Abstract
Prospective studies have shown rapid engraftment using granulocyte–colony-stimulating factor–mobilized peripheral blood stem cells (G-PBSCs) for allogeneic transplantation, though the risks for graft-versus-host disease (GVHD) may be increased. It was hypothesized that the use of G-CSF to prime bone marrow (G-BM) would allow rapid engraftment without increased risk for GVHD compared with G-PBSC. Patients were randomized to receive G-BM or G-PBSCs for allogeneic stem cell transplantation. The study was designed (β < .8) to detect a difference in the incidence of chronic GVHD of 33% (α < .05). The plan was to recruit 100 patients and to conduct an interim analysis when the 6-month follow-up point was reached for the first 50 patients. Fifty-seven consecutive patients were recruited (G-BM, n = 28; G-PBSC, n = 29). Patients in the G-PBSC group received 3-fold more CD34+ and 9-fold more CD3+ cells. Median times to neutrophil (G-BM, 16 days; G-PBSC, 14 days; P < .1) and platelet engraftment (G-BM, 14 days; G-PBSC, 12 days; P < .1) were similar. The use of G-PBSC was associated with steroid refractory acute GVHD (G-BM, 0%; G-PBSC, 32%; P < .001), chronic GVHD (G-BM, 22%; G-PBSC, 80%; P < .02), and prolonged requirement for immunosuppressive therapy (G-BM, 173 days; G-PBSC, 680 days;P < .009). Survival was similar for the 2 groups. Compared with G-PBSC, the use of G-BM resulted in comparable engraftment, reduced severity of acute GVHD, and less subsequent chronic GVHD.
Introduction
The use of granulocyte–colony-stimulating factor (G-CSF) mobilized peripheral blood cells (G-PBSCs) as a source of stem cells for autologous transplantation has resulted in increased yield of CD34+ cells and accelerated engraftment compared to harvested bone marrow (BM).1 Prospective studies have also suggested accelerated engraftment when G-PBSCs are used in allogeneic transplantation.2-10
The major complication of allogeneic stem cell transplantation is graft-versus-host disease (GVHD). The incidence of grades II-IV acute GVHD after HLA-identical sibling donor BM transplantation varied with patient age, sex matching, donor parity, and cyclosporin and methotrexate dose intensities, but it ranges from 30% to 50% in most published series.11-14 T-cell depletion of the graft prevents this complication but results in an increase in the incidence of graft rejection, infection, and disease recurrence.15-18
Approximately 30% of patients surviving beyond day 100 after HLA-identical sibling donor BM transplantation acquire clinical extensive chronic GVHD (cGVHD).19 Risk factors for the development of cGVHD include prior acute GVHD, increasing patient age, and use of a parous female donor for a male recipient.20-22 Prolonged cyclosporin prophylaxis may decrease the occurrence of cGVHD.23 The addition of buffy coat to the marrow inoculum, used successfully to reduce the incidence of graft failure in transfused patients with aplastic anemia, resulted in a significant increase in the incidence of cGVHD.22Chronic GVHD is associated with significant morbidity and mortality and with adverse risk factors at the onset including thrombocytopenia, progressive onset, and elevated bilirubin level.19 24
The dose of T cells infused, therefore, may influence the development and severity of chronic GVHD. G-PBSC results in a 4- to 10-fold increase in the number of T cells compared with BM. Retrospective comparisons of G-PBSC and BM as stem cell sources have suggested an increase in the incidence of extensive chronic GVHD.25
Several reports demonstrate increased progenitor cell yield and accelerated neutrophil and platelet recovery after the harvest of G-CSF–stimulated bone marrow (G-BM) for autologous and allogeneic transplantation.26-31 We hypothesized that the use G-BM may result in rapid engraftment without altering the incidence of acute GVHD but with a reduced risk for cGVHD when compared with G-PBSC.
Patients and methods
Patient accrual and characteristics
Between January 1997 and July 1999, all patients undergoing allogeneic stem cell transplantation from an HLA-identical sibling were asked to participate in a randomized trial of G-PBSC or G-BM as the source of stem cells. All patients and donors signed consent forms approved by the Ethics Committee of the Royal Brisbane Hospital.
Stem cell collections
All donors received G-CSF (Amgen, Thousand Oaks, CA) 10 μg/kg per day as a single evening injection. Randomization to G-BM or G-PBSC was performed before transplantation in permuted blocks of 4 donors, stratified according to the risk for patient disease recurrence (high risk was defined as acute myeloid leukemia [AML] beyond CR1, chronic myeloid leukemia [CML] beyond chronic phase, myelodysplasia, and high-grade or transformed non-Hodgkin lymphoma). Donors randomized to G-BM received G-CSF for 5 days; G-BM harvest was performed on the sixth day (volume, 15-20 mL/kg patient adjusted ideal weight). Donors randomized to G-PBSC received G-GSF for 5 days, with collections performed on the fifth (stored overnight at 4°C) and sixth days, to obtain a minimum CD34 cell count of 2 × 106/kg recipient ideal body weight. A provision was made to perform a third collection if this yield was not achieved. Collections were made with a continuous flow blood cell separator (Cobe Laboratories, Lakewood, CA; volume, 200 mL; collection rate, 1 mL/min; flow rate, 40-70 mL/min). Cells were then pooled and infused on transplantation day 0.
Supportive care
All patients received cyclosporin by 2-hour intravenous infusion at a dose of 5 mg/kg day −1 to day +1, then 3 mg/kg adjusted to trough levels of 100 to 300 μg/mL. Methotrexate was administered on day +1 at a dose of 15 mg/m2 and subsequently on days +3 and +6 at a dose of 10 mg/m2. Each dose was followed 24 hours later by a single dose of 15 mg folinic acid (days +2, +4, +7). In the absence of disease recurrence or active GVHD, cyclosporin was tapered between day +100 and day +180. Use of growth factors after transplantation was limited to those patients with delayed neutrophil recovery at day +21. All patients received 200 mg fluconazole daily from day +1 and 500 mg/m2 acyclovir 3 times daily intravenously while they had cytopenia; subsequently, they received 1 g twice daily valacyclovir once they could tolerate oral therapy. Cytomegalovirus (CMV) surveillance included weekly polymerase chain reaction and pp65 antigenemia testing (until day +100). Treatment for CMV reactivation included 5 mg/kg ganciclovir twice daily for 1 week and then once daily Monday through Friday for 3 weeks. Bactrim was given before transplantation and was restarted after day +21. Fluconazole, Bactrim, and valacyclovir administration were continued until 1 month after the cessation of all immunosuppressive therapy. Acute GVHD grades II-IV was treated with 2 mg/kg prednisone and was tapered at a rate of 0.25 mg/kg per week. Patients with refractory acute GVHD were treated with antithymocyte globulin (75 mg/kg over 5 days), high-dose prednisone (10 mg/kg), and tacrolimus. Chronic GVHD was treated with cyclosporin–tacrolimus with prednisone as the first-line therapy for at least 6 months. Second-line therapy was added at 2 months for treatment failure and included mycophenolate, clofazimine, and thalidomide administered in a sequential manner based on treatment response.
Evaluations and definitions
Stem cell products were analyzed for CD34+ subsets and T-cell subsets by flow cytometry using previously published methods.32 Neutrophil engraftment was defined as having occurred after the first of 3 days with an absolute neutrophil count (ANC) greater than 500/μL after the posttransplant nadir. Platelet engraftment was defined as having occurred on the first of 7 consecutive days with a platelet count greater than 20 000/μL without platelet transfusions. Acute and chronic GVHD were graded by Seattle criteria. Response of acute GVHD to prednisone was defined as sensitive (no flare on prednisone taper), dependent (flare before day +100 on prednisone taper), and refractory (no response or progression after 5 days at 2 mg/kg). Patients who died while in relapse after transplantation were categorized as having died of relapse. Patients who died without disease recurrence were categorized as experiencing nonrelapse mortality.
Statistics
The hypothesis in this study was that the use of G-PBSCs would result in an increase in the incidence of clinical extensive chronic GVHD 6 months after transplantation. A sample of 80 patients would be required to detect a difference of 33% with a power of 80% and a 1-sided α of 0.05. Assuming 80% of patients would survive longer than 100 days, we aimed to enroll 100 patients. The study design included an interim analysis when the first 50 patients survived more than 180 days after transplantation. Study closure rules at this time point included an excess cumulative incidence of severe (grades III-IV) acute GVHD or clinical extensive chronic GVHD, defined byP < .02. Time to engraftment, cumulative incidence of acute and chronic GHVD, duration of immunosuppression therapy, and survival were compared using the log rank test. Death or relapse before day 100 was treated as a competing risk for determination of the cumulative incidence of acute GVHD. Patients alive in remission at day +100 were considered at risk for the development of chronic GVHD. Subsequent death or disease recurrence was treated as a competing risk. Potential risk factors for the development of GVHD (age, CMV serostatus, sex match, donor parity, disease risk, conditioning regime, log CD3, CD34, total nucleated cell dose per kilogram patient weight, and cell source) were included in multivariate models (logistic regression for acute GVHD, Cox regression for cGVHD), where univariate analysis determinedP < .1.
Results
Study population
Patient characteristics are shown in Table1. There was a predominance of patients with AML in CR1 in the G-BM group and with CML in chronic phase in the G-PBSC group. Median follow-up time of surviving patients was 645 days.
Patient demographics
| . | G-BM (n = 28) . | G-PBSC (n = 29) . | P . |
|---|---|---|---|
| Age (y) | 44 (16-60) | 46 (24-58) | .2 |
| High risk (%) | 9 (32) | 11 (38) | .6 |
| Diagnosis | |||
| AA | 1/0 | 0/0 | |
| AL | 8/1 | 1/4 | |
| CML | 2/1 | 8/1 | |
| MDS | 0/5 | 0/4 | |
| MM | 4/0 | 4/0 | |
| MF | 0/0 | 1/0 | |
| NHL/CLL | 4/2 | 4/2 | |
| CMV | .7 | ||
| D−R− (%) | 5 (18) | 3 (10) | |
| D−R+ (%) | 7 (25) | 5 (17) | |
| D+R− (%) | 3 (11) | 4 (14) | |
| D+R+ (%) | 13 (46) | 17 (59) | |
| Sex match | .4 | ||
| M to M (%) | 5 (18) | 5 (17) | |
| M to F (%) | 10 (36) | 6 (21) | |
| F to M (%) | 8 (29) | 8 (28) | |
| F to F (%) | 5 (19) | 10 (35) | |
| PF to M (%) | 5 (18) | 4 (14) | |
| Conditioning | .3 | ||
| CYTBI (%) | 5 (18) | 5 (17) | |
| BUCY (%) | 19 (68) | 21 (72) | |
| Other (%) | 4 (14) | 3 (10) |
| . | G-BM (n = 28) . | G-PBSC (n = 29) . | P . |
|---|---|---|---|
| Age (y) | 44 (16-60) | 46 (24-58) | .2 |
| High risk (%) | 9 (32) | 11 (38) | .6 |
| Diagnosis | |||
| AA | 1/0 | 0/0 | |
| AL | 8/1 | 1/4 | |
| CML | 2/1 | 8/1 | |
| MDS | 0/5 | 0/4 | |
| MM | 4/0 | 4/0 | |
| MF | 0/0 | 1/0 | |
| NHL/CLL | 4/2 | 4/2 | |
| CMV | .7 | ||
| D−R− (%) | 5 (18) | 3 (10) | |
| D−R+ (%) | 7 (25) | 5 (17) | |
| D+R− (%) | 3 (11) | 4 (14) | |
| D+R+ (%) | 13 (46) | 17 (59) | |
| Sex match | .4 | ||
| M to M (%) | 5 (18) | 5 (17) | |
| M to F (%) | 10 (36) | 6 (21) | |
| F to M (%) | 8 (29) | 8 (28) | |
| F to F (%) | 5 (19) | 10 (35) | |
| PF to M (%) | 5 (18) | 4 (14) | |
| Conditioning | .3 | ||
| CYTBI (%) | 5 (18) | 5 (17) | |
| BUCY (%) | 19 (68) | 21 (72) | |
| Other (%) | 4 (14) | 3 (10) |
AA indicates aplastic anemia; AL, acute leukemia; MDS, myelodysplasia; MM, multiple myeloma; MF, myelofibrosis; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; D, donor; R, recipient; M, male; F, female; PF, parous female; CYTBI, 120 mg/kg cyclophosphamide + total body irradiation (12 Gy, 6 fractions); BUCY, 16 mg/kg busulfan + 120 mg/kg cyclophosphamide.
Stem cell products
Uncorrected yields of nucleated cells and of CD34+ and CD3+ cells are shown in Table2. There was little difference in the number of nucleated cells infused, whereas the numbers of CD34+ and CD3+ cells were, respectively, 3-fold and 9-fold greater for the G-PBSC product.
Median numbers of CD34+ progenitors and T cells infused
| . | G-BM . | G-PBSC . | P . |
|---|---|---|---|
| TNC/kg (× 108) | 8.6 (3.7-12.8) | 10.4 (5.0-18.1) | .006 |
| CD34/kg (× 106) | 2.6 (0.8-6.3) | 7.2 (1.9-19.9) | < .0001 |
| CD3/kg (× 106) | 45 (16-77) | 403 (43-714) | < .0001 |
| . | G-BM . | G-PBSC . | P . |
|---|---|---|---|
| TNC/kg (× 108) | 8.6 (3.7-12.8) | 10.4 (5.0-18.1) | .006 |
| CD34/kg (× 106) | 2.6 (0.8-6.3) | 7.2 (1.9-19.9) | < .0001 |
| CD3/kg (× 106) | 45 (16-77) | 403 (43-714) | < .0001 |
Ranges are indicated in parentheses (actual patient weights).
Engraftment
There was a suggestion of more rapid engraftment in the G-PBSC group than in the G-BM group, though the results did not reach significance. Median time to neutrophil recovery was 16 days (range, 12-23 days) using G-BM compared with 14 days (range, 10-23 days) for G-PBSC recipients (P < .1). This analysis excluded 2 patients who died before day 28 without achieving neutrophil recovery (1 from each group). Median time to platelet recovery was 14 days (range, 9-22 days) after G-BM and 12 days (range, 8-25 days) after G-PBSC transplantation (P < .1). Three patients (2 in the G-BM group; 1 in the G-PBSC group) who died before day 28 without reaching platelet recovery were excluded from the analysis. Median numbers of transfused packed cells (3 G-BM, range, 0-15; 3 G-PBSC, range, 0-32; P < .3) and platelet transfusion episodes (5 G-BM, range, 2-22; 3 G-PBSC, range, 1-47; P < .3) between day 0 and day +30 after transplantation were similar for the 2 groups.
Acute graft-versus-host disease
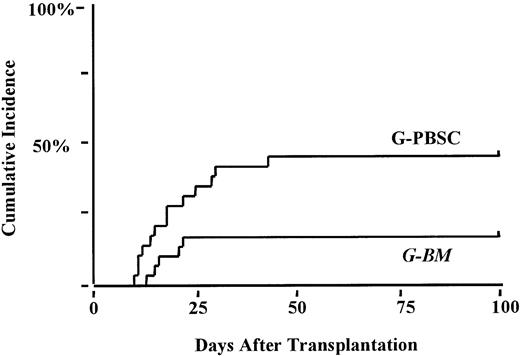
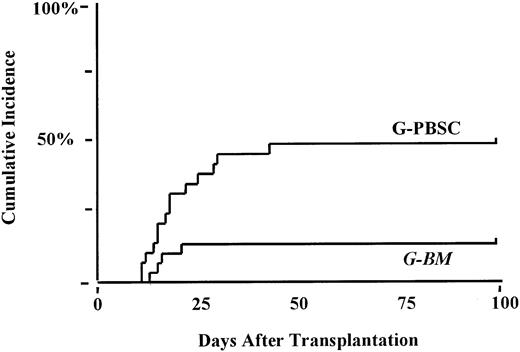
The cumulative incidence of grades II-IV acute GVHD was 52% in the G-BM compared to 54% in the G-PBSC group (P < .6). Five patients (2 in the G-BM group; 3 in the G-PBSC group) died before day 100 without acquiring acute GVHD. The incidence of grades III-IV acute GVHD was 22% in the G-BM group compared with 43% after G-PBSC transplantation (P < .09; Figure1). The proportion of patients with steroid-dependent or refractory acute GHVD (47% G-PBSC; 18% G-BM;P < .02) was significantly increased after G-PBSC transplantation (Figure 2). No other factors were found to be associated with the development of severe or steroid dependent or resistant acute GVHD. The risk for grades III-IV acute GVHD after G-PBSC transplantation was increased when the T-cell dose exceeded 403 × 106/kg (P < .06).
Cumulative incidence of severe grades III-IV acute GVHD.
G-PBSC, 43%; G-BM, 22%; P < .09.
Cumulative incidence of severe grades III-IV acute GVHD.
G-PBSC, 43%; G-BM, 22%; P < .09.
Cumulative incidence of prednisone dependent or refractory acute GHVD.
G-PBSC, 47%; G-BM, 18%; P < .02.
Cumulative incidence of prednisone dependent or refractory acute GHVD.
G-PBSC, 47%; G-BM, 18%; P < .02.
Chronic graft-versus-host disease
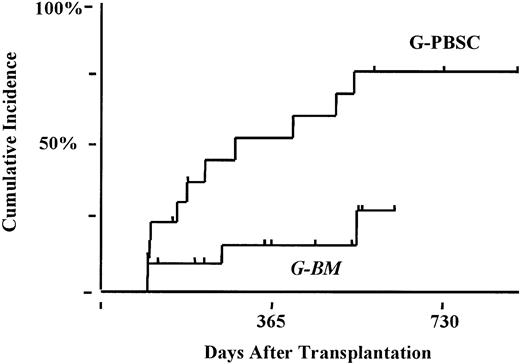
Forty-two patients were alive and in remission at day +100 after transplantation and thus were considered at risk for the development of chronic GVHD. Overall incidence of clinical chronic GVHD (limited and extensive) was significantly higher after G-PBSC transplantation (G-PBSC, 90%; G-BM, 47%; P < .02). The use of G-PBSC was a major risk factor for the development of clinical extensive chronic GVHD (G-PBSC, 80%; G-BM, 22%; P < .002; Figure3). According to multivariate analysis, age greater than 45 years (relative risk [RR], 3.6; confidence interval [CI], 1.2-9.2; P < .02) and use of G-PBSC (RR, 5.1; CI, 1.7-15; P < .004) remained independently predictive for the development of clinical extensive cGVHD.
Cumulative incidence of clinical extensive chronic GVHD.
G-PBSC, 80%; G-BM, 22%; P < .003.
Cumulative incidence of clinical extensive chronic GVHD.
G-PBSC, 80%; G-BM, 22%; P < .003.
There were no differences in the pattern of onset, incidence of thrombocytopenia, or hyperbilirubinemia at the time of development of cGVHD. Duration of immunosuppression therapy (Figure4) was significantly prolonged after G-PBSC transplantation (median, 680 days; range, 173-890+ days) than in the G-BM group (median, 173 days; range, 111-913+ days) (P < .009).
Kaplan-Meier estimates of the percentage of patients remaining on immunosuppression therapy.
Median duration: G-PBSC, 680 days; G-BM, 173 days;P < .009.
Kaplan-Meier estimates of the percentage of patients remaining on immunosuppression therapy.
Median duration: G-PBSC, 680 days; G-BM, 173 days;P < .009.
Relapse and survival
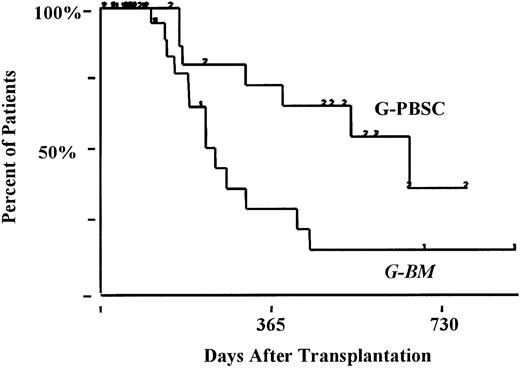
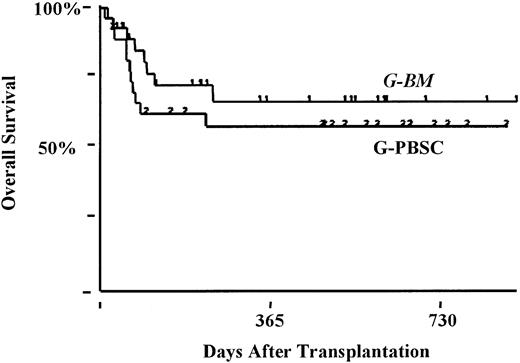
Eight patients had relapses—5 after G-BM (2 with high-risk disease) and 3 after G-PBSC (2 with high-risk disease) transplantation. Sustained remission has followed the withdrawal of immunosuppression therapy (chemorefractory myeloma, G-BM, n = 1) and donor lymphocyte infusion and interferon (AML in CR2, G-BM, n = 1). Overall survival rate at 18 months was 66% ± 6% (standard risk, 75% ± 7%; high risk, 50% ± 11%) and was not affected by stem cell source (G-BM, 67% ± 9%; G-PBSC, 64% ± 9%; P < .9) (Figure 5).
Kaplan-Meier estimates of the percentage of patients surviving at 18 months.
G-PBSC, 64%; G-BM, 67%; P < .9.
Kaplan-Meier estimates of the percentage of patients surviving at 18 months.
G-PBSC, 64%; G-BM, 67%; P < .9.
Discussion
G-PBSCs have replaced BM as the stem cell source of choice for autologous transplantation. This has been based on the ease of collection and the rapidity of engraftment. Interest has now extended to the use of G-PBSC for allogeneic transplantation. Concerns regarding the severity of GVHD because of the increased T-cell load have been expressed; however, retrospective comparisons2,3,5,6,9,25,33 and prospective randomized studies4,7,8,10 have yielded conflicting results. G-BM for allogeneic transplantation has been evaluated in a small series of patients with acceleration of neutrophil and platelet engraftment and has been compared with those of historical controls.30This study shows that the use of G-BM results in rapid, sustained engraftment with a reduced risk for severe acute and subsequent clinical extensive chronic GVHD in comparison with G-PBSC.
There is scant information on the use of G-BM for stem cell transplantation. Studies in mice have suggested a 50% reduction in the number of spleen colony-forming units and granulocyte macrophage–colony-forming units in femoral bone marrow after 4 days of G-CSF at 500 μ/kg, with a return to baseline levels 24 hours after the cessation of therapy. The administration of G-CSF and stem cell factor to splenectomized mice decreased the number of pluripotent hematopoietic stem cells in the bone marrow 4-fold. However, by 14 days after complete injection, the marrow had expanded 10-fold in repopulating ability.34 Studies in humans found an increase in bone marrow cellularity and lineage-restricted myeloid progenitors after mobilization with 5 days of G-CSF. There was no difference in yields of CD34+ cell CFU-GM, and engraftment times were similar to those for historical controls.35 By contrast, Slowman et al36 found an increased yield of CD34+ cells without accelerating time to neutrophil engraftment. Damiani et al26 randomized 55 patients undergoing autologous stem cell transplantation to receive G-BM or G-PBSC, with collections performed after 3 days of G-CSF at 16 μg/kg. There was no difference in the times to neutrophil (G-BM, 12 days; G-PBSC, 11 days) or platelet (G-BM, 13 days; G-PBSC, 11 days) recovery.26 Weisdorf27 randomized patients to receive G-CSF or GM-CSF for 6 days before either BM or PBSC harvests. PBSCs were harvested when the BM was either hypocellular or involved by disease. The source of stem cells did not impact the time to count recovery. Isola et al30 administered G-CSF to healthy donors at a dose of 10 μg/kg for 2 days before harvest. Compared with historical controls of unstimulated bone marrow, G-BM contained similar numbers of nucleated cells, CD34+ cells, and CD3+ cells but an increase in granulocyte macrophage colony-forming units. Engraftment was accelerated compared with unstimulated bone marrow (ANC greater than 1000/μL, 17 vs 26 days; PLT greater than 20 000/L, 20 vs 26 days). A long-term follow-up study confirmed stable donor engraftment.31 Couban et al29 administered G-CSF (median dose, 12 μg/kg) for 4 days before G-BM harvest. Neutrophil (18 days) and platelet (22 days) engraftment were accelerated to control groups receiving G-BM. Serody et al28 compared sequential cohorts receiving G-BM or G-PBSC (G-CSF 10 μg/kg for 4 days) for allogeneic transplantation. GVHD prophylaxis used abbreviated methotrexate, as in our study, though leucovorin was not used. Platelet recovery (G-BM, 16 days; G-PBSC, 13 days), but not neutrophil recovery (G-BM, 16 days; G-PBSC, 17 days), was faster after G-PBSC. The incidence of grades II-IV acute GVHD (G-BM, 27%; G-PBSC, 60%; P < .07) and chronic GVHD (G-BM, 37%; G-PBSC, 68%; P < .05) were increased in the G-PBSC group.
Based on our results, it appears that engraftment times after autologous or allogeneic stem cell transplantation are comparable using G-BM or G-PBSCs as stem cell sources. The optimal dosage and scheduling of G-CSF administration before harvest remain to be determined. It is possible that delayed collection of G-BM34 may further optimize engraftment kinetics. Contaminating peripheral blood contributes significantly to the yield of CD34+ cells; however, in this study preharvest peripheral blood CD34+cell counts were not performed. We also found G-BM harvest times to be greatly reduced compared with standard BM collection, paralleling the observation of others.29 This was particularly useful with overweight donors or those who presented anatomic challenges.
In line with other studies, we found that the incidence of grades II-IV acute GVHD was similar after G-PBSC and G-BM allografting. However, in contrast to these studies, we found that patients who acquired acute GVHD after G-PBSC transplantation were more likely to have severe organ involvement and to respond poorly to prednisone therapy. One suggested mechanism for the increased incidence of severe acute GVHD in the current study is the abbreviated methotrexate schedule used in this study. It has been shown that the risk for grades II-IV acute GVHD for patients undergoing HLA-identical sibling marrow is increased (28% vs 39%; P < .03) with the omission of day 11 methotrexate. The risk for grades III-IV acute GVHD, however, was not affected by day 6 or day 11 methotrexate administration.14In our study, the incidence of grades III-IV acute GVHD in the G-PBSC group was highest when the T-cell dose exceeded 4 × 108/kg. It is possible that the omission of day 11 methotrexate is particularly relevant to the development of grades III-IV acute GVHD in patients receiving high T-cell doses. This observation contrasts with the findings of a study of 160 patients in which CD34 rather than CD3 cell dose was found to correlate with the development of grades II-IV acute GVHD (no variables were found to correlate with the development of grades III-IV acute GVHD).6 Various GVHD prophylaxis regimes were used in this study, though the lowest incidence of grades III-IV GVHD was observed with tacrolimus and mini-methotrexate (5 mg/m2 days 1, 3, 6).
The primary end-point in this study was the development of clinical extensive cGVHD. The study was closed after the initial interim analysis because of the highly significant difference in the incidence of this complication. Retrospective analyses comparing unstimulated BM and G-PBSCs as stem cell sources have suggested an increase in the incidence of cGVHD,25,33 and they parallel Storb's22 original observation of increased cGVHD after the addition of donor buffy coat to promote engraftment in transfused patients with aplastic anemia undergoing allogeneic BM transplantation. Randomized studies have reached differing conclusions, though the French multigroup study, which also used abbreviated methotrexate prophylaxis, found a significantly higher incidence of extensive cGVHD in the G-PBSC group.8
This study was not designed to detect a difference in survival between the 2 study groups, but their survival curves are similar. The development of cGVHD is known to be protective against disease recurrence for patients with acute leukemia and CML.37-40 Prospective randomized studies and a retrospective comparison have suggested improved leukemia-free survival after G-PBSC, restricted to patients with advanced disease4,7 (defined by acute leukemia beyond CR1 and CML beyond chronic phase). This difference was variably attributed to reduced disease recurrence4 and reduced treatment-related mortality.33 Given that a high response rate to donor lymphocyte infusions has been demonstrated for relapsing chronic-phase CML, it seems unlikely that the use of G-PBSC would result in a survival advantage for this group of patients.41-43 The response rate to donor lymphocyte infusions is low for relapsing AML and ALL, and it is likely that the use of G-PBSC would be of advantage for these patients.
In conclusion, we have demonstrated that the use of G-BM results in rapid and sustained engraftment. Compared with G-PBSC, median neutrophil and platelet recovery was delayed by 2 days. Optimal timing of bone marrow harvest after G-CSF administration remains to be determined. Although the incidence of grades II-IV acute GVHD was similar for the 2 groups, patients undergoing G-PBSC transplantation were more likely to acquire severe acute GVHD refractory to prednisone and cGVHD with a prolonged requirement for immunosuppression therapy to control symptoms. We recommend the use of G-BM rather than G-PBSCs, especially for patients in whom disease recurrence can be effectively treated with donor lymphocyte infusions.
This work is a component of a Masters thesis through the Department of Epidemiology, University of Newcastle, NSW, Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
James Morton, Bone Marrow Transplant Unit, Royal Brisbane Hospital, Herston Rd, Herston, Q4029, Australia; e-mail:james_morton@health.qld.gov.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal