Mosquito allergy is characterized by intense local skin symptoms that consist of not only erythema or bulla but also ulcer or scar and general symptoms such as high fever following mosquito bites. Most of the reported cases were from East Asia, and the majority of patients tended to die of hemophagocytic syndrome.1 On the other hand, nearly one third of patients with chronic active Epstein-Barr virus (EBV) infection may also have mosquito allergy and natural killer (NK)–cell proliferation.2-5 However, until recently the relationship between EBV infection and mosquito allergy remained unclear. More recently, we showed a close relationship between mosquito allergy and EBV-infected NK-cell lymphoproliferation.1 One of the unanswered questions is whether EBV-infected NK cell lymphoproliferative disease (LPD) precedes the onset of mosquito allergy among these patients.
We report here a case in which the time of primary EBV infection, serial analyses of lymphocytes subsets, EBV-infected cells, and skin lesions of mosquito allergy were analyzed to clarify this issue. A 6-year-old female was admitted to our hospital for diagnosis and treatment of mosquito allergy that had started 6 months previously. Her clinical history was as follows. At 6 months old, she developed pancytopenia resulting in B-precursor acute lymphoblastic leukemia (ALL) without typical chromosomal abnormalities. Although she entered complete remission following our ALL protocol, continuous chemotherapy became difficult because of severe myelosuppression and repeated infections. Because she had no HLA-matched related or unrelated donors, we decided to perform CD34-positive cell transplantation from her HLA-haploidentical father when she was aged 1 year and 11 months.6 At the time of transplantation she was negative for EBV serology and her father was positive. Preconditioning regimen consisted of total body irradiation (TBI) (12 Gy) and melphalan (140/m2), and 4.54 × 106/kg of CD34-positive cells were infused on February 6, 1996. Tacrolimus was used for graft-versus-host disease (GVHD) prophylaxis. Since her posttransplantation clinical course was uneventful except for delayed platelet recovery, tacrolimus was stopped on day 64. Two months later, due to development of chronic GVHD of the skin, cyclosporine was started. Although tests for sex chromatin by fluorescence in situ hybridization (FISH) showed complete donor engraftment, poor recovery of platelet count still continued. Therefore additional 9.64 × 106/kg of CD34-positive cells from her father were reinfused on day 148, resulting in complete recovery of platelets.
Because EBV serology was still negative after complete engraftment, we considered that there was no contamination of transmissible EBV-infected B cells in the purified CD34-positive cells obtained from her father.
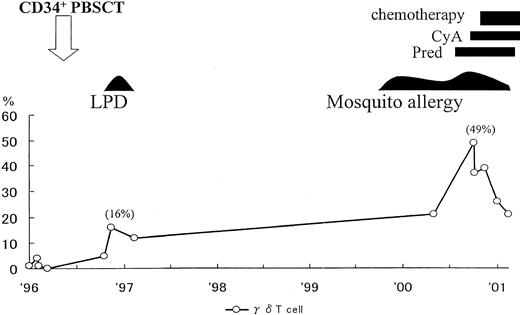
Seven months after transplantation, the patient, aged 2 years and 6 months, developed primary EBV infection with atypical manifestations such as fever, eruptions, hypergammaglobulinemia (IgG, 3030 mg/dL; IgM, 300 mg/dL), liver dysfunction, abdominal lymph nodes swelling, and pancytopenia. Seroconversion against EBV (VCA IgG; 320 × , EA IgG; 10 × , EBNA; < 10 × ) was confirmed, and clinical symptoms as well as laboratory findings were resolved by stopping cyclosporin. Two months later she developed fever and otitis media. Laboratory findings on this occasion revealed that γδ T cells were 16% in the peripheral blood mononuclear cells (healthy control < 5%).
She had been healthy until she developed mosquito allergy at age 5 years and 5 months. Upon admission at this time she had multiple skin lesions especially on the extremities and laboratory findings revealed liver dysfunction and a reactivation pattern of antibodies against EBV.
As shown in Figure 1, γδ T cells gradually increased with time. T-cell receptor γ gene rearrangements were observed and EBV DNA was detected in the sorted γδ T cells. A skin biopsy specimen revealed infiltrating lymphocytes, EBER+ (EBV-encoded RNA), CD3+, CD4−, CD8−, and CD56−, in the skin lesion. This phenotype indirectly suggested γδ T-cell type (data not shown).
Clinical course and changes of γδ T cells in peripheral blood mononuclear cells after CD34-positive cell transplantation.
PBSCT indicates peripheral blood stem cell transplantation; LPD, lymphoproliferative disease; Pred, prednisolone; CyA, cyclosporine A.
Clinical course and changes of γδ T cells in peripheral blood mononuclear cells after CD34-positive cell transplantation.
PBSCT indicates peripheral blood stem cell transplantation; LPD, lymphoproliferative disease; Pred, prednisolone; CyA, cyclosporine A.
Based on these findings, we considered that the patient had developed EBV-infected γδ T-cell proliferation after primary EBV infection. We speculated that the infiltrating cells in the skin may play an important role in the development of mosquito allergy. The patient is currently undergoing chemotherapy and the skin lesions have improved and the number of γδ T cells has been reduced (Figure 1).
Similar to the recent report we are also treating a patient with hydroa vacciniforme accompanying with EBV-infected NK-cell proliferation,7 and considering this patient and the patient in this case report, we speculate that there is a common pathogenesis for chronic active EBV infection, mosquito allergy, and hydroa vacciniforme that may be EBV-associated T/NK-cell LPD. Parallel with human T-cell leukemia virus type 1(HTLV-1)–associated T cell leukemia/lymphoma (ATL), which is a distinct disease entity, we would like to propose EBV-associated T/NK-cell LPD as a disease entity. 8EBV-associated T/NK-cell LPD includes mosquito allergy as well as a variety of other diseases such as chronic active EBV infection (CAEBV), EBV-associated hemophagocytic syndrome (EBV-AHS), peripheral T-cell lymphoma, chronic granular LPD, aggressive NK-cell leukemia, nasal/nasal type lymphoma, and hydroa vacciniforme.
Reduction and elimination of EBV-infected T/NK cells seems to be essential for the treatment of EBV-associated T/NK-cell LPD and it is important to distinguish it from ordinary EBV-associated B-cell LPD.8 9

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal