Abstract
Rapamycin (Rapa), a recently introduced immunosuppressive drug, seems to be effective in preventing acute allograft rejection. Although its antiproliferative effect on T lymphocytes has been investigated extensively, its effect on the initiators of the immune response, the dendritic cells (DCs), is not known. Therefore, the effect of Rapa on monocyte- (mo-DCs) and CD34+-derived DCs in vitro but also on other myeloid cell types, including monocytes and macrophages, was examined. The present study shows that Rapa does not affect phenotypic differentiation and CD40L-induced maturation of mo-DCs. However, Rapa dramatically reduced cell recovery (40%-50%). Relatively low concentrations of Rapa (10−9 M) induced apoptosis in both mo-DCs and CD34+-derived DCs, as visualized by phosphatidylserine exposure, nuclear condensation and fragmentation, and DNA degradation. In contrast, Rapa did not affect freshly isolated monocytes, macrophages, or myeloid cell lines. The sensitivity to Rapa-induced apoptosis was acquired from day 2 onward of mo-DC differentiation. Rapa exerts its apoptotic effect via a reversible binding to the cytosolic receptor protein FKBP-12, as demonstrated in competition experiments with FK506, which is structurally related to Rapa. Partial inhibition of Rapa-induced apoptosis was obtained by addition of ZVAD-fmk, which implies caspase-dependent and caspase-independent processes. The fact that Rapa exerts a specific effect on DCs but not on monocytes and macrophages might contribute to the unique actions of Rapa in the prevention of allograft rejection and other immune responses.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that are specialized in the uptake of antigens and their transport from peripheral tissues to the lymphoid organs. Because of their capacity to stimulate naive T cells, DCs have a central role in the initiation of primary immune responses.1 Human DCs can be grown in vitro from CD34+ progenitors in medium supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor-α2,3 or from monocytes in the presence of GM-CSF and interleukin (IL)-4 or IL-13.4 It has been demonstrated also that in vivo monocytes can differentiate into DCs upon transmigration through endothelial layers.5 6
DCs are thought to play a critical role in diverse facets of immune regulation, ranging from tolerance induction and the prevention of autoimmunity to the induction of antitumor immunity and the protection against infectious agents.1,7-9 A similar dichotomy of DC functions has been proposed to play a role in allograft transplantation. Various experimental models have demonstrated that DCs are critical for the induction of transplant tolerance.10-13 As a consequence DCs have been genetically modified to promote their tolerogenic potential.14 On the other hand, it is clear that the immunostimulatory potential of DCs may also contribute to disease pathogenesis of allograft rejection.15 Donor DCs transplanted en bloc with the allograft migrate to the recipient spleen,16 where they can activate naive T lymphocytes, thereby inducing an immune response via the direct pathway of allorecognition.17 Several studies showed that preventing the direct pathway of allorecognition by DC depletion resulted in prolonged graft survival.18-21 In addition, both transplant injury and local inflammation will attract recipient DCs to the graft,22 which contributes to the indirect pathway of antigen presentation.17 Especially for chronic rejection, it is thought that this indirect pathway plays a critical role. Therefore, DCs might be an important target for immunosuppression to prevent allograft rejection.
Activated T cells, rather than DCs, have been considered the main targets for immunosuppression after transplantation. Immunosuppressive drugs like calcineurin inhibitors (cyclosporin A [CsA] and FK506) and corticosteroids all have profound effects on T-cell activation. Recently, it has been shown that corticosteroids can also inhibit the differentiation and maturation of DCs.23-25 This is in contrast to the calcineurin inhibitors, which only have minor effects on DC function.25-27 Despite good short-term effects for graft survival, current immunosuppression shows limited impact on the incidence of chronic allograft rejection and is characterized by serious side effects. Therefore, there is still a need for the development of alternative drugs.11
Rapamycin (Rapa), a macrolide antibiotic produced by Streptomyces hygroscopicus,28 has been introduced recently as a new effective drug for the prevention of allograft rejection.29-31 Like CsA and FK506, Rapa exerts its effects by binding to immunophilins and interference in the signaling pathways involved in T-lymphocyte proliferation.32 Rapa is structurally related to FK506, and it binds to and competes with FK506 for the same immunophilin FKBP-12. However, only the Rapa-FKBP complex binds and inhibits the function of the serine/threonine kinase mTOR (mammalian target of rapamycin),33 34 which is involved in protein synthesis and cell cycle progression.
Whereas the effects of Rapa are well investigated with respect to the effect on T lymphocytes, the effect of Rapa on antigen-presenting cells is not known. Therefore, we investigated the effect of Rapa on myeloid cell types, including monocytes, macrophages, and monocyte- and CD34+- derived DCs, with respect to differentiation, proliferation, and cell survival. The present study demonstrates that Rapa specifically induces apoptosis in DCs without affecting differentiation and maturation of these cells.
Materials and methods
Reagents
Dexamethasone, (pharmacy, Leiden University Medical Center, The Netherlands), CsA (Novartis Pharma, Basel, Switzerland), and FK506 (Prograft, Fujisawa Benelux, Houten, The Netherlands) were added to the cultures at 1 μM unless indicated otherwise. Rapa (Calbiochem, Cambridge, MA) dissolved in dimethyl sulfoxide (DMSO) was used at the concentrations indicated. Caspase inhibitor ZVAD-fmk (50 μM) was also obtained from Calbiochem. The DNA topoisomerase inhibitor etoposide (Sigma, St Louis, MO) was used at 10 μM final concentration.
Cell lines
The monocytic cell lines U937, THP-1, and the T-cell line Jurkat were grown in RPMI 1640 containing 10% heat-inactivated (Δ) FCS and penicillin/streptomycin (P/S) (all from Gibco/Life Technologies, Breda, The Netherlands). The monocytic cell line MonoMac6 was cultured in RPMI 1640 supplemented with 10 μg/mL insulin, 10 μg/mL transferrin, 10 ng/mL selenium (all Sigma), 50 μM β2-mercaptoethanol, 5% ΔFCS, and P/S. The myeloid cell line KG-1 was grown in Iscoves modified Dulbecco medium (Gibco) containing 20% ΔFCS and P/S.
Generation of dendritic cells
Monocyte-derived DCs.
Monocyte-derived DCs (mo-DCs) were generated as described before.25 In brief, human monocytes were isolated from buffy coats obtained from healthy donors using Ficoll-Hypaque (Sigma) and Percoll (Pharmacia, Uppsala, Sweden) density gradient centrifugation, followed by plastic adherence (2 hours). DCs were generated in 6-well culture plates (Costar, Cambridge, MA) in RPMI 1640 containing 10% ΔFCS and P/S supplemented with 5 ng/mL GM-CSF (Sandoz, Uden, The Netherlands) and 10 ng/mL IL-4 (Peprotech, Rocky Hill, NJ) for at least 6 days.
CD34+-derived DCs.
CD34+ hematopoietic progenitor cells were isolated from umbilical cord blood samples and used in cultures to generate immature DCs as previously described.3 In brief, CD34+cells were isolated from mononuclear fractions through positive selection using anti-CD34–coated microbeads and Midi-Macs separation columns (both from Miltenyi Biotec, Bergish Gladbach, Germany). After cryopreservation, cells were cultured in RPMI 1640 containing 10% ΔFCS, 10 mM HEPES, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, and P/S supplemented with 100 ng/mL GM-CSF (Schering-Plough Research Institute, Kenilworth, NJ), 25 ng/mL stem cell factor (R&D, Abington, United Kingdom), 2.5 ng/mL tumor necrosis factor-α (R&D), and 5% AB+ pooled human serum. For apoptosis experiments, cells were collected after 6 days of culture.
Cell counts were performed by using trypan blue exclusion or by using the Coulter Counter (Coulter Electronics, Mijdrecht, The Netherlands).
Macrophages
Alveolar macrophages.
Human alveolar macrophages were isolated from bronchoalveolar lavage fluid collected from patients who underwent bronchoalveolar lavage for diagnostic reasons and who had no underlying pathological condition in the lung segment from which the lavage fluid was obtained. The cells were seeded in RPMI 1640 supplemented with 10% ΔFCS and P/S. One day after isolation, the nonadherent cells were washed away and apoptosis experiments were initiated.
Monocyte-derived macrophages.
Monocytes were isolated from buffy coats as described above and subsequently cultured for 7 days in RPMI 1640 containing 10% ΔFCS, P/S, and 5 ng/mL GM-CSF.
Activation of mo-DCs
Six-day immature mo-DCs were activated with CD40L. CD40L activation was performed with CD40L-transfected L cells (L-CD40L)35 in a DC:L cell ratio of 4:1. Nontransfected L cells served as control cells (L-Orient). For stimulation, cells were incubated at 0.5 × 106 per well in 24-well plates (Costar) in the presence of IL-4 (10 ng/mL) and GM-CSF (5 ng/mL). Cells were analyzed by flow cytometry after 48 hours.
Analysis of cell surface phenotype by flow cytometry
Cells were harvested and washed in buffer containing 1% BSA, 1% heat-inactivated normal human serum (NHS, Bloodbank, Leiden, The Netherlands), and 0.02% NaN3. Fluorescence-activated cell sorter (FACS) analysis was performed using monoclonal antibodies against the following surface markers: CD1a (Leu-6) and CD14 (Leu-M3) (both Becton Dickinson, San Jose, CA), CD80 (monoclonal antibody 104), CD83 (HB15A; Immunotech, Marseille, France), CD86 (IT2.2; Pharmingen, San Diego, CA), HLA-ABC (w6/32; American Type Culture Collection, Rockville, MD), and HLA-DR (clone B8.11,2). Staining was visualized by using phycoerythrin-conjugated goat-anti–mouse immunoglobulin (Dako, Glostrup, Denmark). Fluorescein isothiocyanate (FITC)-conjugated anti-CD1a (Pharmingen) and phycoerythrin-conjugated anti-CD14 (Leu-M3; Becton Dickinson, San Jose, CA) antibodies were used for double staining of CD34+-derived DCs. The cells were assessed for fluorescence using a FACScan. Data acquisition was performed using Lysis-II software (Becton Dickinson, Mountain View, CA).
Induction and detection of apoptosis
Apoptosis induction experiments were performed in 6- or 24-well culture plates (Costar). CD34-derived DCs, U937, THP-1, MonoMac6, KG-1, Jurkat (all 2 × 105 cells/mL) and peripheral blood monocytes, alveolar macrophages, monocyte-derived macrophages, and mo-DCs (all 3 × 105 cells/mL) were cultured as described above. Cells were treated with several agents for 48 hours unless indicated otherwise, harvested, and assayed for apoptosis.
To determine the morphologic nuclear changes, cells were harvested and fixed with 4% paraformaldehyde on ice. Cytospin preparations were made and stained with Hoechst 33258 (Molecular Probes, Leiden, The Netherlands) for 3 minutes at room temperature. The percentage of apoptotic cells was determined by examining 200 cells and counting the cells that were characterized by nuclear condensation and fragmentation.
Phosphatidylserine exposure was determined using annexin V–FITC (Apoptest FITC kit, Nexins Research, Kattendijke, The Netherlands) in combination with propidium iodide (PI; Molecular Probes). Cells were harvested, washed, labeled with annexin V–FITC for 30 minutes on ice, and subsequently taken up in 1 μg/mL PI. Annexin V/PI staining was conducted on a FACScan and analyzed using Lysis-II software. Data are expressed as percentage of annexin V+ cells in the PI− population or presented otherwise where indicated.
Cell cycle analysis
Cells were harvested, washed in 1 mM ethylenediaminetetraacetic acid/phosphate-buffered saline, and fixed in 90% ethanol for at least 30 minutes at −20°C. The cells were then washed and cellular DNA was stained by treating the cells with 50 μg/mL RNase A (Sigma) and 10 μg/mL PI (Molecular Probes) for 45 minutes at room temperature. Fluorescence intensity was quantified on a per cell basis by FACScan.
Results
Reduced cell yield after mo-DC development in the presence of Rapa
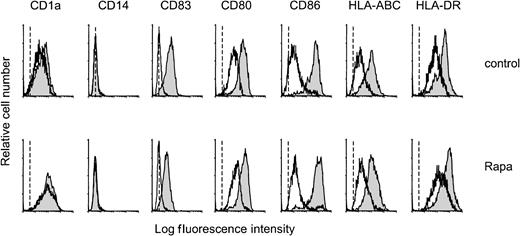
The effect of Rapa on the differentiation of monocytes into mo-DCs was studied by culturing monocytes in the presence of IL-4 and GM-CSF with or without addition of Rapa (10−7 M). After 6 days of culture, both nontreated and Rapa-treated cells displayed morphologic and phenotypical characteristics of DCs. DCs generated in the presence of Rapa showed a relatively low expression of costimulatory and HLA molecules, were positive for CD1a, and were completely negative for CD14 and CD83, as assessed by FACS analysis (Figure1). Activation (48 hours) of these cells with CD40L induced the expression of CD83 and increased the expression of major histocompatibility complex class I, major histocompatibility complex class II, and costimulatory molecules (CD80, CD86) comparable to control DCs (Figure 1).
Rapa does not affect phenotypic differentiation or CD40L-induced maturation.
Monocytes were cultured with IL-4 and GM-CSF in the absence (control) or presence of 10−7 M Rapa. On day 6, cells were harvested, extensively washed, and cocultured with L-Orient or L-CD40L for the subsequent 48 hours. On day 8, cells were harvested and analyzed for several surface markers by flow cytometry. Mean fluorescence intensity of DCs stained only with the secondary antibody was similar for the nonstimulated and the CD40L-stimulated DCs and is represented by the dotted line. Specific staining of nonstimulated DCs is represented by the open histogram, CD40L-stimulated DCs by the filled histogram. The results are representative of 4 independent experiments.
Rapa does not affect phenotypic differentiation or CD40L-induced maturation.
Monocytes were cultured with IL-4 and GM-CSF in the absence (control) or presence of 10−7 M Rapa. On day 6, cells were harvested, extensively washed, and cocultured with L-Orient or L-CD40L for the subsequent 48 hours. On day 8, cells were harvested and analyzed for several surface markers by flow cytometry. Mean fluorescence intensity of DCs stained only with the secondary antibody was similar for the nonstimulated and the CD40L-stimulated DCs and is represented by the dotted line. Specific staining of nonstimulated DCs is represented by the open histogram, CD40L-stimulated DCs by the filled histogram. The results are representative of 4 independent experiments.
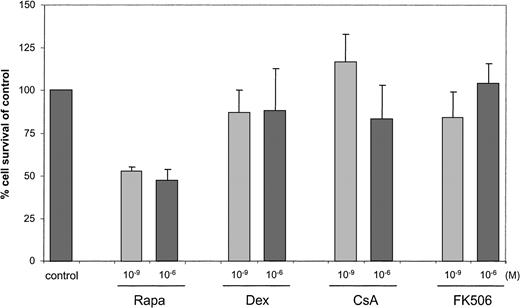
Although Rapa did not affect the phenotype of mo-DCs, viable cell yield at day 6 of culture was 40% to 50% reduced, as assessed by trypan blue exclusion (Figure 2). The immunosuppressive drugs dexamethasone, CsA, or FK506 had only a minor effect on cell recovery (> 86% compared with control).
Reduced cell yield after mo-DC development in the presence of Rapa.
Monocytes were cultured with IL-4 and GM-CSF in the absence (control) or presence of dexamethasone, CsA, FK506, or Rapa (10−9 M or 10−6 M as indicated). After a 6-day culture period with or without immunosuppressive drugs, cells were harvested and counted by trypan blue exclusion or by using the Coulter Counter. DMSO, in a concentration similar to the concentration used to test 10−6 M Rapa, served as a control. Cell survival in at least 4 independent experiments is expressed as mean (± SD) percentage of control DCs.
Reduced cell yield after mo-DC development in the presence of Rapa.
Monocytes were cultured with IL-4 and GM-CSF in the absence (control) or presence of dexamethasone, CsA, FK506, or Rapa (10−9 M or 10−6 M as indicated). After a 6-day culture period with or without immunosuppressive drugs, cells were harvested and counted by trypan blue exclusion or by using the Coulter Counter. DMSO, in a concentration similar to the concentration used to test 10−6 M Rapa, served as a control. Cell survival in at least 4 independent experiments is expressed as mean (± SD) percentage of control DCs.
Rapa induces apoptosis in immature mo-DCs but not in monocytes
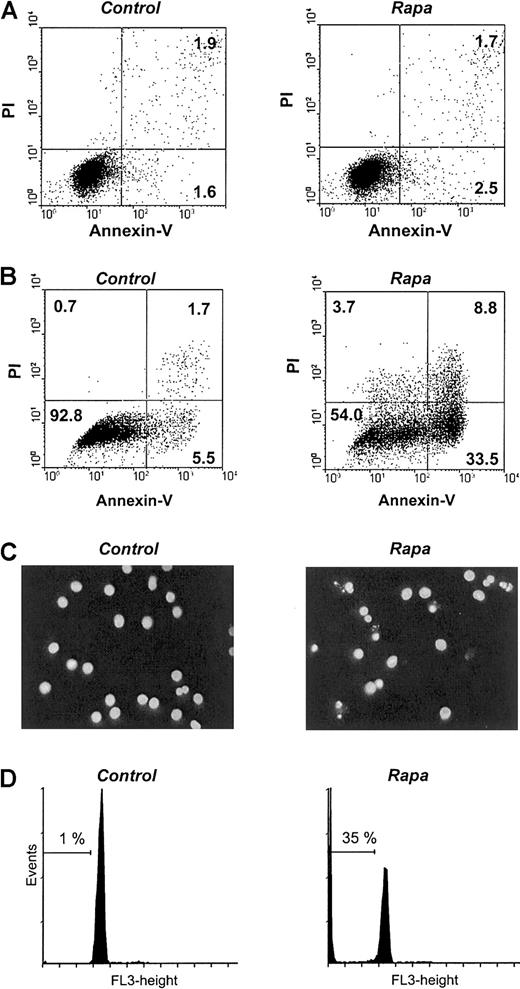
To examine whether Rapa reduced cell recovery by inducing cell death at the start of culture, freshly isolated monocytes were treated with Rapa for 48 hours. Monocyte cultures, without or with the addition of GM-CSF and IL-4, did not show Rapa-induced apoptosis assessed by either Hoechst (data not shown) or annexin V/PI staining (Figure3A). In contrast, treatment of mo-DCs that were generated following the standard 6-day differentiation condition and subsequently treated with Rapa for 48 hours demonstrated a significant degree of apoptosis as shown by an increased phosphatidyl exposure using annexin V–FITC (Figure B), an increased nuclear condensation and fragmentation using Hoechst (Figure 3C), and an increased sub-G0 fraction using DNA staining (Figure 3D). Notably, all mo-DCs are in G0/G1 phase as expected in this nonproliferating cell population.
Rapa induces apoptosis in immature mo-DCs but not in monocytes.
(A) Freshly isolated monocytes were treated with 10−7 M Rapa. After 48 hours of incubation, cells were harvested and analyzed. The percentage of apoptosis was determined by using annexin V/PI staining. The results are representative of 5 independent experiments. (B-D) In vitro–generated mo-DCs were treated with 10−7 M Rapa. After 48 hours of incubation, cells were harvested and analyzed. The percentage of apoptosis was determined by using either annexin V/PI staining (B), Hoechst (C), or DNA staining to determine the sub-G0 fraction (D). The results are representative of 6 independent experiments.
Rapa induces apoptosis in immature mo-DCs but not in monocytes.
(A) Freshly isolated monocytes were treated with 10−7 M Rapa. After 48 hours of incubation, cells were harvested and analyzed. The percentage of apoptosis was determined by using annexin V/PI staining. The results are representative of 5 independent experiments. (B-D) In vitro–generated mo-DCs were treated with 10−7 M Rapa. After 48 hours of incubation, cells were harvested and analyzed. The percentage of apoptosis was determined by using either annexin V/PI staining (B), Hoechst (C), or DNA staining to determine the sub-G0 fraction (D). The results are representative of 6 independent experiments.
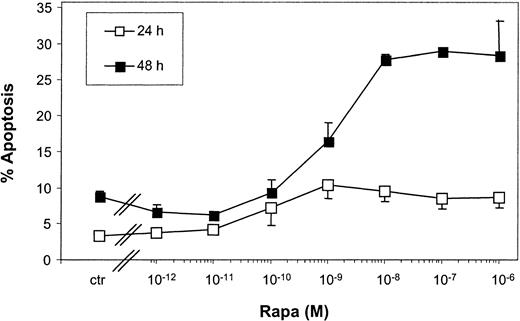
Kinetic experiments revealed that limited apoptosis of mo-DCs was observed after 24-hour incubation with Rapa, which strongly increased after 48 hours (Figure 4). Rapa-induced apoptosis was already observed with 10−9 M Rapa and reached a maximum between 10−8 and 10−6 M.
Rapa-induced apoptosis is regulated in a time- and dose-dependent manner.
Immature mo-DCs were cultured in RPMI–10% FCS supplemented with IL-4 and GM-CSF. Cells were incubated with increasing concentrations of Rapa or the vehicle for 24 or 48 hours. Apoptosis was detected by FACS analysis using annexin V–FITC and PI. The percentage of apoptosis demonstrates the percentage of annexin V+ PI−cells. Data shown are the mean ± SD of duplicate cultures. The results are representative of 2 independent experiments.
Rapa-induced apoptosis is regulated in a time- and dose-dependent manner.
Immature mo-DCs were cultured in RPMI–10% FCS supplemented with IL-4 and GM-CSF. Cells were incubated with increasing concentrations of Rapa or the vehicle for 24 or 48 hours. Apoptosis was detected by FACS analysis using annexin V–FITC and PI. The percentage of apoptosis demonstrates the percentage of annexin V+ PI−cells. Data shown are the mean ± SD of duplicate cultures. The results are representative of 2 independent experiments.
The observed difference between monocytes and mo-DCs suggests that sensitivity to Rapa-induced apoptosis is acquired during differentiation. Daily analysis demonstrated that Rapa sensitivity becomes apparent between day 2 and 3 of differentiation (Figure5).
Sensitivity for Rapa-induced apoptosis is acquired during mo-DC differentiation.
Monocytes were cultured with IL-4 and GM-CSF as described in “Materials and methods.” On different days, Rapa (10−7M) was added for 48 hours. After 48 hours of incubation with or without Rapa, cells were harvested and analyzed using Hoechst. Data shown are the mean (± SD) percentages of apoptosis obtained from duplicate cultures. The results are representative of 4 independent experiments. *P < .05, Student t test for paired samples.
Sensitivity for Rapa-induced apoptosis is acquired during mo-DC differentiation.
Monocytes were cultured with IL-4 and GM-CSF as described in “Materials and methods.” On different days, Rapa (10−7M) was added for 48 hours. After 48 hours of incubation with or without Rapa, cells were harvested and analyzed using Hoechst. Data shown are the mean (± SD) percentages of apoptosis obtained from duplicate cultures. The results are representative of 4 independent experiments. *P < .05, Student t test for paired samples.
Antagonistic effect of FK506 on Rapa-induced apoptosis
Because FK506 and Rapa both bind to FKBP-12, they can act as reciprocal antagonists in systems where only one drug is active.36 37 Different concentrations of Rapa were added to immature mo-DCs in the presence of varying concentrations of the calcineurin inhibitor FK506 or CsA. At least a 10-fold excess of FK506 completely blocked Rapa-induced apoptosis, whereas even 100-fold excess of CsA showed no effect (Figure 6A).
Rapa-induced apoptosis is blocked by FK506.
(A) Immature mo-DCs were cultured in RPMI–10% FCS supplemented with IL-4 and GM-CSF in the absence or presence of 10−7 M Rapa for 48 hours. Before addition of Rapa, the cells were preincubated with CsA or FK506 (10−6 M). Apoptosis was detected by flow cytometry using annexin V/PI staining. The percentage of apoptosis demonstrates the percentage of annexin V+ PI−cells. Data shown are the mean ± SD of duplicate cultures. The results are representative of 3 independent experiments. (B) Immature mo-DCs were cultured in RPMI–10% FCS supplemented with IL-4 and GM-CSF in the absence or presence of 10−7 M Rapa for 48 hours. FK506 (5 × 10−6 M) was added to the cultures at the indicated time points. Apoptosis was detected by flow cytometry using annexin V/PI staining. The percentage of apoptosis demonstrates the percentage of annexin V+ PI− cells. Data shown are the mean ± SD of duplicate cultures. The results are representative of 3 independent experiments.
Rapa-induced apoptosis is blocked by FK506.
(A) Immature mo-DCs were cultured in RPMI–10% FCS supplemented with IL-4 and GM-CSF in the absence or presence of 10−7 M Rapa for 48 hours. Before addition of Rapa, the cells were preincubated with CsA or FK506 (10−6 M). Apoptosis was detected by flow cytometry using annexin V/PI staining. The percentage of apoptosis demonstrates the percentage of annexin V+ PI−cells. Data shown are the mean ± SD of duplicate cultures. The results are representative of 3 independent experiments. (B) Immature mo-DCs were cultured in RPMI–10% FCS supplemented with IL-4 and GM-CSF in the absence or presence of 10−7 M Rapa for 48 hours. FK506 (5 × 10−6 M) was added to the cultures at the indicated time points. Apoptosis was detected by flow cytometry using annexin V/PI staining. The percentage of apoptosis demonstrates the percentage of annexin V+ PI− cells. Data shown are the mean ± SD of duplicate cultures. The results are representative of 3 independent experiments.
Delayed addition of FK506 until 24 hours after the start of Rapa treatment still induced complete inhibition of apoptosis (Figure 6V). This implies that Rapa is reversibly associated with FKBP-12 and that the existence of this complex is required for more than 24 hours to induce strong apoptosis in mo-DCs.
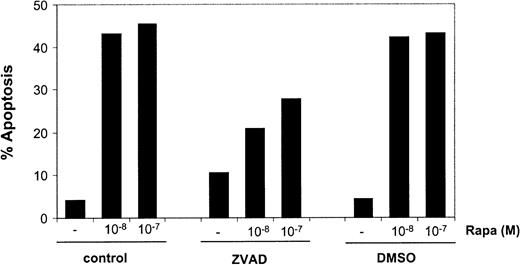
Rapa-induced apoptosis is partially caspase dependent
Next, the involvement of caspase activation in Rapa-induced apoptosis was investigated. Addition of the general caspase inhibitor ZVAD caused a diminished Rapa-induced apoptosis in mo-DCs, as demonstrated by annexin V/PI staining (Figure7) or when analyzed by Hoechst staining (data not shown). The level of inhibition by ZVAD was variable (range 5%-95%), suggesting both caspase-dependent and caspase-independent mechanisms of apoptosis.
Rapa-induced apoptosis is partially blocked by inhibition of caspase activity.
Immature mo-DCs were cultured in RPMI–10% FCS supplemented with IL-4 and GM-CSF in the absence or presence of 10−8 or 10−7 M Rapa for 48 hours. Before addition of Rapa, the cells were preincubated with ZVAD-fmk (5 × 10−5 M) or its solvent, DMSO. Apoptosis was detected by flow cytometry using annexin V/PI staining. The percentage of apoptosis demonstrates the percentage of annexin V+ PI− cells. Data shown are the mean ± SD of duplicate cultures. The results are representative of 5 independent experiments.
Rapa-induced apoptosis is partially blocked by inhibition of caspase activity.
Immature mo-DCs were cultured in RPMI–10% FCS supplemented with IL-4 and GM-CSF in the absence or presence of 10−8 or 10−7 M Rapa for 48 hours. Before addition of Rapa, the cells were preincubated with ZVAD-fmk (5 × 10−5 M) or its solvent, DMSO. Apoptosis was detected by flow cytometry using annexin V/PI staining. The percentage of apoptosis demonstrates the percentage of annexin V+ PI− cells. Data shown are the mean ± SD of duplicate cultures. The results are representative of 5 independent experiments.
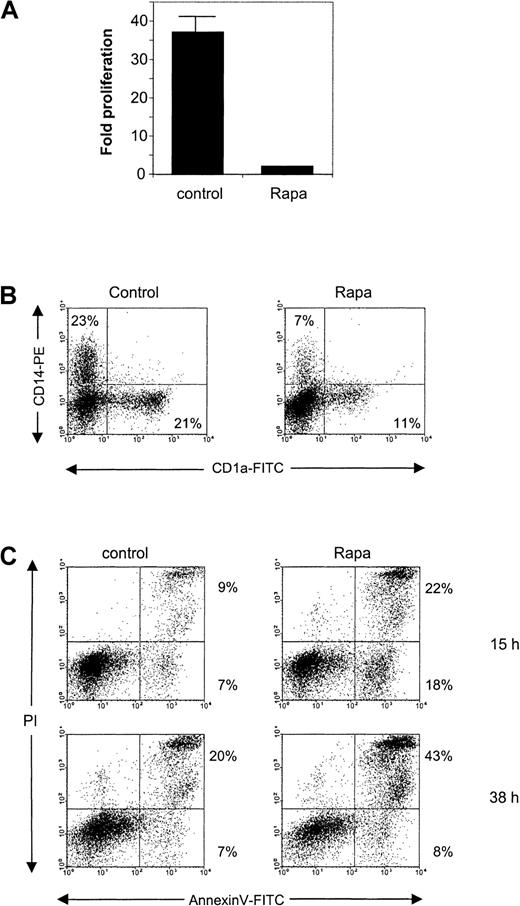
Rapa induces apoptosis in CD34-derived DCs
Next, we tested Rapa on CD34+ cells and on DCs derived from CD34+ precursors. Addition of Rapa to CD34+ cells prevented the strong proliferation, which normally occurs in the first 6 days of culture (Figure8A). This block in proliferation was accompanied by a hampered development of CD14+ and CD1a+ DC populations (Figure 8B).
The effect of Rapa on CD34+ cells and CD34+-derived DCs.
(A) CD34+ cells were cultured for 6 days in the absence or presence of 10−7 M Rapa. The cells were then harvested and analyzed for their proliferation by counting the cells using trypan blue exclusion. Data represented are the mean (± SD) fold of proliferation of 2 different donors. (B) Freshly isolated cord blood CD34+ cells were cultured for 6 days in the absence or presence of 10−7 M Rapa. Differentiation was followed by FACS analysis using anti-CD14 and anti-CD1a directly conjugated antibodies. The results are representative of 2 independent experiments. (C) CD34+-derived DCs were harvested on day 6 and subsequently cultured in the presence of GM-CSF with or without 10−7 M Rapa for 15 or 38 hours. Apoptosis was detected by flow cytometry using annexin V/PI staining. The results shown for both time points are representative of 2 independent experiments.
The effect of Rapa on CD34+ cells and CD34+-derived DCs.
(A) CD34+ cells were cultured for 6 days in the absence or presence of 10−7 M Rapa. The cells were then harvested and analyzed for their proliferation by counting the cells using trypan blue exclusion. Data represented are the mean (± SD) fold of proliferation of 2 different donors. (B) Freshly isolated cord blood CD34+ cells were cultured for 6 days in the absence or presence of 10−7 M Rapa. Differentiation was followed by FACS analysis using anti-CD14 and anti-CD1a directly conjugated antibodies. The results are representative of 2 independent experiments. (C) CD34+-derived DCs were harvested on day 6 and subsequently cultured in the presence of GM-CSF with or without 10−7 M Rapa for 15 or 38 hours. Apoptosis was detected by flow cytometry using annexin V/PI staining. The results shown for both time points are representative of 2 independent experiments.
When normal CD34+-derived DCs at day 6 of culture were exposed to 100 nM Rapa, apoptosis could already be detected after 15 hours of incubation (Figure 8C). After 38 hours of incubation, the percentage of annexin V+ cells increased, mainly found within the PI+ population as a sign of late apoptotic cells (Figure 8C).
Rapa selectively affects DCs but does not influence cell viability or cell cycle progression of other myeloid cell types
To investigate whether Rapa specifically affects DCs, its effect was also tested on other myeloid cells. Monocyte-derived macrophages, alveolar macrophages, U937, THP-1, MonoMac6, KG-1, CD34+-derived DCs, and mo-DCs were incubated with Rapa or etoposide for 48 hours and subsequently analyzed for apoptosis (Table1). Rapa, which was tested in a concentration range of 10−9 M to 10−6 M, was not able to induce apoptosis in any of the myeloid cells tested, except for the DCs. Also, stimulation of freshly isolated monocytes or U937 cells with interferon-γ did not induce Rapa sensitivity (data not shown). Etoposide caused apoptosis in all cell lines but not in the nonproliferating primary cells.
Sensitivity for Rapa-induced apoptosis and cell cycle arrest of different cell types
| Cell types . | Apoptosis, % . | No. of experiments . | G0/G1 fraction, % . | No. of experiments . | |||
|---|---|---|---|---|---|---|---|
| Spontaneous . | Rapa . | Etoposide . | Control . | Rapa . | |||
| Monocytes | 4.3 | 3.5 | 3.8 | 5 | ND | ND | 0 |
| Macrophages (monocyte-derived) | 2.9 | 0 | 0.7 | 4 | ND | ND | 0 |
| Alveolar macrophages | 3.0 | 2.4 | 2.0 | 2 | ND | ND | 0 |
| Dendritic cells (monocyte-derived) | 5.9 | 45.2* | 6.3 | 6 | 100.0 | 100.0 | 2 |
| Dendritic cells (CD34-derived) | 6.8 | 21.5* | ND | 4 | ND | ND | 0 |
| U937 | 2.0 | 2.3 | 74.9* | 3 | 61.0 | 61.1 | 3 |
| THP-1 | 3.9 | 4.8 | 86.2* | 2 | 52.8 | 53.5 | 1 |
| MonoMac6 | 11.5 | 11.0 | 48.1* | 3 | 61.1 | 67.7 | 3 |
| KG-1 | 8.6 | 5.5 | 22.7* | 5 | 53.5 | 50.7 | 4 |
| Jurkat | 2.3 | 2.4 | 71.2* | 3 | 48.7 | 70.8* | 3 |
| Cell types . | Apoptosis, % . | No. of experiments . | G0/G1 fraction, % . | No. of experiments . | |||
|---|---|---|---|---|---|---|---|
| Spontaneous . | Rapa . | Etoposide . | Control . | Rapa . | |||
| Monocytes | 4.3 | 3.5 | 3.8 | 5 | ND | ND | 0 |
| Macrophages (monocyte-derived) | 2.9 | 0 | 0.7 | 4 | ND | ND | 0 |
| Alveolar macrophages | 3.0 | 2.4 | 2.0 | 2 | ND | ND | 0 |
| Dendritic cells (monocyte-derived) | 5.9 | 45.2* | 6.3 | 6 | 100.0 | 100.0 | 2 |
| Dendritic cells (CD34-derived) | 6.8 | 21.5* | ND | 4 | ND | ND | 0 |
| U937 | 2.0 | 2.3 | 74.9* | 3 | 61.0 | 61.1 | 3 |
| THP-1 | 3.9 | 4.8 | 86.2* | 2 | 52.8 | 53.5 | 1 |
| MonoMac6 | 11.5 | 11.0 | 48.1* | 3 | 61.1 | 67.7 | 3 |
| KG-1 | 8.6 | 5.5 | 22.7* | 5 | 53.5 | 50.7 | 4 |
| Jurkat | 2.3 | 2.4 | 71.2* | 3 | 48.7 | 70.8* | 3 |
The different cell types were cultured as described in “Materials and methods.” Cells were cultured with or without 10−7 M Rapa or 10−5 M etoposide. Except the CD34+-derived DCs, which were analyzed after 24 hours, cells were harvested after 48 hours and analyzed for apoptosis using annexin V/PI staining and Hoechst or cell cycle progression using DNA staining. Cell cycle analysis for the percentage of cells present in G0/G1 was performed by excluding cells containing fewer than 2N DNA.
ND indicates not determined.
Significant differences induced by Rapa/etoposide (P < .05, Student t test for paired samples).
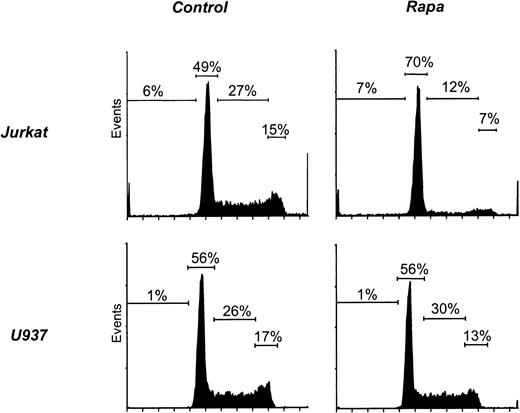
Because Rapa induces a G1 arrest in T cells, we analyzed cell cycle progression in myeloid cell lines. As a positive control, exposure of Jurkat to Rapa resulted in an accumulation of cells in the G0/G1 phase but no increase in the sub-G0 fraction (Figure 9, Table 1). In contrast, the cell cycle of U937 (Figure 9) or any of the other myeloid cell lines (Table 1) was not affected by treatment with Rapa.
Rapa induces a G1 arrest in Jurkat but not in myeloid cell lines.
Jurkat and U937 were cultured as described in “Materials and methods.” Cells were treated with 10−7 M Rapa for 48 hours and subsequently harvested and analyzed by flow cytometry for cell cycle progression using DNA staining. Results shown are representative of 3 independent experiments.
Rapa induces a G1 arrest in Jurkat but not in myeloid cell lines.
Jurkat and U937 were cultured as described in “Materials and methods.” Cells were treated with 10−7 M Rapa for 48 hours and subsequently harvested and analyzed by flow cytometry for cell cycle progression using DNA staining. Results shown are representative of 3 independent experiments.
Discussion
This is the first study demonstrating that Rapa can evoke apoptosis in human DCs. Both the monocyte-derived as well as the CD34+-derived DCs underwent strong apoptosis in the presence of Rapa in a time- and dose-dependent manner. Interestingly, monocytes, macrophages, either monocyte-derived or freshly isolated alveolar macrophages, and myeloid cell lines were completely insensitive for Rapa-induced apoptosis. Furthermore, the antiproliferative effect of Rapa, as shown using Jurkat cells, was absent when using the myeloid cell lines. This suggests a unique action of Rapa on some but not all myeloid cells.
Rapa causes a G1-phase cell cycle arrest in T lymphocytes and some other cell types.34,38-41 In these cases Rapa complexes with FKBP-12, thereby inhibiting the serine/threonine kinase activity of mTOR and subsequent growth factor–induced protein synthesis and cell cycle progression.42-46 Translation of proteins important in cell cycle progression, such as cyclin D1, has been shown especially to be a target of mTOR function.46-48 In addition, the Rapa-induced increase in the expression of Cdk inhibitor p27 (KIP1) regulates G1-phase cell cycle arrest.49 Growing evidence suggests that apoptosis is frequently associated with cells in the G1 phase of the cell cycle, and arrest in late G1 or S phase can accelerate or induce apoptosis. Interestingly, the effect of Rapa we demonstrate on DCs seems to be independent of cell cycle regulation because mo-DCs are nonproliferating. Nevertheless, the activity of Rapa is dependent on prolonged interaction with FKBP-12 as shown by the antagonistic effect of FK506. Thus, the mechanism used by Rapa-FKBP complexes to induce apoptosis in DCs still has to be elucidated.
A proapoptotic effect of Rapa has been demonstrated on murine T cells,50 human B lymphoma cells,51 and rhabdomyosarcoma cells.42 However, in all these studies apoptosis had to be induced by other signals, such as cytotoxic agents, which was further augmented by Rapa. In those cases, no information is provided about the mechanism of apoptosis. In the present study, we show that apoptosis is partially dependent on caspase activation. However, we also clearly identified a caspase-independent pathway. Recently, a number of other situations, including HLA-DR–mediated apoptosis of DCs, has been described in which caspase-independent apoptosis occurs.52-55
At present, immunosuppression used to prevent allograft rejection includes a combination of corticosteroids with a calcineurin inhibitor such as CsA. Despite the benefits of CsA in the prevention of acute rejection, it appears to have little impact on chronic graft loss. The latter might be caused by the fact that treatment with high doses of CsA prevents T-lymphocyte apoptosis and tolerance induction56 or by the fact that calcineurin inhibitors CsA and FK506 are nephrotoxic.57,58 Rapa is a powerful immunosuppressive drug that is not nephrotoxic. In a model of heart transplantation, Rapa, but not CsA, enhances apoptosis of activated T cells, which allows the induction of peripheral tolerance.59 Therefore, several clinical trials are focused on a combination therapy of Rapa and relatively low doses of calcineurin inhibitors.60 61 In those studies, Rapa is administered in higher doses than FK506, which prevents possible antagonism because FK506 has to be given in at least a 10-fold excess to reach a complete antagonistic effect.
The fact that Rapa, next to the antiproliferative effect on T cells, selectively induces apoptosis in DCs might interrupt effective presentation of alloantigens to naive T cells and, thereby, initiation of an alloimmune response both by the direct and indirect pathway. Although the exact mechanism of Rapa-induced apoptosis and the importance in vivo has to be elucidated, induction of DC apoptosis might contribute to the unique actions of Rapa in the prevention of allograft rejection and other immune responses.
The authors thank the collaborators of the Department of Pulmonology (Leiden University Medical Center) for providing human alveolar macrophages, C. Massacrier (Laboratory of Immunological Research, Schering-Plough, Dardilly, France) and A. B. Kruisselbrink (Leiden University Medical Center) for technical assistance, and C. Caux and F. Briere (Schering-Plough) for critical discussions about this manuscript.
C.v.K. is a fellow of the Royal Netherlands Academy of Arts and Sciences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cees van Kooten, LUMC, Dept of Nephrology, C3-P, Albinusdreef 2, 2333 ZA Leiden, The Netherlands; e-mail:kooten@lumc.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal