Abstract
Dendritic cells (DCs) are specialized antigen-presenting cells that have the unique ability to initiate a primary immune response. The effect of physiologic stress on circulating blood DCs has thus far not been studied. In this study, we applied a recently developed method of counting blood DCs to test the hypothesis that significant stress to the body such as surgery and exercise might induce measurable changes in the DC numbers, subsets, phenotype, and function. Twenty-six patients scheduled for elective laparoscopic cholecystectomy, 4 for elective hysterectomy, 56 controls, and 5 volunteers who underwent a stress exercise test were enrolled in the study. Absolute DC counts increased acutely (71.7% ± 11% [SEM],P = .0001) in response to the stress of surgery and dropped below preoperative levels (−25% ± 14% [SEM],P = .05) on days 2-3. The perioperative DC subset balance remained constant. Interestingly, DC counts changed independently of monocyte counts. Exercise also induced a rise in DC counts but coincidentally with monocyte counts. Surprisingly, no phenotypic or functional activation of DCs was seen in either stress situations in vivo. DCs are rapidly mobilized into the circulation in response to surgical and exercise stress, which may serve to prepare the host's immune defenses against trauma. The independent regulation of the DC and monocyte counts reinforces the distinction between these 2 cell populations.
Introduction
Dendritic cells (DCs) are the key specialist antigen-presenting cells that initiate and direct immune responses.1,2 They are produced in the bone marrow, circulate via the blood, and enter the body tissues and mucosal surfaces to act as immune sentinels. When tissues are damaged by trauma or infection or are altered by malignant transformation, inflammatory cytokines and other cellular products are released. These stimuli, whether from the tissues (endogenous) or from microbial products (exogenous), activate resident DCs and also promote recruitment of circulating DCs to the site.3 DCs actively capture and process antigens and then migrate via the lymphatics to draining nodes. Here, they present processed antigens to T and B lymphocytes to initiate primary or stimulate secondary (memory) immune responses. As such, they play a central role in immune surveillance, an essential part of the defense and protective mechanism against infections and malignancy.4
Surgical stress as a result of tissue injury and exposure to anesthesia evokes alterations in metabolic, immune, and endocrine functions.4,5 Endogenous secretion of glucocorticoids and catecholamines can be measured by a rise in serum cortisol, adrenaline, and noradrenaline. Damaged tissues release inflammatory cytokines, which include interleukin 1β (IL-1β), tumor necrosis factor-α, and IL-6. These can be detected locally and systemically. The stress response is also accompanied by a general peripheral blood leucocytosis with various changes in the leukocyte differential and lymphocyte subsets.6,7 A similar stress response is observed during physical exercise.7 8 These changes have been implicated in the generalized immunosuppressive effect of surgical stress and exercise.
Circulating DCs are scarce and represent less than 1% of peripheral blood mononuclear cells.2,9 They fall within the lineage-negative (Lin−) population of peripheral blood mononuclear cells and express HLA-DR in high density (HLA-DR+). Two distinct DC subsets (identified as CD11c+CD123dim/− and CD11c−CD123+) that may produce different outcomes in their interaction with T lymphocytes have been described.10,11 Until recently, their rarity and the lack of a DC-specific marker have made the study and enumeration of DCs in peripheral blood difficult. The production of the monoclonal antibody (mAb) CMRF-44 has facilitated the study and development of a “routine” DC counting method based on the number of CMRF-44+ CD14−CD19−cells.9,12 13 This test now provides an opportunity to collect clinical data on this important leukocyte population.
We hypothesized that a significant physiologic stress such as that induced by surgery or exercise would result in measurable changes in peripheral blood DC counts. This study monitored the changes in DC counts in patients undergoing a standard surgical procedure and exercise and related these changes to other leukocyte counts.
Patients, materials, and methods
Study subjects
Twenty-six patients scheduled for elective laparoscopic cholecystectomy (9 males and 17 females; mean age, 47.1 years; range, 24-72) participated in the study. All patients had a history of biliary colic and ultrasonographic evidence of cholelithiasis. None had acute cholecystitis, jaundice, or any other complications at the time of surgery or a history of any malignant conditions. Two patients who underwent vaginal hysterectomies and 2 who underwent transabdominal hysterectomies also participated in the study. Nine healthy individuals (5 females and 4 males; mean age, 40.4 years; range, 20-50) served as controls for this group. Fifty-six healthy individuals (28 males and 28 females; mean age, 38.5 years; range, 21-76) volunteered for DC counts to establish our normal range. Five healthy volunteers (4 males and 1 female; mean age, 42.2 years; range, 37-49) were recruited to perform treadmill exercise using the Bruce protocol.14 Informed consent was obtained in each case, and the study protocol was approved by the Mater Adult Hospital Research Ethics Committee.
Study design
Peripheral venous blood samples were collected from each patient before, during, and after the surgical procedure. Two samples were collected before surgery, one at the preadmission clinic (PreAdm) a week prior to surgery and another on the morning of the surgery (PreOp). The intraoperative (IntraOp) sample was collected half an hour after the start of the skin incision. Postoperatively, a (Recovery) sample was taken within 2 hours of closure, while the patient was in the recovery room. Further samples were taken on day 1 following surgery and thereafter. The usual follow-up period was 2 to 7 days and, depending upon patient cooperation, further samples were obtained several weeks after the operation.
Each control individual provided blood samples for DC counts at time points that were representative of the times when the surgical patients typically had their samples collected. The PreAdm sample was collected at noon. The samples on the day of surgery were collected at 8am, 12 pm, and 2 pm to correspond to the PreOp, IntraOp, and Recovery times, respectively. All subsequent collections were between 8 am and 9 am. All other healthy volunteers provided blood samples between 8 amand 9 am.
Volunteers performing the treadmill exercise test had blood samples collected prior to (PreEx), immediately after (PostEx 0 hours), and 2 hours (PostEx 2 hours) and 4 hours (PostEx 4 hours) after the exercise and the following day. Heart rate, blood pressure, and continuous 12-lead electrocardiogram measurements were taken throughout the procedure.
Blood DC counting assay
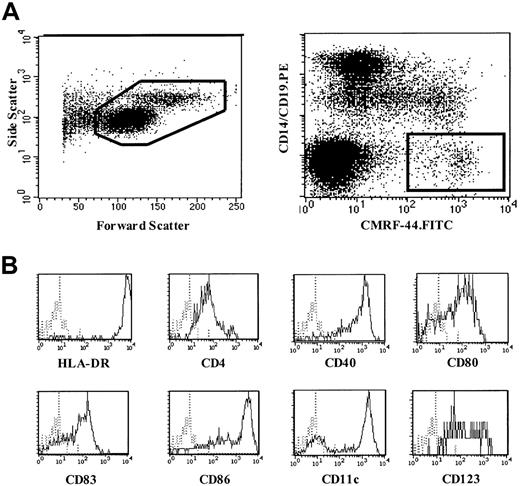
The method of blood DC counting using the CMRF-44 mAb and flow cytometry has been described elsewhere.9 Briefly, 4 mL blood was collected into a standard ethylenediaminetetraacetic acid blood tube. Mononuclear cells (MNCs) were isolated by Ficoll-Hypaque density gradient centrifugation and cultured in U-bottom tubes for 16 to 24 hours in RPMI 1640/10% fetal calf serum at 37°C and 5% CO2 to allow for the maximal up-regulation of the CMRF-44 antigen on DCs.9 Using standard immunolabeling technique, cells were incubated sequentially with the CMRF-44 mAb and fluorescein isothiocyanate–conjugated sheep antimouse immunoglobulin (FITC-SAM). After blocking with 10% mouse serum, phycoerythrin (PE)-conjugated CD14 and CD19 mAb were added. Propidium iodide (PI) was added, and cells were analyzed using a FACSVantage flow cytometer (Becton Dickinson, San Jose, CA). Live MNCs were gated on forward and side light-scatter characteristics (Figure 1A, left) and PI exclusion. DCs were defined as CMRF-44+CD14−CD19− events (Figure 1A, right). Absolute DC counts (106/L) were calculated from the number of MNCs per liter of blood (as determined by the automated cell counter) multiplied by the percentage of DCs (mean of triplicates). Any adherent cells detached from the tube after vigorous 2 mM ethylenediaminetetraacetic acid/phosphate-buffered saline pipetting were negligible (< 1000 cells or 0.01% of the original cell number recovered) and, when analyzed, fell outside the Lin−HLA-DR+ gate (data not shown). In some experiments, fresh MNCs were also used.
Blood DC counting method using CMRF-44 mAb.
(A) A representative forward-side scattergram (left) of MNCs following overnight culture and dot plot (right) demonstrating the gating strategy used to identify blood DCs using CMRF-44 mAb. (B) Phenotype of CMRF-44+, CD14−, and CD19− blood DCs analyzed using 3-color FACS analysis.
Blood DC counting method using CMRF-44 mAb.
(A) A representative forward-side scattergram (left) of MNCs following overnight culture and dot plot (right) demonstrating the gating strategy used to identify blood DCs using CMRF-44 mAb. (B) Phenotype of CMRF-44+, CD14−, and CD19− blood DCs analyzed using 3-color FACS analysis.
Analysis of DC phenotype, activation, costimulatory molecules, and subsets
Three-color flow cytometry was performed for the following analyses. To determine immunophenotype of the CMRF-44+ DCs, cultured MNCs were stained with biotinylated CMRF-44 mAb followed by PE–cyanin 5-conjugated streptavidin, and FITC-conjugated mAb, specific for CD14 and CD19 in combination with PE-conjugated mAb specific for HLA-DR, CD4, CD40, CD80, CD83, CD86, CD11c, and CD123. For the analysis of the expression of DC activation and costimulatory molecules, fresh MNCs were used. MNCs were first stained with specific lineage marker mAb, CD3, CD14, CD16, and CD19, followed by FITC-SAM. After blocking with 10% mouse serum, the cells were stained with PE–cyanin 5-conjugated HLA-DR mAb and PE-conjugated CD40, CD86, and CD11c.
In a separate experiment, MNCs were pretreated with media containing hydrocortisone (10−4 M and 10−6 M) for 2 hours and washed extensively before the overnight culture and blood DC counting assay.
Clinical measurements
Full differential blood count measurements were performed using automated counters Advia 120, Haematology System, or Technicon H.3 RTX (Bayer, Tarrytown, NY) used for routine clinical analyses at the Mater Adult Hospital. Serum cortisol was measured using fluorescence polarization immunoassay (Abbott TDx, Abbott Laboratories, Abbott Park, IL).
Allogeneic mixed leukocyte reaction
The ability of MNCs (with different DC numbers) isolated at the various time points perioperatively to stimulate purified allogeneic T lymphocytes was tested in a mixed leukocyte reaction (MLR). A titration of patient MNCs (5 × 103 to 3 × 105cells) was incubated with T lymphocytes (5 × 104 cells) for 5 days in 96-well U-bottom plates. Sixteen hours prior to harvesting the cells, 18.5 kBq of 3H-thymidine was added to each well. Then, 3H-thymidine uptake was counted in a liquid β-scintillation counter (MicroBeta Trilux Scintillation Counter, Wallac, Turku, Finland).
Monoclonal antibodies and reagents
The following monoclonal antibodies were used: unconjugated and biotinylated mAb CMRF-44 (immunoglobulin M [IgM]), CMRF-75 (IgM), CD14 (CMRF-31, IgG2a), and IgG2a (isotype control) produced in our laboratory; CD3 (OKT3, IgG2a, American Type Culture Collection, Rockville, MD); CD16 (HuNK2, IgG2a) and CD19 (FMC63, IgG1), gifts from Prof H. Zola, Adelaide, Australia; PE-conjugated IgG2a (isotype control), IgG2b (isotype control), CD86 (24F, IgG1), and CD123 (9F5, IgG2b), (PharMingen International, San Diego, CA); PE-conjugated CD3 (SK7, IgG1), CD4 (SK3, IgG1), CD11c (S-HCL-3, IgG2a), CD14 (leuM3, IgG2b), CD16 (leu11c, IgG1), CD19 (leuM12, IgG1), CD80 (L307.4, IgG1), and HLA-DR (L243, IgG2a) (Becton Dickinson); CD83 (HB15a, IgG2b, Immunotech, Marseille, France); FITC-conjugated HLA-DR (MCA672F, IgG1, Serotec, Oxford, United Kingdom); PE–cyanin 5-conjugated IgG1 (PharMingen); and HLA-DR (Immu375, IgG1, Immunotech).
The following reagents were used: FITC-SAM (Amrad Biotech, Melbourne, Australia); PE–cyanin 5-conjugated streptavidin (Dako, Carpinteria, CA); mouse serum and PI (Sigma, St Louis, MO); RPMI 1640 and fetal calf serum (Life Technologies, Melbourne, Australia); and water-soluble hydrocortisone (Pharmacia and Upjohn, Sydney, Australia).
Statistical analysis
Changes in absolute and percentage DC count triplicates between measurement times were analyzed using a hierarchic linear model using the Mixed procedure in SAS software (SAS Institute, Cary, NC).15 Within the model, the intercept and time effects are treated as both fixed and random effects.
Results
Blood DC counting method in normal individuals using CMRF-44 mAb
The CMRF-44+ DCs as measured in this method are HLA-DR++, CD4+, CD40+, CD80+, CD83+, and CD86+. They include cells from the 2 DC subsets (CD11c+CD123dim/− and CD11c−CD123+) (Figure 1B).
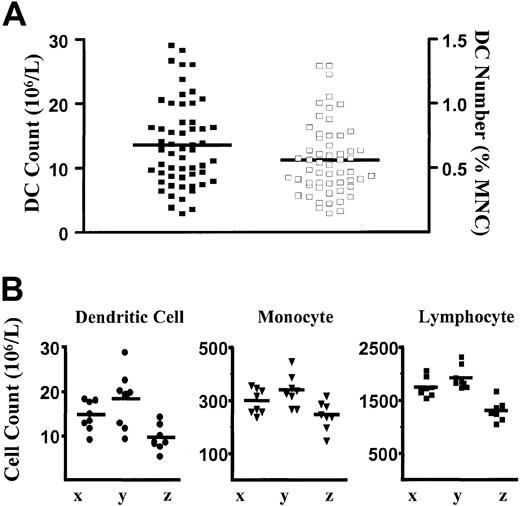
The CMRF-44 mAb was used to establish a flow cytometry–based DC counting method and to define an adult normal range elsewhere.9 Using this method, we obtained a new normal range from 56 healthy adults drawn from hospital, laboratory staff, and the general public. The mean DC count and DC percentages were 13.6 × 106/L (± 6.6 × 106/L [SD], range 2.8 × 106 to 29.0 × 106/L) and 0.56% (± 0.28% [SD], range 0.14% to 1.29%), respectively (Figure 2A). These results were similar to those obtained previously9 and verify the reproducibility of the method. Intraindividual variation was assessed by performing serial DC counts on 3 healthy volunteers on 8 separate occasions over a period of 5 months. Samples were drawn during the same time each morning on each occasion. Although some intraindividual variation in DC count is observed, the counts tend to cluster together to reflect the overall baseline normal level for each individual (Figure 2B). When counts of other MNC populations (lymphocytes and monocytes) were analyzed, similar variations were also observed for each individual (Figure 2B).
Blood DC, monocyte, and lymphocyte counts in healthy individuals.
(A) Scatter plot of the distribution of DC numbers in healthy individuals (n = 56) expressed as absolute DC count (filled squares, left axis) and as a percentage of MNCs (open squares, right axis). Lines represent the mean values. (B) Absolute blood DC, monocyte, and lymphocyte counts in 3 individuals (x, y, and z) measured on 8 separate occasions over a 5-month period. The means are shown as lines. The monocyte and lymphocyte counts were obtained from the automated hematology cell counter.
Blood DC, monocyte, and lymphocyte counts in healthy individuals.
(A) Scatter plot of the distribution of DC numbers in healthy individuals (n = 56) expressed as absolute DC count (filled squares, left axis) and as a percentage of MNCs (open squares, right axis). Lines represent the mean values. (B) Absolute blood DC, monocyte, and lymphocyte counts in 3 individuals (x, y, and z) measured on 8 separate occasions over a 5-month period. The means are shown as lines. The monocyte and lymphocyte counts were obtained from the automated hematology cell counter.
Blood DC counts are altered by surgical and exercise stress
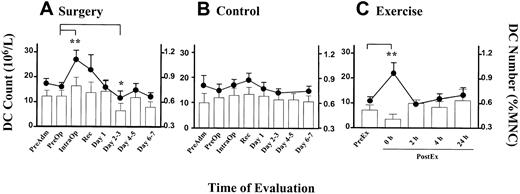
Serial DC counts were performed on patients undergoing elective laparoscopic cholecystectomy. DC counts rose in all but one patient, peaking either during surgery (IntraOp) in 15 (58%) patients, immediately after (Recovery) in 6 (23%) patients, or on day 1 after surgery in 4 (15%) patients. The mean DC count recorded a significant rise (P = .0001) from 16.0 × 106/L (± 1.7 × 106/L [SEM]) at PreOp to 27.0 × 106/L (± 3.7 × 106/L [SEM]) at IntraOp (Figure 3A, line graph, left axis). The change in DC counts (106/L) was closely reflected in the DC numbers as a percentage of MNCs (Figure 3A, column, right axis). The DC counts were observed to be lowest in the day 2-3 time point (P = .05, Figure 3A). A small (statistically nonsignificant) increase in DC counts was detected in the control group, possibly reflecting a circadian variation (Figure 3B).
Alteration in blood DC counts in surgery and exercise.
Time course of DC count (line graph, left axis) and DC number (percentage of MNCs, bar graph, right axis) in (A) patients undergoing laparoscopic cholecystectomy (n = 26), (B) healthy individuals at equivalent time points to the patient group (n = 9), and (C) healthy volunteers during exercise (n = 5). Each point represents the mean value with the error bars showing SEM. (**P < .01, *P = .05).
Alteration in blood DC counts in surgery and exercise.
Time course of DC count (line graph, left axis) and DC number (percentage of MNCs, bar graph, right axis) in (A) patients undergoing laparoscopic cholecystectomy (n = 26), (B) healthy individuals at equivalent time points to the patient group (n = 9), and (C) healthy volunteers during exercise (n = 5). Each point represents the mean value with the error bars showing SEM. (**P < .01, *P = .05).
To compare the effect of another type of stress in the form of exercise, we monitored the DC counts in healthy volunteers who undertook the treadmill exercise program according to the Bruce protocol. All subjects reached stage 5 of the protocol and achieved their maximum predicted heart rate prior to completing the exercise. A rise in the blood DC count (106/L) measured immediately after the exercise was observed in all subjects. The mean DC count at the peak was 22.0 × 106/L (± 4.2 × 106/L [SEM], P = .0004; Figure3C, line, left axis). Two hours after the cessation of exercise, the DC counts returned to baseline levels. Interestingly, the DC proportion (ie, percentage of MNCs) did not rise (Figure 3C, column, right axis).
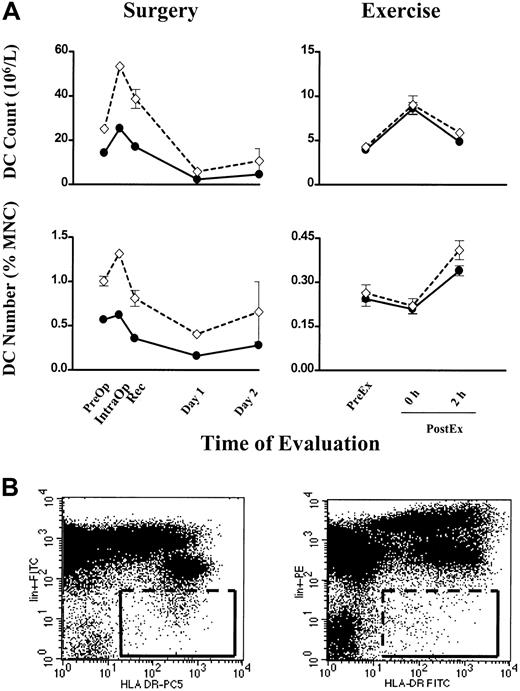
In six individuals, Lin− HLA-DR+ cells were measured concurrently with our method using the CMRF-44 mAb. A close correlation was seen in each case for DC count (106/L) and number (percentage of MNCs) in both surgery and exercise (Figure4A). The discreteness of the CMRF-44+ CD14−CD19− events facilitates a reasonably consistent gating strategy (Figure 1A, right), while the distinction between Lin− and Lin+cells is often less clear and makes the gating for the Lin− HLA-DR+ cells more uncertain (Figure 4B, left). The increase in absolute values with the latter methodology may reflect in part the variable operator dependent Lin−HLA-DR+ gating and possibly the inclusion of more CD123+ DCs. Using a different combination of fluorochromes (PE for lineage markers and FITC for HLA-DR) did not improve the definition of the Lin− HLA-DR+ cells (Figure4B, right).
Individual blood DC counts in surgery and exercise.
(A) Blood DC count (106/L) and DC number (percentage of MNCs) are plotted (●) for 2 individuals, one undergoing surgery (left column) and the other exercise (right column). For comparison, Lin− HLA-DR+ cells measured concurrently are plotted (⋄). Error bars show SEM. (B, left) A representative FITC versus PE–cyanin 5 dot plot showing the gating for Lin−HLA-DR+ cells used in the comparison above. (B, right) A dot plot for Lin− HLA-DR+ cells using the PE versus FITC combination of fluorochromes.
Individual blood DC counts in surgery and exercise.
(A) Blood DC count (106/L) and DC number (percentage of MNCs) are plotted (●) for 2 individuals, one undergoing surgery (left column) and the other exercise (right column). For comparison, Lin− HLA-DR+ cells measured concurrently are plotted (⋄). Error bars show SEM. (B, left) A representative FITC versus PE–cyanin 5 dot plot showing the gating for Lin−HLA-DR+ cells used in the comparison above. (B, right) A dot plot for Lin− HLA-DR+ cells using the PE versus FITC combination of fluorochromes.
Blood DC counts change independently of monocyte counts during surgical stress but not exercise
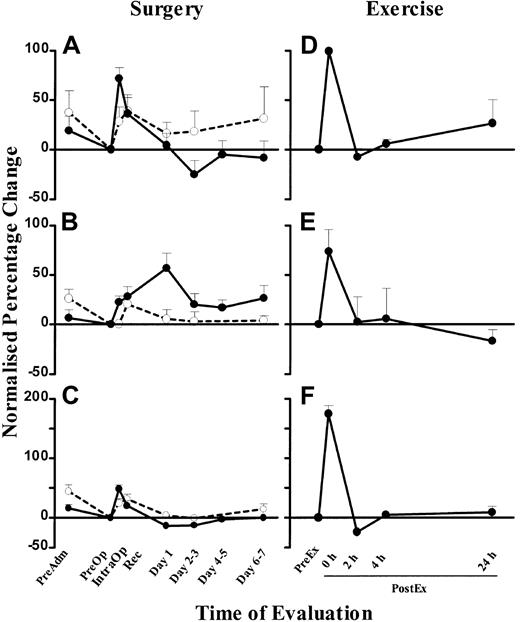
To compare the changes between DC, monocyte, and lymphocyte counts, we expressed the changes as a percentage change normalized to the PreOp counts. The mean percentage IntraOp rise in blood DCs was 71.7% ± 11% (SEM) (Figure 5A), and it fell 25% ± 14% (SEM) below PreOp values on days 2-3. DC counts in control individuals showed a small rise over the matched time course, which may reflect a circadian change, but the rise in surgical patients was significantly greater than in the controls (P < .05; Figure 5A). Blood DC counts were increased 100% ± 10% (SEM) immediately after exercise compared with the PreEx value (Figure 5D).
Normalized percentage change in blood DC, monocyte, and lymphocyte numbers in surgery and exercise.
Normalized percentage change of blood DC (A), monocyte (B), and lymphocyte (C) counts in patients undergoing laparoscopic cholecystectomy (n = 26, ●) and healthy controls at corresponding time points (n = 9, ○). Normalized percentage change of blood DC (D), monocyte (E), and lymphocyte (F) counts in healthy volunteers who underwent the exercise program (n = 5). The PreOp (A-C) or PreEx (D-F) cell counts were used as the baseline for comparison with all other time points for each study group. Each point represents the mean + SEM. The monocyte and lymphocyte counts were obtained from the automated hematology cell counter.
Normalized percentage change in blood DC, monocyte, and lymphocyte numbers in surgery and exercise.
Normalized percentage change of blood DC (A), monocyte (B), and lymphocyte (C) counts in patients undergoing laparoscopic cholecystectomy (n = 26, ●) and healthy controls at corresponding time points (n = 9, ○). Normalized percentage change of blood DC (D), monocyte (E), and lymphocyte (F) counts in healthy volunteers who underwent the exercise program (n = 5). The PreOp (A-C) or PreEx (D-F) cell counts were used as the baseline for comparison with all other time points for each study group. Each point represents the mean + SEM. The monocyte and lymphocyte counts were obtained from the automated hematology cell counter.
Monocyte counts (measured by the clinical automated counter) peaked at 57% ± 15% (SEM) above baseline levels on day 1, at a time when DC counts had already normalized levels (Figure 5B). Similar results were obtained using a CD14 mAb flow cytometry technique (data not shown). In healthy individuals, monocyte counts showed minimal changes over the matched time course. In comparison, exercise induced a greater (74% ± 22% [SEM]) rise, but in contrast to surgery, the rise paralleled the rise in DC counts (Figure 5E).
Total lymphocyte counts (measured by the clinical automated counter) rose by 48% ± 7% (SEM) during surgery, coincidental with the rise in blood DCs (Figure 5C). In healthy individuals, lymphocyte counts also showed a rise over the matched time course. Exercise also induced a concomitant rise of 175% ± 14% (SEM), greater in lymphocyte count than that seen in surgery (Figure 5F).
Blood DC activation molecules are not induced by surgical stress or exercise in vivo
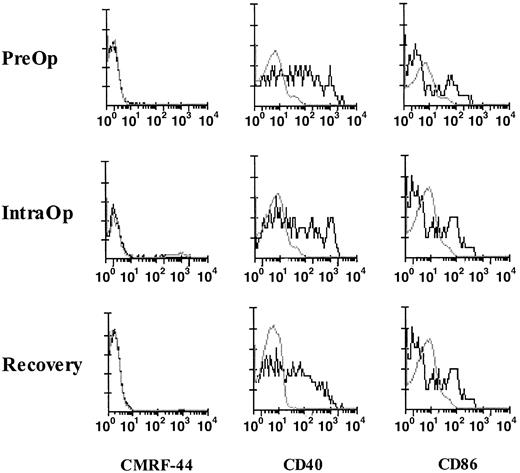
Cytokines, such as IL-1β, tumor necrosis factor-α, and IL-6, released during surgery are known to induce the in vitro up-regulation of the CD40, CD80, and CD86 molecules on blood DCs; therefore, we investigated the activation status of fresh blood DCs. The CMRF-44 antigen is rapidly up-regulated on DCs in vitro without added activation stimuli; however, negligible CMRF-44+ DCs were identified in fresh MNCs during surgery (Figure6). A proportion of fresh Lin− HLA-DR+ DCs from blood collected PreOp expressed CD40 and CD86, but no further up-regulation of either of these molecules occurred during surgery (Figure 6). No up-regulation of CMRF-44, CD40, and CD86 expression was noted during exercise.
Activation and costimulatory molecule expression of fresh DCs in surgery.
FACS analyses of fresh MNCs from a patient collected PreOp, IntraOp, and Recovery. DCs, defined and gated as Lin−HLA-DR+ cells, were analyzed for the expression of CMRF-44 (left), CD40 (middle), and CD86 (right). This result is representative of experiments from 4 patients.
Activation and costimulatory molecule expression of fresh DCs in surgery.
FACS analyses of fresh MNCs from a patient collected PreOp, IntraOp, and Recovery. DCs, defined and gated as Lin−HLA-DR+ cells, were analyzed for the expression of CMRF-44 (left), CD40 (middle), and CD86 (right). This result is representative of experiments from 4 patients.
Blood DC subsets and allostimulatory function are not altered by surgery or exercise
The Lin− HLA-DR+ DCs were also analyzed to determine the proportion of CD11c+ and CD11c− subsets. The percentage of CD11c+ and, conversely, CD11c− (predominantly CD123+) DCs remained stable during surgery (n = 8) and exercise (n = 2) (Table1). The percentage of CD123+DCs was more variable, 2 rising, 3 falling, and 1 constant in surgery (PreOp = 28.8 ± 8.0 [SEM], IntraOp = 23.9 ± 7.1, Recovery = 32.5 ± 7.8, n = 6). The rise in DC counts observed during surgery did not result in an obvious increase in the ability of the MNCs to stimulate a MLR.
Variation of DC subsets during surgery and exercise
| Surgery . | Exercise . | ||||
|---|---|---|---|---|---|
| Time points . | DC subsets . | Time points . | DC subsets . | ||
| CD11c+ . | CD11c− . | CD11c+ . | CD11c− . | ||
| PreOp | 45.7 ± 3.4 | 54.3 ± 3.4 | PreEx | 40.5 ± 2.0 | 59.5 ± 2.0 |
| IntraOp | 48.4 ± 4.7 | 51.6 ± 4.7 | PostEx (0 h) | 41.2 ± 6.8 | 58.8 ± 6.8 |
| Recovery | 44.8 ± 5.2 | 55.2 ± 5.2 | PostEx (2 h) | 47.8 ± 4.8 | 52.2 ± 4.8 |
| Surgery . | Exercise . | ||||
|---|---|---|---|---|---|
| Time points . | DC subsets . | Time points . | DC subsets . | ||
| CD11c+ . | CD11c− . | CD11c+ . | CD11c− . | ||
| PreOp | 45.7 ± 3.4 | 54.3 ± 3.4 | PreEx | 40.5 ± 2.0 | 59.5 ± 2.0 |
| IntraOp | 48.4 ± 4.7 | 51.6 ± 4.7 | PostEx (0 h) | 41.2 ± 6.8 | 58.8 ± 6.8 |
| Recovery | 44.8 ± 5.2 | 55.2 ± 5.2 | PostEx (2 h) | 47.8 ± 4.8 | 52.2 ± 4.8 |
Mean percentage ± SEM; n = 8 (surgery) and n = 2 (exercise).
The rise in blood DC count correlates with serum cortisol levels in surgical stress and exercise
The mean normalized IntraOp serum cortisol increased to 140% ± 50% (SEM) and peaked in Recovery (238% ± 48% [SEM]) before falling to near baseline levels one day after surgery. However, the stress of exercise induced a rise in serum cortisol levels in 2 of 5 individuals, which peaked 2 hours after cessation of exercise. In both situations, the peak serum cortisol occurred after the peak DC counts.
Pharmacologic steroid administration may influence DC function.16 To determine if stress corticosteroid levels influenced the expression of CMRF-44 and hence the enumeration of blood DCs in stress situations, the effect of hydrocortisone on CMRF-44 expression of DCs was tested in blood samples obtained from healthy individuals. A 2-hour preincubation period with hydrocortisone (10−6 M or 10−4 M) followed by extensive washing prior to the usual overnight culture did not appear to affect the expression of CMRF-44 molecule on blood DCs.
Discussion
We report here the first “routine” application of a method to enumerate circulating DCs in a group of patients undergoing routine surgery in a standard clinical setting. We document a rapid surgical stress-induced rise in blood DC counts and make the important physiologic observation that this in vivo rise is independent of monocyte counts, the cell suggested to be an in vitro precursor of DCs. Studies on physical exercise were also instructive—DC counts again rising but less rapidly and in parallel with monocyte counts. Taken together, these data indicate that blood DC and monocyte levels are regulated differently depending on the physiologic circumstances. We also observed a discrete (statistically nonsignificant) rise of the DC counts in samples from healthy individuals collected at various time points. This pattern may represent circadian variation of blood DC counts, a phenomenon established for other leukocyte populations.
DC counts rose early in surgery, and this was followed by a temporary depression of postoperative DC counts. This depression was limited in this relatively minor surgery but may prove more significant in major surgery. Two patients had their laparoscopic surgery converted to open cholecystectomy, where their blood DC counts peaked at 31 × 106/L and 29.6 × 106/L and fell to a nadir of 6.9 × 106/L and 3.2 × 106/L, respectively. The former had a persistently low DC count, ranging from 2.9 × 106 to 6.9 × 106/L during a 3-week follow-up period. The patient subsequently developed a chest infection and was treated with intravenous antibiotics in the hospital for 2 days, and only at week 16 did the follow-up DC count return to the preoperative level. The magnitude of the serum cortisol increases in our patients confirmed a significant stress response to surgery comparable to earlier reports.17 The well-documented effects of glucocorticoids on the immune system may be attributed to the immunosuppression seen after surgery and injury.4 The alterations in blood DC numbers after surgery may be a highly relevant factor to consider in analyzing postoperative infection risk.
DCs require defined signals to induce their differentiation and activation.2 We anticipated that the variety of inflammatory mediators released during surgery might lead to DC activation. Curiously, we found no in vivo up-regulation of the sensitive DC activation CMRF-44 antigen on fresh DCs. Similarly, no up-regulation of CD40 or CD86 was observed. This suggests that although circulating DC numbers are increased, possibly to supply damaged peripheral sites with DCs, these cells remain tightly regulated in terms of their costimulatory capacity while in circulation. This may reflect one of the mechanisms for maintaining self-tolerance and is consistent with the hypothesis that DCs are activated in peripheral tissues upon contact with the right signals and undergo further maturation as they migrate to the lymph nodes.2
The rapid increase in blood DCs during surgery suggests they are preformed and mobilized from existing “storage” sites. Inflammatory cytokines and hormones released during surgery and exercise may contribute to this phenomenon.7,8,18 There is no evidence yet to suggest blood DCs are marginated on blood vessels, but the splanchnic vasculature (including the spleen and liver) or possibly the bone marrow may be sources. DC mobilization from the tissues is unlikely, because these traffic to lymph nodes. Despite the rise in DCs during surgery, we did not observe an obvious change in the ability of the MNCs to stimulate proliferation in the MLR. This is most likely to be due to the lack of sensitivity of the MLR assay or the result of other cell types in limiting the action of DCs. Exercise induces a significant and reproducible increase in DC count, and the mediators involved may be of interest as potential mobilizers of DCs for immunotherapeutic programs. Interestingly, although the stress of surgery and exercise cannot be directly compared, similar changes on immunologic and endocrine parameters have been documented.7,18 The consistent rise in the CD11c+ DC subset (and parallel but individually inconsistent changes in CD123+ subset) in surgery contrasts with the significant changes in DC subsets observed in other situations11 (S.V., unpublished data, June 2000).
The ontogenic relationship between DCs and monocytes has been a long-standing issue and remains unresolved. Data reported here and elsewhere19 suggest that blood DC and monocyte levels are regulated differently depending on the physiologic circumstances. However, it is notable that the DC counts rise in a coordinated fashion with lymphocytes and, at face value, this might be interpreted as a coordinated production/mobilization of these 2 key interacting components of a cognate immune response.
In summary, we have validated a new test for counting blood DCs and have shown that these vital cells increase in number as part of the acute response to surgical and physical stress response. Our additional experience with the method suggests valuable clinical data will be obtained by studying other patient groups, and interesting differences are already being observed. The availability of this routine method to monitor blood DC numbers may open a new window in our understanding of the immune system and has the potential to assist the management of patients. DC counts may, for example, have prognostic value after surgery or infection. Blood DC counts will certainly facilitate the planning of any DC immunotherapy protocols.
We thank the patients and volunteers who participated in the study and thank our surgical colleagues for their contribution. We thank Dalia Khalil and Len Brown for technical support on the flow cytometer. We are grateful to Dr Maria Gilleece for critical reading of the manuscript and Tracy Heywood for help in preparing this manuscript. We especially acknowledge the assistance of Sullivan Nicolaides Pathology, Queensland, Australia, for the out-of-hospital collection of patient specimens.
Supported by a Mater Medical Research Institute grant. C.S.K.H. supported by the HIH Winterthur-Raelene Boyle Scholarship from the Royal Australasian College of Surgeons.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Derek N. J. Hart, Mater Medical Research Institute, Aubigny Place, Raymond Terrace, South Brisbane, Qld 4101, Australia; e-mail: dhart@mmri.mater.org.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal