Abstract
The interferon (IFN)–inducible chemokines, specifically, IFN-γ–inducible protein-10 (IP-10), monokine induced by IFN-γ (Mig), and IFN-inducible T-cell α-chemoattractant (I-TAC), share a unique CXC chemokine receptor (CXCR3). Recently, the highly specific membrane-bound protease and lymphocyte surface marker CD26/dipeptidyl peptidase IV (DPP IV) was found to be responsible for posttranslational processing of chemokines. Removal of NH2-terminal dipeptides by CD26/DPP IV alters chemokine receptor binding and signaling, and hence inflammatory and anti–human immunodeficiency virus (HIV) activities. CD26/DPP IV and CXCR3 are both markers for Th1 lymphocytes and, moreover, CD26/DPP IV is present in a soluble, active form in human plasma. This study reports that at physiologic enzyme concentrations CD26/DPP IV cleaved 50% of I-TAC within 2 minutes, whereas for IP-10 and Mig the kinetics were 3- and 10-fold slower, respectively. Processing of IP-10 and I-TAC by CD26/DPP IV resulted in reduced CXCR3-binding properties, loss of calcium-signaling capacity through CXCR3, and more than 10-fold reduced chemotactic potency. Moreover, IP-10 and I-TAC cleaved by CD26/DPP IV acted as chemotaxis antagonists and CD26/DPP IV–truncated IP-10 and Mig retained their ability to inhibit the angiogenic activity of interleukin-8 in the rabbit cornea micropocket model. These data demonstrate a negative feedback regulation by CD26/DPP IV in CXCR3-mediated chemotaxis without affecting the angiostatic potential of the CXCR3 ligands IP-10 and Mig.
Introduction
Chemokines constitute a family of low molecular mass proteins that regulate the directed migration of specific subclasses of leukocytes during normal and inflammatory processes.1-3 The cellular specificity of chemokines is determined by the restricted expression of chemokine receptors on various leukocyte cell types.4 Chemokines are divided into subfamilies depending on the position of the first 2 cysteines in their primary sequence. The CC subfamily, with 2 adjacent cysteines, contains more than 20 different proteins that regulate the migration of monocytes, eosinophils, basophils, B and T lymphocytes, natural killer (NK) cells, and dendritic cells. The CXC chemokine subfamily, with 2 cysteines separated by one other amino acid, contains several proteins with a Glu-Leu-Arg (ELR) motif in front of the first cysteine. These ELRCXC chemokines all attract neutrophilic granulocytes to sites of inflammation. The CXC chemokines without an ELR motif can attract monocytes and B or T lymphocytes. Three of the known non-ELRCXC chemokines, specifically, interferon-γ (IFN-γ)–inducible protein-10 (IP-10 or CXCL10), monokine induced by IFN-γ (Mig or CXCL9), and IFN-inducible T-cell α-chemoattractant (I-TAC or CXCL11) recognize a single CXC chemokine receptor (CXCR), namely CXCR3.5-8 IP-10, Mig, and I-TAC attract monocytes and activated memory Th1, but not Th2, lymphocytes.9-11Furthermore, eosinophils and subclasses of B and NK cells express CXCR3.11,12 In addition to their role in leukocyte migration, chemokines play a role in angiogenesis.13-16CXCR2 is an important receptor for the angiogenic activity of ELRCXC chemokines.17,18 In contrast, the molecular mechanism underlying the angiostatic activity of the non-ELRCXC chemokines IP-10, Mig, and platelet factor 4 (PF-4 or CXCL4) is not completely understood.15 In addition to CXCR1 and CXCR2 (both receptors for angiogenic ELRCXC chemokines), CXCR3 (the receptor for the angiostatic chemokines IP-10 and Mig) has also been detected on microvascular endothelial cells.19 Recently, expression of CXCR3 on microvascular endothelial cells has been reported to be dependent on the cell cycle.20 Proliferating microvascular endothelial cells in the S/G2-M phase of their cell cycle express CXCR3. Moreover, Mig and IP-10 inhibited endothelial cell proliferation in vitro. These findings point to a possible down-regulatory role for CXCR3 in angiogenic processes.
The NH2-terminal region of most chemokines is crucial for receptor binding and signaling activities. Some chemokines become chemotactic only when processed at the NH2-terminus, for example, truncation of platelet basic protein (PBP) into NAP-2 (CXCL7).21 Others, for example, the monocyte chemotactic proteins MCP-1 (CCL2), MCP-2 (CCL8), and MCP-3 (CCL7), lose their chemotactic activity when NH2-terminal amino acids are cleaved off.22,23 Aminopeptidases or endopeptidases that process chemokines at the NH2-terminus play an important role in the up-regulation or down-regulation of chemokine activities. One of these enzymes, the membrane-associated protease dipeptidyl peptidase IV (DPP IV, EC3.4.14.5) is highly specific. It cleaves off dipeptides from polypeptides with a proline, alanine, or hydroxyproline at the second position. DPP IV, which is expressed on fibroblasts and epithelial and endothelial cells, is identical to the lymphocyte surface glycoprotein and T-cell activation marker CD26.24The extracellular domain of CD26/DPP IV also exists as a soluble and proteolytically active form in plasma and in cerebrospinal and seminal fluids. CD26/DPP IV interacts with CD45 (a protein tyrosine phosphatase) and with adenosine deaminase, has costimulatory activity in T-cell immune responses, plays a role in immune processes such as allograft rejection, suppresses malignant transformation, and has been implicated in the regulation of insulin secretion.24,25Recently, CD26/DPP IV was found to cleave a number of chemokines, but not cytokines.26 For example, removal by CD26/DPP IV of the 2 NH2-terminal amino acids from stromal cell–derived factor-1 (SDF-1 or CXCL12) resulted in significantly reduced chemotactic and calcium-signaling activity due to a decreased affinity for CXC chemokine receptor 4 (CXCR4).27 28 Accordingly, SDF-1(3-68) processed by CD26/DPP IV lacks antiviral activity against T-tropic human immunodeficiency virus type 1 (HIV-1) strains.
CD26 is highly expressed on Th1 cells and its expression is up-regulated by IFN-γ, a typical Th1 cytokine.29Moreover, one of the chemokine receptors that has been identified as a Th1 cell marker, in addition to CCR5, is CXCR3.9-11 Here we report that CD26/DPP IV efficiently cleaved all 3 CXCR3 ligands and demonstrate that the protease differently affects the receptor signaling, lymphocyte chemotactic, and antiangiogenic activities of these chemokines.
Materials and methods
Reagents and cell lines
Recombinant human chemokines, that is, IP-10, Met-IP-10 (IP-10 including an extra NH2-terminal methionine), Mig, and I-TAC and recombinant human interleukin-2 (IL-2) were purchased from PeproTech (Rocky Hill, NJ) or R & D Systems (Abingdon, United Kingdom). Natural IL-8 was purified to homogeneity from conditioned medium of monocytes and contained an equimolar mixture of IL-8(1-77), IL-8(5-77) and IL-8(6-77).21 Soluble natural human CD26/DPP IV, without membrane anchor and starting at amino acid Gly31, was obtained from total seminal plasma and purified to homogeneity by anion exchange followed by affinity chromatography on immobilized adenosine deaminase as described.30 Human CXCR3 was stably expressed in Chinese hamster ovary K1 (CHO-K1) cells (American Type Culture Collection [ATCC], Manassas, VA: CCL-61) and the cells were cultured in HAM F-12 medium (Biowhittaker Europe, Verviers, Belgium) supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, and 400 μg/mL geneticin.
In vitro truncation of chemokines by CD26/DPP IV
To obtain efficient truncation, chemokines were treated with soluble CD26/DPP IV for 18 hours at 37°C. Subsequently, they were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as previously described.31NH2-terminal truncation was verified by Edman degradation on a pulsed liquid phase 477A/120A or Procise 491cLC protein sequencer after electroblotting 0.5 to 2 μg processed chemokine from the gel to Problot membranes (Applied Biosystems, Foster City, CA). Alternatively, truncated chemokines were purified by C8 reverse-phase high-performance liquid chromatography (RP-HPLC) on a Brownlee Aquapore RP-300 column (50 × 1 mm; PerkinElmer, Norwalk, CT) and the averageMr was determined by electrospray ion trap mass spectrometry (Esquire-LC; Bruker Daltonic, Bremen, Germany).
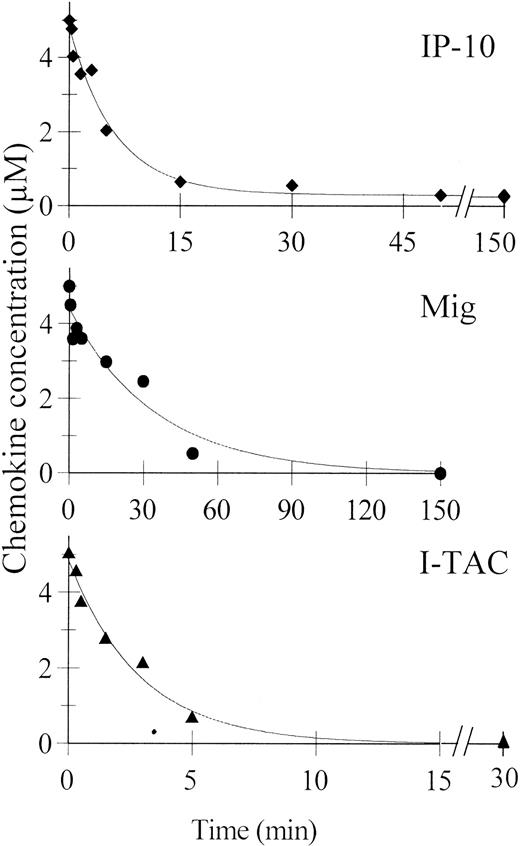
To determine the time course of the NH2-terminal truncation, IP-10, Mig, and I-TAC (5 μM) were incubated with soluble CD26/DPP IV (250, 25, 2.5, and 0.25 U/L) in 15 μL 50 mM Tris buffer, pH 7.5, supplemented with 1 mM EDTA. The specificity of the reaction was checked by incubating the chemokines with Tris buffer alone. Samples (5 μL) were withdrawn after 5, 15, and 30 minutes, and the reaction was stopped by addition of trifluoroacetic acid (final concentration of 0.1%). The samples were desalted on a C18 ZipTip (Millipore, Bedford, MA) and the relative amounts of the NH2-terminally truncated chemokines were determined by ion trap mass spectrometry. The time course of the truncation (shown in Figure 1) was constructed by normalizing the incubation times, taking into account that 25 U/L is close to the normal serum concentration of DPP IV.32
Time course of the truncation of CXCR3 ligands by CD26/DPP IV.
IP-10 (diamonds), Mig (circles), and I-TAC (triangles) at 5 μM were incubated for different time intervals with 25 U/L soluble CD26/DPP IV as indicated in “Materials and methods.” The remaining concentration of the intact CXCR3 ligands determined by mass spectrometry is indicated in the graphs.
Time course of the truncation of CXCR3 ligands by CD26/DPP IV.
IP-10 (diamonds), Mig (circles), and I-TAC (triangles) at 5 μM were incubated for different time intervals with 25 U/L soluble CD26/DPP IV as indicated in “Materials and methods.” The remaining concentration of the intact CXCR3 ligands determined by mass spectrometry is indicated in the graphs.
Chemotaxis assays
Peripheral blood mononuclear cells were purified from buffy coats from healthy volunteers as previously described.33Mononuclear cells were stimulated with anti-CD3 antibodies (OKT3: ATCC CRL-8001) in RPMI 1640 (Biowhittaker Europe) with 10% FBS for 2 days before use. Alternatively, mononuclear cells were cultured in RPMI 1640 with 10% FBS and treated with phytohemagglutinin (PHA; 2 μg/mL) for 3 days, washed with RPMI 1640, and kept in culture for 2 to 3 weeks in RPMI 1640 supplemented with 10% FBS and 50 U/mL IL-2 before use in the chemotaxis assay.7
Lymphocyte chemotaxis was performed in Boyden microchambers (Neuro Probe, Cabin John, MD) with fibronectin-coated, polyvinylpyrrolidone-free polycarbonate membranes (5-μm pore size, Corning Separations Division, Acton, MA). Lymphocytes were suspended in Hanks balanced salt solution (HBSS) plus 0.1% (wt/vol) human serum albumin (HSA) at 2 × 106 cells/mL and were allowed to migrate for 2 hours at 37°C. Before chemotaxis, CXCR3-transfected CHO cells were resuspended in HBSS plus 0.1% HSA and diluted to 1.5 × 106 cells/mL. Boyden chamber chemotaxis experiments were performed for 2 hours at 37°C with 8-μm pore size polyvinylpyrrolidone-free polycarbonate membranes. To study antagonism, truncated chemokines were added at inactive concentrations together with the active substance to the bottom well of the Boyden chambers. Cells that migrated through the membrane were stained with DiffQuick (Merck, Darmstadt, Germany) and counted microscopically in 10 oil immersion fields (× 500 magnification). The chemotactic index was calculated as the number of cells migrated to the sample divided by the number of cells spontaneously migrated to the sample dilution medium (HBSS + 0.1% HSA).
Calcium-signaling and receptor-binding assays
Alterations in intracellular calcium concentration ([Ca++]i) in response to chemokines were monitored by fluorescence spectrometry. Briefly, CXCR3-transfected cells were loaded with the fluorescent dye Fura-2-am for 30 minutes at room temperature as previously described.31Cells were washed with buffer containing 125 μM probenecid (ICN Biomedicals, Costa Mesa, CA), kept at 4°C, and preincubated for 10 minutes at 31°C before use. [Ca++]i were measured in an LS50B spectrofluorimeter (PerkinElmer) at a final cell concentration of 106 cells/mL in buffer containing 125 μM probenecid. During desensitization experiments, CD26/DPP IV–truncated CXCR3-ligands were first added at inactive concentrations followed by intact CXCR3 ligands. The increase in [Ca++]iafter desensitization with the truncated chemokines was compared with the increase in [Ca++]i after the addition of an equal amount of dilution buffer to calculate the percentage desensitization.
Competition for 125I-labeled I-TAC binding was measured on freshly isolated peripheral blood mononuclear cells or on CXCR3-transfected cells as described.34 Briefly, 2 × 106 cells were incubated for 2 hours at 4°C with 0.06 nM 125I I-TAC (Amersham Pharmacia Biotech, Uppsala, Sweden) and varying concentrations of unlabeled chemokine. Cells were centrifuged and washed 3 times with 2 mL phosphate-buffered saline (PBS) supplemented with 2% (wt/vol) bovine serum albumin (BSA) and the radioactivity present on the cells was measured in a gamma counter.
In vivo test for antiangiogenic activity of chemokines
Chemokines were tested for their angiogenic or angiostatic activity in the rabbit cornea micropocket model.16Briefly, 32 mg sucralfate (Merck) was dissolved in 72 μL PBS. Then, 4 μL of this sucralfate solution was mixed with 4 μL Hydron solution (12% Hydron in ethanol; Interferon Sciences, New Brunswick, NJ) and 5-μL pellets were allowed to dry under UV light for 20 minutes. Subsequently, different concentrations of chemokines or dilution buffer (negative control) were dried on the pellets. One pellet was implanted 1 mm from the limbus into a corneal micropocket of each eye of an anesthetized New Zealand white rabbit. Neovascularization of the cornea was scored daily from day 4 to day 8 after implantation of the pellet. Maximal neovascularization was obtained between days 5 and 7. This maximal neovascularization was used for comparison.
Results
Processing of CXCR3 ligands by CD26/DPP IV
The NH2-terminal sequence analysis on commercially available recombinant IP-10 from various companies revealed that most proteins contained an extra methionine at the NH2-terminus (Met-IP-10). Automated Edman degradation on Mig and I-TAC (derived from PeproTech or R&D Systems), and on IP-10 from a batch without NH2-terminal methionine (PeproTech), confirmed the correct NH2-terminus for these recombinant chemokines (Table1). Incubation of IP-10, I-TAC, and Mig with CD26/DPP IV resulted in the effective removal (> 95%) of the NH2-terminal dipeptide. After 18 hours of incubation with CD26/DPP IV, no remaining intact chemokine was detectable by Edman degradation. Proteolysis beyond the penultimate proline or proteolysis of Met-IP-10 was not observed, confirming the purity and specificity of CD26/DPP IV. For biologic evaluation, proteolytically cleaved CXCR3 ligands were purified by C8 RP-HPLC. The purity andMr of the cleaved chemokines were confirmed by mass spectrometry, which excludes carboxy-terminal or internal processing (Table 1).
NH2-terminal sequence analysis and mass spectrometry of CD26/DPP IV–treated chemokines
| Chemokine . | NH2-terminal sequence* . | Reduction inMr . | ||
|---|---|---|---|---|
| Untreated . | CD26/DPP IV–treated . | Theoretical . | Observed† . | |
| Met-IP-10 | MVPLSRTVRCTC | MVPLSRTVRCTC | 0 | 0 |
| IP-10 | VPLSRTVRCTC | LSRTVRCTC | 196.25 | 196.9 ± 0.4 |
| Mig | TPVVRKGRCSC | VVRKGRCSC | 198.22 | 197.7 ± 0.4 |
| I-TAC | FPMFKRGRCLC | MFKRGRCLC | 244.29 | 243.9 ± 0.3 |
| Chemokine . | NH2-terminal sequence* . | Reduction inMr . | ||
|---|---|---|---|---|
| Untreated . | CD26/DPP IV–treated . | Theoretical . | Observed† . | |
| Met-IP-10 | MVPLSRTVRCTC | MVPLSRTVRCTC | 0 | 0 |
| IP-10 | VPLSRTVRCTC | LSRTVRCTC | 196.25 | 196.9 ± 0.4 |
| Mig | TPVVRKGRCSC | VVRKGRCSC | 198.22 | 197.7 ± 0.4 |
| I-TAC | FPMFKRGRCLC | MFKRGRCLC | 244.29 | 243.9 ± 0.3 |
Single-letter amino acid abbreviations used.
The NH2-terminal sequence of untreated and CD26/DPP IV–treated (18-hour incubation) chemokines was determined by automated Edman degradation.
The average (± SD) difference between theMr of intact and truncated CXCR3 ligands was calculated from mass measurements on 4 different incubations (between 2 and 30 minutes) with CD26/DPP IV (25 U/L).
Mass spectrometry was used to study the time course of chemokine processing (Figure 1). When 5 μM IP-10 was incubated with serum concentrations (25 U/L) of CD26/DPP IV, 50% of the chemokine was truncated within the first 5 minutes and after 20 minutes less than 10% of the IP-10 proteins remained intact. In contrast, the kinetics of Mig processing were about 4 times slower (50% conversion in 20 minutes) than that of IP-10, whereas 50% and 90% of intact I-TAC was converted into I-TAC(3-73) within 1.5 and 6 minutes, respectively. Thus, CD26/DPP IV processed I-TAC more than 10-fold faster than Mig.
Impaired lymphocyte chemotactic activity of IP-10 after CD26/DPP IV cleavage
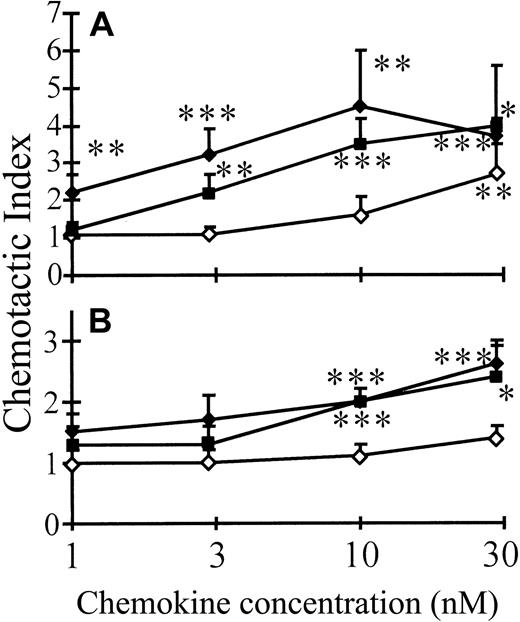
The chemotactic activity of Met-IP-10 and intact and CD26/DPP IV–truncated IP-10 was compared on activated (by PHA or anti-CD3) lymphocytes. A dose-dependent chemotactic effect was obtained with intact IP-10 from 1 nM onward on PHA-stimulated lymphocytes (Figure 2A). Compared to IP-10(1-77), about 3-fold higher concentrations of Met-IP-10 were required for a comparable chemotactic response. IP-10(3-77), however, was still inactive at concentrations as high as 10 nM. Thus, processing of IP-10 by CD26/DPP IV resulted in a 30-fold reduction in lymphocyte chemotactic activity. Similar results were observed with truncated compared to intact IP-10 on anti-CD3–activated lymphocytes (Figure2B), although these cells were less sensitive to IP-10–induced chemotaxis than PHA-stimulated lymphocytes. This observation correlates with the reported lower expression of CXCR3 on anti-CD3–stimulated cells.5
Effect of CD26/DPP IV on the lymphocyte chemotactic activity of IP-10.
The chemotactic activity of Met-IP-10 (filled squares), intact (filled diamonds), and CD26/DPP IV–truncated (open diamonds) IP-10 for PHA-stimulated (A) or anti-CD3–stimulated (B) lymphocytes was measured in the Boyden chamber assay. Results represent the mean (± SEM) chemotactic index of 4 or more independent experiments with cells from different donors. The Student ttest was used for statistical analysis (* = P < .1; ** = P < .05; *** = P < .01 for a significantly positive chemotactic response compared to buffer controls).
Effect of CD26/DPP IV on the lymphocyte chemotactic activity of IP-10.
The chemotactic activity of Met-IP-10 (filled squares), intact (filled diamonds), and CD26/DPP IV–truncated (open diamonds) IP-10 for PHA-stimulated (A) or anti-CD3–stimulated (B) lymphocytes was measured in the Boyden chamber assay. Results represent the mean (± SEM) chemotactic index of 4 or more independent experiments with cells from different donors. The Student ttest was used for statistical analysis (* = P < .1; ** = P < .05; *** = P < .01 for a significantly positive chemotactic response compared to buffer controls).
Effect of CD26/DPP IV on the chemotactic and calcium signaling capacities of IP-10, Mig, and I-TAC on CXCR3-transfected cells
Only one receptor for IP-10, that is, CXCR3, has been identified.6 Therefore, the chemotactic potencies of Met-IP-10, IP-10(1-77) and IP-10(3-77) were compared on CHO cells transfected with CXCR3 (Figure 3). For IP-10, a dose-dependent (minimal effective concentration of 3 nM) chemotactic response was observed, whereas for CD26/DPP IV–truncated IP-10(3-77) the minimal effective concentration (100 nM) was 30-fold higher. Even at 100 nM, Met-IP-10 failed to induce a significant chemotactic response. Intact Mig and I-TAC induced a significant chemotactic response on CXCR3-transfected cells at 10 nM (P < .01). In contrast, truncated Mig(3-103) and I-TAC(3-73) were inactive at concentrations as high as 100 nM. Although their NH2-terminal amino acid is different, removal of 2 amino acids (including the penultimate proline) resulted in a similarly impaired chemotactic activity of all 3 CXCR3 agonists.
Comparison of the chemotactic activity of intact and truncated chemokines on CXCR3-transfected cells.
Met-IP-10 (filled squares), intact (filled symbols), and CD26/DPP IV–truncated (open symbols) IP-10 (diamonds), Mig (circles), and I-TAC (triangles) were tested for their ability to induce a chemotactic response on CXCR3-transfected CHO cells. Results represent the mean (± SEM) chemotactic index of 4 or more independent experiments. The Student t test was used for statistical analysis (* = P < .1; ** = P < .05; *** = P < .01 for a positive chemotactic response compared to buffer controls).
Comparison of the chemotactic activity of intact and truncated chemokines on CXCR3-transfected cells.
Met-IP-10 (filled squares), intact (filled symbols), and CD26/DPP IV–truncated (open symbols) IP-10 (diamonds), Mig (circles), and I-TAC (triangles) were tested for their ability to induce a chemotactic response on CXCR3-transfected CHO cells. Results represent the mean (± SEM) chemotactic index of 4 or more independent experiments. The Student t test was used for statistical analysis (* = P < .1; ** = P < .05; *** = P < .01 for a positive chemotactic response compared to buffer controls).
Furthermore, it was observed that intact IP-10 and I-TAC induced a significant increase in [Ca++]i at concentrations higher than 0.3 nM, whereas 30-fold higher concentrations of Mig were required to obtain a detectable calcium response in CXCR3-transfected CHO cells (Figure4). Met-IP-10 was about 3-fold less potent compared to IP-10(1-77) and CD26/DPP IV–truncated IP-10 lacked the calcium-signaling capacity through CXCR3. Indeed, although intact IP-10 induced calcium mobilization at 1 nM, IP-10(3-77) was inactive in the calcium assay at concentrations as high as 60 nM. Truncation of I-TAC by CD26/DPP IV resulted in a 30- to 100-fold reduced calcium signaling capacity for this CXCR3 ligand. In contrast, no significant reduction in the weak calcium mobilizing capacity of Mig through CXCR3 could be observed for Mig(3-103). In conclusion, although none of the 3 ligands possessed CXCR3-mediated chemotactic activity after NH2-terminal truncation by CD26/DPP IV, Mig, but not IP-10 or I-TAC, retained its rather weak calcium-signaling potency through CXCR3.
Comparison of the calcium-mobilizing capacity of intact and truncated chemokines on CXCR3-transfected cells.
Met-IP-10 (filled squares), intact (filled symbols), and CD26/DPP IV–truncated (open symbols) IP-10 (diamonds), Mig (circles), and I-TAC (triangles) were tested for their ability to increase the [Ca++]i in CXCR3-transfected CHO cells. Results represent the mean (± SEM) increase of the [Ca++]i of 5 or more independent experiments. The detection limit for the increase of the [Ca++]i is indicated by the dashed line.
Comparison of the calcium-mobilizing capacity of intact and truncated chemokines on CXCR3-transfected cells.
Met-IP-10 (filled squares), intact (filled symbols), and CD26/DPP IV–truncated (open symbols) IP-10 (diamonds), Mig (circles), and I-TAC (triangles) were tested for their ability to increase the [Ca++]i in CXCR3-transfected CHO cells. Results represent the mean (± SEM) increase of the [Ca++]i of 5 or more independent experiments. The detection limit for the increase of the [Ca++]i is indicated by the dashed line.
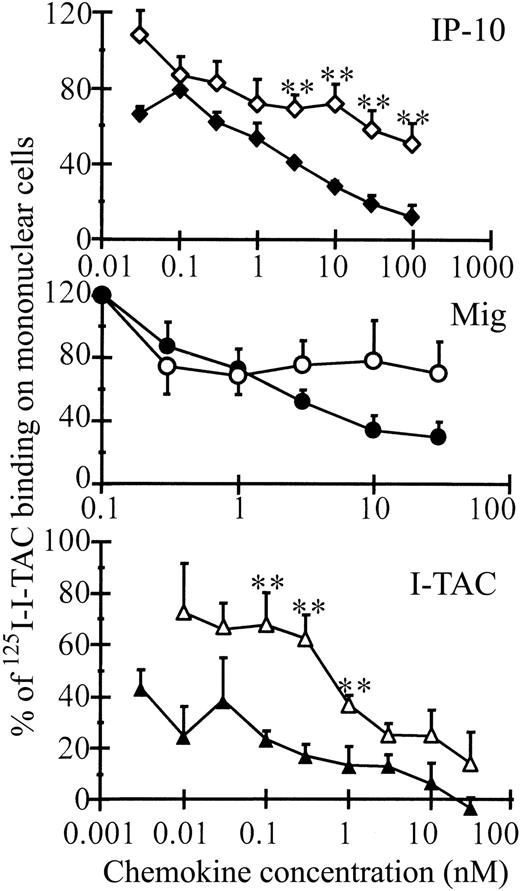
Binding properties of intact and CD26/DPP IV–truncated CXCR3 ligands
The capacity of truncated IP-10 to compete for binding of125I-labeled intact I-TAC to peripheral blood–derived mononuclear cells was significantly decreased (Figure5). 125I-labeled intact I-TAC was preferred over 125I-labeled intact IP-10 for the binding studies, because the commercially available125I-labeled IP-10 has the extra NH2-terminal methionine. At concentrations of 100 nM, IP-10(3-77) displaced only 49% of the labeled I-TAC from the cells, whereas the displacement with 100 nM intact IP-10 was 88%. A comparable difference in binding competition capacity with 125I-labeled I-TAC to mononuclear cells was observed between intact and truncated I-TAC. On CXCR3-transfected cells, the binding affinity of IP-10(3-77) and I-TAC(3-73) was also clearly reduced (Figure6). No significant difference in binding competition capacity to mononuclear cells was observed between both Mig forms although a tendency for reduced binding potency for the truncated Mig was observed. In addition, intact and truncated Mig could not significantly compete for 125I-I-TAC binding to CXCR3-transfected cells.
Receptor-binding properties of intact and CD26/DPP IV–truncated CXCR3 agonists on mononuclear cells.
Intact (filled symbols) and CD26/DPP IV–truncated (open symbols) IP-10 (diamonds), I-TAC (triangles), and Mig (circles) were tested for their ability to compete for 125I-labeled I-TAC binding to peripheral blood–derived mononuclear cells. Results represent the mean (± SEM) percent of 125I-I-TAC that binds to the cells compared to the amount of labeled I-TAC that binds the cells without addition of cold ligands (3 or more independent experiments). The Student t test was used for statistical analysis (** = P < .05 for a significant difference between the intact and truncated chemokines).
Receptor-binding properties of intact and CD26/DPP IV–truncated CXCR3 agonists on mononuclear cells.
Intact (filled symbols) and CD26/DPP IV–truncated (open symbols) IP-10 (diamonds), I-TAC (triangles), and Mig (circles) were tested for their ability to compete for 125I-labeled I-TAC binding to peripheral blood–derived mononuclear cells. Results represent the mean (± SEM) percent of 125I-I-TAC that binds to the cells compared to the amount of labeled I-TAC that binds the cells without addition of cold ligands (3 or more independent experiments). The Student t test was used for statistical analysis (** = P < .05 for a significant difference between the intact and truncated chemokines).
Receptor binding properties of intact and CD26/DPP IV–truncated CXCR3 agonists on CXCR3-transfected cells.
Intact (filled symbols) and CD26/DPP IV–truncated (open symbols) IP-10 (diamonds), I-TAC (triangles), and Mig (circles) were tested for their ability to compete for 125I-labeled I-TAC binding to CXCR3-transfected CHO cells. Results represent the mean (± SEM) percent of 125I-I-TAC that binds to the cells compared to the amount of labeled I-TAC that binds the cells without addition of cold ligands (3 or more independent experiments). The Studentt test was used for statistical analysis (** = P < .05 and *** = P < .01 for a significant difference between the intact and truncated chemokines).
Receptor binding properties of intact and CD26/DPP IV–truncated CXCR3 agonists on CXCR3-transfected cells.
Intact (filled symbols) and CD26/DPP IV–truncated (open symbols) IP-10 (diamonds), I-TAC (triangles), and Mig (circles) were tested for their ability to compete for 125I-labeled I-TAC binding to CXCR3-transfected CHO cells. Results represent the mean (± SEM) percent of 125I-I-TAC that binds to the cells compared to the amount of labeled I-TAC that binds the cells without addition of cold ligands (3 or more independent experiments). The Studentt test was used for statistical analysis (** = P < .05 and *** = P < .01 for a significant difference between the intact and truncated chemokines).
Calcium desensitization and chemotaxis antagonism with truncated CXCR3 ligands
The CXCR3-transfected CHO cells were used to investigate whether inactive CD26/DPP IV–truncated IP-10(3-77) and I-TAC(3-73) could desensitize calcium signaling through CXCR3. Addition of 1 nM IP-10(1-77) 0.3 nM I-TAC(1-73) or 50 nM Mig(1-103) to the CXCR3-transfected cells resulted in an increase in [Ca++]i of at least 100 nM (Figure 4). When 100 seconds prior to these stimuli, 10 nM of inactive IP-10(3-77) or 3 nM of inactive I-TAC(3-73) was added to the cells, the increase in [Ca++]i was reduced by 30% to 40% (Table2). Thus, truncated inactive CXCR3 ligands partially desensitize CXCR3 in calcium-signaling experiments.
Desensitization of CXCR3 by CD26/DPP IV–truncated chemokines
| First stimulus* . | Second stimulus . | Desensitization†(%) . |
|---|---|---|
| 10 nM IP-10(3-77) | 1 nM IP-10(1-77) | 37 |
| 0.3 nM IP-10(1-77) | 39 | |
| 50 nM Mig(1-103) | 34 | |
| 0.3 nM I-TAC(1-73) | 29 | |
| 3 nM I-TAC(3-73) | 0.3 nM IP-10(1-77) | 31 |
| 0.3 nM I-TAC(1-73) | 32 |
| First stimulus* . | Second stimulus . | Desensitization†(%) . |
|---|---|---|
| 10 nM IP-10(3-77) | 1 nM IP-10(1-77) | 37 |
| 0.3 nM IP-10(1-77) | 39 | |
| 50 nM Mig(1-103) | 34 | |
| 0.3 nM I-TAC(1-73) | 29 | |
| 3 nM I-TAC(3-73) | 0.3 nM IP-10(1-77) | 31 |
| 0.3 nM I-TAC(1-73) | 32 |
The first stimulus did not induce an increase of the intracellular calcium concentration.
Compared to CXCR3-transfected CHO cells that were treated with calcium buffer as a first stimulus (average percent desensitization of 2-4 independent experiments).
Because IP-10(3-77) and I-TAC(3-73) failed to induce chemotactic and calcium-signaling responses, but retained some receptor-binding properties, these truncated chemokines were tested as antagonists in chemotaxis assays on CXCR3-transfected cells (Figure7). Addition of an inactive concentration of IP-10(3-77) (30 nM) to the lower well of the Boyden chamber resulted in 70% reduction in chemotactic response toward 30 nM intact IP-10 and in complete inhibition of the chemotactic activity of 10 nM intact IP-10. However, truncated IP-10 failed to inhibit the chemotactic response toward comparable concentrations of intact I-TAC. In contrast, addition of truncated I-TAC(3-73) (30 nM) to intact I-TAC (10 nM or 30 nM) resulted in a 50% reduced chemotactic index.
Inhibition of chemotaxis by truncated IP-10(3-77) and I-TAC(3-73).
The antagonistic activity of CD26/DPP IV–truncated IP-10 and I-TAC was tested on CXCR3-transfected cells in the Boyden microchamber. Intact agonistic IP-10 or I-TAC (at 10 or 30 nM) was added with or without 30 nM truncated chemokine [IP-10(3-77) or I-TAC(3-73)] to the bottom well of the chemotaxis chamber. Results represent the mean (± SEM) percent inhibition of the chemotactic index (CI) toward intact chemokine alone from 4 to 5 independent experiments. The Studentt test was used for statistical analysis comparing the CI with and without addition of truncated chemokine (** = P < .05; *** = P < .01).
Inhibition of chemotaxis by truncated IP-10(3-77) and I-TAC(3-73).
The antagonistic activity of CD26/DPP IV–truncated IP-10 and I-TAC was tested on CXCR3-transfected cells in the Boyden microchamber. Intact agonistic IP-10 or I-TAC (at 10 or 30 nM) was added with or without 30 nM truncated chemokine [IP-10(3-77) or I-TAC(3-73)] to the bottom well of the chemotaxis chamber. Results represent the mean (± SEM) percent inhibition of the chemotactic index (CI) toward intact chemokine alone from 4 to 5 independent experiments. The Studentt test was used for statistical analysis comparing the CI with and without addition of truncated chemokine (** = P < .05; *** = P < .01).
Antiangiogenic activities of IP-10 processed by CD26/DPP IV
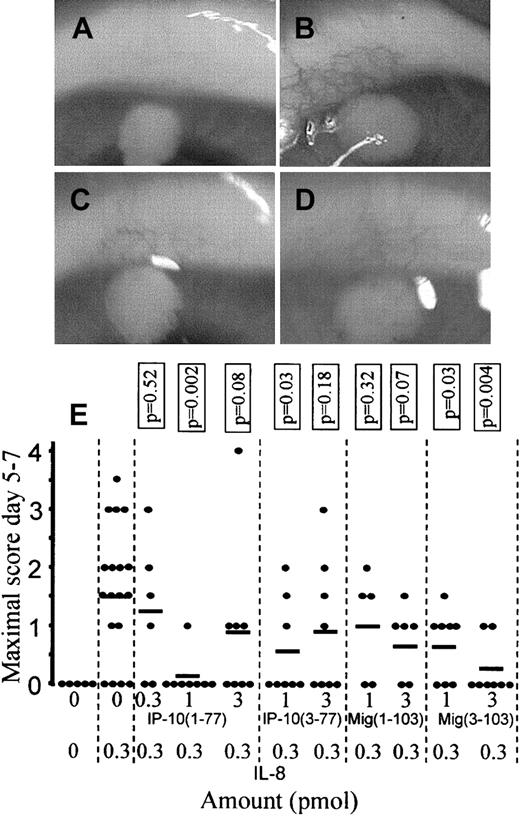
When pellets containing 0.3 pmol natural human IL-8 were implanted into corneal micropockets on rabbit eyes, the induced neovascularization was maximal between days 5 and 7. Maximal inhibition of the angiogenic effect of IL-8 (0.3 pmol) was obtained by addition of 1 pmol intact IP-10 (Figure 8). CD26-truncated IP-10(3-77) equally inhibited IL-8–induced angiogenesis. At 1 and 3 pmol/pellet, no significant differences (Mann-Whitney U test, P > .2) between the antiangiogenic activities of intact and truncated IP-10 were observed. Comparable results were obtained when intact Mig(1-103) or CD26-truncated Mig(3-103) were used as angiogenesis inhibitors. Both intact and truncated Mig inhibited IL-8–induced angiogenesis. These data indicate that, in contrast to the inflammatory effect (chemotaxis), the angiostatic potential of IP-10 and Mig is either not mediated through CXCR3 or implicates alternative CXCR3-triggered signal transduction pathways.
Comparison of the antiangiogenic activity of intact and CD26/DPP IV–truncated CXCR3 ligands.
Hydron pellets were implanted in a corneal micropocket on a rabbit eye. Hydron pellets contained dilution buffer (A), 0.3 pmol natural human IL-8 (B), 0.3 pmol natural human IL-8 and 1 pmol intact IP-10(1-77) (C), or 0.3 pmol natural human IL-8 and 1 pmol of IP-10(3-77) (D). Neovascularization was scored daily (score 0-4) from days 4 to 8 and the maximal neovascularization (occurring between days 5 and 7) obtained with IL-8 with or without intact or CD26/DPP IV–truncated IP-10 or Mig is shown in panel E (each dot represents an independent experiment). The mean values are indicated by the lines and statistical analysis (comparison with the positive control that contained 0.3 pmol IL-8) was performed using the Mann-Whitney U test. The photographs in panels A to D are representative examples for the mean neovascularization scores obtained with IL-8 and IP-10 as indicated in panel E.
Comparison of the antiangiogenic activity of intact and CD26/DPP IV–truncated CXCR3 ligands.
Hydron pellets were implanted in a corneal micropocket on a rabbit eye. Hydron pellets contained dilution buffer (A), 0.3 pmol natural human IL-8 (B), 0.3 pmol natural human IL-8 and 1 pmol intact IP-10(1-77) (C), or 0.3 pmol natural human IL-8 and 1 pmol of IP-10(3-77) (D). Neovascularization was scored daily (score 0-4) from days 4 to 8 and the maximal neovascularization (occurring between days 5 and 7) obtained with IL-8 with or without intact or CD26/DPP IV–truncated IP-10 or Mig is shown in panel E (each dot represents an independent experiment). The mean values are indicated by the lines and statistical analysis (comparison with the positive control that contained 0.3 pmol IL-8) was performed using the Mann-Whitney U test. The photographs in panels A to D are representative examples for the mean neovascularization scores obtained with IL-8 and IP-10 as indicated in panel E.
Discussion
During the past 2 decades, more than 50 human chemokines have been identified. Chemokines and chemokine receptors form a complex network that controls leukocyte migration during normal cell homing as well as inflammatory processes. The receptor specificity of chemokines determines the pattern of target cells. The expression of chemokines and chemokine receptors is regulated at the transcriptional level by different inducers. IFN-γ, a prototypic Th1 cytokine, has been reported to enhance the production of the non-ELRCXC chemokines IP-10, Mig, and I-TAC,7,35-38 but it down-regulates the production of neutrophil chemotactic chemokines such as the ELRCXC chemokines IL-8 and ENA-78.39 All 3 IFN-inducible non-ELRCXC chemokines interact with CXCR3. This receptor is highly expressed on activated memory T cells (CD45RO+ cells) and has been detected on a small portion of B cells and NK cells.5,11 Higher CXCR3 expression levels were detected on Th1 lymphocytes compared to Th2 cells and IP-10 has been reported to attract Th1 but not Th2 cells.9 Additional regulatory mechanisms of chemokine activity and receptor specificity were shown at the posttranscriptional level, mainly by NH2-terminal chemokine processing. For example, limited NH2-terminal truncation of IL-8 (eg, by gelatinase B) enhanced its chemotactic potency, whereas platelet basic protein was found to become chemotactic only when truncated into NAP-2.21,40 In contrast, small modifications of the NH2-terminal residues of MCP-1, MCP-2, and MCP-3 decreased the biologic activity of these CC chemokines.22 23
Recently, the highly specific protease CD26/DPP IV has been reported to process a number of chemokines at the NH2 terminus with different effects on their chemotactic and antiviral activities.24,26 CD26/DPP IV is expressed on a wide variety of cells including fibroblasts and epithelial and endothelial cells. Moreover, CD26/DPP IV expression on CD45RO+ Th1 lymphocytes is further increased during activation. This protease cleaves GCP-2, SDF-1, RANTES, eotaxin, macrophage-derived chemokine (MDC), and the macrophage inflammatory protein-1α (MIP-1α) isoform LD78β into NH2-terminally truncated forms.24,26 Although MCP-1, MCP-2, MCP-3, and MIP-1β also possess the prerequisite penultimate proline, they are not processed by CD26/DPP IV. MCPs are protected from the proteolytic activity by their NH2-terminal pyroglutamic acid.41 Here we report that CD26/DPP IV is able to efficiently cleave the NH2-terminal dipeptide of all 3 CXCR3 ligands, IP-10, Mig, and I-TAC. Time-course experiments showed that with physiologic serum concentrations of CD26/DPP IV, the majority of the chemokine is processed within a few minutes even when high concentrations of chemokine (5 μM) are present. This rapid processing of the CXCR3 ligands at physiologic concentrations of CD26/DPP IV (25 U/L) is indicative of an important functional role of the interaction between this ubiquitous protease and these chemokines. Recently, we compared the kinetic parameters for the truncation of different chemokines by CD26/DPP IV. IP-10 and I-TAC, together with the CXC chemokine SDF-1α and the CC chemokine MDC, were the best substrates for this protease with a half-life of less than 10 minutes at physiologic CD26/DPP IV concentrations.42
The chemotactic potency of the CXC chemokine SDF-1 and of the CC chemokines RANTES, eotaxin, and MDC was drastically reduced on NH2-terminal truncation by CD26/DPP IV.26Accordingly, the binding affinity and calcium-signaling capacity of truncated SDF-1, eotaxin, and MDC for their respective receptors CXCR4, CCR3, and CCR4 decreased. Moreover, inhibition of CD26/DPP IV resulted in prolonged protein kinase B activation by SDF-1 in T cells.43 In contrast, treatment of the CC chemokine LD78β with CD26/DPP IV generated a highly potent monocyte and lymphocyte chemoattractant that retained strong anti–HIV-1 activity.44 The limited NH2-terminal processing of IP-10 by CD26/DPP IV described here resulted in 30-fold reduced lymphocyte chemotactic activity. All 3 truncated CXCR3 ligands, that is, IP-10, Mig, and I-TAC, lost chemotactic activity mediated through CXCR3. The calcium-signaling capacity of IP-10 and I-TAC through CXCR3 was also abolished on NH2-terminal truncation. CD26/DPP IV–truncated IP-10 and I-TAC retained weak CXCR3-binding properties, but higher doses of the truncated variant versus intact chemokine were necessary to remove 125I-I-TAC from its receptor. Finally, on CXCR3-transfected cells, truncated IP-10 acted as a chemotaxis antagonist for intact IP-10 but not for intact I-TAC. In contrast, truncated I-TAC partially inhibited the chemotactic response toward intact I-TAC. These results are not surprising because I-TAC is known to be a stronger CXCR3 ligand that binds, in contrast to IP-10 and Mig, to both coupled and uncoupled CXCR3 receptors.45
So far, limited information is available on the role of CXCR3 in the angiostatic activity of IP-10. The reported expression of CXCR3 on microvascular endothelial cells is dependent on the cell cycle.19,20 Only proliferating, and not resting, microvascular endothelial cells are CXCR3+. However, no firm proof of the necessity of CXCR3 for the antiangiogenic effect of IP-10 and Mig has been given (eg, in receptor neutralization assays). Surprisingly, CD26/DPP IV–truncated IP-10 and Mig retained antiangiogenic activity in the rabbit cornea micropocket model. Consequently, CXCR3-mediated chemotactic activity and calcium signaling are no prerequisites for the angiostatic activity. Recently, also the murine, but not the human, CC chemokine 6C-kine (CCL21) has been reported to inhibit metastasis of human lung cancer cells in severe combined immunodeficient mice and to reduce tumor vascularity.46 Because murine, and not human, 6C-kine is a ligand for human CXCR3, these results suggest that CXCR3 is directly involved in the antiangiogenic effects of its ligands IP-10 and Mig. Thus, if CXCR3 is required for the antiangiogenic activity of IP-10, then the antiangiogenic and leukocyte chemotactic pathways use different signal transduction mechanisms. In fact, recent reports have shown that depending on the cell type multiple signal transduction pathways may be activated through CXCR3 including extracellular signal-regulated kinase (ERK), Src, and phosphatidylinositol-3 kinase (PI3K) signal cascades.47 48 Our results suggest that truncated IP-10 and Mig could activate some (those that are important for the antiangiogenic effect) but not all CXCR3 signal transduction pathways.
Because IFN-γ production (the main inducer of the CXCR3 ligands), CD26/DPP IV expression, and CXCR3 expression are hallmarks for activated memory Th1 cells, processing of the 3 chemokines by CD26/DPP IV may constitute an important physiologic regulatory mechanism during the attraction of leukocytes in a Th1 response. Indeed, in the case of a typical Th1 response, it is crucial to dampen the cellular reaction after influx of sufficient cells. Chemoattracted Th1 lymphocytes, laden with CD26/DPP IV, cleave the angiostatic chemokines IP-10, Mig, and I-TAC, and the angiogenic chemokine SDF-1α, resulting in down-modulation of their chemotactic activity. If the angiostatic effects would also be reduced, then the balance would be in favor of angiogenesis, which may bring the local vessels and the circulating leukocytes closer to the Th1 response site. Obviously, the latter effect does not occur and angiostasis thus helps to keep the circulating lymphocytes out of the field of attraction and to halt the inflammation. As a consequence, the balance between CD26/DPP IV activity and CXCR3 ligands may play an important role in disease processes such as multiple sclerosis,49-51 B-cell leukemia,52 hepatocellular carcinoma,53 and skin diseases36,54 55 in which disease expression of CXCR3 and its ligands have been reported.
The authors thank René Conings, Jean-Pierre Lenaerts, Michel Op de Beeck, and Willy Put for technical assistance. Fresh human buffy coats were kindly provided by the Blood Transfusion Centers of Antwerp and Leuven, Belgium.
Supported by the Fund for Scientific Research of Flanders (FWO-Vlaanderen), the Concerted Research Actions of the Regional Government of Flanders, the InterUniversity Attraction Pole initiative of the Belgian Federal Government (IUAP), and the BIOMED Program of the European Community. P.P., A.W., S.S., P.M., and J.N. hold fellowships of the FWO-Vlaanderen.
M.D. is employed by Euroscreen SA, whose product, CHO-K1 cells transfected with CXCR3, is studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul Proost, Laboratory of Molecular Immunology, Rega Institute for Medical Research, K. U. Leuven, Minderbroedersstraat 10, B-3000 Leuven, Belgium; e-mail:paul.proost@rega.kuleuven.ac.be.


![Fig. 4. Comparison of the calcium-mobilizing capacity of intact and truncated chemokines on CXCR3-transfected cells. / Met-IP-10 (filled squares), intact (filled symbols), and CD26/DPP IV–truncated (open symbols) IP-10 (diamonds), Mig (circles), and I-TAC (triangles) were tested for their ability to increase the [Ca++]i in CXCR3-transfected CHO cells. Results represent the mean (± SEM) increase of the [Ca++]i of 5 or more independent experiments. The detection limit for the increase of the [Ca++]i is indicated by the dashed line.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/13/10.1182_blood.v98.13.3554/6/m_h82411847004.jpeg?Expires=1763481957&Signature=MdByVWopmHz517VpX5iCWnAh16QHvZqw2Tj53vq7BGo72pb3TLDKq2WNIKG8IvCb90HoJsAoLpnSML8qwWTboqxcKf9MRv~znY0g~Vti1nBhwWn3bBQkFYKn76wGthIoGb3O0UoNOsR4HzQUze~Y8Oib6tU1IDzTUfJJ7uTp1IHNrAouFMvuA3HSJ0B43xrgOz76z1sjummxkjHKNjKtmrnEv6k4aR0UudpBYNiFeVcNSGdSYqrE~kRi4Zfxfg~386x6xyoE~UoGK-ItuUj014SNyAhfo6qJIJRA9P7EpqZH61-zLzvY8bpTJuYXH-u3rGifOBOgQThLYiSb3GL-WQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 7. Inhibition of chemotaxis by truncated IP-10(3-77) and I-TAC(3-73). / The antagonistic activity of CD26/DPP IV–truncated IP-10 and I-TAC was tested on CXCR3-transfected cells in the Boyden microchamber. Intact agonistic IP-10 or I-TAC (at 10 or 30 nM) was added with or without 30 nM truncated chemokine [IP-10(3-77) or I-TAC(3-73)] to the bottom well of the chemotaxis chamber. Results represent the mean (± SEM) percent inhibition of the chemotactic index (CI) toward intact chemokine alone from 4 to 5 independent experiments. The Studentt test was used for statistical analysis comparing the CI with and without addition of truncated chemokine (** = P < .05; *** = P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/13/10.1182_blood.v98.13.3554/6/m_h82411847007.jpeg?Expires=1763481957&Signature=0t7ICSkGz~nRaKXG31VdRTPbNArFYEkl1lAtKbafnoR9mnkpOvnqImUZmzq3hoLAG0I6hyfyCZLHu8AIU5FisrieI3ZIerGbPjU7-QkTpRnhX1Sw26DHHtMoCfvCTg4jWuCeOVnf9HRiVuE033Tp2il-o5zTHsR~yUMHJd5t6Pw71IDJueJnA5Fm8caSoE0NWwT1wp6umLi5LISnU~3Y1fwuWaPX1NiMnUVHvLr2cpyPYOnGhla3gv7eiik6s~1KvZoG6LTX6617RDnjWgFpLKpmXX2xZogyJHH3SyoXP6Bq8JViDA1aUMf4mpOEWKmywqcb21QD3tTrbkP~26N1cw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal