Abstract
Multiple myeloma is a B-cell malignancy characterized by the accumulation of plasma cells in the bone marrow and the development of osteolytic bone disease. The present study demonstrates that myeloma cells express the critical osteoclastogenic factor RANKL (the ligand for receptor activator of NF-κB). Injection of 5T2MM myeloma cells into C57BL/KaLwRij mice resulted in the development of bone disease characterized by a significant decrease in cancellous bone volume in the tibial and femoral metaphyses, an increase in osteoclast formation, and radiologic evidence of osteolytic bone lesions. Dual-energy x-ray absorptiometry demonstrated a decrease in bone mineral density (BMD) at each of these sites. Treatment of mice with established myeloma with recombinant osteoprotegerin (OPG) protein, the soluble decoy receptor for RANKL, prevented the development of lytic bone lesions. OPG treatment was associated with preservation of cancellous bone volume and inhibition of osteoclast formation. OPG also promoted an increase in femoral, tibial, and vertebral BMD. These data suggest that the RANKL/RANK/OPG system may play a critical role in the development of osteolytic bone disease in multiple myeloma and that targeting this system may have therapeutic potential.
Introduction
Multiple myeloma is a B-cell neoplasm characterized by the clonal expansion of plasma cells in the bone marrow. A major clinical feature of multiple myeloma is the development of osteolytic bone lesions characterized by the presence of bone pain, hypercalcemia, and pathologic fractures. The mechanisms that lead to the development of myeloma bone disease are unclear. However, histomorphometric studies have demonstrated that the bone disease is characterized by an uncoupling of the normal process of bone remodeling.1-4 Bone resorption is increased and associated with the presence of increased numbers of osteoclasts, whereas bone formation is reduced.1-4 This uncoupling of resorption and formation, in association with an increase in the frequency of bone remodeling units, leads to rapid bone loss and the development of osteolytic bone lesions.
The factors responsible for the increase in osteoclast formation are unclear. Early studies demonstrated that myeloma cell lines and tumor cells from patients with myeloma were able to produce factors that could stimulate an increase in osteoclastic bone resorption in vitro.5,6 However, the specific factors that mediate this increase in bone resorption remain unclear. Studies have implicated interleukin-1β, tumor necrosis factor-α,7,8 and lymphotoxin8-10 in this activity, although these data are conflicting and are often confounded by studying cell lines or whole bone marrow samples.11 Indeed, studies of purified myeloma cells, or single-cell analyses, may argue against the involvement of some of these factors.12,13 More recently, a number of local growth factors have been identified and implicated in this process, including hepatocyte growth factor14,15 and macrophage inflammatory protein 1-α.16 The precise role of these factors in promoting osteoclastic bone resorption in myeloma is currently being investigated; however, other factors may also play a role.
The receptor activator of NF-κB ligand (RANKL,17also known as osteoprotegerin [OPG] ligand,18 osteoclast differentiation factor,19 or TRANCE [TNF-related activation-induced cytokine]20) has been shown recently to play a critical role in normal osteoclast development.21 RANKL is expressed by stromal cells and osteoblasts in the local bone marrow microenvironment, where it can bind to its receptor RANK17 on the surface of osteoclast precursors. The binding of RANKL to RANK plays an important role in promoting osteoclast differentiation and bone resorption. RANKL has also been reported to activate mature osteoclasts to increase bone resorption.22 A soluble decoy receptor known as osteoprotegerin (OPG, also known as osteoclastogenesis inhibitory factor23) has also been identified.24 OPG binds RANKL, inhibiting its interaction with RANK and preventing osteoclast formation. The importance of this system in normal bone remodeling and osteoclast formation can be seen in transgenic mice. Mice with overexpression of OPG or deficiency in RANKL have decreased osteoclast formation and develop osteopetrosis,21,24 whereas mice deficient in OPG have reduced bone mass and develop osteoporosis.25 26
Given the critical nature of this system in normal osteoclast development, it is likely that diseases characterized by abnormal osteoclastic bone resorption, including multiple myeloma, may alter the balance of the RANKL system in the local bone marrow microenvironment in favor of bone resorption. Therefore, the aim of the present study was to determine whether myeloma cells themselves express RANKL and whether targeting this system with recombinant OPG could inhibit the development of osteolytic bone disease induced by myeloma cells in vivo in a model of established multiple myeloma.
Materials and methods
The 5T2MM model
The 5T2MM murine model of multiple myeloma originated spontaneously in elderly C57BL/KaLwRij mice.27,28Subsequently, 5T2MM cells have been propagated in vivo by the intravenous (IV) transfer of diseased bone marrow into young syngeneic mice. Male C57BL/KaLwRijHsd mice were obtained from Harlan CPD (Horst, The Netherlands) and were 6 weeks of age when used. Animals were housed under conventional conditions and had free access to tap water. All procedures involving mice were approved by the local ethics committee. 5T2MM cells were isolated from the bone marrow of disease-bearing animals, purified, and injected through the tail vein into recipient mice, as described previously.29 Progression of multiple myeloma in diseased animals was assessed by measuring the presence of a serum paraprotein using standard electrophoretic techniques.30
Treatment of 5T2MM-bearing mice with recombinant OPG
Mice were injected with 5T2MM cells or left uninjected (control). Serum samples were obtained from animals injected with 5T2MM cells after 8 weeks, and paraprotein concentrations were determined. Once all injected mice had detectable serum paraprotein, they were treated with either recombinant human Fc-OPG31 (5T2 + OPG, 30 mg/kg IV, 3 times per week, n = 13) or a vehicle control (5T2 + vehicle, phosphate-buffered saline, 3 times per week, n = 14). The binding affinity of human OPG to murine and human RANKL was shown to be similar (data not shown). No pharmacokinetic data are available for Fc-OPG in the mouse. However, in the rat, the half-life of the terminal elimination phase of this molecule is approximately 15 hours (unpublished data, January 1998); in cynomolgus monkeys, this has been reported to be 16 hours32; whereas in humans, this is approximately 40 hours.33
Treatment with Fc-OPG or vehicle continued for a further 4 weeks, after which time all animals were killed. The femora, tibiae, and lumbar vertebrae were dissected free of soft tissues and processed for flow cytometric analysis of the bone marrow or fixed in 4% formalin before processing for histologic analysis. Blood samples were also obtained for determination of serum paraprotein concentrations.
Reverse transcriptase–polymerase chain reaction analysis of 5T2MM cells
Expression of the mRNA for RANKL in purified 5T2MM cells and whole bone marrow was determined by reverse transcriptase–polymerase chain reaction (RT-PCR). Bone marrow from control mice or mice bearing 5T2MM cells was flushed from the tibia and femur, and 5T2MM cells were purified as described previously.29 Purity was determined by staining 5T2MM cells with an anti-idiotype antibody and assessing proportions by flow cytometry (see below). In these studies, populations of 5T2MM cells were shown to be 90% to 95% pure. Total RNA was isolated with TriZol (Life Technologies, Glasgow, United Kingdom), precipitated with isopropanol, and resuspended in diethylpyrocarbonate-treated water. Contaminating DNA was removed by incubating RNA samples with DNase I at 37°C for 1 hour. RNA was reverse transcribed to cDNA using Moloney murine leukemia virus reverse transcriptase (Life Technologies) primed with random hexamers (Pharmacia Biotech, Uppsala, Sweden). PCR was performed under standard conditions with 34 cycles of amplification using Amplitaq DNA polymerase (Applied Biosystems, Cheshire, United Kingdom). Following amplification, products were examined by agarose gel electrophoresis and sequenced to confirm their identity. The primers used to amplify murine RANKL were 5′-CAGAATTGCCCGACCAG-3′ and 5′-TCCCATAAAGTCACTCTGTCC-3′.
Flow cytometric analysis of 5T2MM cells
Expression of RANKL on the surface of 5T2MM cells was determined by flow cytometry. The bone marrow from 5T2MM-bearing mice was flushed from the tibia and femur, and mononuclear cells were stained with an anti-5T2MM idiotype-specific murine monoclonal antibody30 and with goat anti-RANKL (IgG, C-20; Santa Cruz Biotechnology, Santa Cruz, CA). After washing, cells were incubated with rat anti–mouse IgG1 conjugated with phycoerythrin (PE; Becton Dickinson, Mountain View, CA) to identify 5T2MM-positive cells, and with rabbit anti–goat IgG conjugated to fluorescein isothiocyanate (FITC; Sigma Chemical, St Louis, MO) to identify RANKL-positive cells. Purified goat IgG (Sigma, Poole, United Kingdom) isolated from pooled normal goat serum was used as a negative control for goat anti–RANKL staining. Expression of RANKL on 5T2MM idiotype-positive and -negative cells was determined and compared with staining with the appropriate isotype control. All samples were analyzed on a FACSort flow cytometer (Becton Dickinson). Samples were analyzed for their forward and side light scatter, as well as FITC and PE fluorescence. Dead or dying cells and cell debris were excluded using appropriate scatter gating.
Radiographic and bone densitometric analysis
Femora, tibiae, and lumbar vertebrae were radiographed using a Faxitron x-ray system (Hewlett Packard, McMinnville, OR). X-rays were scanned using a UMAX PowerLook 1100 Scanner (Umax Systems, Willich, Germany). Images were enlarged, and the numbers of lytic bone lesions in the tibiae and femora were counted manually. Total bone mineral density (BMD) of the femur, tibia, and lumbar vertebrae was measured by dual-energy x-ray absorptiometry using a PIXImus scanner (Lunar, Madison, WI) with dedicated small-animal software. The coefficient of variation for femoral BMD, obtained after scanning 30 femurs 5 times each following repositioning between scans, was found to be 2.7%. Total BMD and morphometric indices of cortical bone were also determined in the distal femoral metaphysis by peripheral quantitative computed tomography (pQCT) (Stratec, Pforzheim, Germany). Five 70-μm slices, 3 mm from the distal end of the femur and separated by 1 mm, were analyzed, and the mean total BMD, cortical width, endosteal length, and periosteal length were determined.
Histologic and histochemical analysis
Tibiae and femora were decalcified in EDTA and embedded in paraffin, and 3-μm sections were cut and stained with hematoxylin and eosin. Cancellous bone area as a proportion of the total area was determined in the distal femoral metaphysis and proximal tibial metaphysis, in an area of 0.64 mm2 and 0.25 mm from the growth plate, using a Leica QWin image analysis system (Leica Microscope Systems, Milton Keynes, United Kingdom). Sections of both tibiae and femora were also stained for the presence of tartrate-resistant acid phosphatase (TRAP) to identify osteoclasts and counterstained with Gill hematoxylin. Because infiltration of the bone marrow by 5T2MM cells resulted in almost complete removal of cancellous bone, only osteoclasts lining corticoendosteal bone surfaces were assessed. The number of osteoclasts present on 3 mm of each corticoendosteal surface, beginning 0.5 mm from the growth plate, was counted (number/mm).
Statistical analysis
Radiographic, densitometric, and histomorphometric analyses were performed in all 5T2MM-bearing animals (5T2MM + vehicle, n = 14; and 5T2MM + OPG, n = 13). All data are expressed as the mean ± SEM. Comparison between groups was performed by the Mann-Whitney U test.
Results
Myeloma cells express RANKL on the cell membrane
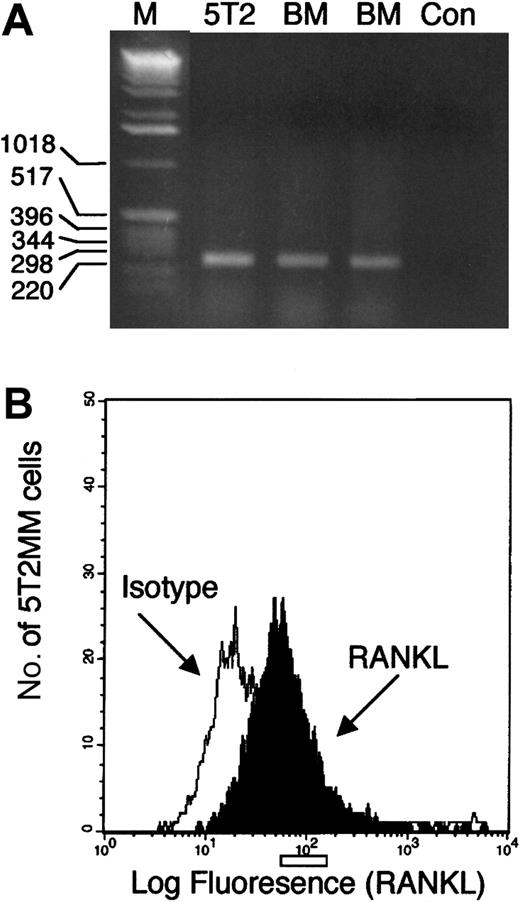
RT-PCR analysis of RANKL expression in purified 5T2MM cells (90% to 95%) and normal bone marrow mononuclear cells resulted in the amplification of a single product (Figure1A). This product was sequenced and confirmed to be RANKL. Flow cytometric analysis of bone marrow mononuclear cells isolated from mice bearing 5T2MM cells demonstrated expression of RANKL on idiotype-positive 5T2MM cells (Figure1B).
Studies of RANKL expression by 5T2MM cells.
(A) RT-PCR analysis of RNA isolated from purified 5T2MM cells (5T2) or bone marrow mononuclear cells from the vertebrae (BM1) or femora (BM2) of control, uninjected mice. M represents a lane containing a DNA standard, and Con is a negative control. (B) Flow cytometric analysis of idiotype-positive 5T2MM cells isolated from the bone marrow of mice bearing 5T2MM cells. Black histogram shows RANKL staining, and white histogram shows staining with an isotype-matched control antibody.
Studies of RANKL expression by 5T2MM cells.
(A) RT-PCR analysis of RNA isolated from purified 5T2MM cells (5T2) or bone marrow mononuclear cells from the vertebrae (BM1) or femora (BM2) of control, uninjected mice. M represents a lane containing a DNA standard, and Con is a negative control. (B) Flow cytometric analysis of idiotype-positive 5T2MM cells isolated from the bone marrow of mice bearing 5T2MM cells. Black histogram shows RANKL staining, and white histogram shows staining with an isotype-matched control antibody.
IV injection of 5T2MM myeloma cells leads to the development of osteolytic bone disease
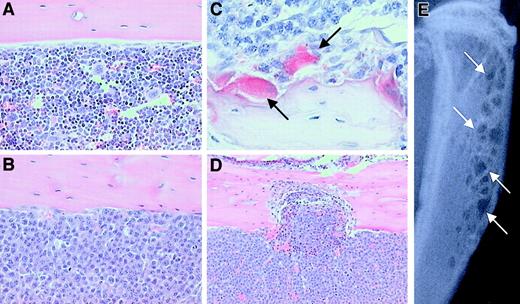
5T2MM myeloma cells were injected into the tail vein of C57BL/KaLwRijHsd mice, and development of the myeloma disease was monitored by measuring serum paraprotein. After 8 weeks, all animals injected with tumor cells had detectable serum paraprotein, confirming the presence of tumor cells. After a further 4 weeks, animals were killed and tissues were removed for examination. Histologic analysis of the distal femur, proximal tibia, and lumbar vertebrae demonstrated that 5T2MM cells had homed to the bone marrow and largely replaced the normal bone marrow (Figure 2A,B). 5T2MM cells were found closely associated with large numbers of TRAP-positive osteoclasts that lined the cancellous bone, when present, and the corticoendosteal bone surface (Figure 2C). The presence of large numbers of TRAP-positive osteoclasts resulted in a significant increase in bone resorption and erosion through the cortex (Figure 2D). These histologic changes were readily detected radiographically as small discrete osteolytic lesions in both the femora and the tibiae (Figure 2E).
The effect of 5T2MM cells on the development of bone disease in C57BL/KaLwRijHsd mice.
(A) Histologic section of the tibia from a control animal showing cortical bone and the presence of normal marrow. Original magnification × 20. (B) Histologic section of the tibia from a 5T2MM-bearing animal showing cortical bone and demonstrating the complete replacement of normal marrow by 5T2MM cells. Original magnification × 20. (C) Section reacted for TRAP activity showing the presence of 5T2MM cells found closely associated with TRAP-positive osteoclasts (arrows) lining the bone surface. Original magnification × 40. (D) Section of the tibia from an animal injected with 5T2MM cells showing resorption through the cortical bone. Original magnification × 10. (E) Radiograph of the tibia of an animal bearing 5T2MM cells showing the presence of lytic bone lesions (arrows).
The effect of 5T2MM cells on the development of bone disease in C57BL/KaLwRijHsd mice.
(A) Histologic section of the tibia from a control animal showing cortical bone and the presence of normal marrow. Original magnification × 20. (B) Histologic section of the tibia from a 5T2MM-bearing animal showing cortical bone and demonstrating the complete replacement of normal marrow by 5T2MM cells. Original magnification × 20. (C) Section reacted for TRAP activity showing the presence of 5T2MM cells found closely associated with TRAP-positive osteoclasts (arrows) lining the bone surface. Original magnification × 40. (D) Section of the tibia from an animal injected with 5T2MM cells showing resorption through the cortical bone. Original magnification × 10. (E) Radiograph of the tibia of an animal bearing 5T2MM cells showing the presence of lytic bone lesions (arrows).
Treatment of 5T2MM-bearing mice with OPG prevents the development of lytic bone lesions
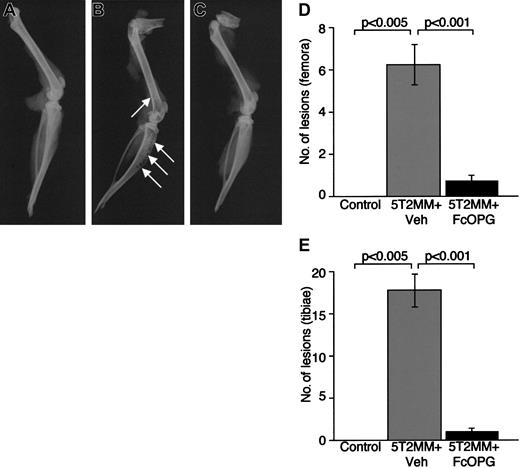
It is clear that 5T2MM myeloma cells can induce the development of osteolytic bone lesions in vivo. Thus, targeting the RANKL system with specific antagonists may inhibit the development of such lesions. Therefore, we examined the effect of recombinant OPG on the development of myeloma bone disease in mice with established myeloma. As expected, radiographic analysis of control animals (no 5T2MM cells) demonstrated the absence of osteolytic lesions in the tibiae or femora (Figure3A,D,E). In contrast, mice bearing 5T2MM cells (5T2MM vehicle) had large numbers of lesions in both the tibiae and femora (Figure 3B,D,E). However, treatment of mice bearing 5T2MM cells with OPG resulted in a significant decrease in the numbers of lytic bone lesions induced by 5T2MM cells (Figure 3C-E). The numbers of lesions in both tibiae and femora were reduced from 17.7 ± 2.0 to 1.1 ± 0.3 and 6.2 ± 0.9 to 0.8 ± 0.3, respectively. The numbers of lesions in 5T2MM-bearing mice treated with OPG were not significantly different from those in control, uninjected animals.
The effect of Fc-OPG on lytic bone lesions induced by 5T2MM cells.
(A) Radiograph of the tibia and femur from a control, uninjected animal. (B) Radiograph of the tibia and femur of an animal injected with 5T2MM cells and vehicle, showing the presence of lytic lesions (arrows). (C) Radiograph of the tibia and femur of an animal injected with 5T2MM cells and OPG. (D) Number of osteolytic bone lesions in the femora of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG. (E) Number of osteolytic bone lesions in the tibiae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG.
The effect of Fc-OPG on lytic bone lesions induced by 5T2MM cells.
(A) Radiograph of the tibia and femur from a control, uninjected animal. (B) Radiograph of the tibia and femur of an animal injected with 5T2MM cells and vehicle, showing the presence of lytic lesions (arrows). (C) Radiograph of the tibia and femur of an animal injected with 5T2MM cells and OPG. (D) Number of osteolytic bone lesions in the femora of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG. (E) Number of osteolytic bone lesions in the tibiae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG.
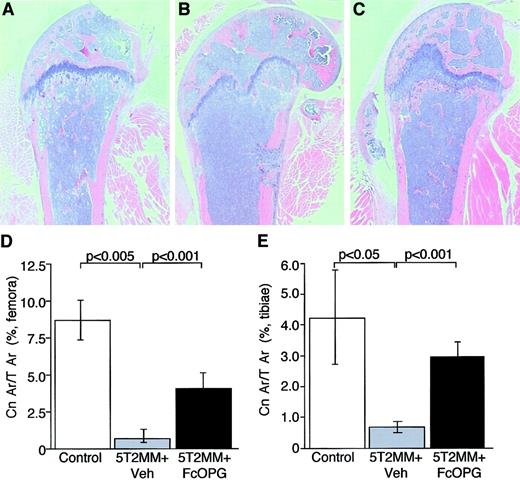
Injection of 5T2MM cells caused a significant decrease in cancellous bone volume in the proximal tibial metaphysis and the distal femoral metaphysis (Figure 4B,D,E). In many cases, the presence of 5T2MM resulted in the complete loss of cancellous bone in the metaphysis. Treatment of mice bearing 5T2MM cells with OPG resulted in a partial prevention of the decrease in cancellous bone induced by the presence of 5T2MM cells (Figure 4C-E). In the tibiae, cancellous bone area in the OPG-treated mice was not significantly different from that in control, uninjected animals (3.0 ± 0.4 and 4.2 ± 1.5, respectively), although in the femora, this remained significantly lower that that observed in uninjected animals (4.2 ± 0.8 and 8.7 ± 1.3, respectively;P < .05).
The effect of OPG on cancellous bone area in mice bearing 5T2MM myeloma cells.
(A) Section of the femur from a control, uninjected animal. (B) Section of the femur from an animal injected with 5T2MM cells and vehicle, showing the decrease in cancellous bone area. (C) Section of the femur from an animal injected with 5T2MM cells and treated with OPG. (D) Cancellous bone area as a percentage of total area in the femora of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG. (E) Cancellous bone area as a percentage of total area in the tibiae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG. Original magnification × 1.6.
The effect of OPG on cancellous bone area in mice bearing 5T2MM myeloma cells.
(A) Section of the femur from a control, uninjected animal. (B) Section of the femur from an animal injected with 5T2MM cells and vehicle, showing the decrease in cancellous bone area. (C) Section of the femur from an animal injected with 5T2MM cells and treated with OPG. (D) Cancellous bone area as a percentage of total area in the femora of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG. (E) Cancellous bone area as a percentage of total area in the tibiae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG. Original magnification × 1.6.
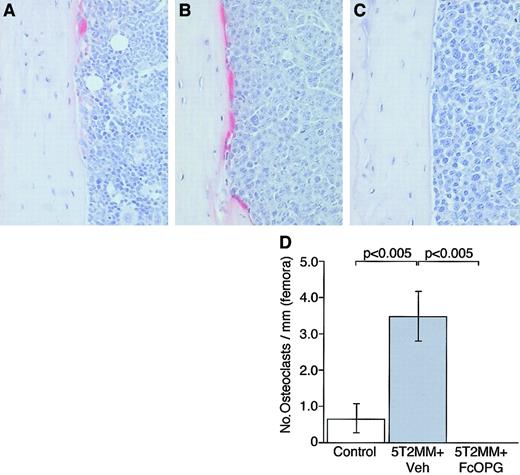
The presence of 5T2MM cells in the tibiae and femora was associated with a significant increase in the numbers of osteoclasts. Because 5T2MM cells caused a dramatic reduction in cancellous bone volume, it was not possible to assess osteoclasts associated with cancellous bone; however, infiltration of 5T2MM cells resulted in a significant increase in the numbers of TRAP-positive osteoclasts present on the corticoendosteal bone surface. In control animals, few osteoclasts were found on this surface (Figure 5A,D); however, in animals injected with 5T2MM cells, large numbers of TRAP-positive cells were observed (Figure 5B,D). In contrast, when 5T2MM-bearing animals were treated with OPG, there was a complete absence of osteoclasts in the tibiae (Figure 5C,D) and femora (data not shown).
The effect of OPG on TRAP-positive osteoclast formation in mice bearing 5T2MM myeloma cells.
(A) Section of the tibia from a control, uninjected animal. (B) Section of the tibia from an animal injected with 5T2MM cells and vehicle, showing large numbers of TRAP-positive cells lining the endocortical surface area. (C) Section of the femur from an animal injected with 5T2MM cells and treated with OPG. (D) Osteoclast numbers in the tibiae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and treated with OPG. Original magnification × 20.
The effect of OPG on TRAP-positive osteoclast formation in mice bearing 5T2MM myeloma cells.
(A) Section of the tibia from a control, uninjected animal. (B) Section of the tibia from an animal injected with 5T2MM cells and vehicle, showing large numbers of TRAP-positive cells lining the endocortical surface area. (C) Section of the femur from an animal injected with 5T2MM cells and treated with OPG. (D) Osteoclast numbers in the tibiae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and treated with OPG. Original magnification × 20.
Treatment of 5T2MM-bearing mice with OPG is associated with an increase in BMD
Bone densitometric analyses demonstrated that OPG treatment of mice with established myeloma not only prevented the development of osteolytic lesions, but also was associated with a significant increase in total BMD. Injection of 5T2MM cells caused a significant decrease in total BMD in tibiae, femora, and lumbar vertebrae (Figure6). Treatment of mice bearing 5T2MM cells with OPG prevented the decrease in total BMD induced by the presence of 5T2MM cells. BMD was significantly greater in mice bearing 5T2MM cells treated with OPG than in those animals bearing 5T2MM cells and treated with vehicle (Figure 6). Interestingly, OPG treatment resulted in an increase in BMD when compared with control animals (Figure 6). OPG treatment caused a significant increase in femoral total BMD (control 62.8 ± 0.9 versus OPG 66.4 ± 1.34), tibial total BMD (control 47.3 ± 0.7 versus OPG 51.2 ± 0.6), and lumbar vertebral total BMD (control 50.6 ± 0.8 versus OPG 61.7 ± 1.2) (P < .05, P < .005, andP < .001, respectively).
The effect of OPG on total BMD, assessed by dual-energy x-ray absorptiometry, in mice bearing 5T2MM myeloma cells.
(A) Total BMD in the tibiae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG. (B) Total BMD in the femora of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and treated with OPG. (C) Total BMD in the lumbar vertebrae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG.
The effect of OPG on total BMD, assessed by dual-energy x-ray absorptiometry, in mice bearing 5T2MM myeloma cells.
(A) Total BMD in the tibiae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG. (B) Total BMD in the femora of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and treated with OPG. (C) Total BMD in the lumbar vertebrae of control animals, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and OPG.
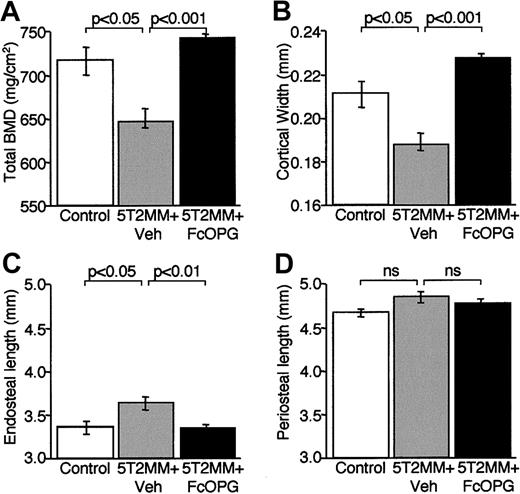
The decrease in total BMD in the femora induced by injection of 5T2MM cells was confirmed by pQCT analysis (Figure7A). Although treatment with OPG resulted in a significant increase in total BMD when compared with animals injected with vehicle, this was not significantly greater than control (Figure 7A). Morphometric analysis of femoral cortical bone revealed that injection of 5T2MM cells was associated with a significant decrease in cortical width that was prevented by treatment with OPG (Figure 7B). The reduction in cortical width appeared to be the result of an increase in size of the bone marrow cavity as endosteal length, but not periosteal length, increased significantly in animals bearing 5T2MM cells (Figure 7C,D). The changes in cortical width and endosteal length were prevented by treatment with OPG. These data are consistent with the demonstration of increased osteoclast numbers present on the corticoendosteal surface in mice bearing 5T2MM cells (Figure 5).
The effect of OPG treatment on total BMD and morphometric indices of cortical structure in the femora of 5T2MM-bearing mice.
BMD and cortical morphometry were assessed by pQCT in control mice, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and treated with OPG. (A) Total BMD. (B) Cortical width. (C) Endosteal length. (D) Periosteal length.
The effect of OPG treatment on total BMD and morphometric indices of cortical structure in the femora of 5T2MM-bearing mice.
BMD and cortical morphometry were assessed by pQCT in control mice, animals injected with 5T2MM cells and vehicle, and animals injected with 5T2MM cells and treated with OPG. (A) Total BMD. (B) Cortical width. (C) Endosteal length. (D) Periosteal length.
Treatment of 5T2MM-bearing mice with OPG is associated with a decrease in serum paraprotein
OPG is clearly effective at preventing the development of myeloma bone disease in the 5T2MM model. Because inhibiting bone resorption may result in an alteration in the local bone marrow microenvironment in which 5T2MM cells grow, it is possible that this may modulate the growth and survival of tumor cells in this environment. To indirectly assess the effect of OPG on tumor burden, we determined serum paraprotein concentrations after treatment. The mean paraprotein concentration in 5T2MM-bearing animals treated with vehicle was 0.66 ± 0.06 g/dL. Serum paraprotein concentrations were 25% lower (0.5 ± 0.03 g/dL) in the 5T2MM-bearing animals treated with OPG; however, this was not statistically significant.
Discussion
RANKL has been reported to play a critical role in normal osteoclast development. Diseases characterized by the presence of increased osteoclast activity, which include multiple myeloma, may mediate this effect by altering the balance of the RANKL/RANK/OPG system in the local bone environment in favor of increased bone resorption. In the present study, we used RT-PCR analysis to demonstrate expression of the mRNA for RANKL in purified 5T2MM murine myeloma cells. Importantly, flow cytometric analysis confirmed that idiotype-specific myeloma cells isolated from the bone marrow of mice with established myeloma stained positively for cell surface RANKL. These data are consistent with a previous report that demonstrated that the mRNA for RANKL was also expressed by 5TGM1 cells,34 a murine myeloma cell line generated from the same strain of mouse as 5T2MM. In addition, we have recently demonstrated that myeloma cells isolated from the bone marrow of patients with myeloma also express RANKL on the cell surface, whereas human myeloma cell lines maintained in vitro express the mRNA for RANKL but do not express RANKL on the cell surface.35 In these studies, staining of myeloma cells for RANKL was inhibited by the peptide used to raise the antibody and by soluble recombinant RANKL. This suggests that the RANKL antibody is binding to RANKL. Taken together, these data suggest that myeloma cells express RANKL on the cell surface, although interactions in the local bone marrow microenvironment may be important in promoting expression. The demonstration that myeloma cells express RANKL raises the possibility that myeloma cells could promote bone resorption directly. However, the possibility that these cells promote osteoclast formation indirectly must also be considered. Indeed, in vitro studies have demonstrated that murine myeloma cells can up-regulate RANKL mRNA expression in ST2 stromal cells, a process dependent on the interaction between the α4β1integrin expressed by myeloma cells and vascular cell adhesion molecule 1 (VCAM-1) on ST2 cells.34 Furthermore, increased expression of RANKL has also been reported in bone marrow biopsy specimens of patients with myeloma,36 and this expression may be associated with bone marrow stromal cells rather than myeloma cells.
Injection of 5T2MM cells into the tail vein of recipient C57BL/KaLwRij mice resulted in the homing to, and growth of, 5T2MM cells in the bone marrow. 5T2MM cells completely replaced the normal bone marrow and were found closely associated with TRAP-positive multinucleated osteoclasts. Osteoclasts could be found on cancellous bone surfaces, when present, and were observed to line the corticoendosteal surface. There was no evidence of an increase in osteoclast numbers on periosteal surfaces. The proximity of 5T2MM cells to osteoclasts, and the demonstration that they express RANKL, would support the suggestion that they could promote bone resorption directly. This could be achieved by promoting osteoclast recruitment and differentiation and/or the activity of individual osteoclasts. The increase in osteoclast number was associated with almost complete removal of cancellous bone in both femora and tibiae. In addition, morphometric data obtained by pQCT analysis demonstrated a reduction in cortical bone thickness in animals bearing 5T2MM cells. This decrease in cortical thickness was associated with an increase in endosteal circumference without any corresponding change in periosteal length. This would suggest that growth of 5T2MM results in a local increase in osteoclast formation, the resorption of cancellous bone, and expansion of the marrow cavity, which occurs at the expense of cortical bone. Indeed, at some locations, osteoclastic activity was so pronounced that it resulted in significant resorption into the cortex and the development of lytic bone lesions visible by radiography.
Because myeloma cells express RANKL, it is likely that they have the capacity to promote osteoclast formation directly. Inhibiting the interaction between RANKL and RANK on osteoclasts and their precursors therefore represents a therapeutic approach to manage this aspect of myeloma. Treatment of mice with established myeloma with recombinant OPG resulted in a significant reduction in the number of osteolytic lesions observed in both femora and tibiae. Histologic analysis demonstrated that OPG treatment was also associated with a partial preservation of cancellous bone volume. However, OPG did not completely prevent the loss of cancellous bone induced by the presence of 5T2MM cells. This may suggest that treatment only partially inhibited the induction of osteoclast activity or that a proportion of the cancellous bone may have been lost before treatment with OPG. To establish which of these possibilities is more likely, we performed histologic examination of osteoclast numbers. These studies demonstrated that OPG treatment completed inhibited the development of TRAP-positive osteoclasts, suggesting that incomplete inhibition of osteoclast activity is unlikely to account for this discrepancy. This would therefore support the suggestion that a certain amount of cancellous bone had been lost before treatment with OPG. Indeed, it is likely that there would have to be a significant expansion of the tumor clone, and possibly associated cancellous bone loss, before it was possible to detect serum paraprotein. Treatment with OPG was also associated with preservation of total BMD at each of the 3 sites examined when compared with animals injected with tumor cells alone. Interestingly, BMD remained higher in the OPG-treated animals than in uninjected control animals. This suggests that OPG treatment was able to inhibit both the age-related bone loss that is seen in the control animals and the bone loss induced by the 5T2MM myeloma cells. Alternatively, this could reflect an effect of OPG on the growth-related accumulation of primary spongiosa at the growth plate, as is seen in young, rapidly growing animals.
In addition to inhibiting the development of myeloma bone disease, OPG treatment resulted in a small decrease in serum paraprotein. Although this was not statistically significant, this raises the possibility that OPG may have either direct or indirect antitumor effects. Further studies are therefore required to establish the effect of inhibiting bone resorption with OPG or other antagonists of the RANKL system on the growth and survival of myeloma cells in the bone marrow microenvironment.
The data presented in this study show that myeloma cells express RANKL, a factor critical in normal osteoclast formation. The presence of this molecule on myeloma cells raises the possibility that these cells may be able to stimulate osteoclastic bone resorption directly. Targeting the RANKL/RANK system with recombinant OPG, the soluble decoy receptor, is able to prevent the development of osteolytic bone disease in established myeloma. These observations suggest that OPG may represent a novel approach to the treatment of myeloma bone disease.
We thank Angelo Willems for expert technical assistance.
Supported by the Leukaemia Research Fund, the Medical Research Council (United Kingdom), the Fonds voor Wetenschappelijk (FWO)-Vlaanderen, Onderzoeksraad-Free University Brussels, the Vlaamse Internationale Samenwerkingsprojecten, and the British Council. P.I.C. is a Leukaemia Research Fund Bennett Senior Fellow and K.V. is a postdoctoral fellow of the FWO-Vlaanderen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter I. Croucher, Nuffield Department of Orthopaedic Surgery, University of Oxford, Nuffield Orthopaedic Centre, Headington, Oxford, OX3 7LD, United Kingdom; e-mail:peter.croucher@orthopaedic-surgery.oxford.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal