Abstract
CD8 T cells are classified as naı̈ve, effector, or memory cells on the basis of CD45RA, CD62L, and CCR7 expression. Sequential engagement of cell-surface CD62L and CCR7 receptors is required for efficient trafficking to lymphoid tissue by means of high endothelial venules. Naı̈ve CD8 T cells are CCR7+CD62L+ CD45RA+, whereas long-term memory cells are CCR7+CD62L+CD45RA−. Effector cytotoxic T cells are thought to be CCR7−CD45RA+. The distribution of CD8 subsets and cytolytic protein expression in healthy donors and donors seropositive for human immunodeficiency virus (HIV) were compared. In HIV-infected subjects, CCR7− CD8 T cells expanded at the expense of naı̈ve and long-term memory cells. In both healthy donors and HIV-infected donors, CCR7+ CD8 T cells were uniformly negative for perforin. In all subsets, perforin and granzyme A were not coordinately expressed, with perforin expression being more tightly regulated. The properties of CD8 T cells specific for cytomegalovirus, Epstein-Barr virus (EBV), and HIV were studied by staining with major histocompatibility complex peptide tetramers. Antigen-specific cells for chronic infections with these viruses were uniformly CCR7− and predominantly CD62L−. In 2 HIV-seropositive donors, 3- to 4-fold fewer EBV-tetramer–positive cells were present in lymph nodes compared with blood. Antigen-specific CD8 T cells are therefore preferentially excluded from lymphoid sites, even when infection is primarily in lymphoid tissue. This may protect lymphoid tissues from immunopathological changes but compromise immune defense against viruses, such as HIV and EBV, that target lymphocytes. HIV-specific CD8 T cells do not express CD45RA, whereas EBV- and CMV-specific CD8 T cells are heterogeneous in CD45RA+expression. Lack of CD45RA expression may indicate incomplete differentiation of HIV-specific CD8 T cells to cytotoxic T cells.
Introduction
The development of a class I–restricted CD8 T-cell response in vivo is associated with changes in the cell-surface phenotype of CD8 T cells that reflect alterations in the migration and functional capability of these cells. Naı̈ve T cells continuously recirculate from blood to lymphoid tissue (lymph nodes [LNs]) through specialized high endothelial venules (HEVs). Sequential engagement of L-selectin (CD62L) and lymphocyte function–associated antigen (LFA) 1 on naı̈ve T cells to their respective ligands, peripheral node addressin and intercellular adhesion molecule (ICAM) 1 and ICAM-2, on HEV, sets into motion tethering, rolling, and firm adhesion of T cells to HEVs—steps that are prerequisite for transmigration of these cells into the surrounding LNs.1,2 A chemokine receptor, CCR7, has also been found to be an important determinant of T-cell homing to LNs.3-5CCR7 on T cells interacts with secondary lymphoid tissue chemokine on HEVs and regulates LN homing, presumably by delivering an activation signal required for LFA-1 binding to ligand.5-7 When the naı̈ve cell encounters antigen, it proliferates and differentiates into effector and memory cells in the specialized microenvironment of the LNs. In keeping with the requirement of effector and memory cells to exert their cytolytic and cytokine secretion function at peripheral sites of infection, previously activated CD8 T cells have different homing properties. They up-regulate adhesins, including CD44 and β1, β2, and β7 integrins, which contribute to their preferential homing to inflamed tissues, from which naı̈ve cells are normally excluded.8-11 The LN homing receptors L-selectin and CCR7 are thought to be strongly down-modulated on effector cells but heterogenously expressed on memory cells, thus suggesting that some memory cells recirculate through LNs as part of immune surveillance.12
Apart from their expression of adhesins, naı̈ve, effector, and memory cells can also be distinguished by their expression of proteins important for their activation and function.13Naı̈ve cells express the RA isoform of CD45, are CD28+ and CD27+, and do not express the cytolytic effector molecules, perforin and granzymes. Although most previously activated T cells are CD45RO+ and CD45RA−, a subset of terminally differentiated CD8 T cells with immediate ex vivo cytolytic ability expresses CD45RA. Data have suggested that, in fact, there may be 3 distinct subsets of antigen-primed CD8 T cells, distinguished by their expression of CCR7 and CD45RA.12 These have been classified as a long-lived CCR7+CD45RA− “central memory” subset, a CCR7−CD45RA+ “effector” subset, and a CCR7−CD45RA− “effector memory” subset. The functional differences between effector cells and effector memory cells are unknown, although the latter are thought to require further differentiation for cytotoxic function.
Infection with human immunodeficiency virus (HIV) stimulates one of the strongest known human antiviral CD8 T-cell responses, with tremendous expansion of previously activated CD8 T cells and a high frequency of circulating HIV-specific CD8 T cells.14-18 Paradoxically, despite their high frequency (estimated to be orders of magnitude greater than the number of HIV-replicating targets19), CD8 T cells are unable to eradicate the virus or halt progressive immunodeficiency in most subjects with HIV infection not receiving antiviral drugs. CD8 T cells in HIV-infected subjects are phenotypically heterogeneous.20 This heterogeneity likely reflects each cell's unique history of antigen exposure (in a setting of ongoing viral replication and chronic inflammation) and functional capability. Some alterations in CD8 T cells may interfere with effective antiviral immunity. Because HIV infection is concentrated in LNs, lack of expression of molecules that target effector cytotoxic T lymphocytes (CTLs) to the LNs might interfere with their ability to provide protection. In fact, one study found a lack of perforin expression, which is required for effective CTL-mediated lysis of HIV-infected cells, in the LNs of HIV-infected subjects.21
In an effort to understand the reasons for the inadequate immune protection in HIV infection, we compared the distribution and functional potential of naı̈ve, memory, and effector subsets of CD8 T cells in healthy and HIV-infected subjects. We found that, in HIV infection, there is a paucity of LN-homing naı̈ve and long-term memory cells, which is more profound in patients with acquired immunodeficiency syndrome (AIDS). Antigen-specific CD8 T cells from HIV-infected donors as well as those from healthy volunteers, stained with tetramers for 3 viruses—HIV, Epstein-Barr virus (EBV), and cytomegalovirus (CMV)—that cause chronic infections, uniformly lacked CCR7 expression and generally did not express CD62L.
Patients and methods
Subjects
Subjects were healthy volunteers and patients who were seropositive for HIV-1 and had various stages of disease. Informed consent was obtained from each subject, and the study was approved by the Center for Blood Research Institutional Review Board and the human investigation committees of the local hospital sites. Blood samples were either freshly obtained or cryopreserved by using a programmed cell freezer (model 9000; Gordinier, Roseville, MI). Flow cytometry results obtained with thawed cells were comparable to those achieved with freshly isolated cells in 2 samples studied, as long as peripheral blood mononuclear cells (PMBC) were frozen within 2 hours of Ficoll-Hypaque density separation. LN aspirates were obtained from palpably enlarged peripheral LNs (PLNs) by using a 20-gauge needle with a 10-mL syringe attached to an aspiration syringe gun.
Tetramers
Tetramers were produced by us or by the National Institute of Allergy and Infectious Diseases Major Histocompatibility Complex (MHC) Tetramer Core Facility (Yerkes Regional Primate Research Center, Atlanta, GA) as described previously.17 22 The tetramers used in this study are listed in Table 1. We are able to characterize tetramer-positive (tetramer+) cells if they are present at a frequency above 0.01% of CD8 T cells.
Tetramers used in the study
| Virus/protein . | Amino acids . | Sequence . | Restricting element . | References . | HIV-seropositive donors . | Healthy donors . | ||
|---|---|---|---|---|---|---|---|---|
| Subjects with recognition of tetramer . | Frequency of CD8 T cells, range (%) . | Subjects with recognition of tetramer . | Frequency in CD8 T cells, range (%) . | |||||
| HIV/gag | 77-85 | SLYNTVATL | A0201 | 17, 23, 24 | 2/5 | 0.21-0.22 | 0 | |
| HIV/RT | 309-317 | ILKEPVHGV | A0201 | 17, 24, 25 | 2/4 | 0.36-0.95 | 0 | |
| HIV/env | 593-600 | YLKDQQLL | B8 | 26 | 3/7 | 0.05-0.11 | 0 | |
| CMV/pp65 | 495-503 | NLVPMVATV | A0201 | 27-29 | 4/5 | 0.18-11 | 3/8 | 0.25-4.85 |
| EBV/BMLF1 | 280-288 | GLCTLVAML | A0201 | 30, 31 | 4/5 | 0.02-0.16 | 4/8 | 0.08-0.12 |
| EBV/BZLF1 | 190-197 | RAKFKQLL | B8 | 30, 31 | 6/6 | 0.18-3.06 | 1/1 | 0.37 |
| Virus/protein . | Amino acids . | Sequence . | Restricting element . | References . | HIV-seropositive donors . | Healthy donors . | ||
|---|---|---|---|---|---|---|---|---|
| Subjects with recognition of tetramer . | Frequency of CD8 T cells, range (%) . | Subjects with recognition of tetramer . | Frequency in CD8 T cells, range (%) . | |||||
| HIV/gag | 77-85 | SLYNTVATL | A0201 | 17, 23, 24 | 2/5 | 0.21-0.22 | 0 | |
| HIV/RT | 309-317 | ILKEPVHGV | A0201 | 17, 24, 25 | 2/4 | 0.36-0.95 | 0 | |
| HIV/env | 593-600 | YLKDQQLL | B8 | 26 | 3/7 | 0.05-0.11 | 0 | |
| CMV/pp65 | 495-503 | NLVPMVATV | A0201 | 27-29 | 4/5 | 0.18-11 | 3/8 | 0.25-4.85 |
| EBV/BMLF1 | 280-288 | GLCTLVAML | A0201 | 30, 31 | 4/5 | 0.02-0.16 | 4/8 | 0.08-0.12 |
| EBV/BZLF1 | 190-197 | RAKFKQLL | B8 | 30, 31 | 6/6 | 0.18-3.06 | 1/1 | 0.37 |
HIV indicates human immunodeficiency virus; CMV, cytomegalovirus; and EBV, Epstein-Barr virus.
Flow cytometry
For external staining, PBMC (2-10 × 105/tube) were stained as previously described22 with the following fluorescein isothiocyanate–conjugated (FITC), phycoerythrin (PE)-conjugated, or Cy5-conjugated monoclonal antibodies (mAbs): CD8 (mAb B9.11; Immunotech, Marseille, France), CD45RA (mAb ALB11; Immunotech), or CD62L (mAb DREG-56; Pharmingen, San Diego, CA), or IgG-FITC, IgG-PE–conjugated, and IgG-Cy5–conjugated, isotype-matched controls (Immunotech). For CCR7 assessment, cells were stained with α-human CCR7 mAb 7H12 (IgG2b produced to recombinant protein at LeukoSite, Cambridge, MA) and then with PE-conjugated F(ab′)2 goat antimouse Ig (Immunotech). After external staining, cells were resuspended in 50 μL Hanks balanced salt solution and permeabilized by using a Fix and Perm Kit (Caltag Laboratories, Burlingame, CA) according to the manufacturer's protocol before staining with FITC or PE-conjugated α- granzyme A (GzmA) mAb (CB9; Pharmingen) or α-perforin mAb (δG9; Pharmingen). For tetramer staining, PBMC (2 × 106) were resuspended in 500 μL fluorescence-activated cell-sorter (FACS) buffer and stained for 40 minutes at 4°C with 0.5 μg/mL streptavidin PE-conjugated tetramers. The cells were washed and resuspended in FACS buffer for costaining with the mAbs described above. Stained cells were resuspended in 50 μL FACS buffer and 2% formaldehyde for analysis on a CD8bright tightly gated lymphocyte population (FACScalibur; Becton Dickinson, Mountain View, CA). Gates were defined by requiring that fewer than 1% of the isotype-control antibody-stained cells were positive.
Statistical analysis
Data from donors with HIV infection were analyzed for significant differences from data from healthy volunteers by using the 2-tailed Student t test and the nonparametric Mann-Whitney test. All analyses were also done to determine whether there were significant correlations with Centers for Disease Control (CDC)–defined disease stage32 and plasma viremia.
Results
Reduction of naı̈ve and long-term memory cells and expansion of effector and effector memory T cells in HIV infection
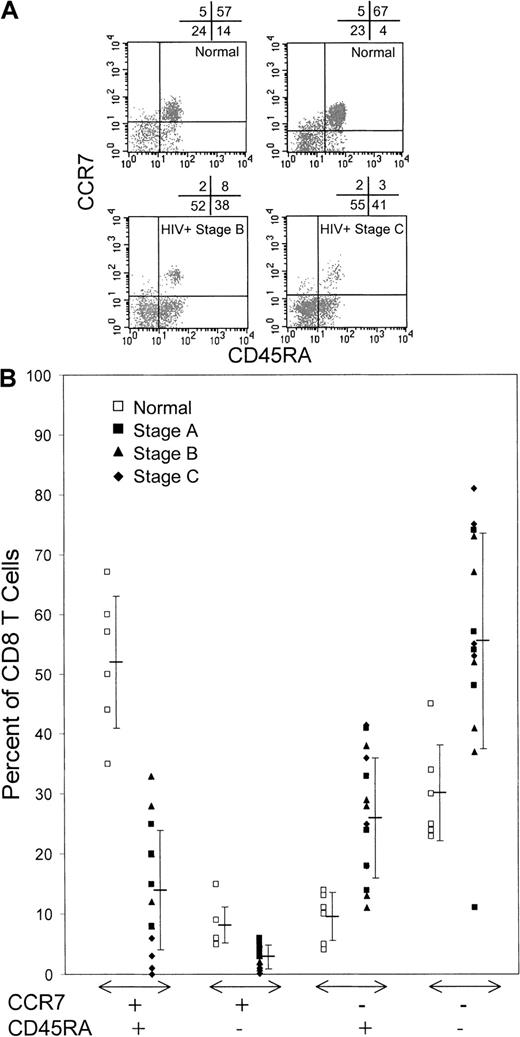
We compared the CD8 T-cell subset distribution, defined by CCR7, CD45RA, and CD62L expression, in 14 HIV-infected subjects at various stages of disease (5 with CDC stage A, 5 with stage B, and 4 with stage C) with that in healthy donors (Figure1). There was a marked reduction in circulating CCR7+ CD8 T cells in HIV-infected donors compared with healthy donors, particularly in patients with AIDS. Whereas 60% ± 9% of healthy donor CD8 T cells were CCR7+, only 17% ± 11% of CD8 T cells from HIV-infected donors expressed this LN-homing molecule (P < .0001). The proportions of CCR7+ CD8 T cells were similar in infected donors with stage A disease and those with stage B (21% ± 6% and 22% ± 9%, respectively) but decreased considerably in those with advanced stage C disease (4% ± 4%).
HIV-infected donors have few CCR7+naı̈ve (CCR7+CD45RA+) or long-term memory (CCR7+CD45RA−) CD8 T cells but compensate with expansions of effector (CCR7−CD45RA+) and effector memory (CCR7−CD45RA−) cells.
(A) Representative dot plots of gated CD8bright lymphocytes from healthy donors and HIV-infected donors classified according to disease stages determined in 1993 by the CDC.32 (B) Summary of CCR7 and CD45RA costaining data from healthy donors and HIV-infected donors. The proportion of CCR7+ naı̈ve and long-term memory cells was lowest in patients with stage C AIDS. The differences between healthy donors and HIV-infected donors are significant.
HIV-infected donors have few CCR7+naı̈ve (CCR7+CD45RA+) or long-term memory (CCR7+CD45RA−) CD8 T cells but compensate with expansions of effector (CCR7−CD45RA+) and effector memory (CCR7−CD45RA−) cells.
(A) Representative dot plots of gated CD8bright lymphocytes from healthy donors and HIV-infected donors classified according to disease stages determined in 1993 by the CDC.32 (B) Summary of CCR7 and CD45RA costaining data from healthy donors and HIV-infected donors. The proportion of CCR7+ naı̈ve and long-term memory cells was lowest in patients with stage C AIDS. The differences between healthy donors and HIV-infected donors are significant.
Previous studies showed a reduced proportion of circulating naı̈ve CD8 T cells (defined as CD62L+CD45RA+) in HIV infection, which becomes more pronounced with advanced disease.33 In our study, the proportions of both CCR7+CD45RA+ naı̈ve cells and CCR7+CD45RA− long-term memory cells were reduced significantly in HIV infection. Whereas most circulating CD8 T cells in healthy donors were CCR7+CD45RA+(52% ± 12%), the numbers of naı̈ve cells were decreased substantially in subjects with HIV infection (14% ± 10%;P < .0001). This reduction was apparent in all stages of disease but was most pronounced in patients with late-stage disease (stage A, 17% ± 6%; stage B, 20% ± 9%; and stage C, 2% ± 2%). Although healthy donors had few circulating CCR7+CD45RA− long-term memory cells (8% ± 4%), the long-term memory pool was even smaller in donors with HIV infection (all HIV-infected donors, 3% ± 2% [P < .0004]; donors with stage A disease, 5% ± 1%; donors with stage B, 2% ± 1%; and donors with stage C, 2% ± 2%). In subjects with HIV infection, there was a reciprocal increase in tissue-homing CD8 T cells, including both CCR7−CD45RA+ terminally differentiated effector cells (all HIV-infected donors, 26% ± 10%; healthy donors, 10% ± 4%; P < .002) and CCR7−CD45RA− CD8 T cells, whose functional role is unclear (all HIV-infected donors, 55% ± 18%; healthy donors, 30% ± 8%; P < .006). The size of these CCR7− populations did not change significantly with disease stage.
A similar analysis was done for coexpression of CCR7 and CD62L in circulating CD8 T cells. Two thirds of CD8 T cells in HIV-infected donors (63% ± 15%) lacked both LN-homing molecules, whereas fewer than one third in healthy donors were CCR7−CD62L− (28% ± 13%;P < .0001). Similarly, there were twice as many CCR7−CD62L+ CD8 T cells in HIV-infected donors than in healthy donors (24% ± 15% versus 11% ± 7%;P < .05).
GzmA and perforin are not coordinately expressed in CD8 T cells
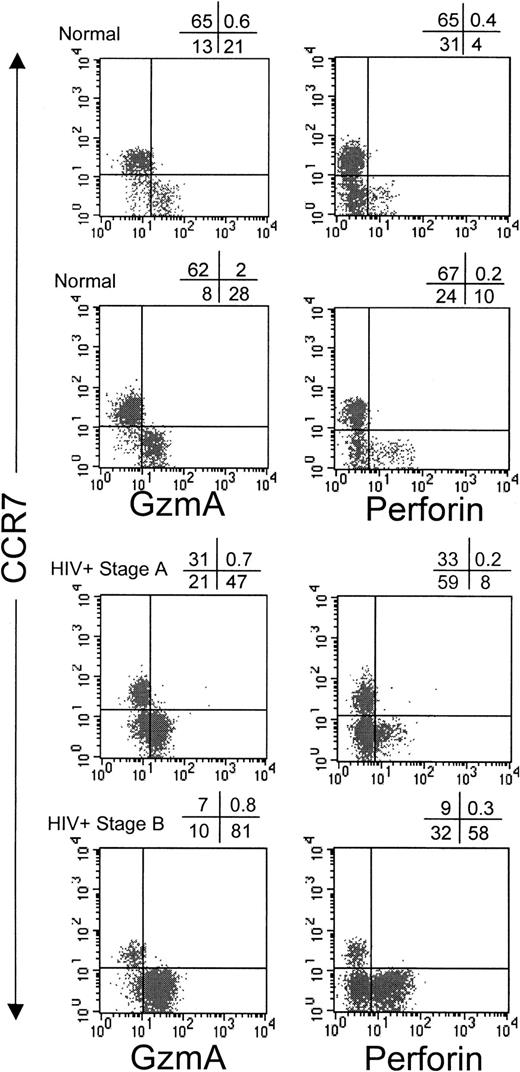
Perforin expression and granzyme expression have generally been thought to be up-regulated in parallel when CD8 T cells differentiate into effector CTLs.34 It is likely that GzmA, the most abundant granzyme in CTLs, continues to be expressed in many previously activated cells, since GzmA was found to be expressed in circulating CD8 T cells from healthy donors in proportion to the numbers of circulating CD8 T cells that were not naı̈ve.35However, the kinetics of perforin expression after effector cell differentiation has not been studied. We therefore assessed perforin and GzmA expression in circulating CD8 T cells costained for CCR7, CD45RA, and CD62L (Figure 2, Figure3, and Table2). In 5 healthy donors, there was a 4.5-fold excess of GzmA-positive (GzmA+) CD8 T cells compared with perforin-positive (perforin+) CD8 T cells; 27% ± 8% of CD8 T cells were GzmA+, whereas only 6% ± 2% were perforin+ (P < .01). Most CD8 T cells that were not naı̈ve (because they no longer expressed CCR7, CD45RA, or CD62L) stained for GzmA. Of CCR7− cells, 69% ± 7% were GzmA+. Similarly, 63% ± 9% of CD45RA− cells and 84% ± 2% of CD62L− cells were GzmA+. CCR7+ CD8 T cells, which include naı̈ve and long-term memory cells, uniformly did not express perforin in samples from healthy donors. Only 0.4% ± 0.4% of CCR7+ cells were perforin+, whereas 3.2% ± 1.6% were GzmA+. Therefore, the cells that homed to the LNs had no immediate cytotoxic capability.
Circulating CCR7+ CD8 T cells in healthy donors and HIV-infected donors do not express GzmA or perforin.
Representative dot plots show costaining of gated CD8brightlymphocytes with CCR7 and GzmA or perforin.
Circulating CCR7+ CD8 T cells in healthy donors and HIV-infected donors do not express GzmA or perforin.
Representative dot plots show costaining of gated CD8brightlymphocytes with CCR7 and GzmA or perforin.
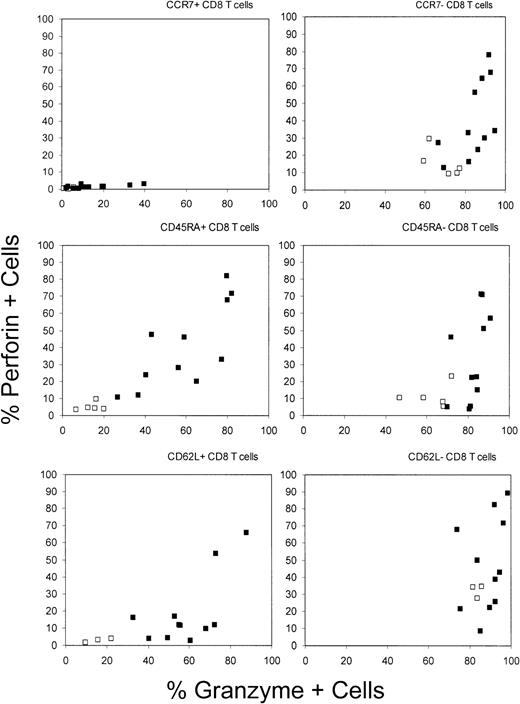
GzmA and perforin are differentially expressed in CD8 T-cell subsets defined by CCR7, CD45RA, or CD62L expression.
GzmA was expressed in many more cells than perforin. CCR7+cells from healthy donors and HIV-infected donors did not express perforin. In HIV-infected donors, higher numbers of GzmA-expressing and perforin-expressing cells were found in all subsets except LN-homing CCR7+ CD8 T cells. □ indicates NI; •, HIV+.
GzmA and perforin are differentially expressed in CD8 T-cell subsets defined by CCR7, CD45RA, or CD62L expression.
GzmA was expressed in many more cells than perforin. CCR7+cells from healthy donors and HIV-infected donors did not express perforin. In HIV-infected donors, higher numbers of GzmA-expressing and perforin-expressing cells were found in all subsets except LN-homing CCR7+ CD8 T cells. □ indicates NI; •, HIV+.
CD8 T cells of each phenotype that expressed granzyme A (GzmA) or perforin
| Donor group . | CD8 T cells . | CCR7+ . | CCR7− . | CD45RA+ . | CD45RA− . | CD62L+ . | CD62L− . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | |
| Healthy (n = 5) | 6 ± 2 | 27 ± 8 | 0.4 ± 0.4 | 3 ± 2 | 16 ± 7 | 69 ± 7 | 5 ± 2 | 14 ± 5 | 11 ± 6 | 63 ± 9 | 3 ± 1 | 16 ± 5 | 32 ± 3 | 84 ± 2 |
| HIV-seropositive (n = 11) | 34 ± 20 | 67 ± 20 | 1.5 ± 1.0 | 15 ± 12 | 40 ± 22 | 84 ± 9 | 40 ± 25 | 59 ± 20 | 34 ± 26 | 82 ± 6 | 19 ± 21 | 59 ± 16 | 47 ± 27 | 89 ± 8 |
| Stage A (n = 5) | 28 ± 18 | 71 ± 14 | 1.0 ± 0.9 | 14 ± 11 | 34 ± 18 | 85 ± 9 | 35 ± 20 | 55 ± 15 | 34 ± 26 | 83 ± 7 | 19 ± 18 | 58 ± 12 | 47 ± 20 | 88 ± 7 |
| Stage B (n = 4) | 35 ± 18 | 56 ± 24 | 1.7 ± 0.7 | 9 ± 4 | 41 ± 20 | 80 ± 8 | 37 ± 24 | 54 ± 21 | 27 ± 22 | 80 ± 6 | 9 ± 5 | 50 ± 10 | 43 ± 27 | 87 ± 9 |
| Stage C (n = 2) | 48 ± 21 | 81 ± 5 | 2.3 ± 0.7 | 30 ± 10 | 54 ± 24 | 91 ± 1 | 58 ± 24 | 79 ± 1 | 47 ± 24 | 86 ± 1 | 39 ± 27 | 80 ± 8 | 56 ± 34 | 94 ± 4 |
| Donor group . | CD8 T cells . | CCR7+ . | CCR7− . | CD45RA+ . | CD45RA− . | CD62L+ . | CD62L− . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | Perforin . | GzmA . | |
| Healthy (n = 5) | 6 ± 2 | 27 ± 8 | 0.4 ± 0.4 | 3 ± 2 | 16 ± 7 | 69 ± 7 | 5 ± 2 | 14 ± 5 | 11 ± 6 | 63 ± 9 | 3 ± 1 | 16 ± 5 | 32 ± 3 | 84 ± 2 |
| HIV-seropositive (n = 11) | 34 ± 20 | 67 ± 20 | 1.5 ± 1.0 | 15 ± 12 | 40 ± 22 | 84 ± 9 | 40 ± 25 | 59 ± 20 | 34 ± 26 | 82 ± 6 | 19 ± 21 | 59 ± 16 | 47 ± 27 | 89 ± 8 |
| Stage A (n = 5) | 28 ± 18 | 71 ± 14 | 1.0 ± 0.9 | 14 ± 11 | 34 ± 18 | 85 ± 9 | 35 ± 20 | 55 ± 15 | 34 ± 26 | 83 ± 7 | 19 ± 18 | 58 ± 12 | 47 ± 20 | 88 ± 7 |
| Stage B (n = 4) | 35 ± 18 | 56 ± 24 | 1.7 ± 0.7 | 9 ± 4 | 41 ± 20 | 80 ± 8 | 37 ± 24 | 54 ± 21 | 27 ± 22 | 80 ± 6 | 9 ± 5 | 50 ± 10 | 43 ± 27 | 87 ± 9 |
| Stage C (n = 2) | 48 ± 21 | 81 ± 5 | 2.3 ± 0.7 | 30 ± 10 | 54 ± 24 | 91 ± 1 | 58 ± 24 | 79 ± 1 | 47 ± 24 | 86 ± 1 | 39 ± 27 | 80 ± 8 | 56 ± 34 | 94 ± 4 |
Values are means ± SD percentages.
Perforin staining results do not support the designation of effector and effector memory populations based on CD45RA expression
When perforin and GzmA staining were done in conjunction with CD45RA staining in samples from healthy donors, there were many more GzmA+ cells than perforin+ cells in both the CD45RA+ and CD45RA− subsets. We found 3 times as many GzmA+ CD45RA+ cells as perforin+ CD45RA+ cells and 6 times as many in the CD45RA− subset. Therefore, although a larger proportion of the GzmA+ CD45RA+ cells were perforin+, at least two thirds of the GzmA+CD45RA+ cells did not stain for perforin and are therefore unlikely to be cytolytic without additional changes in protein expression. The highest concentration of perforin+ CD8 T cells in healthy donors were CD62L− (32% ± 3%; Figure3 and Table 2). These results suggest that the assignment of CCR7−CD62L−CD45RA+ T cells as effector CTLs13 20 may be somewhat justified but is an oversimplification. Because perforin expression is absolutely required for granule-mediated cytolysis, some CCR7−CD45RA− cells are effector CTLs, whereas most CCR7−CD45RA+ CD8 T cells are unlikely to be immediately cytotoxic without additional differentiation.
Increase of GzmA+ and perforin+ CD8 T cells in HIV infection
The proportion of GzmA- and perforin-expressing CD8 T cells in HIV-infected donors was substantially greater than that in healthy donors, again with more cells expressing GzmA than perforin. In donors with HIV infection, 67% ± 21% of CD8 T cells were GzmA+ (P < .001 versus results in healthy donors), whereas 34% ± 21% were perforin+(P < .01 versus results in healthy donors). The number of perforin+ CD8 T cells appeared to increase with disease severity, but the differences were not significant in this small sample. As in samples from healthy donors, virtually no CCR7+ CD8 T cells expressed perforin (1.5% ± 1.0%). However, a small proportion of CCR7+ CD8 T cells in samples from HIV-infected donors expressed GzmA (15% ± 12% versus 3% ± 2% in healthy donors; Figures 2 and 3 and Table 2). This suggests that long-term memory cells have down-modulated perforin but that GzmA expression is less tightly regulated and may persist in some central memory cells as they traffic to the LNs. In healthy donors, the distribution of perforin- and GzmA-expressing cells analyzed for coexpression of the other LN-homing marker CD62L was similar to that for CCR7. CD62L+ cells generally did not express perforin, although the results were not as uniform as those in CCR7+cells. However, in samples from HIV-infected donors, most CD62L+ cells were GzmA+ and, in some of these donors, a good proportion (19% ± 21%) also expressed perforin.
Perforin was expressed in many CD45RA+ and CD45RA− CD8 T cells in samples from donors with HIV infection (means for perforin, 40% and 34% of cells, respectively; and means for GzmA, 59% and 82% of cells). Thus, CD45 isoform expression may not differentiate between effector and effector memory subsets, particularly in inflammatory settings such as HIV infection.
Antigen-specific CD8 T cells do not express CCR7 and most are also CD62L−
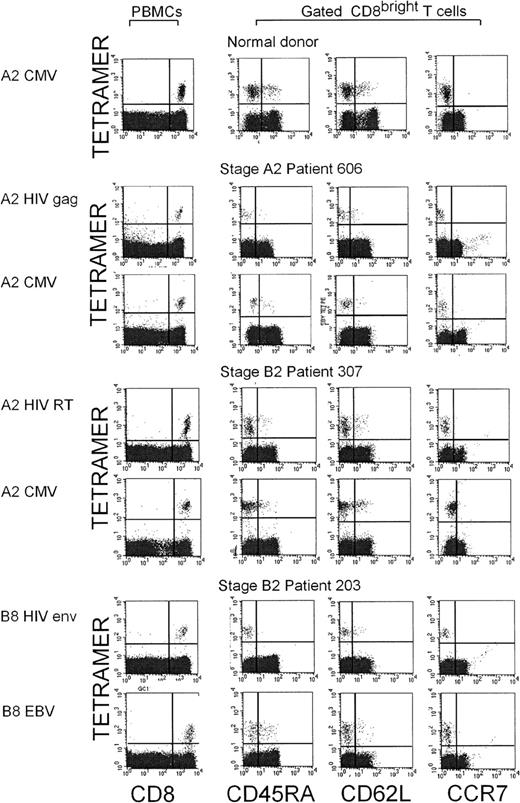
We used tetramer staining to examine cell-surface expression of the LN-homing receptors on antigen-specific CD8 T cells that recognize HIV, CMV, or lytic EBV antigens in the context of HLA A0201 or B8. The tetramers used for this analysis were for HIV A0201–restricted gag and RT epitopes and a B8-restricted env epitope, a CMV A0201–restricted pp65 epitope, and EBV A0201–restricted BMLF1 and B8-restricted BZLF1 epitopes (Table 1). PBMC from 6 HIV-infected subjects positive for A0201 and 7 positive for B8 (various disease stages) and from 8 healthy donors positive for A0201 and one positive for B8 were analyzed. Costaining for homing receptors was done if a discrete population of tetramer+ cells above background levels could clearly be identified, which was possible if the frequency of tetramer+ CD8 T cells was greater than 1 of 10 000. Representative flow cytometry plots are shown in Figure4. The proportion of donors who met this criterion for each epitope and the frequencies of tetramer+cells are shown in Table 1. Generally, the proportion of donors with staining above background levels and the frequency of tetramer+ cells was higher for the EBV and CMV tetramers than for the HIV tetramers used in this study. This may reflect the fact that the epitopes for HIV tetramers used in this and other studies are rarely immunodominant.36 In the HIV-infected donors, there was no relation between the frequencies of specific cells and HIV disease stage, nor was there a difference between healthy donors and HIV-infected donors in the range of frequencies for EBV- and CMV-tetramer+ cells. However, the numbers of subjects with recognition of any particular epitope studied were small.
Antigen-specific CD8 T cells from healthy donors and HIV-seropositive donors (various disease stages), stained with A0201 and B8 tetramers for HIV, CMV, and EBV epitopes, do not express LN-homing receptors.
Representative dot plots for costaining of the indicated tetramers with CCR7, CD62L, and CD45RA are shown. Although the pattern of CCR7 and CD62L staining for tetramer+ CD8 T cells responding to all 3 viruses was similar, CD45RA staining was reduced in HIV-specific T cells compared with cells specific for the other viruses causing chronic infections.
Antigen-specific CD8 T cells from healthy donors and HIV-seropositive donors (various disease stages), stained with A0201 and B8 tetramers for HIV, CMV, and EBV epitopes, do not express LN-homing receptors.
Representative dot plots for costaining of the indicated tetramers with CCR7, CD62L, and CD45RA are shown. Although the pattern of CCR7 and CD62L staining for tetramer+ CD8 T cells responding to all 3 viruses was similar, CD45RA staining was reduced in HIV-specific T cells compared with cells specific for the other viruses causing chronic infections.
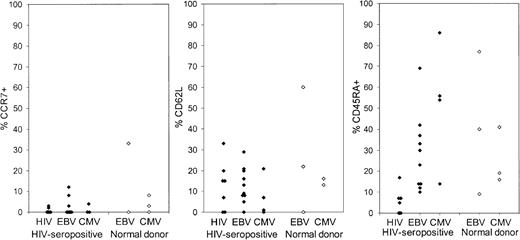
Few tetramer+ CD8 T cells recognizing HIV, EBV, or CMV antigens stained for the chemokine receptor CCR7 (Figure5). Of 25 samples costained for tetramer and CCR7, 15 had no tetramer+ cells staining for CCR7. Only one sample (for an EBV tetramer in a sample from a healthy donor) had any substantial number of cells with detectable CCR7 expression. The other LN-homing molecule, CD62L, was more often expressed on tetramer+ cells but was still only present in fewer than one third of tetramer+ cells, except in the sample with a higher frequency of CCR7 staining. In that sample, 33% of tetramer+ EBV-specific CD8 T cells expressed CCR7 and 60% expressed CD62L. The pattern of CD62L staining on samples from healthy donors was similar to that on samples from HIV-seropositive donors.
Antigen-specific CD8 T cells for HIV, CMV, and EBV uniformly do not express CCR7.
The proportion of tetramer+ CD8 T cells that stained for CCR7, CD62L, and CD45RA is shown for samples from 12 HIV-infected donors and 6 healthy donors who had recognition of at least one of the tetramers listed in Table 1. Expression of CCR7 and CD62L was similar in HIV and other viral-specific cells and in samples from HIV-seropositive donors and healthy donors. However, CD45RA was conspicuously not expressed only in HIV-specific CD8 T cells.
Antigen-specific CD8 T cells for HIV, CMV, and EBV uniformly do not express CCR7.
The proportion of tetramer+ CD8 T cells that stained for CCR7, CD62L, and CD45RA is shown for samples from 12 HIV-infected donors and 6 healthy donors who had recognition of at least one of the tetramers listed in Table 1. Expression of CCR7 and CD62L was similar in HIV and other viral-specific cells and in samples from HIV-seropositive donors and healthy donors. However, CD45RA was conspicuously not expressed only in HIV-specific CD8 T cells.
HIV-specific CD8 T cells, unlike EBV- and CMV-specific T cells, do not express CD45RA
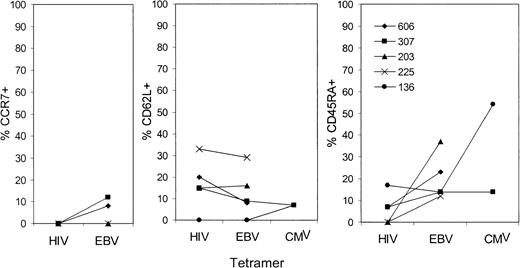
Because CD45RA expression may be linked to effector CTL function, we also stained tetramer+ cells for CD45RA. In HIV-seropositive donors, only 5.1% ± 5.7% of HIV-tetramer+ cells expressed CD45RA, whereas 35.3% ± 22.8% of EBV- or CMV-specific cells from the same subject group were CD45RA+ (Figure 5); the difference was significant (P < .004). The proportion of EBV- and CMV-specific cells that were CD45RA+ in samples from healthy donors (33.7% ± 22.8%) was similar to that in samples from HIV-seropositive donors. Because only 5 of the 13 HIV-infected subjects studied had tetramer-staining cells above background levels for both HIV and non-HIV tetramers, we also graphed CCR7, CD62L, and CD45RA expression in the tetramer+ cells from those 5 donors (Figure 6). Although levels of CCR7 and CD62L expression for HIV-specific and non–HIV-specific cells were comparable, there was substantially more CD45RA expression in the EBV- and CMV-specific cells than in the HIV-specific cells when each donor was considered individually.
Comparison of the proportion of tetramer+CD8 T cells of different phenotypes recognizing different viruses in the same HIV-infected donor.
Subject 606 had CDC stage A2 disease; subjects 307, 203, and 136, stage B2 disease; and subject 225, stage B3 disease. There were no obvious differences in trafficking phenotype according to disease stage. CD45RA was not expressed on HIV-specific tetramer+ cells, but otherwise the expression patterns for cells recognizing different viruses were similar in the same donor.
Comparison of the proportion of tetramer+CD8 T cells of different phenotypes recognizing different viruses in the same HIV-infected donor.
Subject 606 had CDC stage A2 disease; subjects 307, 203, and 136, stage B2 disease; and subject 225, stage B3 disease. There were no obvious differences in trafficking phenotype according to disease stage. CD45RA was not expressed on HIV-specific tetramer+ cells, but otherwise the expression patterns for cells recognizing different viruses were similar in the same donor.
Antigen-specific CD8 T cells are decreased in PLN compared to blood
Because tetramer+ cells did not express CD62L and CCR7, molecules required for interaction with PLN HEVs, we investigated whether tetramer+ cells were reduced in peripheral lymph nodes (PLN) compared with blood. Paired blood and PLN aspirate samples were obtained from 2 HLA B8-positive, HIV-seropositive asymptomatic subjects not currently receiving antiretroviral drugs. Samples from both donors stained for B8 EBV tetramers but not for B8 HIV tetramers. We therefore compared the distribution of total CD8 T cells and EBV-tetramer+ cells in PBMC and PLN. CD62L− and CCR7− CD8 T cells were more than 2-fold more frequent in PBMC than in PLN (Figure7). Correspondingly, there were only half as many CD8+ T cells in PLN as were found in PBMC. These results indicate that although CD62L+CCR7+ CD8 T cells preferentially home to PLN, CD62L−CCR7− cells are selectively impaired in reaching PLN. This was also strikingly reflected in the distribution of tetramer+ cells. There were proportionately 3- to 4-fold fewer EBV-tetramer+ CD8 T cells in PLN than in PBMC (Figure 7). Even within the CD8 subset, there were approximately 2-fold fewer tetramer+ cells in PLN than PBMC (0.4% versus 0.8% in sample A and 0.5% versus 0.8% in sample B; data not shown). Expression of CD62L, CCR7, CD45RA, CD27, and CD28 by EBV-tetramer+ cells was comparable in the 2 sets of PLN and PBMC samples (data not shown).
Selective reduction in EBV-tetramer+ cells in LN aspirates compared with blood from 2 HIV-seropositive donors.
Paired peripheral blood and LN aspirate samples from 2 HIV-seropositive HLA B8+ donors not receiving antiretroviral drugs ([A] CD4 count, 348, and plasma HIV RNA, 68 618; [B] CD4 count, 518, and plasma HIV RNA, 175 135) were compared by analyzing a tightly gated lymphocyte population costained for CD8 and EBV B8 tetramer (second column), CCR7 (third column), and CD62L (last column). In the LNs compared with the peripheral blood, there was a 2-fold reduction in CD8 T cells compared with the total lymphocyte population and a 3- to 4-fold reduction in EBV-tetramer+ CD8 T cells. The proportion of cells staining for CCR7 and CD62L in LNs was much higher than in the blood.
Selective reduction in EBV-tetramer+ cells in LN aspirates compared with blood from 2 HIV-seropositive donors.
Paired peripheral blood and LN aspirate samples from 2 HIV-seropositive HLA B8+ donors not receiving antiretroviral drugs ([A] CD4 count, 348, and plasma HIV RNA, 68 618; [B] CD4 count, 518, and plasma HIV RNA, 175 135) were compared by analyzing a tightly gated lymphocyte population costained for CD8 and EBV B8 tetramer (second column), CCR7 (third column), and CD62L (last column). In the LNs compared with the peripheral blood, there was a 2-fold reduction in CD8 T cells compared with the total lymphocyte population and a 3- to 4-fold reduction in EBV-tetramer+ CD8 T cells. The proportion of cells staining for CCR7 and CD62L in LNs was much higher than in the blood.
Discussion
Although HIV infection stimulates one of the strongest known human antiviral CD8 T-cell responses,14-17 CD8 T cells fail to control disease progression in most infected people. Our results suggest a possible important factor contributing to the inability of CD8 T cells to control HIV: failure of HIV-specific CD8 T cells to express the homing receptors required for efficient trafficking into LNs where most HIV replication occurs. This lack of LN-homing molecules is not restricted to HIV-specific CD8 T cells or to HIV infection, since CMV- and EBV-specific cells from both healthy donors and HIV-infected donors had the same trafficking receptor patterns of expression. Therefore, antigen-experienced CD8 T cells in the setting of chronic infection with persistent antigenemia may be selectively excluded from the LNs, even when the infection is localized primarily to those tissues.
The initial activation of naı̈ve CD8 T cells occurs in T-cell zones of LNs where the naı̈ve T cell encounters dendritic cells bearing the peptide antigen that fits its antigen receptor. Therefore, naı̈ve T cells are equipped with adhesion molecules such as L-selectin and CCR7, whose sequential engagement are required for access to LNs. However, on activation, T cells down-regulate L-selectin and up-regulate adhesion molecules such as β1 and β2 integrins, which are required for homing to peripheral tissues. Thus, a redirection of migration potential from LNs to peripheral tissues is a hallmark of activated T cells. CCR7 has been identified as a key chemokine receptor necessary for lymphocytes to cross the HEV barrier to enter LNs. Moreover, CCR7 also appears to be involved in trafficking within LNs and possibly in regulating cellular entry into afferent lymph.37 MHC-tetramer+ HIV-specific CD8 T cells are uniformly negative for L-selectin and CCR7. In fact, only 15% of all CD8 T cells from subjects seropositive for HIV expressed CCR7, whereas more than 60% of CD8 T cells from healthy subjects expressed this molecule. This may reflect the fact that most CD8 T cells in HIV-seropositive subjects are activated cells that are being chronically restimulated with HIV and other antigens. Thus, HIV-specific CD8 T cells may be preferentially excluded from the principal sites of viral replication and pathological changes in LNs.
In the absence of L-selectin and CCR7, CD8 T cells generally do not bind to HEVs. In HIV infection, where there is a reduction in CCR7+CD62L+ circulating CD8 T cells, one might expect that relatively fewer CD8 T cells would traffic to the LNs. In fact, in 2 subjects we tested, the frequency of CD8 T cells in PLN was only half of that in PBMC, and the number of EBV-tetramer+cells in PLN was reduced even further—to a quarter of the tetramer+ cell numbers in PBMC. Most tetramer+cells in LNs, like those in the circulation, were also negative for L-selectin and CCR7. Clearly, the exclusion of CCR7− T cells from the LNs in HIV infection is leaky, particularly in the presence of LN inflammation. Although entry by means of the HEVs will be largely blocked, CCR7− CD8 T cells can also enter the LNs through the afferent lymphatics. The CCR7−CD62L− EBV-tetramer+ cells in the LN aspirates probably represented recirculating cells from tissue that reached PLN by means of the afferent lymph. Infections with EBV and HIV are localized primarily to LNs. Thus, it is likely that most HIV-specific CD8 T cells, like EBV-specific cells, may also be preferentially excluded from the principal sites of viral replication and pathological changes in LNs. Studying tetramer+ cells in LNs is technically challenging because tetramer staining cannot be done on fixed samples and the frequency of tetramer+ cells, which is low in the peripheral blood, is probably even lower in the LNs. However, understanding the immune response at the site of infection is important for understanding disease pathogenesis.
The exclusion of effector cells from LNs, which in normal circumstances provides efficient division of labor among CD8 T cells and may protect LNs from immunopathological damage by inflammatory cytokines and cytolytic enzymes, may work to the detriment of the host in HIV infection by converting LNs into relatively immunologically privileged sites. A study of acute simian immunodeficiency virus (SIV) infection in macaques also suggested that SIV-specific CD8 T cells are preferentially excluded from the LNs.38 In blood and LN samples from monkeys obtained 13 and 21 days after SIV infection, there were proportionately 4-fold fewer SIV-tetramer+ cells in LNs than in the circulation (P < .007). This is remarkably similar to what we found for EBV-tetramer+ cells in 2 subjects with chronic HIV infection. Clearly, lack of CCR7 and CD62L is not an absolute bar to LN trafficking. In fact, gene-marked, HIV-specific CD8 T-cell clones adoptively transferred into HIV-infected subjects were shown to traffic to LN,39 but how efficiently they can do this is uncertain, since billions of T cells were infused in this clinical study. It is not clear whether the infused cells expressed L-selectin and CCR7, but such expression is unlikely after prolonged in vitro culture.
The distribution of naı̈ve and memory CD8 T-cell subsets is grossly altered in HIV-seropositive subjects. Naı̈ve CD8 T cells, which express CD45RA, L-selectin, and CCR7, were decreased markedly in such subjects and nearly absent in patients with stage C disease. On the basis of differential expression of homing receptors, different subsets of human memory CD8 T cells have been proposed: L-selectin–positive, CD45RA−CCR7+ central memory cells; L-selectin–variable, CD45RA−CCR7− effector memory cells; and L-selectin–variable, CD45RA+CCR7− terminal effector cells.12 The presumably long-lived central memory cells exhibit no immediate effector function but are capable of rapid recall responses. Such cells, which comprised about 8% of CD8 T cells in healthy donors, were reduced to about 3% in HIV-seropositive subjects. This memory pool may become exhausted in HIV infection because of chronic antigen persistence and inflammation. We found that the frequency of naı̈ve cells plummeted in circulating cells from patients with stage C AIDS, whereas the proportion of central memory cells was markedly reduced in PBMC from HIV-seropositive donors with either stage B or stage C disease. Declines in these T-cell populations likely affect the ability to protect against opportunistic infections, which becomes impaired in these stages of HIV disease.
In contrast to naı̈ve and central memory cells, both CD45RA+CCR7− and, especially, CD45RA−CCR7− cells were dramatically expanded in subjects seropositive for HIV. The functional role of CD45RA isoform expression in previously activated CD8 T cells is not clear. The simple designation of CD45RA+ cells as effector cells and CD45RA− cells as effector memory cells does not seem to be valid, judging from perforin expression. Because CD45 is unlikely to contribute directly to effector function, evaluation of GzmA and perforin coexpression is more accurate for distinguishing effector from other antigen-experienced CD8 T cells. Unfortunately, there is no cell-surface marker that unambiguously identifies effector CTLs. Lack of CD27 or presence of CD56 cell surface expression may be better indicators of effector CTLs.13 40-42
Although CD45RA expression may be an imperfect marker for effector CTLs, our finding that CD45RA is virtually absent from HIV-specific CD8 T cells but not from EBV- or CMV- specific cells in the same HIV-infected donors raises the possibility that terminal differentiation into fully competent effector cells may be defective in HIV-specific CD8 T cells. This idea is in accord with our earlier observation that CD8 T cells in HIV-seropositive subjects have defects in HIV-specific cytolytic ability,35 a finding that was confirmed by subsequent studies.22,40 Moreover, perforin is selectively not expressed in LNs during acute and chronic HIV infection, although GzmA is.21 Consistent with the hypothesis of incomplete differentiation is the finding that HIV-tetramer+ cells in the periphery also lack perforin expression and do not down-regulate expression of CD27.22 40
An important observation in this study is that perforin and granzymes were not coordinately expressed. In both healthy and HIV-infected donors, there was a large excess of CD8 T cells that expressed GzmA but not perforin. The granule-mediated pathway, which is critical for immune defense against many viral infections, depends absolutely on the presence of perforin. Mice that have been rendered genetically deficient in perforin succumb to infections with such viruses as lymphocytic choriomeningitis virus.43 Moreover, HIV-infected primary T cells are lysed exclusively by the perforin-dependent granule-mediated pathway.44 Therefore, although perforin and granzyme expression are up-regulated in tandem during the initial differentiation of naı̈ve CD8 T cells into effector CTLs,34 their subsequent expression is not tightly linked and perforin expression is likely to be the controlling factor for cytotoxicity. A report of a striking lack of perforin expression in HIV-specific CD8 T cells compared with other CD8 T cells suggests that perforin expression might be distinctively affected by HIV infection.40 However, this finding must be confirmed.
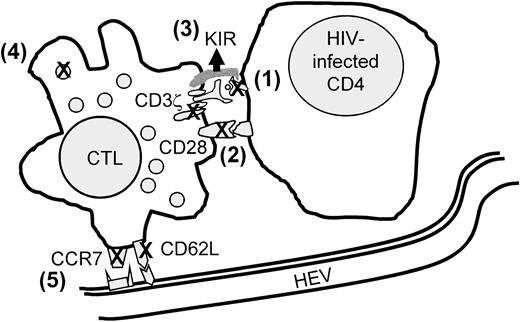
The lack of effective immune surveillance by antigen-specific CD8 T cells in HIV infection is likely to be multifactorial (Figure8). Some researchers have postulated the importance of defects in target cell recognition by HIV-specific CTLs because of down-modulation of MHC class I molecules on infected cells by nef or because of viral mutations of CTL epitopes to evade recognition or inhibit a functional response.45,46 Several possible molecular mechanisms on HIV-specific CD8 T cells that may contribute to lack of an effective response when an HIV-infected cell is recognized have also been described. These are (1) down-modulation of the key T-cell signaling molecules CD3ζ and CD28 on HIV-specific CD8 T cells with corresponding anomalies in signaling recognition of antigen,35,47-52 (2) up-regulation of natural killer inhibitory receptors on HIV-specific CD8 T cells to block triggering,53 and (3) defective differentiation into perforin+ CD45RA+CD27− killer cells so that the specific cells lack the machinery to deliver the lethal hit.22,40 To this list we now add potential impairment of HIV-specific CD8 T cells to traffic efficiently and localize at the principal sites of HIV infection in the LNs. Many of these CD8 T-cell properties, including CD3ζ and CD28 down-modulation and down-modulation of LN-homing receptors, are part of normal immune regulation of these potentially dangerous “serial killers” to limit immunopathological changes.54 It remains to be confirmed whether the lack of perforin expression is a special property of HIV-specific CD8 T cells.21 40 A better understanding of the reasons for these defects and of the normal pathways for regulation of CD8 T-cell function might enable development of more effective therapy for HIV infection.
Model of known factors that may contribute to impaired CD8 T-cell protection in HIV infection.
Contributory factors include (1) inefficient antigen presentation by HIV-infected cells because of nef-mediated down-modulation of MHC class I molecules or viral peptide escape mutation, (2) impaired or incomplete T-cell-receptor signaling because of CD3ζ and CD28 down-modulation, (3) up-regulation of NK inhibitory receptors, (4) lack of perforin for cytolysis, and (5) inefficient homing to lymphoid sites of infection. Many of these molecular events may not be unique to HIV infection but are integral to the regulated CD8 T-cell response to antigen.
Model of known factors that may contribute to impaired CD8 T-cell protection in HIV infection.
Contributory factors include (1) inefficient antigen presentation by HIV-infected cells because of nef-mediated down-modulation of MHC class I molecules or viral peptide escape mutation, (2) impaired or incomplete T-cell-receptor signaling because of CD3ζ and CD28 down-modulation, (3) up-regulation of NK inhibitory receptors, (4) lack of perforin for cytolysis, and (5) inefficient homing to lymphoid sites of infection. Many of these molecular events may not be unique to HIV infection but are integral to the regulated CD8 T-cell response to antigen.
We thank Brooke Harnisch and Zhan Xu for excellent technical help, Michael Lederman and the Case Western Reserve Center for AIDS Research for assistance in obtaining LN samples, and the National Institute of Allergy and Infectious Diseases Reagent and Reference Repository for providing us with most of the tetramers used in this study.
Supported by National Institutes of Health grants AI-42519 and AI-45406 to J.L. and AI-45306 to P.S.
G.C. and P.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Judy Lieberman, Center for Blood Research, 800 Huntington Ave, Boston, MA 02115; e-mail:lieberman@cbr.med.harvard.edu.

![Fig. 7. Selective reduction in EBV-tetramer+ cells in LN aspirates compared with blood from 2 HIV-seropositive donors. / Paired peripheral blood and LN aspirate samples from 2 HIV-seropositive HLA B8+ donors not receiving antiretroviral drugs ([A] CD4 count, 348, and plasma HIV RNA, 68 618; [B] CD4 count, 518, and plasma HIV RNA, 175 135) were compared by analyzing a tightly gated lymphocyte population costained for CD8 and EBV B8 tetramer (second column), CCR7 (third column), and CD62L (last column). In the LNs compared with the peripheral blood, there was a 2-fold reduction in CD8 T cells compared with the total lymphocyte population and a 3- to 4-fold reduction in EBV-tetramer+ CD8 T cells. The proportion of cells staining for CCR7 and CD62L in LNs was much higher than in the blood.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/1/10.1182_blood.v98.1.156/6/m_h81311229007.jpeg?Expires=1769265555&Signature=0ck-CEDSzyMbpgjmLOYDVDaoyltiFFtUW1Zw4H5uViZrHwZTcfC01QzG81v74Ofh3LT-4Eluvglg6aVwMvUZ8T8kkNxE76IhwJb-bHgogx-BtMhY9UHZKLX60d9U8s6bC61otuzdAR9niKyHsY8qlyA34oHWJ-05z8XOj2X~VIART9Ur-6VfWvFZkm3HAZ6ppbIq8qUFd4Z25q4dnry4HDt5uEk4YP00rd~VYpWPVmecSNKWzX97jeqd8j8p1caC1GysW6nUsln-94zE~1PATs8oMN44~0dX8XhMvbjh0GBdZ95IYKVxESjWfbc6Yo8ByvszB9xw5H4xR3k-Ak4U9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal