Abstract
In chronic inflammation, macrophages and neutrophils, which are derived from bone marrow, play a pivotal role. Therefore, reconstitution of bone marrow with anti-inflammatory stem cells may modify inflammation. In this study, transplantation-based gene therapy was applied to glomerular inflammation for a long-lasting suppression of the glomerular damage seen in chronic nephritis. Bone marrow cells were harvested from male donor mice, which had received 5-fluorouracil 3 days previously, and transduced with an interleukin 1 (IL-1) receptor antagonist (IL-1Ra) or a mock gene using a retrovirus vector. After confirmation that transduced cells possessed the transgene at approximately 0.7 copies per cell and secreted recombinant IL-1Ra, these cells were infused into sublethally irradiated (6 Gy) female recipients once daily for 4 consecutive days. These female recipient mice had the male Y antigen in bone marrow, liver, and spleen, and 10% to 20% of their spleen cells possessed the transgene even 8 weeks after transplantation. Glomerulonephritis was then induced in these mice. Renal function and histology were retarded in the mice whose bone marrow was reconstituted with IL-1Ra–producing cells compared with mock transduced cells. In situ hybridization using a Y painting probe revealed that transplanted donor cells were recruited into the glomerulus upon induction of nephritis, suggesting therapeutic effects were channeled through the secretion of IL-1Ra from these cells. Furthermore, the survival rate after a second challenge with nephrotoxic antibody was significantly improved in the IL-1Ra chimera. These results suggest that reconstitution of bone marrow for continuous supply of anti-inflammatory cells may be a useful strategy for the treatment of chronic inflammation.
Introduction
Over the past decade, bone marrow transplantation has been considered an ideal therapeutic strategy for the treatment of certain diseases affecting the hematopoietic system, such as primary immunodeficiencies1 and hemophilia,2 and several clinical trials have been done.3 Such approaches have been possible because of the isolation of hematopoietic stem cells, their genetic manipulation to insert a therapeutic gene, and their return to affected subjects. Recently, the potential for this kind of approach has increased with the discovery of other stem cells (or progenitor cells), such as endothelial4 and neural5 stem cells, and several therapeutic benefits have been reported.6 7 Although this strategy has traditionally been used for the treatment of lethal diseases, we speculated that it could also be applied to the treatment of nonlethal but much more common diseases that are resistant to conventional therapies and affect a large number of people physically and even financially.
Chronic inflammation may be one target for transplantation-based gene therapy because macrophages and neutrophils, which are derived from hematopoietic stem cells, have been mentioned as key determinants in the development of this type of disease process. Bone marrow reconstitution via transplantation may confer resistance against inflammation and provide relief to patients who currently have to continuously take anti-inflammatory drugs.
Glomerulonephritis is a disease causing glomerular inflammation, which progresses slowly or rapidly toward end-stage renal failure, despite active treatment with drugs such as steroid hormones. Throughout the world many patients have had to undergo dialysis as a result of this disorder. In the United States, more than 200 000 patients receive dialysis each year, which constitutes a greater than $15 billion industry.8 This suggests the need for a next-generation therapy against glomerulonephritis that can reduce the problems encountered in conventional therapy. We therefore focused on glomerulonephritis as a target for transplantation-based gene therapy.9,10 We previously used bone marrow cells to establish an inflamed site-specific gene delivery system.9Before transplantation, bone marrow cells were differentiated into monocyte-lineage cells ex vivo to express CD11b and CD18, both of which are ligands of intercellular adhesion molecule-1 (ICAM-1),11 so that these cells could be recruited to sites of ICAM-1 expression. Using this system, we retarded glomerular inflammation during the development of glomerulonephritis by delivering an anti-inflammatory cytokine.10 This system achieved one of the longest controllable periods (up to 2 weeks) among the glomerulus-targeted gene delivery systems; however, it is still not adequate for clinical applications because chronic glomerulonephritis usually progresses over a span of several years. Therefore, we further modified this system to prolong the time window for gene delivery to apply it to chronic glomerulonephritis. Bone marrow cells genetically modified to possess an anti-inflammatory cytokine via a retrovirus were transplanted so that anti-inflammatory mononuclear cells could be continuously supplied from reconstituted bone marrow and long-lasting suppression of local inflammation achieved. In this study, we applied transplantation-based gene therapy to a mouse model of glomerulonephritis, analogous to the human Goodpasture syndrome, and specifically examined the suppression of the proinflammatory cytokine, interleukin 1β (IL-1β), which contributes to the progression of glomerular inflammation in various ways,12-15 via a naturally occurring inhibitor, IL-1 receptor antagonist (IL-1Ra).16
Here, we propose a novel therapeutic strategy for chronic glomerulonephritis using transplantation-based gene therapy, which confers resistance to chronic inflammation. This therapeutic strategy may apply not only to glomerulonephritis but to other chronic inflammatory diseases such as rheumatoid arthritis, for which patients have to take long-term anti-inflammatory drugs such as oral steroids with their attendant side effects.
Materials and methods
Experimental design
Bone marrow of female recipient mice was reconstituted with male bone marrow that had been genetically modified to possess the IL-1Ra or a human glucocerebrosidase (GC) gene using retrovirus. Glomerulonephritis was induced in the IL-1Ra chimera (n = 10) and GC chimera (n = 10) using anti–glomerular basement membrane (anti-GBM) serum 8 weeks after primary bone marrow transplantation. Serum creatinine and urea nitrogen levels, urine albumin secretion, and renal histology were examined to assess the therapeutic effect. To confirm that male donor cells were recruited in the inflamed glomeruli, fluorescence in situ hybridization (FISH) was performed on kidney and spleen sections of chimeric mice with or without nephritis (day 3) (4 per group). To understand how long the reconstituted bone marrow acts as a reservoir of anti-inflammatory cells, anti-GBM serum was again injected into these mice (10 per group) 4 weeks after the first injection (ie, 12 weeks after primary bone marrow transplantation), and the percentage survival rate was compared between the 2 groups.
Animals
Seven-week-old-female DBA/2j mice were purchased from Nippon Crea (Tokyo, Japan). All animals used in this study were maintained in our animal facility on standard laboratory chow.
Bone marrow reconstitution
Male donor mice were treated with 150 mg/kg 5-fluorouracil (Sigma Chemical, St Louis, MO) by intravenous injection 5 days before bone marrow harvest. Bone marrow cells were flushed from the femur, tibia, and pelvis and washed with a basal bone marrow medium: Dulbecco modified Eagle medium (Nikken, Tokyo Japan) with 15% heat-inactivated fetal bovine serum (Gibco, Gaithersburg, MD), penicillin G (100 U/mL), streptomycin (100 μg/mL), and amphotericin B (0.25 μg/ml). Bone marrow cells were suspended in the basal bone marrow medium supplemented with 200 U/mL murine IL-3 (Genzyme, Cambridge, MA), 200 U/mL human IL-6 (kindly donated by Kirin Brewery, Tokyo, Japan), and 100 ng/mL murine stem cell factor (Kirin Brewery). After cultivation in a humidified atmosphere of 5% CO2 at a concentration of 1 × 108 cells per 10-cm dish for 48 hours, the bone marrow cells were transduced with mouse IL-1Ra or the human GC gene via a retrovirus as described below. Female syngeneic recipient mice were prepared for transplantation with a sublethal dose of irradiation (6 Gy). Genetically modified bone marrow cells (5 × 106 per mouse) were infused into recipient mice via the tail vein once daily for 4 consecutive days. Bone marrow cells were freshly prepared for each injection. Eight weeks later, DNA from the bone marrow, liver, and spleen was subjected to polymerase chain reaction (PCR) to confirm successful bone marrow reconstitution.
Recombinant retrovirus preparation and infection
Bone marrow cells were transduced with IL-1Ra or GC via a retrovirus using a centrifuge method.17 As described, ψCRIP packaging cells18 producing an MFG-GC vector (1 × 106 colony-forming units [CFU] per milliliter) expressing human GC were constructed,19 and those producing an MFG–IL-1Ra vector containing the mouse IL-1Ra were purchased from the Riken DNA Bank (Ibaraki, Japan). Retroviral vector–containing supernatant was collected from confluent monolayers of these packaging cells grown in Dulbecco modified Eagle medium with 10% calf serum (Gibco). These supernatants were filtered (0.45 μm) and centrifuged at 14 000g for 2 hours at 4°C to concentrate the virus20 and then resuspended in one-tenth the volume of culture medium. After prestimulation with IL-3, IL-6, and murine stem cell factor for 48 hours, bone marrow cells (1 × 108/mL culture medium) were mixed with the same amount of concentrated retroviral vector and 8 μg/mL polybrene and were centrifuged at 1500g and 20°C for 2 hours once daily for 3 days.17 Freshly prepared retroviral vector–containing supernatant was used for each period of infection. Between centrifugations, the cells were incubated with culture medium in the presence of IL-3, IL-6, and murine stem cell factor without transfer from the centrifuge tube. After the third centrifugation, bone marrow cells were washed and resuspended in phosphate-buffered saline (PBS) for transplantation.
Western blot analysis
Western blot analysis was performed to measure the secretion of IL-1Ra from transfected bone as described.10Briefly, after the transduction with the MFG–IL-1Ra vector, bone marrow cells were cultured for 48 hours in a 6-well plate. Supernatants were harvested, and 2 μg protein was subjected to electrophoresis in a 12.5% sodium dodecyl sulfate–polyacrylamide gel and then transferred to nitrocellulose membrane. The membrane was blocked with 5% dried milk, 0.1% Tween 20, and PBS and incubated with a rabbit polyclonal antibody to human IL-1Ra (R&D Systems, Minneapolis, MN). It was then incubated with donkey antirabbit immunoglobulin horseradish peroxidase conjugate. Antigen-antibody complexes were visualized by chemiluminescence reagents (Amersham, Buckinghamshire, United Kingdom).
Southern blot analysis
To examine the copy number of the transgene integrated in transduced cells, Southern blot analysis was performed for IL-1Ra complementary DNA (cDNA) in bone marrow cells transduced with MFG–IL-1Ra compared with diluted control IL-1Ra cDNA. DNA (20 μg) from transduced bone marrow cells was digested overnight withKpnI, a restriction enzyme that digests the proviral DNA. As a control, IL-1Ra cDNA, which was diluted in mouse genomic DNA (gDNA) isolated from NIH3T3 cells, was also digested with KpnI. The restriction fragments were resolved by electrophoresis in 1% agarose gel and transferred to a methylcellulose membrane. Hybridization was performed with the NcoI/BamHI fragment of the IL-1Ra gene as a probe labeled with digoxigenin by the random primer method using a DIG DNA labeling kit (Roche Diagnostics, Mannheim, Germany). Immunologic detection of labeled biotin was performed using a DIG nucleic acid detection kit (Roche Diagnostics) according to the manufacturer's protocol.
PCR for the Y-specific gene and IL-1Ra gene
Y chromosome–specific PCR was performed as described.21 Briefly, bone marrow, spleen, and liver samples were harvested from mice, suspended in a lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 10 mM ethylenediaminetetraacetic acid), and incubated with proteinase K (100 μg/mL) in 0.1% sodium dodecyl sulfate for 60 minutes at 50°C. The samples were then extracted with phenol-chloroform and ethanol-precipitated. The extracted DNA was used as a template for PCR amplification. Primers for the first PCR were located in the mouse Y chromosome–specific sequence of the sex-determining region22(5′-TGGGACTGGTGACAATTGTC-3′ and 5′-GAGTCAGGTGTGCAGCTCTA-3′).21 The Taq polymerase and reaction buffer were obtained from Sawady (Tokyo, Japan). PCR conditions were as follows: an initial denaturation at 94°C for 5 minutes, then 30 cycles of amplification (denaturation: 94°C, 60 seconds; annealing: 54°C, 30 seconds; elongation: 72°C, 90 seconds). Amplified DNA was again used as a template for nested PCR amplification. Primers for the second PCR were located just inside the first primers (5′-ATGTCAAGCGCCCCATGAAT-3′, 5′-GCCTGTATGTGATGGCATGT-3′). Twenty cycles of amplification were performed under the same conditions as for the primary PCR.
For semiquantitative PCR of the transgene, DNA from spleen was amplified with the human IL-1Ra sequence. Primers for PCR were located in the human IL-1Ra sequence (5′-TAGACATGGTGCCTATTGAC-3′ and 5′-GAGGCTCACAGGACGGTCAG-3′).23 PCR conditions were as follows: an initial denaturation at 95°C for 9 minutes, then 21 cycles of amplification (denaturation: 95°C, 45 seconds; annealing: 57°C, 60 seconds; elongation: 72°C, 45 seconds).24 As standards, different amounts of IL-1Ra cDNA, corresponding to 1, 0.5, 0.2, 0.1, and 0.05 copies per cell, were amplified under the same conditions. After electrophoresis in a 2% agarose gel, amplified products were visualized with ethidium bromide staining.
Induction of anti-GBM glomerulonephritis
Glomerulonephritis was induced using an anti-GBM nephrotoxic serum as described previously.25 In brief, GBM was prepared from Wister Kyoto rats (Charles River Japan, Kanagawa, Japan) by a differential sieving technique, followed by sonication and centrifugation. Rabbit anti-GBM nephrotoxic serum was raised by repeated immunization of a Japanese White rabbit (Kitayama Labs, Nagano, Japan) with particulate GBM. The anti-GBM serum was pooled and decomplemented at 56°C for 30 minutes. To induce anti-GBM glomerulonephritis, DBA/2j mice were intraperitoneally injected with 0.5 mg/20 g body weight of normal rabbit immunoglobulin G (Cappel, West Chester, PA) and complete Freund adjuvant (1:1 dilution; Difco, Detroit, MI). The cross-reactivity of this serum with murine GBM was certified by immunohistochemistry, by which the rabbit immunoglobulin G was detected on the glomeruli 3 days after injection (data not shown). For comparison of the percentage survival rate, anti-GBM antiserum (16 μL/20 g body weight) was repeatedly injected into IL-1Ra and GC chimera 4 weeks after the first challenge.
Renal histology
Ten mice from each group were killed to obtain kidneys 28 days after induction of nephritis. Harvested kidneys were fixed in 10% buffered formaldehyde and embedded in paraffin. Then, 4-μm sections were stained with periodic acid–Schiff (PAS) using a standard method. To semiquantify the renal damage, these sections were subjected to immunohistochemical analyses for the glomerular expression of type I collagen, laminin, and fibronectin. Briefly, paraffin-embedded sections were deparaffinized through a graded alcohol series. When staining for laminin, the sections were digested with a 0.05% solution of protease (Bacillus licheniformis; Sigma) in 50 mM Tris-HCl (pH 7.6) for 8 minutes at 37°C in a water bath. Thereafter, endogenous peroxidase activity was blocked in 0.3% H2O2 in methanol for 20 minutes at room temperature. After being washed in PBS, the sections were incubated with the primary antibodies overnight at 4°C in a humid chamber. Dilution of primary antibodies was at 1:100 for the type I collagen polyclonal antibody (Chemicon, Temecula, CA), at 1:50 for laminin (ICN Biomedicals, Aurora, OH), or at 1:100 for fibrinogen (Dako, Glostrup, Denmark). After washing in PBS, the sections were incubated with biotinylated rabbit antimouse immunoglogulin G (American Quolex, La Mirada, CA) for 60 minutes at room temperature, rinsed in PBS, and incubated with an avidin-biotin-perosidase complex (Vector ABC Elite staining kit, Vector Laboratories, Burlingame, CA). The peroxidase was developed with a diamonobenzidine substrate solution (peroxidase substrate kit, Vector Laboratories). Both kits were used according to the manufacturer's instructions. Finally, sections were counterstained with methyl green reagent. The intensity of glomerular expression of these molecules was scored as previously described26: 0, negative staining; 1, slight staining; 2, moderate staining, 3; marked staining. Scores were assessed in the high-power field (× 400) of 20 consecutive glomerulus cross sections for each animal, and the data are presented as averages of scores from 10 mice in each group.
FISH of the male Y antigen
To trace the transplanted male donor cells in chimeric mice, FISH using a male Y painting probe with spleen and kidney sections from mice with or without nephritis was performed. Kidney and spleen cryosections (6 μM) were mounted on poly-L lysine-coated glass slides (Matunami, Tokyo, Japan) and air-dried at room temperature for an hour. Sections were fixed in freshly prepared 4% paraformaldehyde for 20 minutes and incubated in 0.2N HCl for 10 minutes at room temperature and in proteinase K (10 μg/mL) (Boehringer Mannheim, Mannheim, Germany) in 50 mM Tris, 10 mM ethylenediaminetetraacetic acid, and 10 mM NaCl for 10 minutes at 37°C. The proteinase K reaction was stopped by immersing the slides in 0.2% glycine in PBS for 10 minutes. Before being incubated with the slides, proteinase K was preincubated at 50°C for 1 hour to remove any nucleases. Sections were further fixed with 4% paraformaldehyde for 5 minutes followed by incubation in 0.25% acetic anhydride per 0.1 M triethanolamine for 10 minutes. To inhibit nonspecific staining due to endogenous biotin during immunohistochemical detection, sections were treated with 0.1% avidin and 0.01% biotin for 10 minutes each using a Biotin Blocking System (Dako, Carpinteria, CA). Denaturation of DNA was carried out by incubating sections in 70% formamide in 2 × SSC for 10 minutes at 80°C, dehydrating them in cold graded ethanol (70%, 95%, and 100%) and, finally, air-drying them. The hybridization solution contained a mixture of a biotin-labeled mouse Y painting probe and hybridization buffer (Cambio, Cambridge, United Kingdom) and was denatured at 65°C for 10 minutes. Aliquots of 15 μL were dropped onto each slide and distributed by applying a small coverslip. Hybridization was carried out at 42°C for 16 hours. After hybridization, slides were immersed in 2 × SSC for 5 minutes at 42°C to remove the coverslips. Subsequently, slides were washed twice in 50% formamide, 2 × SSC for 5 minutes, and twice in 0.1% SSC for 5 minutes at 42°C. Immunologic detection of labeled biotin was performed using a biotin (Congo red) detection kit (Cambio), according to the manufacturer's protocol. The sections were observed under a fluorescence photomicroscope, and pictures were taken using an auto-exposure camera (Zeiss, Thornwood, NY).
Serum creatinine and blood urea nitrogen levels
Concentrations of plasma creatinine and blood urea nitrogen were measured using a vision analyzer kit (Abbott Laboratories, North Chicago, IL) according to the manufacturer's instructions.
Urine albumin level
The concentration of albumin in urine was measured by single radial immunodiffusion as previously described9 using a specific antibody for mouse albumin.27 In brief, samples were applied to a 1% agarose gel containing antimouse albumin and incubated for 48 hours. The diameter of the expressed rings on the gel was measured, and concentrations were assessed by comparison with a standard curve.
Statistical analysis
Data were expressed as the means ± SE. Statistical analysis was performed using the 2-sample t test to compare data in different groups. P < .05 was taken as significant.
Results
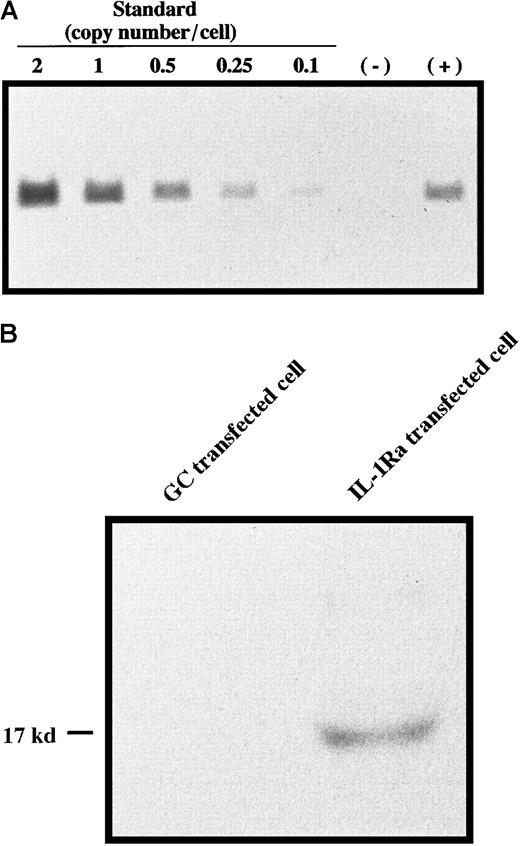
Bone marrow cells harvested from male donor mice were retrovirally transduced with concentrated MFG–IL-1Ra or with MFG-GC as a control using a centrifugation method. Gene copy number analysis revealed that gDNA from transfected bone marrow cells contained the expected fragment of IL-1Ra cDNA generated by restriction digest with KpnI in an amount corresponding to approximately 0.7 copies per cell (Figure1A), and Western blot analysis showed that these cells were able to secrete IL-1Ra protein (Figure 1B), indicating successful gene transfer and expression. These cells were then transplanted into female recipients. Eight weeks later, DNA from the liver, spleen, and bone marrow of each recipient was subjected to nested PCR using the Y-specific antigen. As shown in Figure2A, female recipients had the male Y antigen at 8 weeks after transplantation. Semiquantitative PCR revealed that 10% to 20% of these cells still possessed the transgene (Figure2B). Although we could not identify the type(s) of donor cells in the liver, it was reported that monocytes derived from bone marrow enter the liver and differentiate into Kupffer cells28,29 and, more recently, that hepatocytes may be differentiated from bone marrow stem cells,30 suggesting that these are mainly Kupffer cells and possibly hepatic cells. FISH for Y-specific antigen revealed 71.1 ± 8.9% of the recipient's bone marrow cells to be reconstituted with donor cells at this time point (data not shown). Because hematopoietic stem cells and progenitor cell engraft within 2 weeks of bone marrow transplantation,31 this result indicated successful bone marrow reconstitution. Clonogenic assays for transduced bone marrow cells also showed that 75.8 ± 3.1% of burst-forming units–erythroid (BFU-E), 69.4 ± 8.1% of CFU-macrophage, and 72.8 ± 6.6% of CFU-granulocyte possessed the transgene, and there was no significant difference in the susceptibility to retroviral infection between lineages, suggesting that primitive cells were susceptible to retroviral infection (data not shown).
Retroviral transfection into bone marrow cells.
(A) Gene copy number analysis. DNA from bone marrow cells transfected with (+) or without (−) MFG–IL-1Ra was digested with KpnI and subjected to Southern blot analysis using theNcoI/BamHI fragment of the IL-1Ra gene as a probe. As a standard, different amounts of IL-1Ra cDNA, corresponding to 2, 1, 0.5, 0.25, and 0.1 copies per cell, were also subjected to Southern blotting. (B) Secretion of IL-1Ra from transfected cells. Bone marrow cells transfected with MFG–IL-1Ra or MFG-GC were cultured for 48 hours, and supernatants were subjected to Western blot analysis of IL-1Ra expression.
Retroviral transfection into bone marrow cells.
(A) Gene copy number analysis. DNA from bone marrow cells transfected with (+) or without (−) MFG–IL-1Ra was digested with KpnI and subjected to Southern blot analysis using theNcoI/BamHI fragment of the IL-1Ra gene as a probe. As a standard, different amounts of IL-1Ra cDNA, corresponding to 2, 1, 0.5, 0.25, and 0.1 copies per cell, were also subjected to Southern blotting. (B) Secretion of IL-1Ra from transfected cells. Bone marrow cells transfected with MFG–IL-1Ra or MFG-GC were cultured for 48 hours, and supernatants were subjected to Western blot analysis of IL-1Ra expression.
Bone marrow reconstitution using a retrovirus.
Bone marrow from female recipient mice was reconstituted with genetically engineered male donor cells using a transplantation-based method. Eight weeks later, DNA was extracted from the bone marrow, spleen, and liver of these mice and subjected to PCR for mouse Y antigen (395 base pairs [bp]). A representative photograph from 9 chimeric mice is shown (A). DNA from spleen was also subjected to semiquantitative PCR for the human IL-1Ra gene. This pair of primers may yield both cDNA (263 bp) and gDNA (1045 bp).24 As a standard, different amounts of IL-1Ra cDNA, corresponding to 1, 0.5, 0.2, 0.1, and 0.05 copies per cell, were used. A representative photograph from 4 individual mice is shown. M: DNA marker (φ × 174/HaeIII digest) (B).
Bone marrow reconstitution using a retrovirus.
Bone marrow from female recipient mice was reconstituted with genetically engineered male donor cells using a transplantation-based method. Eight weeks later, DNA was extracted from the bone marrow, spleen, and liver of these mice and subjected to PCR for mouse Y antigen (395 base pairs [bp]). A representative photograph from 9 chimeric mice is shown (A). DNA from spleen was also subjected to semiquantitative PCR for the human IL-1Ra gene. This pair of primers may yield both cDNA (263 bp) and gDNA (1045 bp).24 As a standard, different amounts of IL-1Ra cDNA, corresponding to 1, 0.5, 0.2, 0.1, and 0.05 copies per cell, were used. A representative photograph from 4 individual mice is shown. M: DNA marker (φ × 174/HaeIII digest) (B).
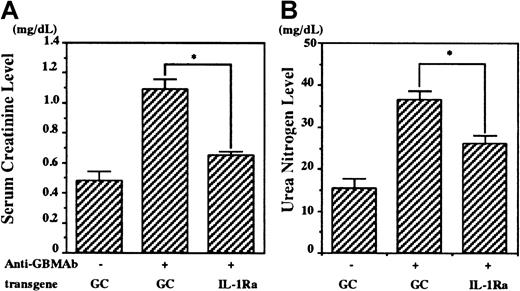
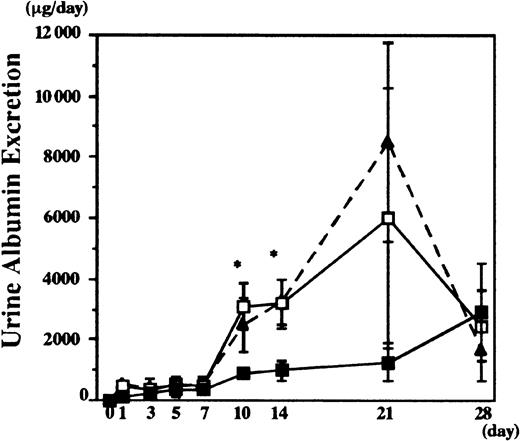
Nephritis was induced in the chimeric mice 8 weeks after transplantation. Following anti-GBM serum administration, serum creatinine and urea nitrogen levels were elevated after 28 days in control mice (Figure 3), showing anti-GBM serum-induced renal failure, whereas lower serum creatinine and urea nitrogen levels were observed in the IL-1Ra chimeras (1.0 ± 0.1 mg/mL in the GC chimeras vs 0.6 ± 0.1 mg/mL in the IL-1Ra chimeras, and 37 ± 2 mg/mL in the GC chimeras vs 27 ± 1 mg/mL in IL-1Ra chimeras on day 28, respectively) (Figure 3). Urine albumin excretion levels also rose progressively in the GC control chimeras, compared with age-matched wild-type female mice (Figure 4). These increases were significantly suppressed in the IL-1Ra chimeras up to 2 weeks (997 ± 321 mg/d in the IL-1Ra chimera vs 3199 ± 796 mg/d in the GC chimera on day 14). However, these differences were not significant at later time points, presumably because the urine was not collected properly because of the reduction in urine volume (< 0.5 mL) in mock chimeras with renal failure.
Effect of bone marrow reconstitution with IL-1Ra on renal function after the induction of anti-GBM glomerulonephritis.
The bone marrow of female recipients was reconstituted with male cells, which possessed the mouse IL-1Ra gene (IL-1Ra chimera) or human GC gene (GC chimera). Anti-GBM glomerulonephritis was induced in these chimeric mice (10 per group), and serum creatinine levels (left) and urea nitrogen levels (right) were measured before and 28 days after the induction. Data are presented as means ± SE. Asterisks indicate significant differences (P < .05) between the 2 groups.
Effect of bone marrow reconstitution with IL-1Ra on renal function after the induction of anti-GBM glomerulonephritis.
The bone marrow of female recipients was reconstituted with male cells, which possessed the mouse IL-1Ra gene (IL-1Ra chimera) or human GC gene (GC chimera). Anti-GBM glomerulonephritis was induced in these chimeric mice (10 per group), and serum creatinine levels (left) and urea nitrogen levels (right) were measured before and 28 days after the induction. Data are presented as means ± SE. Asterisks indicate significant differences (P < .05) between the 2 groups.
Effect of bone marrow reconstitution with IL-1Ra on urine albumin secretion after nephritis induction.
An anti-GBM antibody was infused into IL-1Ra and GC chimera, and urine albumin was measured by single radial immunodiffusion. As a control, urine albumin secretion from wild-type female mice administered with anti-GBM antibody was also measured (dotted line). Data are presented as means ± SE. Asterisks indicate significant differences (P < .05) between the 2 groups. GC chimera (n = 10), ■; IL-1Ra chimera (n = 10), ▪.
Effect of bone marrow reconstitution with IL-1Ra on urine albumin secretion after nephritis induction.
An anti-GBM antibody was infused into IL-1Ra and GC chimera, and urine albumin was measured by single radial immunodiffusion. As a control, urine albumin secretion from wild-type female mice administered with anti-GBM antibody was also measured (dotted line). Data are presented as means ± SE. Asterisks indicate significant differences (P < .05) between the 2 groups. GC chimera (n = 10), ■; IL-1Ra chimera (n = 10), ▪.
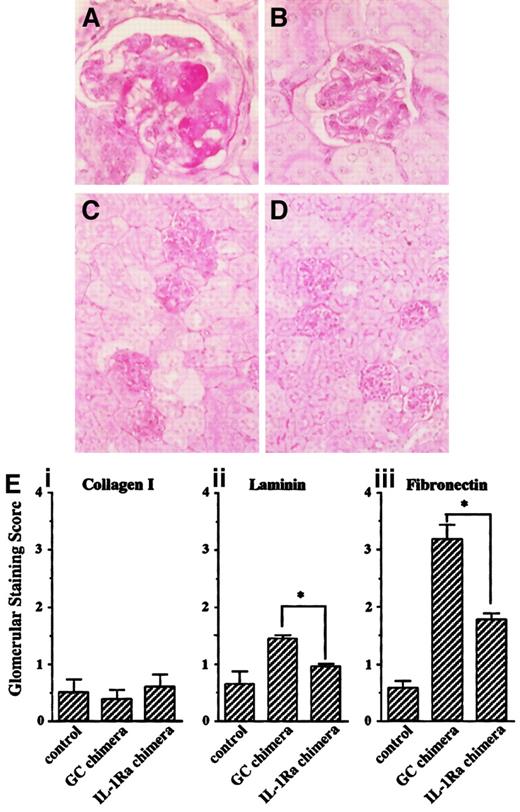
Histologic analysis revealed that anti-GBM serum induced glomerulonephritis in GC chimeras (Figure5A). These lesions included homogeneous deposition of PAS-positive material in the capillary loops, intravascular coagulation, endothelial cell swelling, and necrosis, which were consistent with previous reports,32 33 and similar damage was observed in other glomeruli (Figure 5C). In contrast, the effect was significantly attenuated in the IL-1Ra chimeras (Figure 5B,D). To semiquantify these differences, immunohistochemical analyses were performed on the glomerular expression of type I collagen, laminin, and fibrinogen. Although the glomerular expression of type I collagen was unchanged after induction of nephritis, that of laminin and fibrinogen were significantly enhanced 28 days after anti-GBM serum injection in the GC chimeras, whereas this expansion was markedly suppressed in the IL-1Ra chimeras (Figure 5E). Taken together, these results suggest that bone marrow reconstitution with IL-1Ra confers resistance to glomerular inflammation for at least 12 weeks after primary bone marrow transplantation.
Histology of kidney after nephritis induction in IL-1Ra and GC chimera (day 28).
Kidney sections from nephritis-induced chimeric mice were subjected to PAS staining. (A,C) GC chimera; (B,D) IL-1Ra chimera (original magnification: × 400 for panels A and B, × 100 for panels C and D). (E) To semiquantify the severity of glomerular damage, relative amounts of glomerular staining of type I collagen (i), laminin (ii), and fibrinogen (iii) were scored as described in “Materials and methods.” Data are shown as means ± SE. Asterisks indicate significant differences (P < .05) between the 2 groups.
Histology of kidney after nephritis induction in IL-1Ra and GC chimera (day 28).
Kidney sections from nephritis-induced chimeric mice were subjected to PAS staining. (A,C) GC chimera; (B,D) IL-1Ra chimera (original magnification: × 400 for panels A and B, × 100 for panels C and D). (E) To semiquantify the severity of glomerular damage, relative amounts of glomerular staining of type I collagen (i), laminin (ii), and fibrinogen (iii) were scored as described in “Materials and methods.” Data are shown as means ± SE. Asterisks indicate significant differences (P < .05) between the 2 groups.
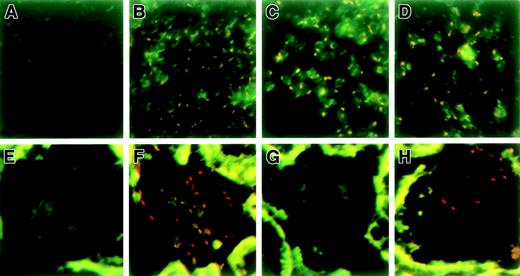
Furthermore, to trace the transplanted donor cells, spleen and kidney sections were subjected to FISH using a Y painting probe because only donor cells possess the Y antigen. There were no significant differences in the number of donor cells in the spleen (75.9% ± 11.4% of spleen cells from the control chimera possessed the Y antigen [Figure 6C] vs 77.6% ± 9.6% from the nephritis-induced chimera [Figure 6D]). On the other hand, Y-positive cells were recruited into the glomerulus upon induction of nephritis (Figure 6H). Because control experiments using male spleen sections did not identify 100% Y-positive nuclei, we may have underestimated the proportion of donor cells (Figure 6B). Western blot analysis revealed that the supernatant of glomeruli isolated from IL-1Ra chimera 3 days after nephrotoxic serum injection contained high levels of IL-1Ra protein. Serum IL-1Ra from both groups was below the detectable limit of Western blot analysis (< 10 pg/mL) (data not shown). Together with the fact that 10 000-fold more IL-1Ra is needed to suppress IL-1 action34 and that the serum IL-1β level was elevated to 27.4 ± 11.7 pg/mL on induction of nephritis as described before,35 these results suggest that the therapeutic effect was not due to the systemic delivery of IL-1Ra but to local suppression of IL-1 within the inflamed glomeruli.
Identification of transplanted donor cells in chimeric mice using FISH.
To trace the transplanted donor cells, spleen and kidney sections from chimeric mice with or without nephritis were subjected to FISH using a Y painting probe (4 per group). Representative photographs are shown. (A) Spleen of control female mouse; (B) spleen of control male mouse; (C) spleen of untreated chimeric mouse; (D) spleen of chimeric mouse treated with anti-GBM serum (day 3); (E) kidney of control female mouse; (F) kidney of control male mouse; (G) kidney of untreated chimeric mouse; and (H) kidney of chimeric mouse treated with anti-GBM serum (day 3). Red dots show Y antigen–positive donor cells.
Identification of transplanted donor cells in chimeric mice using FISH.
To trace the transplanted donor cells, spleen and kidney sections from chimeric mice with or without nephritis were subjected to FISH using a Y painting probe (4 per group). Representative photographs are shown. (A) Spleen of control female mouse; (B) spleen of control male mouse; (C) spleen of untreated chimeric mouse; (D) spleen of chimeric mouse treated with anti-GBM serum (day 3); (E) kidney of control female mouse; (F) kidney of control male mouse; (G) kidney of untreated chimeric mouse; and (H) kidney of chimeric mouse treated with anti-GBM serum (day 3). Red dots show Y antigen–positive donor cells.
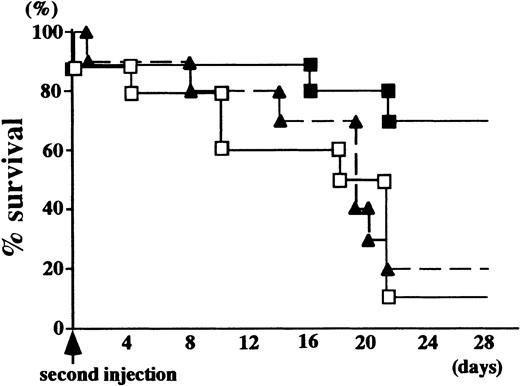
Finally, because our preliminary findings suggested that repeated injection of anti-GBM serum (16 mg/20 g body weight) causes fatal renal damage, the survival rate after the second injection was compared between the 2 groups to examine how long the therapeutic effect persisted. As shown in Figure 7, 90% of the GC chimeras died within 28 days of the second injection, whereas 80% of the IL-1Ra chimera survived to this time point, suggesting that donor cells were continuously supplied from the reconstituted bone marrow and secreted anti-inflammatory cytokines. The therapeutic effect lasted until 4 months after primary bone marrow reconstitution.
Percentage survival curve of IL-1Ra and GC chimeric mice after a second challenge with the anti-GBM antibody.
The anti-GBM antibody was repeatedly injected into chimeric mice 28 days after the primary injection, and survivors were counted (10 per group). As a control, the percentage survival curve of age-matched wild-type female mice is also shown. GC chimera (n = 10), ■; IL-1Ra chimera (n = 10), ▪; wild-type female mice (n = 10), ▴.
Percentage survival curve of IL-1Ra and GC chimeric mice after a second challenge with the anti-GBM antibody.
The anti-GBM antibody was repeatedly injected into chimeric mice 28 days after the primary injection, and survivors were counted (10 per group). As a control, the percentage survival curve of age-matched wild-type female mice is also shown. GC chimera (n = 10), ■; IL-1Ra chimera (n = 10), ▪; wild-type female mice (n = 10), ▴.
Discussion
Chronic inflammation is a basic symptom of several common diseases such as rheumatoid arthritis and glomerulonephritis, from which many people throughout the world continue to suffer despite advances in the understanding of pathophysiology. No satisfactory nontoxic long-lasting therapy is currently available, and a novel therapeutic strategy is therefore required. Recently, transplantation-based gene therapy has been reported to be promising for the treatment of hematologic disorders,1,2 and because it is evident that macrophages and neutrophils are derived from hematopoietic stem cells and play a major role in disease progression, chronic inflammation may be treatable with this strategy. We examined the possible application of transplantation-based gene therapy to chronic inflammation using glomerulonephritis as an inflammation model.9 10 In the present study, we showed that bone marrow reconstitution with stem cells transduced with an anti-inflammatory retroviral vector confers resistance to glomerular inflammation, and that the therapeutic effect lasts at least 16 weeks.
To prolong the therapeutic effect, several modifications were made. In the previous model, the bone marrow cells infused into recipients had been differentiated into monocyte-lineage cells ex vivo,9 whereas in the present study, undifferentiated primitive marrow cells were used for transplantation. This was done so that modified transplanted cells could retain the potential for self-renewal as well as differentiation in vivo. Also, we used an adenoviral vector to transfect foreign genes in the previous system because an adenovirus can achieve a high level of gene transfer into ubiquitous cell types,36 including monocyte-lineage cells.37 A retroviral vector was used for the present system because it can integrate foreign genes into the gDNA of target cells, with the result that every cell from reconstituted bone marrow possesses the transgene.
We previously succeeded in transducing the GC gene via a retrovirus into hematopoietic stem cells and demonstrated increased enzymatic activity in murine macrophages 7 months after transplantation.19 On the basis of this original method, we first tried to transduce a foreign gene using a retrovirus. Unfortunately, the IL-1Ra delivered by this method was not able to suppress glomerular inflammation, probably because the retroviral long terminal repeat is a much weaker promoter than the CAG (cytomegalovirus enhancer-chicken β-actin hybrid) promoter38 used for the previous study.10 We then made further modifications and tried several different methods, finally concluding that the most efficient approach was repeated transplantation of transduced marrow cells into sublethally irradiated mice using a centrifugation method19 with concentrated viral supernatant.20 This modification resulted in the highest efficacy among our retrovirus-based transduction attempts. However, Western blot analysis showed that the supernatant from bone marrow cells transduced with IL-1Ra via a retrovirus with our modification contained less recombinant IL-1Ra than that from adenovirally transduced cells (data not shown). Nevertheless, retrovirally transduced bone marrow cells significantly suppressed glomerular inflammation. This can be explained by the differences in the number of anti-inflammatory cells and duration of their occupancy at the site of inflammation. With the previous method, circulating bone marrow cells competed for a limited area of ICAM-1 expression with native macrophages. Also, the short half-life of differentiated monocyte cells macrophage resulted in only a transient effect on the inflammation. On the other hand, native macrophages per se had anti-inflammatory properties in the present system and did not necessarily compete with other cells. These cells may also be continuously supplied from reconstituted bone marrow so that inflammation is suppressed throughout its progression. These factors may also explain why the amount of IL-1Ra protein secreted from transplanted donor cells was not large enough to increase the serum IL-1Ra level, but suppressed the local inflammation, and overcome the disadvantages of using a retrovirus for gene transfer.
There are, however, several problems to be solved before this system can be applied clinically. Because IL-1 is a multipotent cytokine, systemic suppression of IL-1 by IL-1Ra may induce several unexpected side effects. However, no changes were observed in hematologic parameters or hormonal and biochemical indicators in healthy volunteers given a 3-hour intravenous infusion of IL-1Ra (up to 10 mg/kg) in a phase I trial.39 Although we could not exclude the possibility that a much higher concentration of IL-1Ra may affect homeostasis, the evidence suggests that less than 10 pg/mL IL-1Ra in serum does not have detectable toxicity. Furthermore, we focused on IL-1β as a key proinflammatory cytokine because it has been reported to mediate the progression of inflammation and the blockade of IL-1 action by IL-1Ra suppresses inflammation, at least in experimental anti-GBM glomerulonephritis.10 12 However, inflammation is not a monogenic disease, and many factors contribute to its progression. Advances are needed to understand the physiologic mechanisms and identify the key molecule(s) in human inflammation to develop clinical applications.
Another problem is cell specificity. Because unfractionated marrow cells, including multipotent stem cells, were used to reconstitute bone marrow, cells other than monocyte-lineage cells may be produced and have an unpredictable effect in nontargeted organs. In fact, clonogenic assay revealed that there was no significant difference in the susceptibility to retroviral infection between hematopoietic lineages, including BFU-E, CFU-erythrocyte, and CFU-M (data not shown). Therefore, an on/off switching system, by which participants in inflammation are activated exclusively at the inflamed site, will be required. In this regard, we recently succeeded in inflamed site-specific activation of monocyte-lineage cells in vitro using an IL-1β promoter combined with a Cre/loxP system40 so that transgene could be induced in the cells and the environment where IL-1β was produced (T. Yokoo, unpublished data, October 2001). Currently, the in vivo utility of this regulation is being examined in our laboratory.
In this study, we could not induce nephritis before bone marrow reconstitution, because it takes more than 4 weeks for transplanted donor cells to settle down in the recipient organ and our anti-GBM serum induces severe nephritis and progresses to end-stage renal failure within 4 weeks. There is a need to confirm whether this strategy is feasible in preestablished inflammatory diseases as a next step. We are now trying to clarify this issue by delivering hepatocyte growth factor (HGF) into a spontaneous mouse model for chronic renal disease, because HGF has been described as a renotrophic factor and continuous injection of high dose HGF may prevent manifestation of renal dysfunction in congenital renal failure.41
In conclusion, we applied stem cell transplantation–based gene therapy to the treatment of glomerulonephritis and succeeded in long-term suppression of glomerular inflammation. Although the application of gene therapy for nonlethal diseases is controversial,42 43this success may strengthen the rationale for gene therapy as a treatment for glomerulonephritis. We have only begun this research, and many problems must be solved before making major investments toward clinical use. We still believe that these trials represent the next step in research aimed at the discovery of a novel therapeutic strategy that will solve the problems encountered in conventional therapy.
We thank Dr Paul Robbins (University of Pittsburgh) for providing pMFG retroviral vector and Crip cells and thank Ms T. Murata for immunohistochemical analysis.
Supported by a grant from the Ministry of Health and Welfare of Japan and the Bio-Venture Research Fund Project Aid grant from the Ministry of Education, Science and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Takashi Yokoo, Dept of Internal Medicine (II), Jikei University School of Medicine, 3-25-8 Nishi-shimbashi, Minato-ku, Tokyo, Japan 105-8461; e-mail: tyokoo@jikei.ac.jp.

![Fig. 2. Bone marrow reconstitution using a retrovirus. / Bone marrow from female recipient mice was reconstituted with genetically engineered male donor cells using a transplantation-based method. Eight weeks later, DNA was extracted from the bone marrow, spleen, and liver of these mice and subjected to PCR for mouse Y antigen (395 base pairs [bp]). A representative photograph from 9 chimeric mice is shown (A). DNA from spleen was also subjected to semiquantitative PCR for the human IL-1Ra gene. This pair of primers may yield both cDNA (263 bp) and gDNA (1045 bp).24 As a standard, different amounts of IL-1Ra cDNA, corresponding to 1, 0.5, 0.2, 0.1, and 0.05 copies per cell, were used. A representative photograph from 4 individual mice is shown. M: DNA marker (φ × 174/HaeIII digest) (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/1/10.1182_blood.v98.1.57/6/m_h81311232002.jpeg?Expires=1769163788&Signature=g65scjlO0Ws4D7ntXnu0CkncGDV0TO3hT-o1F-gyF3rPo9E265NEi4FdNLJ0HoEMe1ffZzL9dEBA6aB4-CtnKGM6uuvSYA1COtH3ZjxAcGIrC7dZZDvdu3wAcE7oErS-KIBQjhJg3JSuV~zkm0hov51JS4bq99~Mzh9c6Fkd75~vJUzRCIn4~8LpdfLfIp29cs4Mi5b28-ktBoPOJuLZHRefkBes2i5E2YCTzNzZf7puclnF7pMVB78vBXaDsGBtMmpwDsdFtOhmHWU5CPv5MukyWqZQ~AgXHR54be33hVGYO-OLt9mI0P4a-jNrF6Ap0K~60JdgF46YeHLIDlM6EA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal