. In the overlay (C), yellow represents colocalization of the 2 stainings. The figure shows an aggregate of 2 cells. Results were reported from a representative of 3 experiments.
Integrity of the cytoskeleton is required for P-selectin–induced polymorphonuclear leukocyte adhesion and Mac-1 clustering
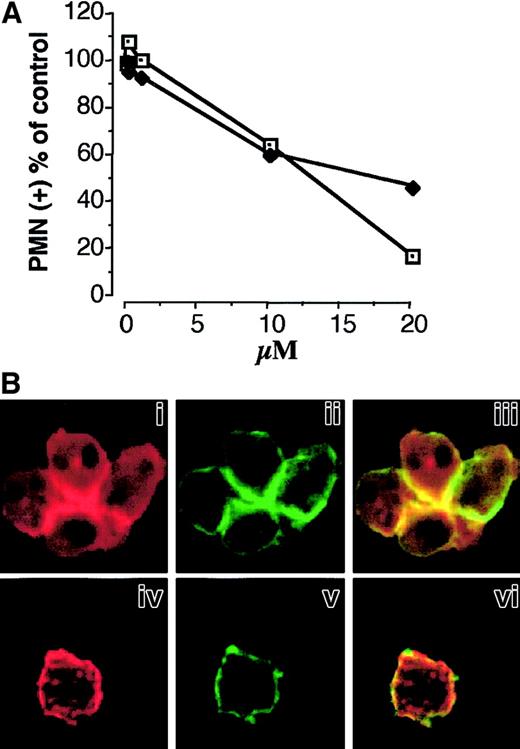
The finding that Mac-1 associates with the cytoskeleton and that Mac-1 clusters colocalize with F-actin patches at the site of cell-cell contact suggested the involvement of actin polymerization in Mac-1 adhesiveness promoted by P-selectin. Therefore, we evaluated the effect of cytochalasin D, an agent that impairs actin filament polymerization, on P-selectin–induced PMNL adhesion and Mac-1 clustering. Treatment with cytochalasin D inhibited PMNL homologous aggregation induced by soluble P-selectin and PMNL adhesion to activated platelets (Figure7A) in a concentration-dependent manner. PMNLs pretreated with 20 μM cytochalasin D and stimulated with P-selectin–IgG chimera showed areas completely devoid of F-actin and areas containing dense clumps or even compact foci of F-actin forming a discontinuous ring beneath the cell membrane (Figure 7Biv). The formation of large clusters of Mac-1, normally localized at the site of cell-cell boundaries (Figure 7Bii), was almost completely prevented by cytochalasin D treatment. Small patches of Mac-1 appear distributed along the cell membrane (Figure 7Bv). This result indicates that the assembly of F-actin is required for the formation and maintenance of the Mac-1 clusters at the site of cell-cell contact and that it plays a key role in Mac-1 adhesiveness triggered by P-selectin.
Cytochalasin D prevents PMNL adhesion to platelets, P-selectin–induced PMNL homologous aggregation, and the colocalization of Mac-1 clusters and F-actin patches in aggregated PMNLs.
(A) HE-loaded PMNLs were preincubated with increasing concentrations of cytochalasin D for 2 minutes at 37°C before the addition of PFA-fixed, BCECF-loaded activated platelets (♦). Mixed-cell suspensions were coincubated in standard conditions, the interaction was stopped at 2 minutes, and samples were processed for FACS analysis. Alternatively, cytochalasin D–pretreated PMNLs were challenged with P-selectin–IgG chimera (10 μg/mL) (■). The reaction was stopped at 2 minutes, and P-selectin–induced PMNL homologous aggregation was evaluated by counting nonaggregated PMNL by optical microscopy. The results are from a representative of 2 different experiments. (B) PMNLs were preincubated for 2 minutes at 37°C with ethanol (i-iii) or 20 μM cytochalasin D (iv-vi), before the addition of P-selectin–IgG chimera (10 μg/mL). Incubation in standard conditions was stopped at 2 minutes, and samples were processed for confocal laser scanning microscopy. Panels i, ii, and iii show an aggregate of 4 cells. Panels i and iv show F-actin staining, and ii and v show CD11b staining. In the overlay (iii, vi), yellow represents colocalization of the 2 stainings. The figure is representative of results obtained in 5 different experiments.
Cytochalasin D prevents PMNL adhesion to platelets, P-selectin–induced PMNL homologous aggregation, and the colocalization of Mac-1 clusters and F-actin patches in aggregated PMNLs.
(A) HE-loaded PMNLs were preincubated with increasing concentrations of cytochalasin D for 2 minutes at 37°C before the addition of PFA-fixed, BCECF-loaded activated platelets (♦). Mixed-cell suspensions were coincubated in standard conditions, the interaction was stopped at 2 minutes, and samples were processed for FACS analysis. Alternatively, cytochalasin D–pretreated PMNLs were challenged with P-selectin–IgG chimera (10 μg/mL) (■). The reaction was stopped at 2 minutes, and P-selectin–induced PMNL homologous aggregation was evaluated by counting nonaggregated PMNL by optical microscopy. The results are from a representative of 2 different experiments. (B) PMNLs were preincubated for 2 minutes at 37°C with ethanol (i-iii) or 20 μM cytochalasin D (iv-vi), before the addition of P-selectin–IgG chimera (10 μg/mL). Incubation in standard conditions was stopped at 2 minutes, and samples were processed for confocal laser scanning microscopy. Panels i, ii, and iii show an aggregate of 4 cells. Panels i and iv show F-actin staining, and ii and v show CD11b staining. In the overlay (iii, vi), yellow represents colocalization of the 2 stainings. The figure is representative of results obtained in 5 different experiments.
Ligand binding to Mac-1 is required for the formation of Mac-1 clusters and F-actin patches in polymorphonuclear leukocytes challenged with P-selectin
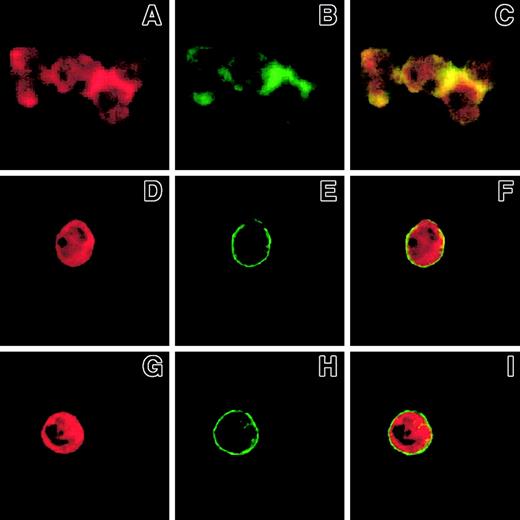
Because mAbs to β2-integrins inhibited SRC tyrosine kinase activation in PMNLs adherent to P-selectin–expressing cells (Figure3), we investigated whether ligand binding to Mac-1 was required for clustering and colocalization with F-actin at cell-cell boundaries. Blockade of either P-selectin by WAPS 12.2 (Figure8D-F) or β2-integrin by IB4 resulted in a complete inhibition of Mac-1 clustering (Figure 8H) and F-actin accumulation (Figure 8G), providing further support for the interpretation that Mac-1 binding to its ligand is an essential preliminary step to switch on the intracellular machinery that mediates the formation of Mac-1 clusters and F-actin accumulation at the adhesion sites.
Ligand binding to Mac-1 is required for the formation of Mac-1 clusters and F-actin patches in PMNLs challenged with P-selectin.
PMNLs, preincubated for 10 minutes at 4°C with 20 μg/mL control antibody (A-C) or anti-CD18 antibody IB4 (G-I) were challenged with 10 μg/mL P-selectin–IgG chimera. Alternatively, PMNLs were challenged with 10 μg/mL P-selectin–IgG chimera preincubated for 10 minutes with 20 μg/mL anti–P-selectin antibody, WAPS (D-F). Incubation in standard conditions was stopped at 2 minutes, and samples were processed for confocal laser scanning microscopy. A, B, and C show an aggregate of 4 cells. A, D, and G show F-actin staining, and B, E, and H show CD11b staining. In the overlay (C, F, I), yellow represents colocalization of the 2 stainings. The figure reports the results from a representative of 3 different experiments.
Ligand binding to Mac-1 is required for the formation of Mac-1 clusters and F-actin patches in PMNLs challenged with P-selectin.
PMNLs, preincubated for 10 minutes at 4°C with 20 μg/mL control antibody (A-C) or anti-CD18 antibody IB4 (G-I) were challenged with 10 μg/mL P-selectin–IgG chimera. Alternatively, PMNLs were challenged with 10 μg/mL P-selectin–IgG chimera preincubated for 10 minutes with 20 μg/mL anti–P-selectin antibody, WAPS (D-F). Incubation in standard conditions was stopped at 2 minutes, and samples were processed for confocal laser scanning microscopy. A, B, and C show an aggregate of 4 cells. A, D, and G show F-actin staining, and B, E, and H show CD11b staining. In the overlay (C, F, I), yellow represents colocalization of the 2 stainings. The figure reports the results from a representative of 3 different experiments.
Discussion
Adhesion of PMNLs to activated platelets proceeds through the coordinated action of P-selectin and Mac-1.2,5-8 In the present study, we report that the activity of tyrosine kinases belonging to the SRC family is required for Mac-1 adhesive function triggered by P-selectin. Blockade of SRC kinase activity by specific inhibitors prevented PMNL-platelet adhesion and tyrosine phosphorylation of P-110, the major protein undergoing phosphorylation in PMNLs adherent to activated platelets.2
SRC tyrosine kinases can be activated in PMNLs as a consequence of stimulation by soluble and particulate agonists41 and after β2-integrin engagement.27 Our study shows, for the first time, that 2 members of the SRC family, LYN and HCK, become activated in PMNLs adherent to P-selectin–expressing cells through the coordinated action of P-selectin and the β2-integrin Mac-1.
Engagement of β2-integrins is accompanied by the association of SRC tyrosine kinases with cytoskeletal proteins and other signaling proteins.42 The recruitment of these tyrosine kinases is essential to promote the rearrangement of actin cytoskeleton and the formation of adhesion-like structures, necessary for PMNL activation. In fact, studies using PMNL from Hck- and Fgr-deficient mice highlighted an essential role of these kinases in PMNL functions, such as spreading, respiratory burst, and degranulation, triggered by integrin engagement.43,44 All these observations demonstrate that SRC kinases hold a key position in the “outside-in” signaling transduced by β2-integrins. In contrast, no experimental evidence has been reported to date indicating that through a SRC-mediated signaling these integrins may also promote their adhesive function. In different cell types, SRC and the other components of the family are essential for the integrin-induced cytoskeletal changes necessary for cell locomotion, as indicated by impaired in vitro migration of Hck- and Fgr-deficient macrophages or of CSK overexpressing rat basophilic leukemia cells.17,39Moreover, recent data obtained in fibroblasts from Src-deficient mice indicated that SRC regulates cell spreading by promoting the reversibility of integrin-cytoskeleton interaction.18
Integrin-cytoskeleton linkage plays a crucial role in β2-integrin function.45-47 In the present study, we found that in PMNL-platelet aggregates and in PMNL-PMNL aggregates induced by soluble P-selectin, Mac-1 was rapidly redistributed to Triton X-100–insoluble cytoskeletal fraction and accumulated at the site of cell-cell contact in large clusters colocalized with polymerized F-actin. These events were all impaired by inhibitors of SRC tyrosine kinase activity, suggesting that in our experimental model these kinases mediate signals responsible for cytoskeleton reorganization and changes of β2-integrin–cytoskeleton interactions. The dynamics of integrin-cytoskeleton interaction regulated by SRCs are necessary to confer the proper cell plasticity required for rapid spreading and migration.18 This suggests that in PMNLs, SRC tyrosine kinases could be required for rapid remodeling of adhesion sites and cell morphology, allowing the appropriate membrane-to-membrane apposition that occurs during the formation of cell aggregates. A rapid remodeling of the adhesion sites could be important in our experimental conditions in which the short duration of membrane to membrane contact during cell-cell collisions is an important factor limiting adhesion efficiency.48
Although it cannot be excluded that P-selectin binding to its receptor(s) could stimulate changes in integrin affinity, the evidence of Mac-1 clustering indicates that increased avidity is involved in Mac-1 adhesiveness triggered by P-selectin. In fact, clustering on the plasma membrane results in high-avidity of integrins that strengthens the interaction with the ligand.22,49,50 Moreover, clustering of integrins on the cell surface colocalizes at the cytoplasmic side signaling molecules, including kinases and their substrates.51 The formation of integrin clusters requires an early phase of integrin release from the cytoskeletal constraints that allows its mobility in the plasma membrane.52-54 Once the integrin has bound ligand, establishment of new connections between integrin and the cytoskeleton may be required for firm adhesion. In our study, treatment of PMNLs with cytochalasin D prevented PMNL-platelet adhesion and PMNL-PMNL aggregation induced by soluble P-selectin and the formation of Mac-1 clusters usually observed at the boundaries of PMNL-PMNL aggregates; only small clusters of Mac-1 randomly distributed along plasma membrane could be observed. These data support the hypothesis that the cytoskeleton plays a key role in Mac-1 adhesiveness triggered by P-selectin. As in P-selectin stimulation, the β2-integrin function induced by the engagement of α4β1 integrin on T lymphocytes55 and Mac-1 activation triggered by immune complexes, but not by fMLP,56 can be abrogated by impairing actin cytoskeletal re-arrangement.
PSGL-1 is the predominant P-selectin ligand in PMNLs.57 In the present study, we showed clustering of Mac-1 and accumulation of F-actin in PMNL aggregates triggered by the engagement of PSGL-1 by a mAb. These data confirm and extend our previous observations showing that cross-linking of PSGL-1 by a mAb was able to trigger tyrosine kinase-dependent Mac-1–mediated homotypic aggregation and protein tyrosine phosphorylation in PMNLs.2 The role of PSGL-1 as a signaling molecule was originally suggested by Hidari et al,58 who showed that the ligation of PSGL-1 by P-selectin and by mAbs results in the activation of the 42-44 kd MAP kinase and in the tyrosine kinase-dependent production of IL-8 in PMNLs. However, the evidence that PSGL-1 can signal activation of human PMNLs is contradictory.14,59 It is important to note that in our experimental conditions, signaling due to P-selectin binding to PSGL-1 and signaling induced by engagement of Mac-1 by its ligand could not be differentiated, in agreement with the hypothesis that the 2 adhesive systems are tightly coordinated with each other. In fact, in our model, the activation of LYN and HCK in PMNLs interacting with P-selectin–expressing cells was prevented by blocking anti-CD18 antibody, indicating that not only P-selectin stimulation but also the binding of Mac-1 to its ligand is essential for kinase activation. In agreement, the blockade of CD18 prevents the formation of Mac-1 clusters and their colocalization with F-actin patches. Thus P-selectin, interacting with its receptor on PMNLs, directly triggers an initial Mac-1 interaction with its ligand responsible for SRC kinase activation; these, in turn, mediate the remodeling of cytoskeleton-integrin linkages, integrin diffusion, and clustering that finally strengthen cell-cell adhesion. A multistep model, in which initial ligand binding stimulates post-receptor events leading to integrin clustering and stabilization of integrin-ligand interaction, has already been proposed for β2- and αIIbβ3 integrin.11,60 61
In summary, the mechanism proposed for Mac-1 activation triggered by P-selectin highlights a new role of SRC tyrosine kinases in a regulatory loop by which the β2-integrin regulates its own adhesive function and suggests a pivotal role for SRC kinases in the recruitment of PMNLs by activated platelets.
We thank Dr S. Smyth (Division of Hematology, Health Science Center, Stony Brook, NY) for helpful discussion and suggestions. We also acknowledge Dr R. Polishchuk for expert help in using confocal laser scanning microscopy and Dr L. Fumagalli (Institute of General Pathology, University of Verona, Italy) for help in setting up the in vitro kinase assay. We thank Drs R. P. McEver, K. L. Moore, M. K. Robinson, and G. Berton and the Ortho Diagnostic System for valuable monoclonal antibodies and the Genetics Institute for the P-selectin–IgG fusion proteins and CHO-P cells.
Supported in part by Fondation Segré, Geneva, Switzerland; Ministero della Sanità, Convenzione n. ICS060.2/RF99.74, IRCCS Aviano; and the Italian National Research Council (Convenzione CNR-Consorzio Mario Negri Sud). R.S. was the recipient of a fellowship by Fondazione Italiana Ricerca sul Cancro.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paola Piccardoni, Department of Vascular Medicine and Pharmacology, “G.Bizzozero” Laboratory of Blood and Vascular Cell Interactions, Consorzio Mario Negri Sud, 66030 Santa Maria Imbaro, Italy; e-mail: piccardo@cmns.mnegri.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal