Abstract
Residual mediastinal masses are frequently observed in patients with Hodgkin disease (HD) after completed therapy, and the discrimination between active tumor tissue and fibrotic residues remains a clinical challenge. We studied the diagnostic value of metabolic imaging by 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) in detecting active mediastinal disease and predicting relapse. Twenty-eight HD patients with a residual mediastinal mass of at least 2 cm after initial therapy or after salvage chemotherapy were prospectively assigned to 29 examinations with FDG PET and were evaluated as 29 “subjects.” Patients were monitored for at least 1 year after examination and observed for signs of relapse. Median follow-up was 28 months (range, 16 to 68 months). A PET-negative mediastinal tumor was observed in 19 subjects, of whom 16 stayed in remission and 3 relapsed. Progression or relapse occurred in 6 of 10 subjects with a positive PET, whereas 4 subjects remained in remission. The negative predictive value (negative PET result and remission) at 1 year was 95%, and the positive predictive value (positive PET result and relapse) was 60%. The disease-free survival for PET-negative and PET-positive patients at 1 year was 95% and 40%, respectively. The difference was statistically significant. A negative FDG PET indicates that an HD patient with a residual mediastinal mass is unlikely to relapse before 1 year, if ever. On the other hand, a positive PET result indicates a significantly higher risk of relapse and demands further diagnostic procedures and a closer follow-up.
Introduction
Hodgkin disease (HD) has become a highly curable neoplasia. The response and survival rates for newly diagnosed patients with HD could be increased in recent years using aggressive polychemotherapy such as the BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone) regimen and radiation. BEACOPP showed a significant increase of the overall survival compared with standard COPP/ABVD (cyclophosphamide, vincristine, procarbazine, prednisone/doxorubicin, vinblastine, bleomycin, dacarbazine) in patients with advanced-stage HD.1 Also, high-dose chemotherapy with autologous stem cell support can cure many relapsed patients of their disease.2 However, in up to 64% of all HD cases, computed tomography (CT) or magnetic resonance imaging (MRI) show remaining tumor masses after completed treatment,3 although only a few of these patients will eventually relapse.4,5 These masses may represent fibrotic tissue or even an active Hodgkin tumor, but discrimination by CT is usually not possible.5 If the tumor is easily accessible, as an enlarged inguinal lymph node, the questionable lesion can be excised and histologically analyzed, whereas a mediastinal tumor can only be accessed by mediastinoscopy or open thoracic surgery. Mediastinoscopy is associated with a mortality risk of up to 0.5%6 and a high failure rate for a pathological diagnosis because of the relatively small amount of tissue that can be gained. Open thoracic surgery is an invasive procedure and thus not desirable for a potentially cured patient. Therefore, posttreatment mediastinal residues usually require follow-up CT scans at short time intervals. A delay in the diagnosis of progression or early relapse may result from this strategy and could mean a loss of precious time for salvage therapy.
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is a relatively new diagnostic method compared with CT or MRI and has been reported to detect active tumors in many cancer entities, especially in lymphomas.7-10 There are 2 clinically relevant indications for FDG PET in patients suffering from HD. First, FDG PET can be used to improve staging techniques at initial diagnosis by detecting lesions not detected by CT. Second, FDG PET may be used in patients with residual tumor masses and could allow discrimination between active lymphoma and nonactive fibrotic tissue. FDG PET for primary staging of HD has been investigated by several groups.9 11-15 The aim of the present study was to assess the diagnostic and prognostic value of FDG PET in HD patients with residual mediastinal masses after completed therapy.
Patients and methods
Patients
Twenty-eight patients with HD and a residual mediastinal mass after chemotherapy as shown by CT (1 patient MRI) were included in our study. All histologies had been confirmed before treatment by one of the reference pathologists of the German Hodgkin's Lymphoma Study Group. Patients were recruited prospectively between November 1994 and January 2000 at the Department of Internal Medicine I of the University of Cologne, Germany. They were assigned to 29 FDG PET scans within 4 months after completion of conventional initial therapy or after salvage therapy with or without high-dose chemotherapy and peripheral blood stem cell transplantation (PBSCT). All patients had a mediastinal mass more than 2 cm in diameter. None of them had received another chemotherapy or radiotherapy after PET before a remission status could be documented to preserve the predictive value of PET. The characteristics at the time of the examinations for 28 eligible patients with 29 FDG PET studies are listed in Table1.
Characteristics of 28 eligible patients
| Characteristics . | No. of patients . |
|---|---|
| Sex | |
| Male | 20 |
| Female | 8 |
| Histologic subtype | |
| Nodular sclerosis | 21 |
| Mixed cellularity | 6 |
| Lymphocyte predominant | 1 |
| Ann Arbor clinical stage | |
| I | 1 |
| II | 9 |
| III | 7 |
| IV | 2 |
| Relapse | 9 |
| B symptoms | |
| Yes | 14 |
| No | 14 |
| Treatment | |
| Chemotherapy alone | 11 |
| Radiotherapy alone | 1 |
| Combined therapy | 8 |
| Salvage chemotherapy without PBSCT | 6 |
| Salvage chemotherapy with PBSCT | 2 |
| Prior mediastinal irradiation | |
| Yes | 18 |
| No | 10 |
| Characteristics . | No. of patients . |
|---|---|
| Sex | |
| Male | 20 |
| Female | 8 |
| Histologic subtype | |
| Nodular sclerosis | 21 |
| Mixed cellularity | 6 |
| Lymphocyte predominant | 1 |
| Ann Arbor clinical stage | |
| I | 1 |
| II | 9 |
| III | 7 |
| IV | 2 |
| Relapse | 9 |
| B symptoms | |
| Yes | 14 |
| No | 14 |
| Treatment | |
| Chemotherapy alone | 11 |
| Radiotherapy alone | 1 |
| Combined therapy | 8 |
| Salvage chemotherapy without PBSCT | 6 |
| Salvage chemotherapy with PBSCT | 2 |
| Prior mediastinal irradiation | |
| Yes | 18 |
| No | 10 |
The patients ranged in age from 18-76 years, with a median age of 36.
FDG PET
PET data of the chest were obtained on an ECAT EXACT scanner (Siemens-CTI, Knoxville, TN) with an axial field of view of 16.2 cm. The measured axial field of view extended over 45 cm (3 bed positions). Forty-seven overlapping slices per bed position were acquired with a full width of 6.0 mm at half-maximal resolution and approximately 5.0-mm slice thickness with a 3.75 mm center-to-center distance in the axial direction. Emission data were reconstructed by filtered back-projection with a Hanning filter and a cut-off frequency of 0.4 Nyquist. Corrections for attenuation were based on measured transmission scans. FDG was produced by a standard technique.16 Patients were in the fasting state (> 12 hours) controlled by a blood glucose test before examination (mean value 5.4 mM/L [98 mg/dL]). A dose of 370 MBq FDG was injected intravenously. A focally increased FDG uptake in the mediastinum was considered positive if visually detectable. “Positive” results were also semiquantitatively investigated using the standardized uptake value (SUV). All FDG PET scans were performed at and in collaboration with the PET-Center of the Max Planck Institute for Neurological Research, Cologne, Germany.
Data analysis
For all patients, the last date of a clinically complete remission or the first date of a progression and/or relapse after PET was documented. Follow-up evaluation occurred every 3 months by a hematologist and included a physical examination, a complete laboratory work-up, a chest x-ray, and an abdominal ultrasound. Clinically complete remission with a residual mass was defined by the presence of residual mediastinal masses in an additional follow-up CT/MRI scan, no B symptoms, a normal laboratory test, and an abdominal ultrasound without any signs of progressive disease. Progression was defined as a growth of the mediastinal tumor according to CT/MRI within 3 months after completion of therapy. A relapse was characterized by tumor progression later than 3 months after completed therapy. One patient had 2 FDG PET scans after initial therapy and after salvage chemotherapy, respectively. This patient was evaluated as 2 different subjects in the data analysis.
Statistics
Comparison of the 2 groups (FDG PET–positive or –negative) for the probability of relapse was calculated by Fisher exact tests. The disease-free survival (DFS) was performed with a Kaplan-Meier analysis. The comparison between groups was calculated by a log-rank test. A probability (P value) smaller than .05 was considered statistically significant.
Results
FDG PET and response
During the median follow-up of 28 months (range, 16 to 68 months), 9 (31%) of 29 subjects had a progression/relapse after a median of 1.2 months, with a range of 0.1 to 29 months. Five (56%) subjects progressed/relapsed after initial therapy and 4 (44%) after salvage chemotherapy. A biopsy was performed on all of these subjects and confirmed the initial HD subtype.
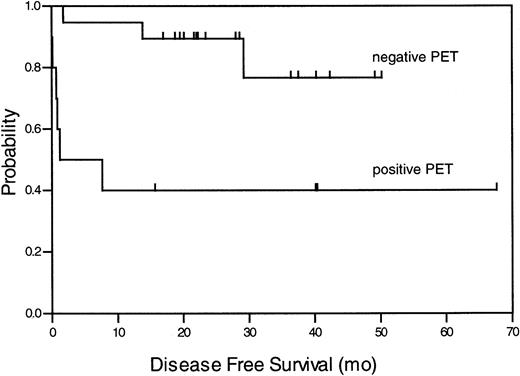
Ten subjects had a positive (34%) and 19 subjects a negative (66%) FDG PET scan. All positive PET examinations showed only increased glucose metabolism in the mediastinal area and not in any other region. The SUV of the positive scans ranged from 1.6 to 6.9 (mean value 3.4). Of the FDG PET positive subjects, 4 (40%) stayed in remission after a median observation time of 40 months (range, 16 to 68 months), whereas 6 (60%) progressed or relapsed in a median time of 0.72 months after therapy (range 0 to 7.5 months). Sixteen (84%) PET-negative subjects stayed in remission during the entire observation time and 3 (16%) relapsed. At 1 year, 95% of all PET-negative patients were disease-free, in contrast to only 40% of patients with a positive PET scan. These findings were significant (P = .032) and are summarized in Table 2. A Kaplan-Meier analysis for the DFS of patients with a negative or positive PET result is presented in Figure 1. The comparison of the groups (DFS at 1 year 95% vs 40% ± 5%) resulted in a significant log-rank test with P = .004. The positive predictive value (defined as “true” positive for relapse) was 60%, and the negative predictive value (defined as “true” negative for clinical remission) for a relapse earlier than 1 year after PET was 95% (P = .003).
Remission status according to PET result
| . | PD/relapse . | CRr . | Predictive value, % . |
|---|---|---|---|
| PET-positive | 6 | 4 | (positive) 60 |
| PET-negative | 3 | 16 | (negative) 84 |
| . | PD/relapse . | CRr . | Predictive value, % . |
|---|---|---|---|
| PET-positive | 6 | 4 | (positive) 60 |
| PET-negative | 3 | 16 | (negative) 84 |
P value for the difference between positive and negative FDG PET is .032.
PD indicates progressive disease; CRr, clinically complete remission with a residual mass.
Kaplan-Meier analysis of DFS for all patients with negative and positive FDG PET result.
The log-rank test produced the probability ofP = .004.
Kaplan-Meier analysis of DFS for all patients with negative and positive FDG PET result.
The log-rank test produced the probability ofP = .004.
Sites of relapse and subsequent outcome
All 6 subjects with a positive PET scan and recurrent disease relapsed only within the detected mediastinal area and not elsewhere. Of the 3 other relapsing subjects who were PET-negative, 2 suffered from a mediastinal relapse and 1 from a combined mediastinal and liver relapse. All 9 subjects with a recurrent HD were followed for at least 15 months or until death. Three relapsing subjects with a negative PET result could be treated successfully and reached complete remission with a follow-up of 18, 20, and 24 months, whereas only 2 of 6 relapsing subjects with a positive PET reached a complete remission with a follow-up of 36 and 50 months. However, these numbers are too small to reach significance for the analysis of whether PET can predict subsequent outcome. Of the 4 remaining subjects, 1 subject achieved a partial remission with a high-dose chemotherapy and is currently treated with a palliative regimen 15 months after the PET examination. Three subjects died due to progressive disease 1, 4, and 23 months after their PET scans.
False-positive PET results
Four of the 10 PET-positive examinations were false positive. The SUV of these mediastinal lesions were 3.3, 3.1, 2.4, and 1.6, respectively. One patient had a postchemotherapeutic thymus hyperplasia, which regressed after another 6 months according to a second CT scan. The reasons for misleading PET data in the 3 remaining patients are unknown; clinical causes such as pneumonitis could be ruled out. The PET examinations had been performed 3 to 4 months after completed therapy. The follow-up was 40, 40, and 68 months. Mediastinoscopy with a large biopsy of 21 g of mediastinal mass was performed on one of these patients and revealed tissue with hyaline sclerosis and a massive histiocytic reaction but without any signs of malignancy. Three of the 4 patients with a false-positive PET result had received chemotherapy and additional radiotherapy to the residual mediastinal masses before PET examinations, as shown in Table3.
Characteristics of patients with mediastinal tumors and false-positive or false-negative FDG PET result
| Patient no. . | Age, y . | Therapy and stage . | Mass in control CT . | Site3-150 . | Time until result, mo3-151 . | Biopsy . | Reason . |
|---|---|---|---|---|---|---|---|
| FDG PET false positive | |||||||
| 1 | 25 | 2× COPP/ABVD + RT (IIB) | Stable | Left hilar | 68 | nd | Unknown |
| 2 | 18 | 2× COPP/ABVD + RT (IIA) | Stable | Ventral med | 40 | Negative | Unknown |
| 3 | 32 | 2× COPP/ABVD + RT (IIA) | Stable | Lower med | 40 | nd | Unknown |
| 4 | 18 | 4× BEACOPP esc + 4× BEACOPP bas (IVB) | Regression | Ventral med | 12 | nd | Thymus hyperplasia |
| FDG PET false negative | |||||||
| 5 | 32 | 8× BEACOPP-14 (IIIB) | Mediastinal | 14 | Positive | Late relapse | |
| 6 | 32 | 3× DexaBEAM (1st relapse, med) | Mediastinal | 29 | Positive | Late relapse | |
| 7 | 33 | 8× BEACOPP esc (IVA) | Mediastinal, abdominal | 2 | Positive | Unknown, PET after 2 mo positive | |
| Patient no. . | Age, y . | Therapy and stage . | Mass in control CT . | Site3-150 . | Time until result, mo3-151 . | Biopsy . | Reason . |
|---|---|---|---|---|---|---|---|
| FDG PET false positive | |||||||
| 1 | 25 | 2× COPP/ABVD + RT (IIB) | Stable | Left hilar | 68 | nd | Unknown |
| 2 | 18 | 2× COPP/ABVD + RT (IIA) | Stable | Ventral med | 40 | Negative | Unknown |
| 3 | 32 | 2× COPP/ABVD + RT (IIA) | Stable | Lower med | 40 | nd | Unknown |
| 4 | 18 | 4× BEACOPP esc + 4× BEACOPP bas (IVB) | Regression | Ventral med | 12 | nd | Thymus hyperplasia |
| FDG PET false negative | |||||||
| 5 | 32 | 8× BEACOPP-14 (IIIB) | Mediastinal | 14 | Positive | Late relapse | |
| 6 | 32 | 3× DexaBEAM (1st relapse, med) | Mediastinal | 29 | Positive | Late relapse | |
| 7 | 33 | 8× BEACOPP esc (IVA) | Mediastinal, abdominal | 2 | Positive | Unknown, PET after 2 mo positive | |
RT indicates radiotherapy; med, mediastinum; nd, not done; esc, escalated; bas, basis.
For FDG PET false-positive patients, this is the tumor localization site; for FDG PET false-negative patients, the site of relapse.
For FDG PET false-positive patients, this is the time after there was clinically complete remission with a residual mass; for FDG PET false-negative patients, the time after which there was progressive disease and/or relapse.
False-negative PET results
A negative PET result was observed in 19 patients, of whom 3 had a progression or relapse. In one of these patients, PET failed to detect a growing mediastinal tumor that appeared larger in a second CT scan that was done shortly afterward. A CT-controlled biopsy of this lesion confirmed the diagnosis of HD. A second PET scan was performed 2 months after the initial PET and was then positive for the tumor. In the other 2 patients, who had a negative FDG PET, relapse occurred 14 and 29 months after the examination, respectively. Both patients relapsed at the initial mediastinal site. None of the 3 patients with a false-negative PET result had any prior mediastinal irradiation. Table3 summarizes the characteristics of the patients with a false positive or false-negative PET result.
Discussion
The analysis of active lymphoma after chemotherapy or radiotherapy has a great clinical impact. Before FDG PET was introduced for the clinical evaluation of patients with lymphoma, tumor activity of residual mediastinal masses after therapy could only be assessed by invasive procedures such as mediastinoscopy or by CT using a “time frame,” eg, repeating the procedure at regular intervals to detect a progressive tumor. MRI has been reported to provide prognostic information by discriminating between active malignant tissue and fibrosis/normal tissue in T2-weighted images.17-19However, MRI has a low sensitivity and is therefore not useful for lymphoma.2067Gallium scintigraphy is a metabolic imaging technique to detect active tumor tissue, but it has disadvantages such as a low spatial resolution, lack of specificity, and difficulty in quantification of uptake.21 Other significant shortcomings of this technique are shown particularly in the posttherapy setting because the sensitivity depends on the subtype and the location of the lymphoma.22,23 FDG PET imaging has the potential to provide information about active tumor sites in staging9,12,14 as well as in monitoring response to therapy. Moreover, it has been reported to be superior to67gallium in respect to sensitivity and specificity.24 FDG PET seems to have a value for HD patients with residual tumor burden.8 25-29 Residual mediastinal masses are frequently observed in patients with HD after completed therapy, and the discrimination between active tumor tissue and fibrotic residues remains a clinical challenge. Therefore, we initiated a prospective study to determine the value of FDG PET in these patients.
The results of this trial clearly indicate that patients with a negative FDG PET result are unlikely to progress or relapse within a year or longer after therapy. The chance for remaining in remission in the first year after therapy is about 95%. However, if FDG PET identifies an active mediastinal area, the information is questionable because the odds for a relapse and a clinical remission are almost even. Statistically, this was reflected by an excellent negative predictive value of 95% at 1 year for all patients with a “true” negative PET finding and a weak positive predictive value of 60% for all patients with a “true” positive result. Nevertheless, even though the predictive value of PET-positive patients was low, the risk of a mediastinal relapse was significantly higher than for PET-negative patients. This fact justifies additional, perhaps invasive, diagnostic procedures and a closer follow-up. Two of the 6 patients with a relapse who were identified by PET could be successfully treated with a subsequent therapy and were in continuous complete remission.
According to our data, false positive FDG uptake seems to be a problem in HD patients. The characteristics of the patients with a false-positive result suggest a younger age compared with the average age in the group of PET-positive patients (23 vs 35 years). Also, they had received initial therapy, 3 of them for a limited and 1 for an advanced stage. The PET examinations had been performed at least 3 months after completed therapy so that treatment-related increased metabolism could be ruled out. The high FDG uptake might be due to immunologic hyperreactivity, especially by the thymus and other lymphatic tissue after chemotherapy or radiotherapy. It is not unusual for HD patients younger than 25 years to present with an enlarged ventral mediastinal mass during the first 6 months after completion of therapy as a sign of thymus hyperplasia.30 This phenomenon may be a reason for false-positive results in FDG PET as described previously.31 In our study, one 18-year-old male had a PET-positive thymus hyperplasia that had decreased in size after another 3 months in a control CT scan. However, the reasons for false-positive PET results in the other 3 patients remain unknown.
Several studies have been published dealing with the role of FDG PET for the evaluation of residual tumor masses. Jerusalem et al27 analyzed 19 patients with HD and 35 with non-Hodgkin lymphoma (NHL) in 2 groups, depending on a positive or a negative CT scan. Thirteen HD patients and 11 patients with NHL had a positive CT scan, ie, residual masses. The involved localizations were divided into “thoracic,” “abdominal,” and “peripheral.” Of 5 patients with a positive FDG PET, all relapsed or progressed. Among the 19 PET-negative patients, 5 progressed and 14 remained in remission. Our data are similar to the PET-negative results; only 1 of 19 patients relapsed after 1 year of follow-up in our study, whereas 18 remained disease-free. In contrast, our data, which are specific for mediastinal masses especially in HD patients, showed only a low positive predictive value for FDG PET.
In another trial, 16 patients with either HD or NHL had a positive PET with residual masses according to routine methods.8 Nine of these patients remained disease-free. However, 4 of them received radiation after PET, and 2 of the patients had FDG uptake outside the questionable sites. In a more recent study, the same group examined a more homogenous group of 37 HD patients with residual masses. All patients underwent CT and FDG PET during and after therapy.32 The investigators found a negative predictive value of 96% and a positive predictive value of 46%. In our study, we excluded all patients who had an additional radiation after FDG PET before a progressive disease could be documented. Also, none of our patients had any questionable lesions outside the mediastinal area according to the complete CT scan and the thoracic PET. We calculated a similar negative predictive value but a higher positive predictive value compared with de Wit et al.32 This difference could be due to the fact that we required a minimum follow-up of 1 year and focused on mediastinal masses exclusively.
The question about future concepts and the role of FDG PET in HD patients with residual masses is not clearly answered. There is an ongoing discussion concerning whether PET is necessary at all for these patients because it is possible to wait for a definite progression of the disease by means of clinical symptoms or a progressive CT result. However, the diagnosis of a relapse should be done as early as possible to ensure a fast therapeutic reaction. Nevertheless, the data of this study point out that one should not rely on a positive FDG PET result exclusively but force pathological diagnosis before any salvage therapy. On the other hand, a clear advantage of FDG PET lies in its high negative predictive value that can reassure both patients and their treating doctors that disease is absent.
This work was supported by the Deutsche Forschungsgemeinschaft and the Deutsche Krebshilfe.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martin R. Weihrauch, Immunologisches Labor Haus 16, Uniklinik Koeln, Joseph-Stelzmann-Str 9, 50924 Koeln, Germany; e-mail: martin.weihrauch@uni-koeln.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal