Abstract
Complex pertubations of hemostasis occur in sickle cell disease (SCD). Although the procoagulant property of sickle erythrocytes in vitro is tied to exposure of phosphatidylserine (PS), no study has directly linked this PS positivity to in vivo thrombin generation. This study was designed to determine if thrombin generation in SCD correlates with erythrocyte PS, or whether platelets play a significant role. PS was quantified on erythrocytes and platelets from 40 patients with SCD (SS genotype = 25; SC genotype = 15) and 11 controls. Markers of thrombin generation (prothrombin fragment F1.2; thrombin-antithrombin or TAT complexes) and fibrin dissolution (D-dimer; plasmin-antiplasmin or PAP complexes) were also evaluated. Thrombin generation and activation of fibrinolysis occurred with elevations in F1.2, TAT, and D-dimer. Although numbers of both PS-positive erythrocytes and platelets were elevated, there was no correlation between PS-positive platelets and any hemostatic markers. In contrast, correlations were noted between PS-positive erythrocytes and F1.2 (P < .0002), D-dimer (P < .000002), and PAP (P < .01). Correlations between F1.2 and D-dimer (P < .0001) demonstrated that fibrinolysis was secondary to thrombin generation. In patients with the SC genotype, abnormalities in coagulation, although present, were of a lesser magnitude than in SS disease. This study suggests that the sickle erythrocyte is the cell responsible for the thrombophilic state in SCD because associations between erythrocyte PS and thrombin generation were observed. No such relationship with platelet PS was noted. The use of erythrocyte PS as a surrogate marker in trials testing new therapeutic modalities may provide insights into the vascular complications of SCD.
Introduction
Although sickle cell disease (SCD) has played a singular role in establishing the field of molecular medicine, this entity remains one in which the bench to bedside translation of biologic concepts is still very much a work in progress. One of the complications of SCD is a thrombophilic state associated with complex pertubations of plasma and cellular hemostatic mechanisms.1,2 These changes include evidence for thrombin generation,3-8 depletion of natural anticoagulants,6-10 the activation of cellular elements including white cells11-13 and platelets,2,14and increased levels of circulating soluble tissue factor and microvascular endothelial cells with a tissue factor phenotype.15-17 Numerous investigations have been conducted to elucidate the mechanisms responsible for the prothrombotic state. Initial studies demonstrated that sickle erythrocytes accelerated the clotting time and stimulated prothrombinase activity.18,19 Subsequently, it was shown that the loss of normal membrane phospholipid asymmetry with the appearance of anionic phosphatidylserine (PS) on the erythrocyte surface, promoted the assembly of clotting factors on the cell membrane leading to the development of a sickle erythrocyte with procoagulant phenotype.18-20 Another link between cause and effect would be the demonstration that in vivo thrombin generation in SCD does in fact correlate with sickle erythrocyte membrane PS exposure and that platelets (which also can provide the catalytic template on which coagulation factors interact)21 would in this circumstance be a secondary player. Our study was designed in an attempt to forge such a link.
The use of annexin V (a calcium-dependent phospholipid-binding protein) and flow cytometry has facilitated the demonstration of PS on cell surfaces.22 23 We have quantified PS exposure on erythrocytes and platelets in SCD by using this technique and have additionally evaluated various hemostatic parameters to assess whether thrombin generation is related to the exposure of PS on the erythrocyte and platelet surfaces. Markers of in vivo thrombin generation included prothrombin fragment F1.2 and thrombin-antithrombin (TAT) complexes. D-dimer (a measure of both cross-linked fibrin formation and degradation by plasmin) and plasmin-antiplasmin (PAP; the irreversible complex between plasmin and its principal physiologic inhibitor α2-antiplasmin) were the main tests of fibrinolysis assessed.
Materials and methods
Materials
Phycoerythrin (PE)–labeled mouse monoclonal antibodies against human glycophorin A, CD235a (anti-CD235a–PE, clone 11E4B7.6 [KC16]), the β-subunit of the human fibrinogen receptor CD61 (anti-CD61–PE, clone PM6/13), and isotypic control antibody (clone 679.1Mc7) were obtained from Immunotec (Beckman-Coulter, Miami, FL) or Serotec (Raleigh, NC). Fluorescein isothiocyanate (FITC)–labeled annexin V was purchased from R&D Systems (Minneapolis, MN).
Collection of blood
Venous blood was obtained from 40 infants and children with SCD in steady state (25 with SS and 15 with SC genotype, aged 6 months to 19 years) and 11 age-matched African American controls. Patients were considered to be in steady state if afebrile, had not been hospitalized or transfused within 8 weeks, and had had no vaso-occlusive episode within at least 14 days. Blood samples (48) were obtained from 40 patients such that 2 blood samples at least 1 year apart were obtained on 6 infants with SS and 2 with the SC genotypes. Because fetal hemoglobin (HbF) levels decrease dramatically during infancy and early childhood, and because an inverse correlation exists between HbF and PS-positive erythrocytes,24 these samples were treated as individual data points for the purpose of hemostatic marker analyses. This study was approved by the Institutional Review Committees for the protection of human subjects at St Christopher's Hospital for Children and at Thomas Jefferson University. Informed consent was obtained or assent, in addition to parental permission, where appropriate. For analyses of PS-positive erythrocytes and platelets, blood (100 μL) was collected in sodium heparin and assessed within 2 hours. For analysis of markers of thrombin generation and fibrinolysis, blood (1 mL) was collected in sodium citrate, plasma was separated, and aliquots were frozen at −80°C until assayed.
Flow cytometric analyses of PS-positive erythrocytes and platelets
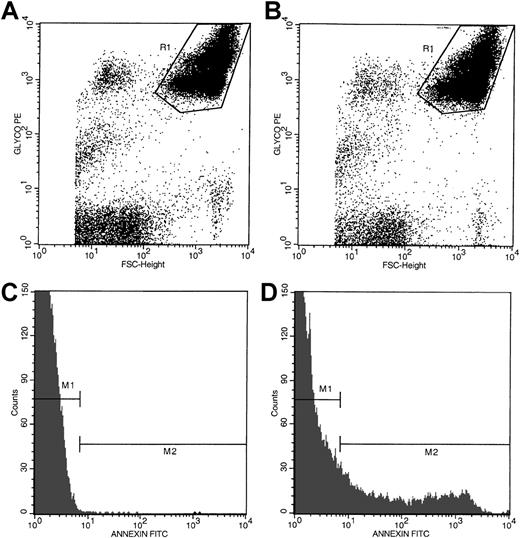
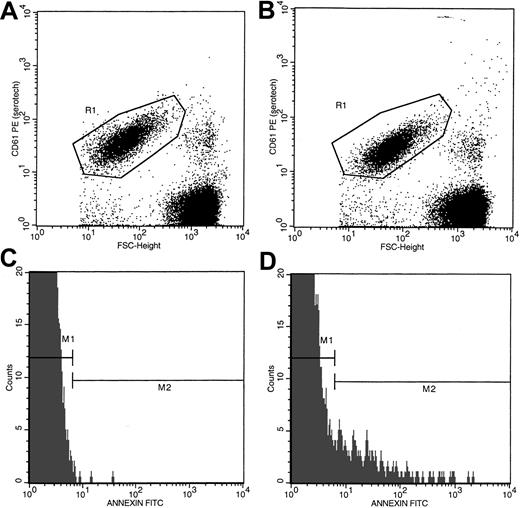
Anticoagulated whole blood (5 μL) was incubated for 30 minutes at room temperature with 10 μL annexin V-FITC and 20 μL of either anti-CD235a–PE or anti-CD61–PE in the presence of either 2.5 mM CaCl2 or EDTA in a total volume of 100 μL adjusted with HBSS-HEPES buffer. Incubation mixtures were then diluted with 1 mL buffer containing either 2.5 mM CaCl2 or EDTA and analyzed in a Becton Dickinson Flow Cytometer (San Jose, CA) formatted for 2-color analyses as previously described.24 Data from 60 000 events, collected at a flow rate of 300 to 500 events per second, were analyzed. Red cells and platelets were separated by size and identified by their distinct immunofluorescence (CD235a-positivity for red cells or CD61-positivity for platelets). As shown in Figure1, panels A and B, using the dot plots of CD235a-PE fluorescence and forward size scatter (FSC), CD235a-positive events (red cell–associated, region marked R1) were separated from CD235a-negative (non–red cell) events. Annexin V–positive cells in the red cell region were then determined by using the histograms of annexin V–FITC fluorescence as shown in panels C and D. Negative (gate M1) and positive histogram regions (gate M2) were set with the use of samples stained with annexin V–FITC and CD235a-PE in the presence of EDTA (Figure 1C). Nonspecific membrane immunofluorescence (gate M2, panel C) was subtracted from the respective sample fluorescence (gate M2, panel D). Data were expressed as the percentage of PS-positive red cells. PS positivity associated with platelets was assessed by using the dot plots of CD61-PE fluorescence and forward size scatter plus the respective histograms of annexin V–FITC fluorescence (Figure2A-D) employing the steps outlined above for red cell analyses.
Flow cytometric analyses of PS-positive erythrocytes in whole blood from a representative patient with SCD.
Dot plots of forward size scatter verses anti-CD235a–PE fluorescence from a blood sample stained in the presence of EDTA and calcium are presented in panels A and B, respectively. Region R1 in these dot plots represents red cell–associated events. Histogram profiles of annexin V–FITC fluorescence of erythrocytes stained in the presence of EDTA and calcium are presented in panels C and D, respectively. Annexin V–negative (gate M1) and –positive (gate M2) histogram regions were set by using the blood sample stained in the presence of EDTA as shown in panel C. The histogram profile presented in panel D demonstrates the presence of a distinct subpopulation of PS-positive erythrocytes (gate M2).
Flow cytometric analyses of PS-positive erythrocytes in whole blood from a representative patient with SCD.
Dot plots of forward size scatter verses anti-CD235a–PE fluorescence from a blood sample stained in the presence of EDTA and calcium are presented in panels A and B, respectively. Region R1 in these dot plots represents red cell–associated events. Histogram profiles of annexin V–FITC fluorescence of erythrocytes stained in the presence of EDTA and calcium are presented in panels C and D, respectively. Annexin V–negative (gate M1) and –positive (gate M2) histogram regions were set by using the blood sample stained in the presence of EDTA as shown in panel C. The histogram profile presented in panel D demonstrates the presence of a distinct subpopulation of PS-positive erythrocytes (gate M2).
Flow cytometric analyses of PS-positive platelets in whole blood from a representative patient with SCD.
Dot plots of forward size scatter verses anti-CD61–PE fluorescence from a blood sample stained in the presence of EDTA and calcium are presented in panels A and B, respectively. Region R1 in these dot plots represents platelet-associated events. Histogram profiles of annexin V–FITC fluorescence of platelets stained in the presence of EDTA and calcium are presented in panels C and D, respectively. Annexin V–negative (gate M1) and –positive (gate M2) histogram regions were set by using the blood sample stained in the presence of EDTA as shown in panel C. The histogram profile presented in panel D demonstrates the presence of a subpopulation of PS-positive platelets (gate M2).
Flow cytometric analyses of PS-positive platelets in whole blood from a representative patient with SCD.
Dot plots of forward size scatter verses anti-CD61–PE fluorescence from a blood sample stained in the presence of EDTA and calcium are presented in panels A and B, respectively. Region R1 in these dot plots represents platelet-associated events. Histogram profiles of annexin V–FITC fluorescence of platelets stained in the presence of EDTA and calcium are presented in panels C and D, respectively. Annexin V–negative (gate M1) and –positive (gate M2) histogram regions were set by using the blood sample stained in the presence of EDTA as shown in panel C. The histogram profile presented in panel D demonstrates the presence of a subpopulation of PS-positive platelets (gate M2).
Analysis of markers for thrombin generation and fibrinolysis
Plasma levels of hemostatic markers, including prothrombin fragment F1.2, TAT, D-dimer, PAP complexes, tissue plasminogen activator (tPA), and plasminogen activator inhibitor-1 (PAI-1), were measured by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Dade Behring, Hollywood, FL, and American Diagnostica, Greenwich, CT).
Data analysis
Statistical evaluation was performed by using the Sigmastat Statistical Package (Jandel Scientific, San Rafael, CA). All results are presented as the mean ± SD. Because analyses of the data related to PS-positive blood cells and coagulation markers showed a nonparametric distribution, significant differences between control and patients were analyzed by using the Kruskal-Wallis test. If theP value for this overall comparison was significant atP < .05, group-wise comparisons were performed, using the Mann-Whitney test. Both Pearson and Spearman correlation tests were used to determine the relationship between 2 variables. Both tests yielded similar results for the same pair of variables analyzed. Values presented for the R and P values were obtained by Pearson tests on log-transformed data.
Results
PS-positive erythrocytes and platelets in SCD
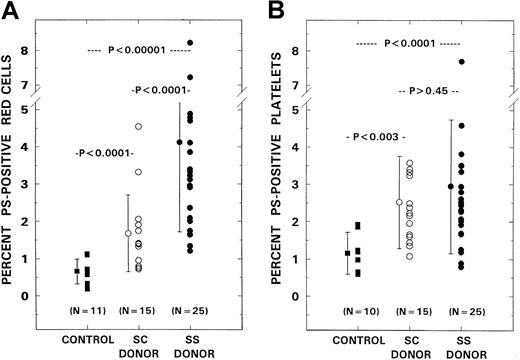
The mean level of PS-positive red cells in the controls was 0.66% ± 0.34% (± SD). Levels in the patient groups with SC and SS genotypes were 1.68% ± 1.03% and 4.12% ± 2.41%, respectively (Figure 3A). Although a moderate increase in PS-positive erythrocytes was noted in SC disease (P < .0001), marked increases were observed in patients with the SS genotype when compared with both SC patients (P < .0001) and controls (P < .0001). Levels of PS-positive platelets in controls and patients with SC and SS genotypes were 1.16% ± 0.56%, 2.52% ± 1.23%, and 2.95% ± 1.79%, respectively (Figure 3B). Although significant differences were noted between controls and both patient groups (P < .004 in SC; P < .0001 in SS), there were no interpatient group differences (P > .45).
PS-positive erythrocytes and platelets in controls and patients with SCD.
PS-positive erythrocytes (panel A) and platelets (panel B) from control donors (n = 11) were compared with those from patients with the SS (n = 25) and SC (n = 15) genotypes. Bars represent mean ± SD values.
PS-positive erythrocytes and platelets in controls and patients with SCD.
PS-positive erythrocytes (panel A) and platelets (panel B) from control donors (n = 11) were compared with those from patients with the SS (n = 25) and SC (n = 15) genotypes. Bars represent mean ± SD values.
Markers of thrombin generation
Mean plasma levels of prothrombin fragment F1.2 in controls and patients with SC and SS genotypes were 0.57, 0.87, and 1.30 nM, respectively (Table 1). Although a minimal but statistically significant increase in the level of F1.2 was noted in the SC patient group (P < .004), levels were markedly elevated in SS disease when compared with either controls (P < .0001) or the SC genotype (P < .02). Mean levels of TAT were 1.6, 4.0, and 5.54 ng/mL in controls, SC disease, and SS disease, respectively. Although significant differences were noted between controls and both patient groups (P < .03 with SC; P < .0001 with SS), there were no significant differences in TAT levels between patient groups (P > .08).
Markers of thrombin generation and fibrinolysis in patients with sickle cell disease
| Marker . | Controls (N = 11) . | SC genotype (N = 15) . | SS genotype (N = 25) . |
|---|---|---|---|
| F1.2 (nM) | 0.57 ± 0.21 | 0.87 ± 0.26* | 1.30 ± 0.53* |
| TAT (ng/mL) | 1.6 ± 0.71 | 4.00 ± 4.44* | 5.54 ± 4.89* |
| D-dimer (μg/mL) | 0.05 ± 0.03 | 0.05 ± 0.04 | 0.17 ± 0.20* |
| PAP (μg/mL) | 0.42 ± 0.14 | 0.33 ± 0.22 | 0.49 ± 0.34 |
| tPA (ng/mL) | 3.98 ± 1.55 | 8.27 ± 6.96* | 6.25 ± 3.11 |
| PAI-1 (ng/mL) | 17.8 ± 9.8 | 21.8 ± 13.3 | 24.8 ± 17.7 |
| Marker . | Controls (N = 11) . | SC genotype (N = 15) . | SS genotype (N = 25) . |
|---|---|---|---|
| F1.2 (nM) | 0.57 ± 0.21 | 0.87 ± 0.26* | 1.30 ± 0.53* |
| TAT (ng/mL) | 1.6 ± 0.71 | 4.00 ± 4.44* | 5.54 ± 4.89* |
| D-dimer (μg/mL) | 0.05 ± 0.03 | 0.05 ± 0.04 | 0.17 ± 0.20* |
| PAP (μg/mL) | 0.42 ± 0.14 | 0.33 ± 0.22 | 0.49 ± 0.34 |
| tPA (ng/mL) | 3.98 ± 1.55 | 8.27 ± 6.96* | 6.25 ± 3.11 |
| PAI-1 (ng/mL) | 17.8 ± 9.8 | 21.8 ± 13.3 | 24.8 ± 17.7 |
TAT indicates thrombin-antithrombin; PAP, plasmin-antiplasmin; tPA, tissue plasminogen activator; PAI-1, plasminogen activator inhibitor-1.
Plasma levels of markers of thrombin generation (F1.2 and TAT) and fibrinolysis (D-dimer, PAP, tPA, and PAI-1) were assessed by using commercially available enzyme-linked immunosorbent assay kits. Values presented are the means ± SD.
Marker levels were significantly different from controls atP < .05 to P < .0001.
Markers of fibrinolysis
Mean levels of D-dimer in the controls and patients with SC and SS disease were 0.05, 0.05, and 0.17 μg/mL, respectively (Table 1). Although there were no differences in levels between controls and SC patients (P> 0.35), levels of this marker were significantly elevated in SS disease when compared with either controls (P < .006) or the SC group (P < .0001). Plasma levels of tPA in controls, SC patients, and SS patients were 3.98, 8.27, and 6.25 ng/mL, respectively. The levels of tPA were elevated in SC patients (P < .008). No significant differences were noted between SS disease and controls (P > .05). PAP and PAI-1 levels were similar in all groups evaluated (P > .5; Table 1). In the patient groups, striking positive relationships were noted between levels of D-dimer and F1.2 (R = 0.53, P < .0001) and between D-dimer and PAP (R = 0.64,P < .000002).
Relationships between hemostatic markers and PS-positive erythrocytes and/or platelets in SCD
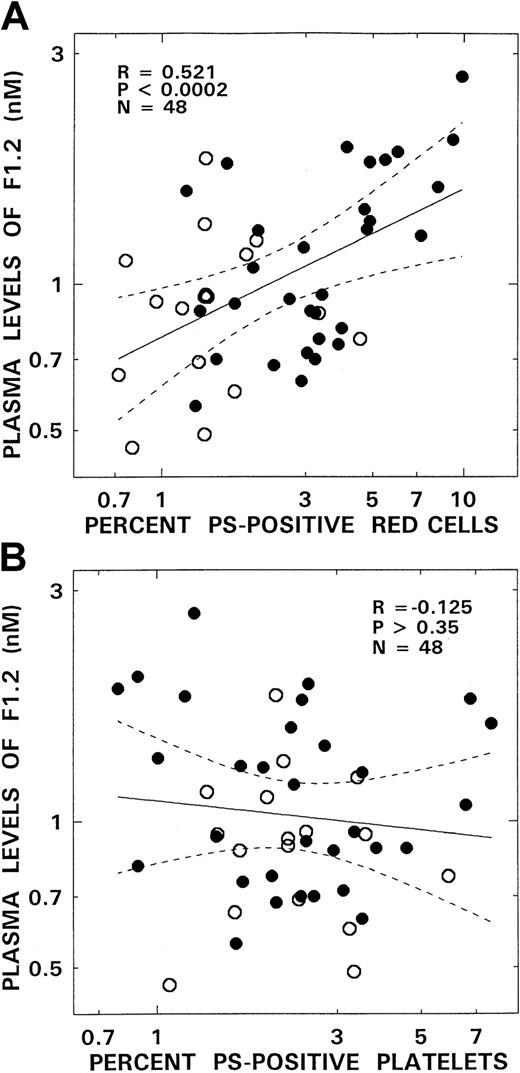
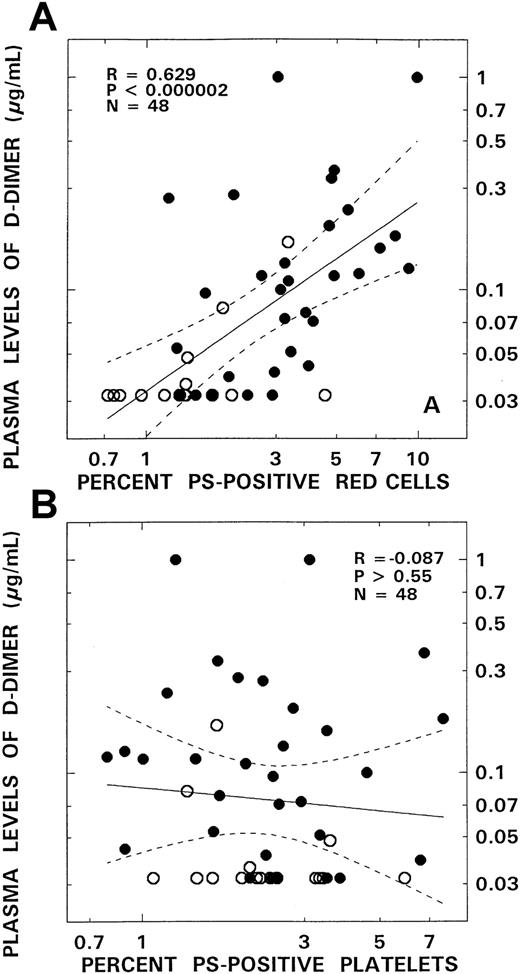
Although no correlations were noted between levels of PS-positive platelets and any of the hemostatic markers evaluated, significant positive correlations occurred between levels of PS-positive red cells and plasma F1.2, D-dimer, and PAP (Table2; Figures4 and5). These correlations were most striking for F1.2 (R = 0.52,P < .0002; Figure 4A) and D-dimer (R = 0.63,P < .000002; Figure 5A). A modest association also existed between PS-positive erythrocytes and levels of PAP (R = 0.37, P < .01) and levels of the thrombin inactivation marker TAT, although the latter association did not attain statistical significance (P > .09).
Relationships between the levels of individual hemostatic markers and presence of phosphatidylserine-positive erythrocytes and platelets in patients with sickle cell disease
| Marker . | Red cell . | Platelet . | ||
|---|---|---|---|---|
| R . | P . | R . | P . | |
| F1.2 | .521 | < .0002 | −.125 | > .35 |
| TAT | .244 | > .09 | −.080 | > .55 |
| D-dimer | .629 | < .000002 | −.087 | > .55 |
| PAP | .372 | < .01 | .015 | > .90 |
| tPA | .028 | > .85 | .045 | > .75 |
| PAI-1 | .241 | > .09 | .189 | > .15 |
| Marker . | Red cell . | Platelet . | ||
|---|---|---|---|---|
| R . | P . | R . | P . | |
| F1.2 | .521 | < .0002 | −.125 | > .35 |
| TAT | .244 | > .09 | −.080 | > .55 |
| D-dimer | .629 | < .000002 | −.087 | > .55 |
| PAP | .372 | < .01 | .015 | > .90 |
| tPA | .028 | > .85 | .045 | > .75 |
| PAI-1 | .241 | > .09 | .189 | > .15 |
TAT indicates thrombin-antithrombin; PAP, plasmin-antiplasmin; tPA, tissue plasminogen activator, PAI-1, plasminogen activator inhibitor-1.
Plasma levels of hemostatic markers were evaluated by using commercially available enzyme-linked immunosorbent assay kits, and circulating levels of phosphatidylserine-positive erythrocytes and platelets were assessed by using annexin V by flow cytometry. Values presented for the R and P values were obtained by using the Pearson test on log-transformed data. Similar results were obtained by using the Spearman test on rank-transformed data.
Relationship between plasma levels of F1.2 and PS-positive red blood cells and PS-positive platelets from patients with SCD.
Panel A depicts the association between the levels of F1.2 and PS-positive red blood cells. Panel B presents correlation between F1.2 and PS-positive platelets. The open and closed circles represent data points from patients with the SC and SS genotypes, respectively. The solid lines represent the linear-regression fit to the data, and the dotted lines represent the 99% confidence interval curves.
Relationship between plasma levels of F1.2 and PS-positive red blood cells and PS-positive platelets from patients with SCD.
Panel A depicts the association between the levels of F1.2 and PS-positive red blood cells. Panel B presents correlation between F1.2 and PS-positive platelets. The open and closed circles represent data points from patients with the SC and SS genotypes, respectively. The solid lines represent the linear-regression fit to the data, and the dotted lines represent the 99% confidence interval curves.
Relationship between D-dimer and PS-positive red blood cells and PS-positive platelets from patients with SCD.
Panel A depicts the association between the levels of D-dimer and PS-positive red blood cells. Panel B presents correlation between D-dimer and PS-positive platelets. The open and closed circles represent data points from patients with the SC and SS genotypes, respectively. The solid lines represent the linear-regression fit to the data, and the dotted lines represent the 99% confidence interval curves.
Relationship between D-dimer and PS-positive red blood cells and PS-positive platelets from patients with SCD.
Panel A depicts the association between the levels of D-dimer and PS-positive red blood cells. Panel B presents correlation between D-dimer and PS-positive platelets. The open and closed circles represent data points from patients with the SC and SS genotypes, respectively. The solid lines represent the linear-regression fit to the data, and the dotted lines represent the 99% confidence interval curves.
Longitudinal hemostatic evaluations
Results of paired blood samples performed at least 1 year apart (mean = 14 months) on the 8 infants previously noted revealed interesting although nonconclusive results. Initial values for F cells (red cells containing HbF), erythrocyte, and platelet PS were 72% ± 21%, 2.7% ± 1.6%, and 1.9% ± 0.7%, respectively. Follow-up evaluations as expected revealed a fall in F cell levels to 50% ± 25% (P < .0001, paired t test), whereas PS levels on erythrocytes and platelets increased to 3.7% ± 1.7% (P = .15) and 2.8% ± 1.9% (P = .14), respectively. Evaluations of hemostatic markers also revealed a trend toward activation with elevated levels of F1.2, D-dimer, and TAT in 6 of 8 paired samples when compared with their initial values [F1.2 = 0.95 ± 0.3 versus 1.29 ± 0.58 nM (P = .17); D-dimer = 0.08 ± 0.06 versus 0.24 ± 0.33 μg/mL (P = .14); TAT = 2.78 ± 1.06 versus 3.83 ± 1.86 ng/mL (P = .29)].
Discussion
The presence of PS on cell surfaces is associated with numerous pathophysiologic consequences. Externalization of PS on platelet membranes following platelet activation is critical to coagulation mechanisms and the efficient propagation of the hemostatic process.20,21 In SCD, the consequences of PS exposure on the red cell membrane include an exacerbation of anemia because of enhanced phagocytic recognition and removal,20 enhanced adhesivity to the vascular wall,25 and the expression of procoagulant activity that can promote assembly on the membrane of both tenase and prothrombinase complexes.19,20 Although studies have demonstrated the surface exposure of PS on the erythrocytes in patients with SCD,22 23 none have sought to assess the effect of this pathologic procoagulant phenotype in relation to thrombin generation and fibrinolysis. Moreover, because platelet activation occurs in SCD, an additional question that is important to address is whether PS exposure on the platelet occurs in these patients and, if so, what is the relative contribution of erythrocyte versus platelet PS to the pathophysiology of thrombin generation.
Results presented elucidate a crucial role for abnormal erythrocyte PS exposure in the thrombophilia of SCD. Striking correlations were noted between erythrocyte PS levels and prothrombin fragment F1.2 (P < .0002; Figure 4), with a similar trend with respect to TAT levels (Table 2). Although additional evidence of abnormal platelet membrane PS exposure was noted, the absence of any correlations between platelet PS and hemostatic markers implies that platelet activation is not the predominant mechanism responsible for thrombin generation. Our longitudinal paired studies (in progress at this time) suggests an additional trend between increasing levels of erythrocyte PS and hemostatic activation in 6 of 8 infants evaluated to date, although the numbers were too small to achieve statistical significance. The inference that red cell PS is one of the major factors controlling coagulation activation is also strengthened by a recent study we performed in infants with SCD.24 Because HbF inhibits polymerization of sickle cell hemoglobin, we hypothesized that, in vivo sickling/desickling with consequent membrane perturbation, procoagulant PS exposure and activation of coagulation mechanisms would be prevented in infants with SCD by the high HbF levels. With progression into early childhood and a decline in HbF levels, PS-positive red cells and coagulation activation would supervene. This scenario was proven to occur in that study with increasing plasma F1.2 levels correlating with increasing erythrocyte PS positivity and decreasing numbers of F cells.24 These 2 reports provide crucial evidence for the promoter effect on coagulation that results from erythrocyte PS exposure. Although acceleration of coagulation via enhanced procoagulant activity is the hallmark of the PS-positive erythrocyte, we must be cognizant that the trigger for hemostatic activation in SCD is presumably related to elevated circulating levels of tissue factor and the presence of microvascular endothelial cells with a tissue factor phenotype that circulate in increased numbers in these patients.15,16 This latter finding appears to be linked to the up-regulation of endothelial nuclear factor κB (NFκB) and potentially other transcription regulators, including activator protein-1 (AP1) by sickled erythrocytes.17 26 Thus, evidence points to the sickle erythrocyte as the central player responsible for the thrombophilic state and the large pool of red cells (when compared with the other circulating cellular elements) perhaps reinforcing the absolute numbers of erythrocytes available in this disease entity to both trigger coagulation activation via enhanced tissue factor availability and accelerate coagulation via the presence of circulating procoagulant PS-positive erythrocytes. An additional factor that needs to be considered is the question of the relative lifespan of platelets versus erythrocytes, and whether the relatively shorter half-life of platelets when compared with erythrocytes complicates the interpretation of our results. In this regard, it is important to note that erythrocyte half-life is markedly shortened in SCD, and that, although patients with both the SS and SC genotypes had similar values for PS-positive platelets, hemostatic markers in these patients did correlate with erythrocyte PS positivity.
Our studies also address the alterations in the fibrinolytic system. No correlations were observed between platelet PS and the fibrinolytic markers assessed. A striking correlation, however, between erythrocyte PS and D-dimer levels (P < .000002; Figure 5) was noted, with a more modest one between red cell PS and PAP levels (P < .01). In addition, a correlation between F1.2 and D-dimer was observed (P < .0001) consistent with the hypothesis that activation of the fibrinolytic system and D-dimer formation is secondary to the generation of thrombin and fibrin deposition. In addition, because leucocytosis and white cell activation has been observed in SCD,11-13 it is logical to ask the question whether proteolytic activity released from these cells could induce fibrinolysis unrelated to the effect of plasmin. The correlation noted, however, between D-dimer and PAP levels (P < .000002) suggests that fibrinolysis occurs mainly because of plasmin generation and not because of white cell proteolytic activity.
Comparison of hemostatic markers between children with the SS and SC genotypes has not been previously reported. Our study documents the same trend that has been observed in the adult8—namely, that, whereas markers of in vivo thrombin production are moderately elevated in children with SC disease, they are more marked in SS disease. PS functions as an essential cofactor for prothrombin activation by factor Xa.19,20 Using this association, Helley et al27 have demonstrated that SS erythrocytes accelerated prothrombin activation to a greater degree than SC red cells. The methodology used, however, removed reticulocytes (a subpopulation of which externalizes PS),23 thus resulting in an underestimation of procoagulant activity in patients with high reticulocyte counts. Our flow cytometric analyses, in which all red cell populations were assessed, clearly show that patients with the SC genotype do indeed demonstrate elevated levels of erythrocyte-annexin V binding, but of a lesser magnitude than in patients with SS disease (Figure 3) and that this correlates with the lesser degree of thrombin generation observed in the SC genotype. The markedly lower incidence of stroke, macrovascular changes, and prothrombotic tendency observed in patients with SC disease28 concomitant with the lesser degree of coagulation activation appear consistent with a role for hemostasis in the pathophysiology of long-term vascular modeling in SCD. In addition, a previous preliminary observation29 that erythrocyte membrane PS and F1.2 levels are most markedly elevated in children with SCD judged to be at high risk of stroke (by Doppler measurements demonstrating cerebral vascular narrowing) suggests that these macrovascular changes seen in the cerebral vessels in patients with stroke or prestroke are directly or indirectly related to sickle erythrocyte PS exposure and abnormal in vivo thrombin generation. Thus, the demonstrated value of chronic transfusion therapy in the prevention of stroke in children with abnormal cranial Doppler flow studies or in reversing the vascular pathology that can occur after a cerebrovascular accident may be related to the ability of a transfusion regimen to normalize thrombin generation when the procoagulant sickle erythrocyte is replaced by a normal red cell in which phospholipid asymmetry is maintained. In fact, it was shown that in a group of chronically transfused children, certain coagulation parameters had normalized after transfusion, with a reduction (although not a normalization) in TAT levels in the small patient cohort evaluated.7 From the analyses presented here and the previous preliminary observation of Styles et al,29 we suggest that the level of erythrocyte PS during such a transfusion regimen may be of relevance in the monitoring of large vessel remodeling in SCD. In addition, because previous evidence points to a role for erythrocyte PS in red cell endothelial adhesion,25 and because adhesion is a significant factor in the propensity to microvascular occlusion, the importance of this biologic marker as a potential indicator of macrovascular and microvessel pertubations in SCD should be further assessed.
In conclusion, we have demonstrated unequivocally the association between red cell PS exposure and thrombin generation in patients with SCD. Although activation of the fibrinolytic system was noted, it appears to be a secondary phenomenon in response to thrombin generation and fibrin deposition. With the availability of transgenic murine models and novel interventive strategies (such as antiadhesion therapy, inhibitors of cellular dehydration, and inducers of HbF other than hydroxyurea), we are on the threshold of a new era of clinical trials in SCD. We suggest that the use of erythrocyte PS as a surrogate marker in such trials could provide valuable insights into disease pathophysiology, especially in relation to the vascular complications of this disease entity.
We thank Surekha Kulkarni and Vijender R. Viadyula, PhD, for technical assistance; Patricia O'Neal, Sandra Moss, and Miriam Gilday for their phlebotomy expertise; and Lorenzo Thomas for secretarial assistance.
Supported by grants HL51497 and 1P60HL62148 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
A recent paper of Tomer et al30 requires discussion in light of our findings. The authors demonstrate that a diet rich in n-3 fatty acids, provided as fish oil, decreased hemostatic activation in a small group (n = 5) of patients with SCD. They also demonstrated a decrease in platelet activation markers and suggested that the inhibition of hemostatic activation provided by fish oil ingestion was directly linked to platelet inhibition. While markedly elevated red cell PS levels were noted at study inception, no concomitant data is provided on red cell annexin V binding (ie, erythrocyte PS levels) after therapy. An alternate explanation is that a decrease in red cell procoagulant activity with a resulting decrease in in vivo thrombin generation could have caused the observed platelet effects. Thus, the data presented are open to various interpretations, with the real possibility that the platelet effects noted were secondary rather than primary.
Author notes
B. N. Yamaja Setty, Thomas Jefferson University, Department of Pediatrics, Medical College Bldg, Suite 727, 1025 Walnut St, Philadelphia, PA 19107; e-mail:yamaja.setty@mail.tju.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal